Abstract
To investigate the abnormal brain activities in the early stage of Parkinson’s disease (PD), the electroencephalogram (EEG) signals were recorded with 20 channels from non-dementia PD patients (18 patients, 8 females) and age matched healthy controls (18 subjects, 8 females) during the resting state. Two methods based on the ordinal patterns of the recorded series, i.e., permutation entropy (PE) and order index (OI), were introduced to characterize the complexity of the cortical activities for two groups. It was observed that the resting-state EEG of PD patients showed lower PE and higher OI than healthy controls, which indicated that the early-stage PD caused the reduced complexity of EEG. We further applied two methods to determine the complexity of EEG rhythms in five sub-bands. The results showed that the gamma, beta and alpha rhythms of PD patients were characterized by lower PE and higher OI, i.e., reduced complexity, than healthy subjects. No significant differences were observed in theta or delta rhythms between two groups. The findings suggested that PE and OI were promising methods to detect the abnormal changes in the dynamics of EEG signals associated with early-stage PD. Further, such changes in EEG complexity may be the early markers of the cortical or subcortical dysfunction caused by PD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a common neurodegenerative disorder, Parkinson’s disease (PD) presents with a series of symptoms and signs known as parkinsonism (Kalia and Lang 2015). In clinics, the symptoms of PD are predominantly characterized by motor dysfunction, such as gait disturbance, bradykinesia, rigidity, resting tremor, and postural instability (Kalia and Lang 2015; Valls-Solé and Valldeoriola 2002; Jankovic 2008). These symptoms could bring about serious disability and a reduction in the quality of life for the patients. More than that, most of PD patients may suffer from autonomic dysfunction, cognitive impairments or psychiatric disturbances (Moore et al. 2005; Lindgren and Dunnett 2012). Nowadays, about 7–10 million individuals worldwide suffers from PD, and most of them are over 50 years of age (Bowman et al. 2016). This degenerative disorder has become not only a medical but also a serious social problem.
The clinical diagnosis for PD is usually based on the patient history and physical examination. There is no standard diagnostic test for PD. Usually, the presence of both cardinal motor dysfunction and response to levodopa are features that support a diagnosis of PD. In this procedure, one of the challenges is to distinguish typical PD from the atypical parkinsonism (Darbin 2012), such as age-related parkinsonism or multiple system atrophy. That is because some of the cardinal signs of parkinsonism are not usually specific to PD. Especially, the motor symptoms are very easily confused with the bodily functions of normal aging, i.e., age-related parkinsonism (Darbin 2012; Kalia and Lang 2015; Valls-Solé and Valldeoriola 2002; Jankovic 2008; Savitt et al. 2006; Miller and O’Callaghan 2015). This makes an accurate diagnosis of PD difficult, particularly in the early stages of PD. Even if carefully examined, the diagnostic accuracy of typical PD in clinics is only around 80–90 % (Miller and O’Callaghan 2015; Gómez et al. 2011). Therefore, it requires new approaches to improve PD detection, especially in its early stages. This is important for the efficient treatment of such degenerative disorder of the nervous system.
Electroencephalogram (EEG) is a commonly used non-invasive technique, which is able to depict brain electric activity with high temporal resolution. The recorded EEG waves have been shown to reflect the functional state of cortical layers and their subcortical driving structures, especially the cortical dysfunction of some etiologies (Freeman and Quiroga 2013; Akar et al. 2015, 2016; Bowman et al. 2016; Keller et al. 2014). In clinical and basic research, EEG has been widely used to investigate the potential markers of neuropsychiatric disorders, including PD, schizophrenia, autism, major depressive disorder, Alzheimer’s disease, epilepsy, and so on (Miller and O’Callaghan 2015; Han et al. 2013; Gandal et al. 2012; Klassen et al. 2011; Hampel et al. 2010; Kheiri et al. 2012; Leuchter et al. 2009). Nowadays, it has become a common tool of auxiliary diagnosis used in neurology and psychiatry (Kalia and Lang 2015; Miller and O’Callaghan 2015; Freeman and Quiroga 2013), which is critical for the early intervention and timely treatment of relevant disorders.
Many studies have focused on the spectral analysis of brain activity in PD patients. The corresponding methods were usually linear measures. Because they belonged to the theory of random process, and the EEG signal was generally considered as a linear stochastic process in this case (Pezard et al. 2001). With spectral analysis, several pathological brain rhythms associated with PD have been identified, especially in beta band. By characterizing the local field potentials (LFPs) recorded in the subthalamic nucleus (STN) or globus pallidus internus (GPi), it was shown that the motor symptoms in PD patients were related to an increased activity in alpha band and more importantly in beta band (Beuter et al. 2014; Brown et al. 2004). By analyzing the resting EEG signals, it was found that there was a decrease of resting beta power (Stam et al. 1994; Pezard et al. 2001) and a remarkable increase in theta and low alpha powers in non-demented PD patients relative to controls (Stoffers et al. 2007; Tanaka et al. 2000). Our previous study (Han et al. 2013) found that such abnormalities in EEG sub-bands already occurred in the early stages of the disease. Further, a number of studies concluded that the most common abnormality of EEG in PD patients was the generalized slowing of brain activity. For instance, Serizawa et al (2008) showed that PD patients exhibited a diffuse slowing in their quantitative EEG in comparison to age-adjusted normal controls; Bosboom et al (2006) found that the PD was characterized by a slowing of resting-state brain activity involving theta, beta and gamma bands, and dementia in PD was associated with a further slowing of resting-state brain activity; Stoffers et al (2007) further confirmed that the slowing of oscillatory brain activity was a stable characteristic of PD without dementia. They also made clear that the widespread slowing of brain activity already occurred in the earliest clinical stages of untreated PD patients (Stoffers et al. 2007). Apart from spectral analysis, there were also investigations that focused on the functional interaction between brain regions. Here the brain was described as a complex network, and graph theory was used to characterize the structure and dynamics of relevant networks. With such methods, Stoffers et al (2008) observed that the increased resting-state functional connectivity in alpha range was a feature of PD from the earliest clinical stages, and the increases in theta or beta bands appeared later in the disease; Utianski et al (2016) recently found that the network alteration and breakdown was the robust attribute of the pathophysiology of PD cortical dysfunction.
Further, nonlinear analysis of brain activity has also revealed reliable information about the underlying dynamics related to cognitive process and brain pathology (Akar et al. 2015, 2016; Chenxi et al. 2016; Bhattacharya and Lee 2016; Gao et al. 2011). One common method was to characterize the complexity of the recorded neurosignals, since it carried important information about the cortical or subcortical dynamics (Akar et al. 2015, 2016; Chenxi et al. 2016). Such nonlinear methods were different from spectral analysis, since they belonged to information theory or dynamical system theory (such as, chaos or fractal). Thus, the complexity of neural signals was usually regarded as the result of nonlinear deterministic dynamics, possibly a chaotic process. With complexity measures, it has been offered valuable predictions about the abnormal dynamics in neurosignals produced by PD (Gómez et al. 2011; Stam et al. 1994, 1995; Darbin et al. 2013; Müller et al. 2001). For example, Stam et al (1994, 1995) used correlation dimension (D2) to investigate the dynamics of resting EEG in the patients with PD and dementia, and found a decreased complexity in both patient groups compared to controls. They also observed that the demented patients had significantly lower D2 and largest Lyapunov exponent (L1) compared to controls, and simultaneously L1 was lower in dementia than that in PD patients (Stam et al. 1995). Contrary to resting state, Müller et al (2001) found that PD patients during the performance of a complex motor task showed higher dimensionality than controls, which indicated more complexity was contained within the EEG recording. The calculation of above complexity measures, i.e., D2 or L1, often requires long, stationary and noiseless signals to obtain meaningful results. However, such condition is usually unable to be achieved for EEG data (Gómez et al. 2011; Eckmann and Ruelle 1992). Recently, Gómez et al (2011) applied another complexity measure, i.e., Lempel-Ziv complexity (LZC), to study the MEG activity of PD, and found that the early-stage PD patients showed lower complexity than controls for all MEG channels. Nevertheless, LZC is based on a coarse-grained measure of the recording. Then, it is possible that some important information from the recorded signal might have been lost with limited symbol conversions (Gómez et al. 2011). These earlier studies suggested that a reduced complexity in resting-state EEG/MEG may be associated with the cortical dysfunction produced by PD.
One alternative approach to determine the complexity of neurosignal is the measure based on the ordinal patterns of recorded times series. Two common methods are permutation entropy (PE) (Bandt and Pompe 2002) and order index (OI) (Ouyang et al. 2010). The former measures the unpredictability of time series (Li et al. 2010; Yi et al. 2014), while the latter measures the predictability of the series. That is, a recorded data with high complexity corresponds to a larger PE and lower OI, whereas a low complexity leads to a lower PE and larger OI. Earlier studies have shown that this kind of complexity measures is particularly suitable for non-stationary time series (Cao et al. 2004; Keller et al. 2014). They have been successfully applied to analyze epileptic and anesthetic EEG data (Ouyang et al. 2010; Cao et al. 2004; Keller et al. 2014; Li et al. 2007, 2008, 2010). In our previous study (Yi et al. 2014), we have also used them to detect the complexity changes of EEG activity associated with manual acupuncture at acupoint ST 36 in healthy subjects. These earlier studies demonstrate that the ordinal pattern based measures, i.e., PE or OI, offer some distinct advantages over classical complexity index. Such as, simplicity, robustness, fast calculation, artifact resistant, and invariance with respect to nonlinear monotonous transformations.
In this study, we examine the performances of both PE and OI in differentiating the complexity of EEG rhythms of early-stage PD patients from that of healthy controls. Our aim is to use EEG to detect the early signs of abnormal brain dynamics produced by this disorder. To our knowledge, this is the first study to apply two methods to investigate the abnormal EEG background activity in the early stages of PD. Based on previous studies mentioned above (Stam et al. 1995; Gómez et al. 2011), we hypothesized here that there would be a reduced PE and increased OI values, i.e., reduced complexity, in the patients in comparison with healthy controls.
Methods
Subjects
The whole recording procedure was performed with the approval of the local ethics committee. 18 patients with a clinical diagnosis of idiopathic PD (10 men, 8 women; mean age 61.1 ± 7.7 years) and 18 age- and sex-matched healthy subjects (10 men, 8 women; mean age 60.8 ± 8.6 years) participated in our study. All the subjects were right handed and without deficits in hearing. They were introduced to the nature of the investigation, and their written informed consent was obtained according to the declaration of Helsinki before EEG recording.
For PD patients, the disease diagnosis was based on the medical history, neurological and physical examinations as well as response to levodopa drugs. The exclusion criteria included atypical parkinsonism, neuroleptic drug use, antidepressants, dopamine blocking agents, alcohol abuse, presence of other neurological or psychiatric conditions, and any other severe illness. The mean duration of PD was 4 ± 2.2 years (range 1–7 years). The Hoehn and Yahr (H–Y) stage was 1–2. Specifically, 8 of them were in stage 1, 6 were in stage 1.5, and 4 were in stage 2. Their scores on the Mini-Mental-Status examination (MMSE) were 28.9 ± 1.7 (range 27–31), which were within normal limits. To reduce the heterogeneity in the medication, the PD patients were receiving levodopa drugs (i.e., Sinemet), and all of them withdrew from levodopa for at least 12 h before the study.
For control group, the age-matched subjects were healthy and intellectually, with no symptoms or history of any neuropsychiatric disorder. All of them were normal on neurological examination, and their scores of the MMSE were 29.3 ± 1.1 (range 28–31).
EEG recording
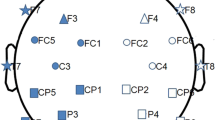
EEG recording was performed in a quiet and dimly illuminated room shielded from outer electrical or magnetic fields. The EEG data was recorded on a Neuroscan system using 20 Ag–AgCl electrodes set on the scalp according to the international 10–20 system. The position of relevant electrode was as shown in Fig. 1a. Linked ears were used as references, and EEG impedances were kept below 5 kΩ. The EEG signals were recorded at a sampling rate of 256 Hz and then bandpass filtered at 0.25–75 Hz.
The subjects were seated upright in a comfortable recliner with their eyes closed to attain a state of relaxed wakefulness. During the recording, all the subjects in either group were instructed and coached to be relaxed and alert. For each subject, the EEG data was recorded for 5 min. After that, 80 s (i.e., 20,480 data points) of multichannel EEG signals free of signs of muscle artifact, ocular movement and other artifacts were selected for further analysis by an experienced technician, who was blind to the diagnosis of PD. Figure 1b gives the examples of the raw EEG for channel C4 and F3 respectively recorded from PD patients and healthy controls.
Data analysis
PE and OI
PE was a common complexity measure for continuous time series, which was calculated by mapping signal onto a symbolic sequence. The process was summarized as follows. For a given scalar series \(\{ x_{1} ,x_{2} ,x_{3} , \ldots ,x_{i} , \ldots ,x_{N} \}\), a vector X i = [x(i), x(i + τ), …, x(i + (m − 1)τ] was formed by embedding the original series to a m-dimensional phase space. Here m was the embedding dimension and τ was the lag time. By arranging X i in decreasing or increasing order, we obtained \(m!\) possible permutations, which was indicated by π. Using \(p_{j} (j = 1,2, \ldots ,m!)\) to denote the probability distribution of permutation π j , the PE for this scalar series was defined as (Cao et al. 2004; Li et al. 2010; Yi et al. 2014)
Based on this definition, the relevant normalized PE of the series can be written as (Cao et al. 2004; Li et al. 2010; Yi et al. 2014)
This index was first proposed by Bandt and Pompe (2002). Like other entropies, PE was a measure of the unpredictability in the frequency distribution of a given time series (Li et al. 2010; Yi et al. 2014). The more regular the series, the smaller the PE was. Once all permutations had the same probability, the value of PE was 1. In this case, the relevant series was completely random.
OI was proposed by Ouyang et al (2010) to quantify the degree of the order of EEG signals. Given a recorded scalar series \(\{ y_{1} , \, y_{2} , \, y_{3} , \ldots , { }y_{i} , \ldots , \, y_{N} \}\), we used a simple algorithm to form a surrogate series \(\{ s_{1} ,s_{2} ,s_{3} , \ldots , { }s_{i} , \ldots ,s_{N} \}\) by randomly shuffling it (Dolan and Spano 2001). Then, all of the original data were included in a completely random order. Although the surrogate data exactly preserved the distribution of the original series, its ordinal patterns were destroyed. After that, we applied embedding procedure to both original and surrogate data to generate two vectors, which were Y i = [y(i), y(i + τ), …, y(i + (m − 1)τ] and S i = [s(i), s(i + τ), …, s(i + (m − 1)τ]. For vector Y i , we computed its probability distribution \(p_{y} (\pi_{i} ) \, (i = 1,2, \ldots ,m!)\) for permutation π i , and then re-sorted it in the order of descending frequency. In this way, a rank-frequency distribution \(p_{y} (\pi_{Ri} ) \, (i = 1,2, \ldots ,m!)\) was formed. The same process was performed on vector S i . Then, the OI of the original series was defined by (Ouyang et al. 2010)
Here p s (π Ri ) was the rank frequency of the surrogate data. To simplify the calculation of OI, Ouyang et al (2010) proposed to assume that each permutation had identical probability of occurrence in surrogate data, and simultaneously not to consider the finite length of the series. Under these conditions, the p s (π Ri ) in surrogate data was a uniform distribution. Then, Eq. (3) became
where \(p_{e} = \left\{ {\frac{1}{m!},\frac{1}{m!}, \ldots ,\frac{1}{m!}} \right\}\). Unlike PE, a large value of OI indicated that the time series contained more predictable information. When there were more random and unpredictable components in the recorded series, the relevant OI will be lower.
Before we performed complexity analysis on two groups, the moving window of size 4 s with a shift step of 1 s was used to partition the recorded EEG into blocks of data sets of short length 1024 points. To track the transient changes of complexity within the recording, we computed the PE and OI for each data subset. By taking the average PE and OI values over all the windows corresponding to each channel, we obtained two complexity measures per channel and subject.
Further, the calculation of each complexity measure was dependent on the embedding dimension m and lag τ (Ouyang et al. 2010; Cao et al. 2004; Keller et al. 2014; Li et al. 2008, 2010). In our previous study (Yi et al. 2014), we have systematically investigated how the values of dimension m, lag τ, and data length L influence PE and OI. It was shown that m = 5 and τ = 1 was suitable for length L = 1024 when detecting the changes of PE or OI of EEG series. We took these values in the following analysis.
Wavelet packet transform
We used wavelet packet transform (WPT) proposed by Coifman et al (1990) to extract five rhythms in relevant frequency band from the original EEG signals. The process of decomposition was a recursive filter operation, which was performed by a low-pass filter l(k) and a high-pass filter h(k). The result of decomposition formed a WPT tree of time series x(t) (Han et al. 2013; Sun and Chang 2002), as shown in Fig. 2 (here level is j = 3). Since the WPT contained the complete decomposition at each level, it was able to achieve a higher multi-resolution for non-stationary EEG data. Using filter l(k) and h(k), the wavelet packet function can be obtained by the following recursive relationships (Han et al. 2013; Sun and Chang 2002; Akansu and Haddad 2001);
Here ψ(t) was the so-called mother wavelet function. The recursive relations of the wavelet packet component signals between the jth level and the (j+1)th level can be written as (Han et al. 2013; Sun and Chang 2002; Akansu and Haddad 2001)
For more details about WPT, please see Akansu and Haddad (2001) or Sun and Chang (2002).
The EEG series recorded from two groups were decomposed by five levels in our study, and there were 32 frequency bands at the 5th level. The frequency resolution was estimated by (Han et al. 2013)
Since the sampling rate was \(f_{S} = 256{\text{Hz}}\) in our study, the relevant frequency resolution was \(\Delta f = 4{\text{Hz}}\). In this case, the range for each frequency band was f 1: [0 Hz, 4 Hz], f 2: [4 Hz, 8 Hz], f 3: [8 Hz, 12 Hz], f 4: [12 Hz, 16 Hz], ……, f 31: [120 Hz, 124 Hz], and f 32: [124 Hz, 128 Hz]. As a result, the delta band of the original EEG was f 1, the theta band was f 2, the alpha band was f 3, the beta band was f 4 − f 8, and the gamma band was f 9 − f 16.
Autoregressive burg method
To further appreciated whether the EEG rhythm extracted by WPT was in the frequency band of interest, we used autoregressive (AR) Burg method (Kay and Marple 1981) to determine the power spectrum of each EEG segment. This was a model-based method. For a given series y(n) (0 ≤ n ≤ N), its AR model can be expressed by
where q = 10 was the model order, which was determined by using Akaike information criterion (Akaike 1974). c i was the AR coefficient and δ(n) was the white noise with variance σ 2. We referred {c 1, c 2, c 3, …, c q , σ 2} as to AR parameters. The power spectral density (PSD) for y(n) was (Yi et al. 2013; Han et al. 2013).
We used Burg method to estimate the coefficients of the AR model. Then, the PSD estimation became (Yi et al. 2013; Han et al. 2013; Kay and Marple 1981; Kay 1988)
Here \(\hat{e}_{q}\) was the total least square error.
In the following study, the relative powers in delta, theta, alpha, beta and gamma bands were obtained by dividing the power in relevant sub-band by the total power.
Statistical analysis
The statistical analysis of the results was performed by a Mann–Whitney U-test to evaluate the differences between PD and control groups. For multiple comparisons, the Bonferroni correction was applied to the P values. The results were considered as significant at the level of P < 0.05/m. Here m was the factor of Bonferroni correction, which referred to the number of outcomes being tested.
Results
PE and OI of the EEG series for PD and control subjects
In this section, the complexity analysis was carried out separately for each EEG channel. Figure 3a, b respectively gave the values of PE and OI versus time for the sample EEG data shown in Fig. 1b. It was observed that channel C4 and F3 for PD patient were both characterized by lower PE and higher OI most of the time compared to healthy control. Figure 3c, d showed the statistical analysis of two measures over all the windows for either channel. It was indicated that the difference of PE or OI between two groups were both statistically significant (P < 0.05, Mann–Whitney U-test with Bonferroni correction).
a, b Respectively give the time course of PE and OI values of the EEG recorded at C4 and F3 channels (as shown in Fig. 1b). c, d Are the relevant PE and OI values of either channel averaged over all the windows. Standard errors are represented with vertical lines. Asterisk indicates that the difference between two conditions is significant (Bonferroni adjusted P < 0.05)
Figure 4 summarized the average PE and OI values estimated for 18 PD patients or 18 control subjects at all EEG channels. One can find that the PD patients displayed lower PE for all EEG channels and higher OI for most channels (except Fp2 and P3) compared to healthy controls, as shown in Fig. 4a. The differences of PE values between two groups were statistically significant for channel Fp1, Fp2, F3, F8, C3, Cz, C4, T5, P3, T6 and O2 (P < 0.05, Mann–Whitney U-test with Bonferroni correction). For OI values, the significant differences between two groups occurred at less channels (P < 0.05, Mann–Whitney U-test with Bonferroni correction), which were respectively F7, Fz, T3, C3, C4, Pz, P4 and O1. We also calculated the average values of applied complexity measure (i.e., PE and OI) over 20 EEG channels for either group. Figure 4b, c respectively gave the corresponding boxplots at the whole brain level for PE and OI values. One can find that the differences of PE and OI values between PD and control subjects were both statistically significant (P < 0.05, Mann–Whitney U-test with Bonferroni correction). These results revealed that the complexity of cortical EEG activity was reduced in the early-stage PD patients than that in control subjects.
a Topographical distribution of PE and OI values for PD patients (left) and healthy controls (center). The right panels show the significant differences of corresponding measure between two groups (Bonferroni adjusted P < 0.05), which are marked by blue dots. The label of each channel has been indicated in Fig. 1a. b, c Are the boxplots of the PE and OI values between two groups averaged over 20 channels. The notches in the box are a graphic confidence interval (95 %) about the median of a sample. The lower and upper lines of the “box” are the 25th and 75th percentiles of the sample, the distance between the top and bottom of the box is the interquartile range, and the line in the middle of the box is the sample median. Outliers (red plus sign) are cases with values that are more than 1.5 times the interquartile range. (Color figure online)
Complexity of five EEG rhythms for PD patients and control subjects
Here we applied two complexity measures to five EEG rhythms extracted by WPT, which were respectively in delta, theta, alpha, beta, and gamma bands. Figure 5 showed the sample EEG waveform in each sub-band decomposed from C4 channel as shown in Fig. 1b for two groups. The right panels gave the corresponding PSD of each rhythm estimated by AR Burg method. With PSD, we further identified the relevant rhythm in the left side was in the frequency band of interest.
a, b Give the examples of five EEG rhythms in gamma, beta, alpha, theta and delta bands for PD and control subjects. They are the component signals of the fifth-level WPT for the original EEG series recorded at C4 channel (as shown in Fig. 1b). The right panels are the PSD of relevant rhythm in the left side
Figure 6 summarized the average PE values of each EEG rhythm estimated for 18 PD patients and 18 healthy controls at all channels. The statistical results for average OI values were summed up in Fig. 7. The average number of EEG channels for PD group with decreased PE or increased OI in each sub-band was given in Table 1. Compared with controls, the gamma, beta and alpha rhythms displayed lower PE value and higher OI value at most EEG channels for PD patients. The differences of PE and OI in three sub-bands between two groups were statistically significant for some channels (P < 0.05, Mann–Whitney U-test with Bonferroni correction), which were marked by blue dots in the right panels of Figs. 6 and 7. This indicated that the complexity of gamma, beta and alpha rhythms was lower in PD patients than that in control subjects. However, there were no obvious and consistent differences between two groups for theta or delta rhythms. One can observe that for some EEG channels, the complexity measure (i.e., PE or OI) of either rhythm in PD patients was higher than controls, which will be lower for other channels. The significant differences between two groups occurred at very few channels.
The statistical results in Fig. 8 confirmed the conclusions shown in Figs. 6 and 7. Compared to controls, the gamma, delta and alpha rhythms for PD patients were characterized by reduced PE value and increased OI value at the whole brain level. The differences of relevant complexity measure between two groups were all statistically significant for three EEG rhythms (P < 0.05, Mann–Whitney U-test with Bonferroni correction). However, no obvious and statistical differences of either complexity measure between two groups were observed in theta or delta sub-bands.
Finally, we investigated the relation of the complexity of EEG rhythm to its power between conditions. Figure 9 showed the relative power in each sub-band at the whole brain level for either group. Compared with controls, an increase in the relative power of delta, theta and alpha bands was observed for the patients, while the relative power of beta or gamma bands was reduced. The differences of the relative power between two groups were statistically significant in theta, alpha and gamma bands (P < 0.05, Mann–Whitney U-test with Bonferroni correction). It was indicated that increasing the power of the EEG rhythm would not be associated with the increased complexity. Then, we were unable to predict a relation between the oscillation and the irregularity of EEG from our recorded data.
Discussion
Complexity is a common measure used to understand the nature of cortical dynamics. There have been several studies on the complexity of EEG/MEG signals in PD patients, whereas it is still a controversial issue. Pezard et al (2001) showed that the local entropy of EEGs was increased in PD patients comparatively to healthy controls. On the contrary, Stam et al (1994) found that the D2 and the L1 of EEG in PD patients were significantly lower than controls; Vaillancourt and Newell (2000) observed that the resting EMGs exhibited lower approximate entropy in PD patients compared to healthy subjects; Gómez et al (2011) reported that the LZC of the resting-state MEG from early-stage PD patients was lower than healthy controls. The last three studies are in accordance with our predictions obtained by PE or OI measures, which demonstrate that the resting EEG/MEG activities in PD patients present an overall loss of complexity compared to controls. Such predictions also provide further support for the proposal that a loss of complexity appears when the nervous system becomes functionally impaired (Stam et al. 1994, 1995; Akar et al. 2015, 2016; Chenxi et al. 2016; Gao et al. 2011; Darbin et al. 2013; Müller et al. 2001; Keller et al., 2014).
In addition to above literatures, there have also been studies on the complexity of EEG rhythms in different sub-bands. Chen et al (2010) used LZ estimator to show that the oscillatory activity in beta band had a reduced complexity. Our group previously found that the wavelet packet entropy in alpha or beta bands was both lower in PD patients than healthy controls. In present study, the results of PE and OI in alpha and beta bands are in accordance with these earlier findings. A reduced complexity of the oscillatory activity in beta band has been shown to be associated with the akinesia and rigidity of PD (Chen et al. 2010; Rosin et al. 2007). Then, the reduced PE or increased OI values in PD group observed by our recorded EEG data may be regarded as potential markers for the motor impairments of the patients.
It has been reported that the non-dementia PD patients displayed an increased synchronization in alpha and beta bands by resting-state EEG/MEG study, especially in alpha band (Palmer et al. 2010; Silberstein et al. 2005; Stoffers et al. 2008). The reduction of complexity in either sub-band observed in our study may underlie their increased synchronized oscillations. Further, the oscillatory activity of gamma band in basal ganglia has been hypothesized to play a pro-kinetic role and to contribute to movement generation (Brown 2003; Brown and Williams 2005; Crone et al. 1998). The abnormal changes in this sub-band have been suggested as a possible basis for the symptoms of bradykinesia and bradyphrenia in PD patients (Brown 2003; Jenkinson et al. 2013; Rowland et al. 2015). Then, the gamma band activity becoming more predictable in our study may be considered as a reflection of the reduced motor and the cognitive decline in PD patients. Although these interpretations are still speculative, they indicate that resting EEG background activity could be available for distinguishing PD patients from elderly healthy controls.
By using Mann-Whitney U-test with Bonferroni correction, we observed that the significant changes in complexity measures between two groups were limited to some specific brain regions, especially at C4, C3, and Cz channels. It is known that C3 and C4 channels are over sensorimotor cortex, and Cz channel is over supplemental motor area. The brain rhythms, such as beta, gamma, or alpha, recorded in these cortical areas are tightly related to the motor action and ability. The abnormities of the oscillatory activity in relevant frequency band have been shown to be associated with both the motor symptoms and the disease progression of PD (Marsden et al. 2001; Rowland et al. 2015). Then, the changes of PE or OI at C3 or C4 channels may be developed into a potential marker of the dyskinesia caused by PD. However, it should be noted that our complexity analysis of cortical activity is performed by a sensor-based approach, which makes the PE or OI results from different EEG channels highly correlated (Stoffers et al. 2007; Olde Dubbelink et al. 2013; Gómez et al. 2011). Then, the changes observed in the regions of sensor space may not directly reflect the physiological changes in relevant regions underlying the sensor. One alternative method for this issue is the source modeling technique (Stoffers et al. 2007; Olde Dubbelink et al. 2013), which allows one to analyze the distribution of reconstructed sources over the cortical areas.
There were no statistically significant differences of two complexity measures in delta or theta bands. However, several spectral studies with EEG/MEG (Olde Dubbelink et al. 2013; Stoffers et al. 2007; Neufeld et al. 1994; Han et al. 2013) have observed an increase in theta or delta powers for PD patients compared to controls. Our results of relative power in Fig. 9 are in accordance with these earlier predictions. Further, our group has used wavelet packet entropy to show that the complexity of delta and theta rhythms is both reduced in PD patients (Han et al. 2013). These findings suggest that the abnormities caused by PD also occur in two low-frequency rhythms even if we were unable to observe significant changes in the PE or OI values of EEG activities in either sub-band.
Finally, we included in the present study only 18 non-demented patients in the early stages of PD. The findings require further supports in a large clinical cohort of the patients. Meanwhile, the reduced complexity has been seen in many neurological disorders. Although the exclusion criteria in our study have included atypical parkinsonism, neuropsychiatric condition and any other severe illness, it is still required longitudinal study to identify whether the observed changes in PE or OI are specific to PD rather than age-related parkinsonism or multiple system atrophy. Moreover, it has been shown that the dopaminergic replacement therapy has long effects on sensory-motor circuitry (George et al. 2013; Herz et al. 2014). All the patients in our study have received levodopa drugs. Although they were off medication for at least 12 h before the recording, it is still unable to exclude the long term effects of treatments. In fact, earlier studies (Ziemann et al. 1997; Dalrymple-Alford et al. 1995) have shown that such drug does not appear to significantly affect electrophysiological indices as well as cognitive ability in the early stages of PD. Then, our predictions are more likely related to the effects of PD than drug.
Conclusion
The present work successfully applied PE and OI methods to detect the abnormalities in the complexity of resting EEG background activity in the early-stage PD patients. It was demonstrated that PD patients showed a widespread decrease in the complexity of cortical activities compared to controls. We also observed that the gamma, beta and alpha rhythms of the patients were characterized by a reduced complexity. These abnormities may be some very early and probably preclinical features of PD, which may be related to the presence of subtle cortical or subcortical dysfunction caused by the disease. The findings suggested that the ordinal pattern based complexity measures may have the potentials to become the markers of the dyskinesia and cognitive decline in PD. The following studies on a large clinical case of PD patients are necessary to confirm that the reduced complexity of resting-state cortical activity is indeed a stable feature for PD. Meanwhile, the longitudinal follow-up of our PD patients will enable us to further identify whether such markers of brain cortical dynamics are specific to the early PD.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Contr 19(6):716–723
Akansu AN, Haddad RA (2001) Multiresolution signal decomposition. Academic Press, Boston
Akar SA, Kara S, Agambayev S, Bilgic V (2015) Nonlinear analysis of EEGs of patients with major depression during different emotional states. Compute Biol Med 67:49–60
Akar SA, Kara S, Latifoglu F, Bilgic V (2016) Analysis of the complexity measures in the EEG of schizophrenia patients. Int J Neural Syst 26(2):1650008
Bandt C, Pompe B (2002) Permutation entropy: a natural complexity measure for time series. Phys Rev Lett 88(17):174102
Beuter A, Lefaucheur JP, Modolo J (2014) Closed-loop cortical neuromodulation in Parkinson’s disease: an alternative to deep brain stimulation? Clin Neurophysiol 125(5):874–885
Bhattacharya J, Lee EJ (2016) Modulation of EEG theta band signal complexity by music therapy. Int J Bifurc Chaos 26(1):1650001
Bosboom JL, Stoffers D, Stam CJ, van Dijk BW, Verbunt J, Berendse HW, Wolters ECh (2006) Resting state oscillatory brain dynamics in Parkinson’s disease: an MEG study. Clin Neurophysiol 117(11):2521–2531
Bowman FD, Drake DF, Huddleston DE (2016) Multimodal imaging signatures of Parkinson’s disease. Front Neurosci 10:131
Brown P (2003) Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord 18(4):357–363
Brown P, Williams D (2005) Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol 116(11):2510–2519
Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, Di Lazzaro V (2004) Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson’s disease. Exp Neurol 188:480–490
Cao Y, Tung W, Gao JB, Protopopescu VA, Hively LM (2004) Detecting dynamical changes in time series using the permutation entropy. Phys Rev E 70(4):046217
Chen CC, Hsu YT, Chan HL, Chiou SM, Tu PH, Lee ST, Tsai CH, Lu CS, Brown P (2010) Complexity of subthalamic 13–35 Hz oscillatory activity directly correlates with clinical impairment in patients with Parkinson’s disease. Exp Neurol 224(1):234–240
Chenxi L, Chen Y, Li Y, Wang J, Liu T (2016) Complexity analysis of brain activity in attention-deficit/hyperactivity disorder: a multiscale entropy analysis. Brain Res Bull 124:12–20
Coifman RR, Meyer Y, Quake S, Wickerhauser MV (1990) Signal processing and compression with wavelet packets. Yale University, New Haven
Crone NE, Miglioretti DL, Gordon B, Lesser RP (1998) Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain 121(Pt 12):2301–2315
Dalrymple-Alford JC, Jamieson CF, Donaldson IM (1995) Effects of selegiline (deprenyl) on cognition in early Parkinson’s disease. Clin Neuropharmacol 18(4):348–359
Darbin O (2012) The aging striatal dopamine function. Parkinsonism Relat Disord 18(5):426–432
Darbin O, Adams E, Martino A, Naritoku L, Dees D, Naritoku D (2013) Non-linear dynamics in parkinsonism. Front Neurol 4(4):211
Dolan KT, Spano ML (2001) Surrogate for nonlinear time series analysis. Phys Rev E 64(4 Pt 2):046128
Dubbelink KTO, Stoffers D, Deijen JB, Twisk JW, Stam CJ, Berendse HW (2013) Cognitive decline in Parkinson’s disease is associated with slowing of resting-state brain activity: a longitudinal study. Neurobiol Aging 34(2):408–418
Eckmann JP, Ruelle D (1992) Fundamental limitations for estimating dimensions and Lyapunov exponents in dynamical systems. Phys D 56(2–3):185–187
Freeman WJ, Quiroga RQ (2013) Imaging brain function with EEG: advanced temporal and spatial analysis of electroencephalographic signals. Springer, Heidelberg
Gandal MJ, Edgar JC, Klook K, Siegel SJ (2012) Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology 62(3):1504–1518
Gao J, Hu J, Tung W (2011) Complexity measures of brain wave dynamics. Cogn Neurodyn 5(2):171–182
George JS, Strunk J, Mak-McCully R, Houser M, Poizner H, Aron AR (2013) Dopaminergic therapy in Parkinson’s disease decreases cortical beta band coherence in the resting state and increases cortical beta band power during executive control. Neuroimage Clin 3:261–270
Gómez C, Olde Dubbelink KT, Stam CJ, Abásolo D, Berendse HW, Hornero R (2011) Complexity analysis of resting-state MEG activity in early-stage Parkinson’s disease patients. Ann Biomed Eng 39(12):2935–2944
Hampel WH, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J, Herholz K, Bokde AL, Jessen F, Hoessler YC, Sanhai WR, Zetterberg H, Woodcock J, Blennow K (2010) Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov 9(7):560–574
Han CX, Wang J, Yi GS, Che YQ (2013) Investigation of EEG abnormalities in the early stage of Parkinson’s disease. Cogn Neurodyn 7(4):351–359
Herz DM, Florin E, Christensen MS, Reck C, Barbe MT, Tscheuschler MK, Tittgemeyer M, Siebner HR, Timmermann L (2014) Dopamine replacement modulates oscillatory coupling between premotor and motor cortical areas in Parkinson’s disease. Cereb Cortex 24(11):2873–2883
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376
Jenkinson N, Kühn AA, Brown P (2013) Gamma oscillations in the human basal ganglia. Exp Neurol 245:72–76
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912
Kay SM (1988) Modern spectral estimation: theory and application. Prentice-Hall, New Jersey
Kay SM, Marple SL (1981) Spectrum analysis-a modern perspective. Proc IEEE 69:1380–1419
Keller K, Unakafov AM, Unakafova VA (2014) Ordinal patterns, entropy, and EEG. Entropy 16(12):6212–6239
Kheiri F, Bragin A, Engel JJ, Almajano J, Winden E (2012) Non-linear classification of heart rate parameters as a biomarker for epileptogenesis. Epilepsy Res 100(1–2):59–66
Klassen BT, Hentz JG, Shill HA, Driver-Dunckley E, Evidente VG, Sabbagh MN, Adler CH, Caviness JN (2011) Quantitative EEG as a predictive biomarker for Parkinson disease dementia. Neurology 77(2):118–124
Leuchter AF, Cook IA, Gilmer WS, Marangell LB, Burgoyne KS, Howland RH, Trivedi MH, Zisook S, Jain R, Fava M, Iosifescu D, Greenwald S (2009) Effectiveness of a quantitative electroencephalographic biomarker for predicting differential response or remission with escitalopram and bupropion in major depressive disorder. Psychiatry Res 169(2):132–138
Li XL, Ouyang GX, Richards DA (2007) Predictability analysis of absence seizures with permutation entropy. Epilepsy Res 77(1):70–74
Li X, Cui S, Voss LJ (2008) Using permutation entropy to measure the electroencephalographic effects of sevoflurane. Anesthesiology 109(3):448–456
Li D, Li X, Liang Z, Voss LJ, Sleigh JW (2010) Multiscale permutation entropy analysis of EEG recordings during sevoflurane anesthesia. J Neural Eng 7(4):046010
Lindgren HS, Dunnett SB (2012) Cognitive dysfunction and depression in Parkinson’s disease: what can be learned from rodent models? Eur J Neurosci 35(12):1894–1907
Marsden JF, Limousin-Dowsey P, Ashby P, Pollak P, Brown P (2001) Subthalamic nucleus, sensorimotor cortex and muscle interrelationships in Parkinson’s disease. Brain 124:378–388
Miller DB, O’Callaghan JP (2015) Biomarkers of Parkinson’s disease: present and future. Metabolism 64(3 Suppl 1):S40–S46
Moore DJ, West AB, Dawson VL, Dawson TM (2005) Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci 28:57–87
Müller V, Lutzenberger W, Pulvermüller F, Mohr B, Birbaumer N (2001) Investigation of brain dynamics in Parkinson’s disease by methods derived from nonlinear dynamics. Exp Brain Res 137(1):103–110
Neufeld MY, Blumen S, Aitkin I, Parmet Y, Korczyn AD (1994) EEG frequency analysis in demented and nondemented parkinsonian patients. Dementia 5(1):23–28
Ouyang G, Dang C, Richards DA, Li X (2010) Ordinal pattern based similarity analysis for EEG recordings. Clin Neurophysiol 121(5):694–703
Palmer SJ, Lee PW, Wang ZJ, Au WL, McKeown MJ (2010) θ, β but not α-band EEG connectivity has implications for dual task performance in Parkinson’s disease. Parkinsonism Relat Disord 16(6):393–397
Pezard L, Jech R, Růzicka E (2001) Investigation of non-linear properties of multichannel EEG in the early stages of Parkinson’s disease. Clin Neurophysiol 112(1):38–45
Rosin B, Nevet A, Elias S, Rivlin-Etzion M, Israel Z, Bergman H (2007) Physiology and pathophysiology of the basal ganglia-thalamo-cortical networks. Parkinsonism Relat Disord 13:S437–S439
Rowland NC, De Hemptinne C, Swann NC, Qasim S, Miocinovic S, Ostrem JL, Knight RT, Starr PA (2015) Task-related activity in sensorimotor cortex in Parkinson’s disease and essential tremor: changes in beta and gamma bands. Front Hum Neurosci 9:512
Savitt JM, Dawson VL, Dawson TM (2006) Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Investig 116(7):1744–1754
Serizawa K, Kamei S, Morita A, Hara M, Mizutani T, Yoshihashi H, Yamaguchi M, Takeshita J, Hirayanagi K (2008) Comparison of quantitative EEGs between Parkinson disease and age-adjusted normal controls. J Clin Neurophysiol 25(6):361–366
Silberstein P, Pogosyan A, Kühn AA, Hotton G, Tisch S, Kupsch A, Dowsey-Limousin P, Hariz MI, Brown P (2005) Cortico-cortical coupling in Parkinson’s disease and its modulation by therapy. Brain 128(Pt 6):1277–1291
Stam KJ, Tavy DL, Jelles B, Achtereekte HA, Slaets JP, Keunen RW (1994) Non-linear dynamical analysis of multichannel EEG: clinical applications in dementia and Parkinson’s disease. Brain Topogr 7(2):141–150
Stam CJ, Jelles B, Achtereekte HA, Rombouts SA, Slaets JP, Keunen RW (1995) Investigation of EEG non-linearity in dementia and Parkinson’s disease. Electroencephalogr Clin Neurophysiol 95(5):309–317
Stoffers D, Bosboom JL, Deijen JB, Wolters EC, Berendse HW, Stam CJ (2007) Slowing of oscillatory brain activity is a stable characteristic of Parkinson’s disease without dementia. Brain 130(Pt 7):1847–1860
Stoffers D, Bosboom JL, Deijen JB, Wolters ECh, Stam CJ, Berendse HW (2008) Increased cortico-cortical functional connectivity in early-stage Parkinson’s disease: an MEG study. Neuroimage 41(2):212–222
Sun Z, Chang CC (2002) Structural damage assessment based on wavelet packet transform. J Struct Eng 128(10):1354–1361
Tanaka H, Koenig T, Pascual-Marqui RD, Hirata K, Kochi K, Lehmann D (2000) Event-related potential and EEG measures in Parkinson’s disease without and with dementia. Dement Geriatr Cogn Disord 11(1):39–45
Utianski RL, Caviness JN, van Straaten EC, Beach TG, Dugger BN, Shill HA, Driver-Dunckley ED, Sabbagh MN, Mehta S, Adler CH, Hentz JG (2016) Graph theory network function in parkinson’s disease assessed with electroencephalography. Clin Neurophysiol 127(5):2228–2236
Vaillancourt DE, Newell KM (2000) The dynamics of resting and postural tremor in Parkinson’s disease. Clin Neurophysiol 111(11):2046–2056
Valls-Solé J, Valldeoriola F (2002) Neurophysiological correlate of clinical signs in Parkinson’s disease. Clin Neurophysiol 113(6):792–805
Yi GS, Wang J, Deng B, Wei XL, Han CX (2013) Modulation of electroencephalograph activity by manual acupuncture stimulation in healthy subjects: An autoregressive spectral analysis. Chin Phys B 22(2):028703
Yi GS, Wang J, Tsang KM, Chan WL, Wei XL, Deng B, Han CX (2014) Ordinal pattern based complexity analysis for EEG activity evoked by manual acupuncture in healthy subjects. Int J Bifurc Chaos 24(2):1450018
Ziemann U, Tergau F, Bruns D, Baudewig J, Paulus W (1997) Changes in human motor cortex excitability induced by dopaminergic and anti-dopaminergic drugs. Electroenceph Clin Neurophysiol 105(6):430–437
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 61372010, 61471265 and 61601320), the Tianjin Municipal Natural Science Foundation (Grant No. 13JCZDJC27900), the China Postdoctoral Science Foundation (Grant No. 2015M580202), and the Specialized Research Fund for the Doctoral Program of Higher Education (Grant No. 20130032110065).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yi, GS., Wang, J., Deng, B. et al. Complexity of resting-state EEG activity in the patients with early-stage Parkinson’s disease. Cogn Neurodyn 11, 147–160 (2017). https://doi.org/10.1007/s11571-016-9415-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-016-9415-z