Abstract
MicroRNAs (miRNAs) are a class of small, noncoding RNAs that play crucial roles in almost all cellular processes. As key post-transcriptional regulators of gene expression, miRNAs mainly induce mRNA degradation or translational repression. Recently computational and experimental studies have identified an abundance of motifs involving miRNAs and transcriptional factors (TFs). Here, we study the functional characteristics of one such motif, a two-node miRNA-mediated double negative feedback loop (MDNFL) in which a TF suppresses an miRNA and the TF itself is negatively regulated by the miRNA. Several examples of this motif are described from the literature. We propose a general computational model for the MDNFL based on biochemical regulations and explore its dynamics by using bifurcation analysis. Our results show that the MDNFL can behave as a bistable switch. This functional feature is in agreement with experimental observations of the widespread appearance of miRNAs in fate decisions such as differentiation during development. Importantly, it is found that under the interplay of a TF and an miRNA, the MDNFL model can behave as switches for wide ranges of parameters even without cooperative binding of the TF. In addition, we also investigate how extrinsic noise affects dynamic behavior of the MDNFL. Interestingly, it is found that when the MDNFL is in the bistable region, by choosing the appropriate extrinsic noise source, the MDNFL system can switch from one steady state to the other and meanwhile the production of either miRNA or protein is amplified significantly. From an engineering perspective, this noise-based switch and amplifier for gene expression is very easy to control. It is hoped that the results presented here would provide a new insight on how gene expression is regulated by miRNAs and further guidance for experiments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Living cells can continuously react to their environment with specific biochemical responses. At the core of this functionality are the interaction networks of biochemical molecules, such as genes, proteins, metabolites, and small molecules. Over the past decade, the structures of a great deal of such networks, including protein–protein interaction, metabolic, signaling, and transcription regulatory networks, have been mapped out. For the transcriptional regulation networks and protein–protein interaction networks of different organisms, large-scale statistical analysis has uncovered that these networks are far from random and contain significantly recurring nontrivial patterns of interconnections termed network motifs, such as negative autoregulation, feed-forward loops, and feedback loops (Shen-Orr et al. 2002; Lee 2002; Wuchty et al. 2003). In addition, several motifs of mixed interaction, including two types of interactions: those between transcription factors and their target genes and those between proteins, have also recently been found in integrated cellular networks of transcription regulation and protein–protein interactions (Yeger-Lotem and Margalit 2003; Yeger-Lotem et al. 2004). Understanding the functionality of these network motifs can help elucidate the design principle and provide insight into the behavior of regulatory networks. Previous work in transcriptional regulatory networks of several species has discovered that each motif has a specified structure and capacity to perform specific information-processing functions (Alon 2007).
Gene expression is under tight regulation at many levels (Shimoni et al. 2007). In the past, it was widely recognized that the regulation of gene expression is a task of regulatory proteins in all organisms. Thus, most research focused mainly on transcriptional and post-translational regulations. However, recent studies have implicated that microRNAs (miRNAs), a family of about 22-nucleotide small noncoding RNAs that regulate stability or translation of mRNA transcripts at the post-transcriptional level, may play crucial roles in modulating almost all cellular processes ranging from development and metabolism to apoptosis, signaling pathways, and diseases such as cancer (Ambros 2004; Bushati and Cohen 2007; Stefani and Slack 2008). As post-transcriptional gene suppressors, miRNAs regulate gene expression by base pairing to target mRNA molecules at conserved sites in the 3′ untranslated regions of the mRNAs, ultimately leading to a reduction in the levels of protein encoded by the target mRNAs (Filipowicz et al. 2008). Extensive evidence suggests that this suppression can occur by either translational repression or mRNA cleavage (Filipowicz et al. 2008; Bartel 2004; Valencia-Sanchez et al. 2006; Guo et al. 2010). In the former, miRNAs act as catalytic factors, preventing the initiation of translation, suppressing the production of proteins. In the latter, miRNAs act in a non-catalytic fashion, leading to the degradation of the target mRNA and the miRNA itself (Wang and Raghavachari 2011). Through either mechanism, miRNAs can keep gene products at extremely low copy numbers. The regulatory roles of miRNAs have been a subject of research for the last several years, both experimentally and theoretically (Shimoni et al. 2007; Wang and Raghavachari 2011; Levine et al. 2007; Levine and Hwa 2008; Mehta et al. 2008; Osella et al. 2011; Mitarai et al. 2009; Bumgarner et al. 2009; Iliopoulos et al. 2009). Although some of the miRNAs have been well studied, the mechanisms of various functions and biological significance of miRNAs are still not well understood (Bumgarner et al. 2009; Iliopoulos et al. 2009; Tsang et al. 2007; Shalgi et al. 2007; Re et al. 2009; Martinez et al. 2008; Martinez and Walhout 2009; Yu et al. 2008; Inui et al. 2010; Ivey and Srivastava 2010; Johnston et al. 2005; Kim 2007; Fazi 2005; Juan et al. 2009; Bracken 2008; Li and Carthew 2005; Visvanathan et al. 2007; Xu et al. 2009; Zhao et al. 2009; Pospisil 2011).
Recently several studies have shown that the transcriptional regulation by transcription factors (TFs) and post-transcriptional regulation by miRNAs are often highly coordinated (Shimoni et al. 2007; Tsang et al. 2007; Shalgi et al. 2007; Re et al. 2009; Martinez et al. 2008; Martinez and Walhout 2009; Yu et al. 2008; Inui et al. 2010; Ivey and Srivastava 2010). Of particular relevance is the accumulating evidence that the interplay of miRNAs and transcriptional regulators such as activators and repressors regulates key developmental events and cell fate decisions (Johnston et al. 2005; Kim 2007; Fazi 2005; Juan et al. 2009; Bracken 2008; Li and Carthew 2005; Visvanathan et al. 2007; Xu et al. 2009; Zhao et al. 2009; Pospisil 2011). These observations imply that the existence of considerable crosstalk between the transcriptional and post-transcriptional layers. Therefore, miRNA functions can be fully understood only by addressing TF and miRNA regulatory interactions together in “mixed” networks. Interestingly, recently computational and experimental studies have identified several recurrent network motifs contained in these mixed networks (Shimoni et al. 2007; Tsang et al. 2007; Shalgi et al. 2007; Re et al. 2009; Martinez et al. 2008; Martinez and Walhout 2009; Yu et al. 2008; Inui et al. 2010; Ivey and Srivastava 2010), such as miRNA-mediated single-input modules in which an miRNA regulates a group of target genes (Shimoni et al. 2007), and miRNA-mediated feedback and feed-forward loops (Tsang et al. 2007; Shalgi et al. 2007; Re et al. 2009; Martinez et al. 2008), as has been observed in transcriptional regulation networks of bacteria and yeast (Shen-Orr et al. 2002; Lee 2002). The simplest motif is a two-node miRNA-mediated double negative feedback loop (MDNFL) in which a TF suppresses an miRNA and the TF itself is negatively regulated by the miRNA.
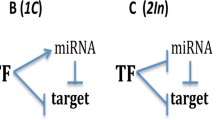
In this paper, we focus on this particular network motif, the MDNFL. The major motivation for this studying is that this specific motif has been reported in several recent experimental studies (Fazi 2005; Juan et al. 2009; Bracken 2008; Li and Carthew 2005; Visvanathan et al. 2007; Xu et al. 2009; Zhao et al. 2009; Pospisil 2011). For example, in human hematopoietic cells, NFI-A and miR-233 function in a double negative feedback loop to control granulocytic differentiation (Fazi 2005; Fig. 1a). In undifferentiated cells, miR-233 level is low and NFI-A level is high; however, upon retinoic acid signaling, miR-233 level increases due to the activation by the TF C/EBPa and NFI-A is then repressed, facilitating the differentiation to the myeloid lineage. This double negative feedback loop ensures mutually exclusive expression of miR-233 and NFI-A, thereby generating a bistable system, i.e., undifferentiated versus differentiated hematopoietic cells (Fazi 2005). Similarly, during skeletal muscle development, Ezh2 and miR-214 form a double negative feedback loop to regulate skeletal muscle cell differentiation (Juan et al. 2009; Fig. 1b). In undifferentiated myoblasts, Ezh2 is highly expressed and represses miR-214. Upon differentiation, MyoD/myogenin expression is activated and promotes transcription of miR-214, which in turn negatively regulates Ezh2 by inhibiting translation of its mRNA and thus reducing Ezh2 expression, ensuring complete differentiation of skeletal muscle cells (Juan et al. 2009). Moreover, in embryologic development, a double negative feedback loop between ZEB1-SIP1 and the miRNA-200 family controls epithelial-mesenchymal transition (Bracken 2008; Fig. 1c). In epithelial cells, a stable state is maintained by a high miR-200 level, which inhibits ZEB1/SIP1 and hence increases the expression of ZEB-repressed epithelial genes. The transition to a mesenchymal state can be induced by TGF-h, which increases the ZEB1-SIP1 level. A high ZEB1-SIP1 level in turn instigates and maintains a mesenchymal state through the repression of miR-200 by ZEB1-SIP1 (Bracken 2008). Likewise, in Drosophila eye development, the reciprocal repression between miR-7 and Yan ensures their mutually exclusive expression pattern: Yan is expressed in progenitor cells and miR-7 is expressed in photoreceptor cells (Li and Carthew 2005; Fig. 1d). The transition can be induced by the EGFR signaling, which transiently triggers Yan degradation. A decrease in Yan level relieves miR-7 from transcriptional repression, subsequently leading to the depletion of Yan in photoreceptor cells (Li and Carthew 2005). Moreover, during embryonic central nervous system development, a double negative feedback loop between the anti-neural REST/SCP1 and proneural miR-124 pathways contributes to a rapid and efficient transition of cellular phenotypes between neural progenitors and post-mitotic neurons (Visvanathan et al. 2007; Fig. 1e). In nonneuronal cells including neural progenitors, the REST/ SCP1 complex transcriptionally represses expression of miR-124 and other neuronal genes. As the REST level decreases during neurogenesis, miR-124 expression is derepressed, and subsequently, miR-124 post-transcriptionally suppresses multiple anti-neural factors including SCP1, resulting in further inhibition of the anti-neural pathway by REST/SCP1. This regulatory loop may represent key mechanisms to sense the intricate balance between proneural and anti-neural cues during development, to coordinate robust neuronal gene expression, and to confer neuronal identity in a timely manner (Visvanathan et al. 2007). In addition, upon initiation of embryonic stem cell differentiation, reciprocal inhibition of miR-145 and OCT4 contributes to irreversible silencing of self-renewal and pluripotency programs (Xu et al. 2009; Fig. 1f). Similar MDNFL motifs are also found in regulating the balance between neural stem cell proliferation and differentiation (Zhao et al. 2009; Fig. 1g), and PU.1-directed macrophage differentiation (Pospisil 2011; Fig. 1h). These experimental observations imply that this basic module may function as a bistable switch to regulate cell fate decisions such as differentiation during development. Actually, it has been shown that double negative feedback loops can act as bistable switches both experimentally and theoretically (Gardner et al. 2000; Ferrell 2002; Ferrell and Xiong 2001; Chatterjee et al. 2008). However, it is not clear whether the MDNFL can behave as a bistable switch. It is also not known the possible functions and biological significance of the miRNA in the MDNFL.
Biological examples of the MDNFL motif. a MDNFL controls granulocytic differentiation in human hematopoietic cells (Fazi 2005). b MDNFL regulates skeletal muscle cell differentiation during skeletal muscle development (Juan et al. 2009). c MDNFL regulates epithelial-mesenchymal transition during embryological development (Bracken 2008). d MDNFL promotes photoreceptor differentiation in the Drosophila eye (Li and Carthew 2005). e MDNFL contributes to a rapid and efficient transition of cellular phenotypes between neural progenitors and post-mitotic neurons during embryonic central nervous system development (Visvanathan et al. 2007). f MDNFL contributes to irreversible silencing of self-renewal and pluripotency programs upon initiation of embryonic stem cell differentiation (Xu et al. 2009). g MDNFL controls the balance between neural stem cell proliferation and differentiation (Zhao et al. 2009). h MDNFL regulates PU.1-directed macrophage differentiation (Pospisil 2011). The solid and dotted lines denote transcriptional and post-transcriptional regulations, respectively
To address these questions, we propose a general computational model for the MDNFL based on biochemical regulations and explore its dynamics by using bifurcation analysis. Our results show that the MDNFL can indeed behave as a bistable switch. This functional feature is in agreement with experimental observations of the widespread appearance of miRNAs in fate decisions during development. Importantly, it is found that under the interplay of a TF and an miRNA, the MDNFL model can behave as switches for wide ranges of parameters even without cooperative binding of the TF. It thus provides a novel mechanism to induce bistability through this combinatorial regulation even without cooperativity in the regulation. In addition, we also investigate how extrinsic noise affects dynamic behavior of the MDNFL. Interestingly, it is found that when the MDNFL is in the bistable region, the MDNFL system can switch from one steady state to the other and meanwhile the production of either miRNA or protein is amplified significantly by choosing the appropriate extrinsic noise source. From an engineering perspective, this noise-based switch and amplifier for gene expression is very easy to control because carefully tuning noise strength is not needed.
Deterministic model and bifurcation analysis for the MDNFL
Deterministic model for the MDNFL
In this paper, we are mainly interested in analyzing the dynamics of the MDNFL and see what can be achieved in the simplest setting. Thus, different cellular compartments and separate concentrations for the nucleus and cytoplasm are not considered and biochemical regulations are modeled by simple rate equations. The proposed MDNFL model is depicted schematically in Fig. 2. It involves two genes g a , g s , their corresponding transcripts, mRNA, miRNA, and a protein A encoded by g a . The protein A regulates transcription of gene g s and the miRNA base pairs with the mRNA to forms the complex C.
Extensive evidences suggest that the mechanisms of microRNA-mediated gene regulation can be translational repression or destabilization of its target mRNAs (Valencia-Sanchez et al. 2006), i.e., miRNAs can either decrease the rate of translation or increase the degradation of its target mRNAs. Here, we choose to model the effect of miRNA regulation by taking the degradation rate of the target mRNA as a function of miRNA concentration. However, we note that the alternative choice of a translation rate of the target mRNAs as a function of miRNA concentration does not qualitatively alter the results below. The time evolution of the concentrations of the species in the MDNFL can be described by the following four deterministic rate equations:
In the above equations, [g s ], [g s : A ], [M s ], [M a ], and [A], respectively, denote the concentration of free gene g s , gene g s with A bound to its promoter, miRNA, mRNA, and protein A. As in Francois and Hakim (2005), here the cellular volume is taken as volume unit and thus the concentrations and the number of the species are equivalent. It is assumed that there is only a single copy of gene g s in the cell, i.e., [g s ] + [g s : A] = 1 mol. Therefore, [g s ] can also represent the probability that gene g s is free without A bound to its promoter. Particularly, A proteins bind to the promoter of gene g s at a rate k θ and when bound they dissociate at a rate k −θ. The miRNA M s is transcribed at a rate k b when A binds to gene g s or at a rate k f otherwise. Thus, k b > k f corresponds to transcriptional activation by protein A and k b < k f to transcriptional repression, which will be focused on in this paper. Since regulation of gene g a is not considered, it is simply assumed that mRNA M a is produced at a given basal rate k a . The mRNA M a is translated into protein A at a rate k A . δ s , δ m , and δ A are the degradation rates for M s , M a , and A, respectively. γ is the base pairing rate of the miRNA with the mRNA. Here we assume that the base pairing is irreversible, consistent with the fact that the complex formed by miRNA and mRNA is extremely stable or rapidly degraded, and thus the complex needs not to be treated as a dynamical variable. Throughout this paper except if otherwise stated, we fix parameters as follows: \(k_{-\theta}=0.04 \, \hbox{min}^{-1}, k_{\theta}=0.001 \, \hbox{mol}^{-1} \hbox{min}^{-1}, k_a=4 \, \hbox{mol} \hbox{min}^{-1}, k_f=35 \, \hbox{mol min}^{-1}, k_b \,= \,2 \, \hbox{mol min}^{-1}, k_A=1 \, \hbox{min}^{-1}, \delta_s \,= \,0.006 \, \hbox{min}^{-1}, \delta_{\rm m} \,= \,0.04 \, \hbox{min}^{-1}, \delta_A=0.01 \, \hbox{min}^{-1}, \) and \(\gamma=0.02 \, \hbox{mol}^{-1} \hbox{min}^{-1}.\) In general, the range of each parameter value above is rather wide and here it is chosen to be typical in vivo value. It should be noted that all the above parameters were either directly taken from literature or estimated based upon analogous biological systems (Shimoni et al. 2007; Levine et al. 2007; Levine and Hwa 2008; Francois and Hakim 2005; Khanin and Vinciotti 2008; Aguda et al. 2008).
Steady states, bistable switches, and one-way switches
To probe the dynamics of the MDNFL, we first investigate how the steady-state value of [A] changes as a function of the different parameters in Eqs.(1)–(4) by performing bifurcation analysis. In the steady state, the rates of change of \(\frac{{\rm d} [g_s]}{{\rm d} t}, \; \frac{ {\rm d} [M_s]}{{\rm d} t}, \; \frac{{\rm d} [M_a]}{{\rm d} t},\) and \(\frac{{\rm d} [A]}{{\rm d} t}\) are zero. A system behaves as a bistable switch if it has two stable equilibria in the appropriate parameter regime. Figure 3a shows the bifurcation diagram of system (1)–(4) as a function of the free g s promoter transcription rate k f . The two saddle-node bifurcation points \(\hbox{SN}_1 (k_f\approx 11.828 \,\hbox{mol\,min}^{-1})\) and \(\hbox{SN}_2 (k_f\approx 67.799 \, \hbox{mol\,min}^{-1})\) enclose a bistable region. For any k f within this region, the system has two stable equilibria and one unstable equilibrium, which are represented by solid and dash-dotted lines, respectively. The two stable equilibria in the bistable region correspond to low and high values of [A], respectively. For convenience, we denote the low/high protein concentration as the off/on state. It can be seen that a region of bistability separates two regions of monostability, bistability exhibits only for intermediate k f values. The two stable state branches monotonically decrease with k f because the miRNA concentration increases with k f and thus the unpaired mRNA concentration decreases and further the production of the protein is reduced. At a specific value of k f in the bistable region, the choice between the stable equilibria is history-dependent, i.e., the final state depends on initial conditions (Ferrell 2002). If the value of k f is initially small, the system ends up in the on state. As k f increases along the upper stable branch, A remains to be in the on state until the bifurcation point \(\hbox{SN}_2\) is reached. When k f is further increased, the on state vanishes, and the system moves towards the lower stable branch, corresponding to the off state. Then, if we decrease k f , the system proceeds along the lower stable branch until \(\hbox{SN}_1\) is reached and another transition occurs, which brings the system back to the upper stable branch, an on state. The system therefore exhibits some hysteresis. Hysteresis is a property of bistable systems and of the potential importance in biological switching. First, it reduces the probability that a switch will repeatedly flip back and forth between two states when the bifurcation parameter is hovering near its threshold value, thereby providing an unambiguous threshold switching mechanism and buffering against the noise (Ferrell and Xiong 2001). Second, it provides a potential mechanism for biochemical memory (Zhang et al. 2007). During the maturation of Xenopus oocyte, for example, the p42 MAPK/Cdc2 system can keep a long-term memory of a transient differentiation stimulus (Xiong and Ferrell 2003). There are now several known systems in which bistability and hysteresis have been observed experimentally (Gardner et al. 2000; Xiong and Ferrell 2003; Acar et al. 2005; Ozbudak et al. 2004; Sha et al. 2003; Pomerening et al. 2003).
Biologically speaking, the above results indicate that when the free transcription rate of the miRNA lies outside the region enclosed by the two saddle-node bifurcation points, a smaller/larger free transcription rate will lead to a higher/lower protein concentration. When the free transcription rate lies in the region, however, a smaller/larger free transcription rate may lead to a lower/higher protein concentration, depending on the initial conditions. As the free transcription rate of the miRNA increases gradually from a small value, the protein concentration will decrease gradually but remain in high level until \(\hbox{SN}_2\) is reached, where it switches to a low level. After that, the protein concentration will stay in the low level unless the free transcription rate is reduced and exceeds \(\hbox{SN}_1.\) This is consistent with the experimental observations that in human hematopoietic cells, the increase of miR-233 level due to the activation by the TF C/EBPa upon retinoic acid signaling, similar to the increase of the free transcription rate, can facilitate undifferentiated hematopoietic cells where miR-233 level is low and NFI-A level is high differentiate to the myeloid lineage (Fazi 2005; Fig. 2a), and the experimental observations that during skeletal muscle development, the increase of miR-214 level promoted by MyoD/myogenin which is activated upon differentiation, ensure undifferentiated myoblasts where Ezh2 is highly expressed complete differentiation (Juan et al. 2009; Fig. 2b). Similarly, as the free transcription rate decreases gradually from a large value, the protein concentration will increase gradually but stay at low level until \(\hbox{SN}_1\) is reached, where it switches to a high level. After that, the protein concentration will remain in the high level if the free transcription rate is not increased.
The bifurcation diagram of system (1)–(4) with k b as a control parameter is shown in Fig. 3b. It indicates that as k b increases along the upper stable branch, A continues to be in the on state until some critical value, \(\hbox{SN}_2\approx 2.635 \, \hbox{mol\,min}^{-1},\) is exceeded, at which a discontinuous jump to the off state occurs and the system become monostable. Then, if k b decreases, A stays at the off state indefinitely, i.e., the transition is irreversible. Such kind of switches is termed irreversible or one-way switches (Tyson et al. 2003). The one-way switch is an extreme manifestation of hysteresis, i.e., its lower stable solution branches into the negative domain but is actually eliminated due to a physically meaningful restriction. Based on such hysteresis and irreversibility, bistable switches can make a reliable decision in many physiological processes, as in the yeast galactose-utilization network (Acar et al. 2005; Ramsey 2006), the MAPK/PKC signaling network (Bhalla and Iyengar 2001; Bhalla et al. 2002), and the p42 MAPK cascade in Xenopus oocytes (Ferrell and Machleder 1998).
Codimension two bifurcation diagrams of the MDNFL model. a k f and k b as the bifurcation parameters. b k f and k a as the bifurcation parameters. c k f and k A as the bifurcation parameters. d k f and γ as the bifurcation parameters. e k f and δ s as the bifurcation parameters. f k f and δ m as the bifurcation parameters. g k f and δ A as the bifurcation parameters. h k f and k −θ as the bifurcation parameters. The regions enclosed by the solid lines are bistable regions, outside of which the model is monostable
The effect of parameter variations on the bistable regime
In order to see whether the MDNFL model can behave as switches for wide ranges of parameters, the codimension two bifurcation diagram of system (1)–(4) is constructed for all the important parameters to determine the range of bistable regime. Figure 4a shows the bifurcation diagram of system (1)–(4) with k f and k b as the bifurcation parameters. It can be seen that the region of bistability becomes wider and the upper threshold of k f increases as k b decreases due to the fact that a larger k f is needed to compensate inefficiency of the miRNA level at a smaller k b so as to produce bistability. Figure 4b illustrates the bifurcation diagram of system (1)–(4) with k f and k a as the bifurcation parameters. It is shown that with increasing k a , the region of bistability becomes wider and the lower and upper thresholds increase moderately because a larger k f is required to compromise efficiency of the mRNA level at a larger k a in order to induce bistability. For a fixed k f , when we increase the basal transcription rate k a , the system undergoes a transition from a monomodal off state to a bistable region and then to a monomodal on state. This is consistent with the experimental observations that increasing the ZEB1-SIP1 level activated by TGF-h, similar to increasing k a , can induce a transition from a epithelial state maintained by a high miR-200 level and a low ZEB1-SIP1 level to a mesenchymal state where the ZEB1-SIP1 level is high and the miR-200 level is low (Inui et al. 2010; Bracken 2008; Fig. 2c). The bifurcation diagram of system (1)–(4) with k f and k A as the bifurcation parameters is shown in Fig. 4c. It can also be seen that with increasing k A , bistable regime becomes wider and the lower and upper thresholds increase moderately. In addition, decreasing the production rate k A from a large value for a fixed k f will shift the system from a monomodal on state, across a bistable region, to a monomodal off state. Figure 4d shows the bifurcation diagram of system (1)–(4) with k f and γ as the bifurcation parameters. It indicates that when there is no the negative post-transcriptional regulation, i.e., at γ = 0, the system is monostable. However, moderately increasing γ from zero can shift the system into bistable regime. Therefore, the negative regulation mediated by the miRNAs can induce bistability. In addition, the region of bistability becomes wider with increasing γ. A larger miRNA-mRNA base pairing strength γ drives the system to the off sate and so enlarges the bistable parameter range. Figure 4e shows the bifurcation diagram of system (1)–(4) with k f and δ s as the bifurcation parameters. With increasing k f , bistability emerges, depending on the values of δ s . In addition, the bistable regime becomes narrow while the lower and upper thresholds increase moderately with increasing δ s . For a fixed δ s , when we increase k f , the system undergoes a transition from a monomodal on state to a bistable regime and then to a monomodal off state. The bifurcation diagram of system (1)–(4) with k f and δ m as the bifurcation parameters is shown in Fig. 4f. Since decreasing the degradation rate of the mRNA δ m has similar effect with increasing the basal transcription rate of the mRNA k a , the region of bistability becomes wider and the upper threshold of k f increases as δ m decreases. While the value of δ m is fixed, increasing the value of k f from zero will shift the system from a monomodal on state, across a bistable region, to a monomodal off state. The bifurcation diagram of system (1)–(4) with k f and δ A as the bifurcation parameters is shown in Fig. 4g. By increasing the degradation rate of the protein δ A from a small value, the transition begins with a monomodal on state to a a bistable region then to a monomodal off state. This is consistent with the experimental observations that the degradation of protein Yan transiently triggered by the EGFR signalling, similar to the increase of δ A , can induce a transition from a progenitor cell state where Yan is expressed to a photoreceptor cell sate where miR-7 is expressed (Li and Carthew 2005; Fig. 2d). The parameter k −θ exhibits a similar influence as the parameter δ A . When we increase k −θ, the system undergoes a transition from a monomodal on state to a a bistable region then to a monomodal off state, as seen in Fig. 4h.
Results for external noise affecting k b with parameter values \(k_b=2.6\,\hbox{mol\,min}^{-1}, k_f=35\,\hbox{mol\,min}^{-1},\) and D = 0.75. a Bifurcation diagram for the concentration of protein A as a function of k b . The dotted vertical line is the parameter for which stochastic simulation was carried out. b Bifurcation diagram for the concentration of miRNA M s as a function of k b . The steady state with a high M s concentration is plotted in the dashed line and corresponds to the right Y axis. The other two states are in the solid line and dash-dotted line, respectively, and corresponds to the left Y axis. c The time evolution of the concentration of protein A. d The time evolution of the concentration of miRNA M s . Stable and unstable states are represented by solid (or dashed) and dash-dotted lines, respectively
In summary, the above codimension two bifurcation diagrams indicate that in the MDNFL model each parameter can be varied individually for a wide range, while preserving bistability. This means that the MDNFL model can behave as switches for wide ranges of parameters. Moreover, it should be pointed out that here we assume that the production of miRNA, M s , is regulated by protein A, through binding of a single protein A to its promoter, g s , that is, without cooperative binding of the TF to its promoter. In this case, the system is always monostable if the miRNA-mediated regulation does not exist (Ferrell and Xiong 2001; Lipshtat et al. 2006) [ also see Fig. 4d]. On the other hand, the miRNA-mediated regulation, if works solely, also leads to monostability (Levine et al. 2007). Interestingly, under the interplay of the TF and miRNA, it is found that the MDNFL model can behave as switches for wide ranges of parameters even without cooperative binding of the TF. It thus provides a novel mechanism to induce bistability through this combinatorial regulation even without cooperativity in the regulation, which is fundamentally distinct from the generation of bistability by intrinsically nonlinear positive feedback regulation, such as protein dimerization and cooperative formation of heterodimers (Ferrell 2002; Ferrell and Xiong 2001; Zhdanov 2009; Liu et al. 2011). The evidence for use of the MDNFL motif as switches has been reported recently, especially on cell fate decisions (Fazi 2005; Juan et al. 2009; Bracken 2008; Li and Carthew 2005; Visvanathan et al. 2007; Xu et al. 2009; Zhao et al. 2009; Pospisil 2011). It is hoped this novel mechanism can be realized in artificial genetic networks in the future.
Stochastic model and noise-induced dynamics for the MDNFL
The deterministic analysis above successfully demonstrates how the steady-state values of the MDNFL system shift as parameter values change. However, cellular processes at the molecular level are inherently stochastic (Balàsi et al. 2011). Numerous studies have revealed the importance of stochastic or probabilistic effects in biological systems where the number of molecules is low enough for noise to be important, and the deterministic chemical kinetic descriptions are limited (Lipshtat et al. 2006; Hasty et al. 2000; Vilar et al. 2002; Rao et al. 2002; Wang et al. 2007; Li and Li 2008). In addition, noise can also have significant effects on the stability and synchronization of genetic regulatory networks as well as neuronal networks (Shi et al. 2008; Wang et al. 2009; Guo 2011; Wu et al. 2012). There are two sources of noise in biological systems: intrinsic noise and extrinsic noise. Intrinsic noise originates from discrete nature of biochemical events such as transcription, translation, multimerization, and protein/mRNA decay processes (Raj and van Oudenaarden 2008). On the other hand, extrinsic noise results from perturbations in the extrinsic environment or the random variations in one or more of the externally set control parameters, such as the rate constant of the transcription of a specific gene (Hasty et al. 2000). In addition, extrinsic and intrinsic noise often exist in biological system simultaneously. In this section, we mainly focus on how extrinsic noise affects the dynamic behavior of the MDNFL. The effect of intrinsic noise on the dynamic behavior of the MDNFL will be analyzed systematically elsewhere.
Stochastic model for the MDNFL
As shown in Fig. 2, the two transcription rate of gene g s , k b and k f , influence the dynamics of the MDNFL. Although the transcription is described as a single biochemical reaction in our model, it actually consists of a complex sequence of reactions (Hasty et al. 2000). It is natural to assume that this part of the gene regulatory sequence is likely to be affected by fluctuations of many internal or external parameters, and it is interesting to study the effect of such noise on the dynamics of the MDNFL. In order to introduce such extrinsic noise into system (1)–(4), we adapt an approach in the spirit of Ref. (Hasty et al. 2000). We vary the transcription rates by allowing the parameters k b and k f in system (1)–(4) to vary stochastically, that is, \(k_b\rightarrow k_b+\xi_1(t)\) and \(k_f\rightarrow k_f+\xi_2(t).\) In such a manner, we obtain the following stochastic model:
where ξ1(t) and ξ2(t) are random terms with zero means \(\langle\xi_1(t)\rangle=0\) and \(\langle\xi_2(t)\rangle=0.\) In order to encapsulate rapid random fluctuations, we make the standard requirement that the autocorrelation be “δ-correlated”, i.e., the statistics of ξ i (t) are such that \(\langle\xi_i(t) \xi_j(t')\rangle=2D\delta_{ij} \delta(t-t'),\) where δ ij is the Kronecker delta, δ(t − t′) is the Dirac function and D is the constant that characterizes the strength of the perturbation. The above stochastic model is numerically simulated using the Euler–Maruyama scheme with the integration time step \(\Updelta {\rm t}=0.0001\) (Higham 2001).
Noise-induced switching and amplifying
To obtain the qualitative effect of the external noises on the dynamics of system (1)–(4), we first use the bifurcation diagram to anticipate the effect of fluctuations allowed in the transcription rates. For the deterministic system, the steady-state values of the concentration of protein A and miRNA M s for different values of parameter k b are shown in Fig. 5a and b. It can be seen that the system undergoes a saddle-node bifurcation at \(k_b\approx2.635 \, \hbox{mol\,min}^{-1}.\) For the values of k b larger than the saddle-node bifurcation point, the system has one unique stable equilibrium with low A and high M s concentrations, whereas for other values of k b , the system has three equilibria: one is unstable and the other two stable, where one stable equilibrium corresponds to low A and high M s concentrations, and the other corresponds to high A and low M s concentrations. In the absence of noise, the concentrations of protein A and miRNA M s will converge to one of the two stable equilibria completely determined by the initial state of the system. A comparison of Fig. 5a and b shows that the upper stable branch in Fig. 5a is steep, while the lower stable branch in Fig. 5b is flat. Therefore, we can anticipate that when the system is in the bistable region and has high A and low M s concentrations, if the parameter k b varies, the variations in the concentration of protein A will be notably large, while the fluctuations in the concentrations of miRNA M s will be small. If the system lies in the other stable equilibrium with low A and high M s concentrations, however, only a mild change in both the concentrations of A and M s takes place due to the flat lower stable branch in Fig. 5a and the flat upper stable branch in Fig. 5b.
In order to verify the above observation quantitatively, we next incorporate extrinsic noise to make k b stochastically vary and investigate the temporal behavior of the system under the fluctuations. We simulated the stochastic model for the MDNFL [Eqs. (5)–(8)] with a large noise intensity, D = 0.75. Initially, we fix the parameter value \(k_b=2.6 \, \hbox{mol\,min}^{-1},\) as indicated by a broken vertical line in Fig. 5a, such that the system is in the bistable region. The simulation results beginning with the concentration of protein A equal to its upper value of approximately 2,000 and miRNA M s concentration about 0 are presented in Fig. 5c and d. At first, the fluctuations in the concentration of protein A are quite large while the concentration of miRNA M s is nearly unchanged as we expected. Then, at around 5,500 min, the concentration of A quickly drops to the lower value. Meanwhile, the concentration of M s quickly jumps to its upper value and remains in there with quite small variations, as anticipated. This phenomenon indicates that the extrinsic noise introduced into the transcription rate is sufficient to induce the system to switch from one stable state to the other by crossing the unstable state. When the number of miRNA M s is randomly fluctuated to a comparable value with that of mRNA M a , most of the mRNA molecules are expected to base pair rapidly with the miRNA molecules and degraded, which will result in a low expression of protein A and thus leave the gene g s unregulated. In this case, the concentration of miRNA M s will soon build up to a rather high values if k f is large enough, i.e., the transition occurs.
On the other hand, a large fluctuation in the concentration of protein A before the switching implies that the amount of the protein is quite sensitive to the variation in the parameter k b if much more protein A exists in the system than miRNA M s . But after the fast transition occurs at around 5,500 min, flat curves in both Fig. 5c and d means that the other steady state is nearly unaffected by the fluctuations in the value of k b and thus is impossible to switch back unless noise intensity increases. This suggests that dynamic behavior of the MDNFL can be either sensitive or robust to the same perturbation affecting k b depending on the specific state at which the MDNFL initially stays (Stelling et al. 2004), and under such perturbation with large enough strength, the MDNFL is very likely to switch to or remain in the robust state with low A and high M s concentrations. It is also noticed that in the fast transition, the concentration of miRNA M s increases abruptly by over three orders of magnitude in a very short time if k f is large enough. This character suggests that the extrinsic noise affecting k b might be used to amplify miRNA M s ’s production.
Similar results can be obtained for the extrinsic noise affecting k f when the value of the parameter k f is initially set to \(12 \, \hbox{mol\,min}^{-1}\) except that the state with high A and low M s concentrations that is sensitive to the fluctuations in k b discussed above is robust to the random variation of k f , and the other state with low A and high M s concentrations which is robust to the fluctuations in k b is sensitive to the random variation of k f , as suggested in Fig. 2a. As a result, if the MDNFL is affected by the perturbation affecting k f , contrary to the above result, the MDNFL is very likely to switch to or remain in the state with high A and low M s concentrations. Likewise, the concentration of protein A may increase significantly during the switching process. This interesting feature of the MDNFL allows us to obtain the desired state switch and amplifying in the MDNFL easily by choosing the appropriate extrinsic noise source. From an engineering perspective, this character makes this type of switch easy to control because accurately tuning the strength and timing of noise has been done in Hasty et al. (2000) is not needed here.
Discussion and conclusion
The importance of post-transcriptional regulation by miRNAs has recently been recognized in diverse biological processes in animals and plants (Ambros 2004; Bushati and Cohen 2007; Stefani and Slack 2008; Inui et al. 2010; Ivey and Srivastava 2010). Moreover their profiles are altered in several human diseases (Alvarez-Garcia and Miska 2005; Esquela-Kerscher and Slack 2006), making miRNAs a major focus of research in nowadays molecular biology. Recently, possible functions and biological significance of miRNAs have been the subject of both experimental and theoretical studies (Shimoni et al. 2007; Wang and Raghavachari 2011; Levine et al. 2007; Levine and Hwa 2008; Mehta et al. 2008; Osella et al. 2011; Mitarai et al. 2009; Bumgarner et al. 2009; Iliopoulos et al. 2009). Some special functional features of miRNAs have been indicated by mathematical modeling and dynamical simulations, e.g., a tunable threshold-linear expression pattern and repression of fluctuation in the silence regime (Levine et al. 2007; Levine and Hwa 2008), better than TF-based regulation at filtering input noise but suffers higher intrinsic noise arising from transcriptional bursting (Mehta et al. 2008), and fine-tuning of a target protein level and an efficient noise control (Osella et al. 2011). However, most of the previous works focus only on how miRNAs post-transcriptionally regulate the target protein levels, work solely, and induce monostability. Recently, several double negative feedback loops involving miRNAs and TFs have been found experimentally in a variety of organisms (Martinez et al. 2008; Johnston et al. 2005; Kim 2007; Fazi 2005; Juan et al. 2009; Bracken 2008; Li and Carthew 2005; Visvanathan et al. 2007; Xu et al. 2009; Zhao et al. 2009; Pospisil 2011). It has been shown that such composite double negative feedback loop (MDNFL) occurs frequently, and hence constitutes a network motif (Tsang et al. 2007; Martinez and Walhout 2009). To our knowledge, the issue about possible functions of this basic motif and the effects of combinational regulation by TFs and miRNAs have not yet been well addressed. To better understand the dynamical properties of this basic motif, we have presented a general computational model for the MDNFL and explored its dynamics by using bifurcation analysis. Our results show that there exist wide ranges of parameters where the MDNFL can behave as switches. These functional features are consistent with the widespread appearance of miRNAs in fate decisions such as differentiation during development. In addition, positive feedback and cooperativity in the regulation of gene expression are generally considered to be necessary for obtaining bistable expression states (Ferrell and Xiong 2001; Lipshtat et al. 2006). Interestingly, here it is found that under the combinatorial regulation by a TF and an miRNA, the MDNFL model can behave as switches for wide ranges of parameters even without cooperative binding of the TF. It thus provides a novel mechanism to induce bistability through this combinatorial regulation even without cooperativity in the regulation. We hope this mechanism can be realized in artificial genetic networks in the future.
On the other hand, cellular processes at the molecular level are inherently stochastic (Balàsi et al. 2011). The origin of stochasticity can be attributed to internal and external noises. It is well known that noise can have great impacts on biological systems’ dynamical properties (Lipshtat et al. 2006; Hasty et al. 2000; Vilar et al. 2002; Rao et al. 2002; Wang et al. 2007; Li and Li 2008), for example, noise can induce sustained stochastic oscillation even though the corresponding deterministic system only yields steady state (Li and Li 2008). In this paper, we have also studied the effect of extrinsic noise on the dynamic behavior of the MDNFL. It is found that if the noise is considered, the MDNFL system can generate some different phenomena not observed in the corresponding deterministic model. More specifically, when the MDNFL system is in the bistable region, extrinsic noise fluctuating the two transcription rates of the miRNAs gene can induce the system quickly switch from one steady state to the other. In addition, any one of the two steady states is robust to one type of the two different extrinsic noises but sensitive to the other. This feature makes this type of switch easy to control because the requirement of carefully tuning noise strength is not considered. Moreover, during the transition process, the concentration of the miRNA (or the protein) is quite low before the switch can be amplified significantly.
The control of cellular functions through the design and manipulation of gene regulatory networks is an intriguing perspective in applications (Wang et al. 2007). Current gene therapy techniques are limited in that transfected genes are typically either in an “on” or “off” state (Hasty et al. 2000). For the effective treatment of many diseases, however, the expression of a transfected gene needs to be regulated in a systematic fashion. Thus, the development of extrinsically controllable noise-based switches and amplifiers for gene expression could have significant clinical implications and the results in this paper suggest that the MDNFL might be quite useful for that. To make this feature of MDNFL clinically applicable, it is important to look for an appropriate noise source. Sets of chemical reactions that affect the transcription of gene g s might be used as the noise input of this switch. Moreover, it has recently been shown that manipulation of miRNAs is readily achievable in vivo and thus holds exciting promise for potential therapeutic applications for diseases associated miRNAs (krützfelt 2005; Elmèn 2008).
It has been widely recognized that a complicated living organism cannot be fully understood by merely analyzing individual components, and that interactions of those components or biomolecular networks in terms of structures and dynamics are ultimately responsible for an organism’s form, rhythms, and functions (Wang et al. 2008). But the intrinsic complexity of biomolecular networks inhibits us to understand them as a whole. Instead, researchers turn to study the building block, or motif, of these networks. Elucidating these motifs’ dynamics and functions would shed light on the whole networks’ behaviors. Computational models based on experimental data have been proven to be quite useful to solve these problems (Alon 2007). In this paper, we have proposed a simple computational model for the miRNA-mediated double negative feedback loop (MDNFL) motif and studied its functional characteristic motivated by this motif appears in regulation fate decisions in a variety of organisms (Martinez et al. 2008; Johnston et al. 2005; Kim 2007; Fazi 2005; Juan et al. 2009; Bracken 2008; Li and Carthew 2005; Visvanathan et al. 2007; Xu et al. 2009; Zhao et al. 2009; Pospisil 2011). Besides the minimal architecture discussed here, miRNA-mediated network motifs with other architectures can be similarly analyzed, such as miRNA-mediated feed-forward loops (Tsang et al. 2007; Shalgi et al. 2007; Re et al. 2009). It is expected that the insight gained from the study of these simple motifs could provide a basis for investigation of more complex networks assembled by simple building blocks. A more clear understanding of the miRNA-mediated motifs is also important for bio-engineering or artificial control of specified components, interactions, and even network functions. It is hoped that the results presented here could provide a new view on how gene expression is regulated and further guidance for experiments.
Finally, it is worth mentioning that a plausible method whereby a system may be experimentally probed for bistability has been proposed in (Hasty et al. 2000). The main idea is that in a bistable system, the threshold value of the control parameter required for the system to be switched from off to on state is different from that for transitioning in the reverse direction [also see Fig. 2]. Since the degradation parameter δ A is easier to manipulate externally (Hasty et al. 2000), according to the suggestion given in (Hasty et al. 2000), one can perform an experiment for the MDNFL system by slowly increasing the degradation rate δ A from a low initial value to see whether the concentration of protein A can abruptly jump to a lower value at some moment after a slow decrease. If so, one then reverses the direction of the experiment to observe whether the concentration of protein A can suddenly switch to a higher value at some point after a slow increase. If it occurs and the downward threshold value differs from that of the upward, then one can conclude that the MDNFL system is a bistable system. Similar methods have been used to probe for bistability in previous experimental studies (Gardner et al. 2000; Xiong and Ferrell 2003; Ozbudak et al. 2004). In addition, it should be pointed out that simple genetic network motifs are always embedded in a larger and more complex network, under some circumstances, it thus might be not easy for us to observe in vivo the phenomena predicted here due to the fact that the MDNFL no longer functions independently, but as pointed out in (Alon 2007) in many cases the functions can be preserved.
References
Acar M, Becskei A, van Oudenaarden A (2005) Enhancement of cellular memory by reducing stochastic transitions. Nature 435:228–232
Aguda BD, Kim Y, Piper-Hunter MG, Friedman A, Marsh CB (2008) MicroRNA regulation of a cancer network: consequences of the feedback loops involving miR-17-92, E2F, and Myc. Proc Natl Acad Sci USA 105:19678–19683
Alon U (2007) Network motifs: theory and experimental approaches. Nat Rev Genet 8:450–461
Alvarez-Garcia I, Miska E (2005) MicroRNA function in animal development and human disease. Development 132:4653–4662
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355
Balàsi G, van Oudenaarden A, Collins JJ (2011) Cellular decision making and biological noise: from microbes to mammals. Cell 144:910–925
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Bhalla US, Iyengar R (2001) Robustness of the bistable behavior of a biological signaling feedback loop. Chaos 11:221–226
Bhalla US, Ram PT, Iyengar R (2002) MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science 297:1018–1023
Bracken CP et al (2008) A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 68:7846–7854
Bumgarner SL, Dowell RD, Grisafi P, Gifford DK, Fink GR (2009) Toggle involving cis-interfering noncoding RNAs controls variegated gene expression in yeast. Proc Natl Acad Sci USA 106:18321–18326
Bushati N, Cohen SM (2007) MicroRNA functions. Annu Rev Cell Dev Biol 23:175–205
Chatterjee A, Kaznessis YN, Hu W (2008) Tweaking biological switches through a better understanding of bistability behavior. Curr Opin Biotech 19:475–481
Elmèn J et al (2008) LNA-mediated microRNA silencing in non-human primates. Nature 452:896–899
Esquela-Kerscher A, Slack FJ (2006) Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer 6:259–269
Fazi F et al (2005) A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPα regulates human granulopoiesis. Cell 123:819–831
Ferrell JE (2002) Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol 14:140–148
Ferrell JE, Machleder EM (1998) The biochemical basis of an all-or-none cell fate switch in Xenopus Oocytes. Science 280:895–898
Ferrell JE, Xiong W (2001) Bistability in cell signaling: how to make continuous processes discontinuous, and reversible processes irreversible. Chaos 11:227–236
Filipowicz W, Bhattacharyya SN, Sonenberg N (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9:102–114
Francois P, Hakim V (2005) Core genetic module: the mixed feedback loop. Phys Rev E 72:031908
Gardner TS, Cantor CR, Collins JJ (2000) Construction of a genetic toggle switch in Escherichia coli. Nature 430:339–342
Guo D (2011) Inhibition of rhythmic spiking by colored noise in neural systems. Cogn Neurodyn 5:293–300
Guo H, Ingolia NT, Weissman JS, Bartel DP (2010) Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466:835–840
Hasty J, Pradines J, Dolnik M, Collins JJ (2000) Noise-based switches and amplifiers for gene expression. Proc Natl Acad Sci USA 97:2075–2080
Higham DJ (2001) An algorithmic introduction to numerical simulation of stochastic differential equations. SIAM Rev 43:525–546
Iliopoulos D, Hirsch HA, Struhl K (2009) An epigenetic switch involving NF-kB, Lin28, Let-7 microRNA, and IL6 links inflammation to cell transformation. Cell 139:693–706
Inui M, Martello G, Piccolo S (2010) MicroRNA control of signal transduction. Nat Rev Mol Cell Biol 11:252–263
Ivey KN, Srivastava D (2010) MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell 7:36–41
Johnston RJ, Chang S, Etchberger JF, Christopher OO, Hobert O (2005) MicroRNAs acting in a double-negative feedback loop to control a neuronal cell fate decision. Proc Natl Acad Sci USA 102:12449–12454
Juan AH, Kumar RM, Marx JG, Young RA, Sartorelli V (2009) Mir-214-dependent regulation of the polycomb protein Ezh2 in skeletal muscle and embryonic stem cells. Mol Cell 36:61–74
Khanin R, Vinciotti V (2008) Computational modeling of post-transcriptional gene regulation by MicroRNAs. J Comput Biol 15:305–316
Kim J et al (2007) A microRNA feedback circuit in midbrain dopamine neurons. Science 317:1220–1224
krützfelt J et al. (2005) Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438:685–689
Lee TI et al (2002) Transcriptional regulatory networks in Saccharomyces cerevisiae. Science 298:799–804
Levine E, Hwa T (2008) Small RNAs establish gene expression thresholds. Curr Opin Microbiol 11:574–579
Levine E, Zhang Z, Kuhlman T, Hwa T (2007) Quantitative characteristics of gene regulation by small RNA. PLoS Biol 5:e229
Li X, Carthew RW (2005) A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell 123:1267–1277
Li D, Li C (2008) Noise-induced dynamics in the mixed-feedback-loop network motif. Phys Rev E 77:011903
Lipshtat A, Loinger A, Balaban NQ, Biham O (2006) Genetic toggle switch without cooperative binding. Phys Rev Lett 96:188101
Liu D, Chang X, Liu Z, Chen L, Wang R (2011) Bistability and oscillations in gene regulation mediated by small noncoding RNAs. PLoS ONE 6:e17029
Martinez NJ, Walhout AJM (2009) The interplay between transcription factors and microRNAs in genome-scale regulatory networks. BioEssays 31:435–445
Martinez NJ, Ow MC, Barrasa MI, Hammell M, Sequerra R (2008) A C. elegans genome-scale microRNA network contains composite feedback motifs with high flux capacity. Genes Dev 22:2535–2549
Mehta P, Goyal S, Wingreen NS (2008) A quantitative comparison of sRNA-based and protein-based gene regulation. Mol Syst Biol 4:211
Mitarai N, Benjamin JM, Krishna S, Semsey S, Csiszovzki Z, Massè E, Sneppen K (2009) Dynamic features of gene expression control by small regulatory RNAs. Proc Natl Acad Sci USA 106:10655–10659
Osella M, Bosia C, Corà D, Caselle M (2011) The role of incoherent microRNA-mediated feedforward loops in noise buffering. PLoS Comput Biol 7:e1001101
Ozbudak EM, Thattai M, Lim HN, Shraiman BI, van Oudenaarden (2004) A multistability in the lactose utilization network of Escherichia coli. Nature 427:737–740
Pomerening JR, Sontag ED, Ferrell JE (2003) Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nat Cell Biol 5:346–351
Pospisil V et al (2011) Epigenetic silencing of the oncogenic miR-17-92 cluster during PU.1-directed macrophage differentiation. EMBO J 30:4450-4464
Raj A, van Oudenaarden A (2008) Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135:216–226
Ramsey SA et al (2006) Dual feedback loops in the GAL regulon suppress cellular heterogeneity in yeast. Nat Genet 38:1082–1087
Rao CV, Wolf DM, Arkin AP (2002) Control, exploitation and tolerance of intracellular noise. Nature 420:231–237
Re A, Cora D, Taverna D, Caselle M (2009) Genome-wide survey of microRNA-transcription factor feed-forward regulatory circuits in human. Mol BioSyst 5:854–867
Sha W, Moore J, Chen K, Lassaletta AD, Yi CS, Tyson JJ, Sible JC (2003) Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proc Natl Acad Sci USA 100:975–980
Shalgi R, Lieber D, Oren M, Pilpel Y (2007) Global and local architecture of the mammalian microRNA-transcription factor regulatory network. PloS Comput Biol 3:e131
Shen-Orr SS, Milon R, Mangan S, Alon U (2002) Network motifs in the transcriptional regulation network of Escherichia coli. Nat Genet 31:64–68
Shi X, Wang Q, Lu Q (2008) Firing synchronization and temporal order in noisy neuronal networks. Cogn Neurodyn 2:195–206
Shimoni Y, Friedlander G, Hetzroni G, Niv G, Altuvia S, Biham O, Margalit H (2007) Regulation of gene expression by small non-coding RNAs: a quantitative view. Mol Syst Biol 3:138
Stefani G, Slack FJ (2008) Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol 9:219–230
Stelling J, Sauer U, Szallasi Z, Doyle FJ, Doyle J (2004) Robustness of cellular functions. Cell 118:675–685
Tsang J, Zhu J, van Oudenaarden A (2007) MicroRNA-mediated feedback and feedforward loops are recurrent network motifs in mammals. Mol Cell 26:753–797
Tyson JJ, Chen KC, Novak B (2003) Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol 15:221–231
Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R (2006) Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20:515–524
Vilar JMG, Kueh HY, Barkai N, Leibler S (2002) Mechanisms of noise-resistance in genetic oscillators. Proc Natl Acad Sci USA 99:5988–5992
Visvanathan J, Lee S, Lee B, Lee JW, Lee S-K (2007) The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev 21:744–749
Wang S, Raghavachari S (2011) Quantifying negative feedback regulation by microRNAs. Phys Biol 8:055002
Wang J, Zhang J, Yuan Z, Zhou T (2007) Noise-induced switches in network systems of the genetic toggle switch. BMC Syst Biol 1:50
Wang R, Li C, Chen L, Aihara K (2008) Modeling and analyzing biological oscillations in molecular networks. Proc IEEE 96:1361–1385
Wang Z, Liu G, Sun Y, Wu H (2009) Robust stability of stochastic delayed genetic regulatory networks. Cogn Neurodyn 3:271–280
Wu H, Liao X, Feng W, Guo S (2012) Mean square stability of uncertain stochastic BAM neural networks with interval time-varying delays. Cogn Neurodyn 6:443–458
Wuchty S, Oltvai ZN, Barabási AL (2003) Evolutionary conservation of motif constituents in the yeast protein interaction network. Nat Genet 35:176–179
Xiong W, Ferrell JE (2003) A positive-feedback-based bistable ‘memory module’ that governs a cell fate decision. Nature 426:460–465
Xu D, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS (2009) MicroRNA-145 regulates OCT4, SOX2,and KLF4 and represses pluripotency in human embryonic stem cells. Cell 137:647–658
Yeger-Lotem E, Margalit H (2003) Detection of regulatory circuits by integrating the cellular networks of protein–protein interactions and transcription regulation. Nucleic Acids Res 31:6053–6061
Yeger-Lotem E, Sattath S, Kashtan N, Itzkovitz S, Milo R, Pinter RY, Alon U, Margalit H (2004) Network motifs in integrated cellular networks of transcription-regulation and protein–protein interaction. Proc Natl Acad Sci USA 101:5934–5939
Yu X, Lin J, Zack DJ, Mendell JT, Qian J (2008) Analysis of regulatory network topology reveals functionally distinct classes of microRNAs. Nucleic Acids Res 36:6494–6503
Zhang XP, Zhang C, Liu F, Wang W (2007) Linking fast and slow positive feedback loops creates an optimal bistable switch in cell signaling. Phys Rev E 76:031924
Zhao C, Sun G, Li S, Shi Y (2009) A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol 16:365–371
Zhdanov VP (2009) Bistability in gene transcription: interplay of messenger RNA, protein, and nonprotein coding RNA. BioSystems 95:75–81
Acknowledgments
We are grateful to the reviewers and the editor for their valuable comments and suggestions.This work was supported by the National Science Foundation of China (Grants No. 10832006, 11172158).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cai, S., Zhou, P. & Liu, Z. Functional characteristics of a double negative feedback loop mediated by microRNAs. Cogn Neurodyn 7, 417–429 (2013). https://doi.org/10.1007/s11571-012-9236-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-012-9236-7