Abstract
We study the effect of colored noise on the rhythmic spiking activity of neural systems in this paper. The phenomenon of the so-called inverse stochastic resonance , that is, noise with appropriate intensity suppresses the spiking activity in neural systems, is clearly observed in a special parameter regime. We find that the inhibition effect of colored noise is stronger than that of Gaussian white noise. Furthermore, our simulation results show that the inhibition effect of colored noise provides a useful mechanism for the generation of synchronized burst in type-2 mixed-feed-forward-feedback loop neuronal network motif, which indicates that such inhibition effect might have some biological implications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Noise is ubiquitous in the brain. Recently, much effort has been devoted to investigating the effects of noise on the dynamics of neuronal ensembles. It has been widely recognized that noise is able to facilitate the information processing and enrich the inherently stochastic dynamics of neural systems (Deco et al. 2009; Destexhe and Contreras 2006). Two fundamental and maybe the most important mechanisms that the neural system may use to enhance its information transmission capability with the help of noise are stochastic resonance (Collins et al. 1995; Gailey et al. 1997; Chialvo et al. 1997; Lee and Kim 1999; Chik et al. 2001; Guo and Li 2009) and coherence resonance (Guo and Li 2009; Pikovsky and Kurths 1997; Lindner et al. 2002; Li and Gao 2008; Sun et al. 2008). For each mechanism, the response of the neural system greatly depends on the noise intensity, and there exists a maximal neural response at an optimal noise level. Furthermore, it has also been observed that noise can enrich the stochastic dynamics of neuronal ensembles and induce many complex behaviors, such as synchronization (Li et al. 2006), deterministic chaos (Hansel and Sompolinsky 2002), and burst firing (Neiman et al. 2007). All these findings are in good agreement with the previous postulate suggesting that noise might play several functional roles in the signal processing of the brain (Adey 1972).
In some recent work (see Gutkin et al. 2009; Tuckwell et al. 2009; Tuckwell and Jost 2010), the authors reported a new inhibition effect of noise on neural systems. It was found that the noise can suppress the spiking activity of a single Hodgkin-Huxley (HH) neuron. Under some specific conditions, there exists a minimal firing rate (or minimal frequency of firing rhythm) corresponding to an optimal noise level. Such phenomenon is the so-called inverse stochastic resonance (ISR), because the behavior in this phenomenon likes the reverse of that in stochastic resonance (Gutkin et al. 2009). More importantly, a similar inhibition effect of noise has also been observed in several real biological neural systems (Paydarfar et al. 2006), which implies that this type of inhibition effect might have some physiological significance. However, so far the relevant studies mainly studied the case of Gaussian white noise. Although another important case—colored noise has been also considered in some previous studies, to the best of our knowledge the inhibition of rhythmic spiking due to colored noise in neural systems has not been thoroughly discussed and the relevant questions still remain unclear. Especially, since the experimental findings have revealed that the colored noise, rather than the Gaussian white noise, provides the best model to simulate the background noise input of real biological neural systems (Destexhe and Contreras 2006; Destexhe et al. 2003), it is necessary to examine how colored noise influences the ISR phenomenon in neural systems as well as explore whether colored noise has some special advantages over Gaussian white noise in terms of ISR.
In this paper, we address the aforementioned questions and provide insights by computational modeling. Following the work of Gutkin et al. (2009) and Tuckwell et al. (2009), we also use the HH neuron model to mimic the action potential firing dynamics of biological neurons. Our goal is to examine how the colored noise influences the spiking activity of the neural system. The main finding of the present work is that the neural system can indeed exploit the colored noise to suppress its spiking activity. Moreover, our simulation results also demonstrate that the inhibition effect of colored noise is stronger than that of Gaussian white noise. Finally, an example is given to elucidate the underlying physiological significance of the ISR phenomenon. All these results may provide a foundation for understanding the ISR phenomenon in the brain.
The rest of this paper is organized as follows. The HH neuron model as well as the numerical simulation method is introduced in Sect. “Model and method”. The main results of the present work are presented in Sects. “ISR in single HH neuron” and “ISR Induced synchronized bursts in a neuronal network motif” Finally, a brief conclusion and discussion of our work are given in Sect. “Conclusion and future work”.
Model and method
We now introduce the HH neuron model used in the present work. Here we only describe the dynamics of a single HH neuron, because the ISR phenomenon is mainly examined in a single HH neuron in this work. The methods about how to model the synaptic connections will be briefly given in “ISR Induced synchronized bursts in a neuronal network motif” The membrane potential of a single HH neuron can be described by the following four differential equations (Hodgkin and Huxley 1952; Gerstner and Kistler 2002):
with X = m, h, n satisfies
Here C is the membrane capacitance per unit area, V represents the membrane potential, G K , G Na, and G L stand for the maximal potassium, sodium, and leakage conductances per unit area, and E K, E Na, and E L denote the corresponding reversal potentials, and I is the total external current injected to the neuron. Three dimensionless parameters m, h, and n are used to control the activation and inactivation of sodium channels and the activation of potassium channels, respectively. α X and β X (X = m, h, n) are rate functions that are given by the following set of equations (Hodgkin and Huxley 1952; Gerstner and Kistler 2002):
Without loss of generality, the parameters of the HH neuron are set as their standard values (Gerstner and Kistler 2002), which are specified in Table 1.
For a single HH neuron, the total external current is given by I = I 0 + μ(t), where I 0 is the external constant current and μ(t) is the noise current. In this work, the noise current is modeled by an Ornstein-Uhlenbeck (OU) process:
Here ξ(t) is a Gaussian white noise with zero mean and unit variance, τ c is the correlation time, and D is a diffusion coefficient used to denote the noise intensity. By its definition, μ(t) corresponds to a zero mean colored noise with correlation function
It should be noted that, for the special case τ c = 0 ms, the above noise current reduces to the Gaussian white noise.
The aforementioned stochastic differential system is numerically solved by using the Euler-Maruyama algorithm (Kloeden et al. 1994), with a fixed step h = 0.005 ms. Notice that the chosen integration step is sufficiently small to ensure the simulation accuracy of the HH neuron. A spike is detected whenever the membrane potential upward crosses a threshold V th = 70 mV. According to the discussion given in the previous work (Chialvo et al. 1997), the membrane potential might fluctuate rapidly several times around the threshold for mid-to-high noise intensities, thus leading to some “false” spikes. To eliminate these false spikes, we introduce a 5-ms pseudo absolute refractory period, in which no threshold crossing is considered as an action potential anymore (Chialvo et al. 1997). In additional stimulations, it turns out that the threshold value can vary in a wide range without altering the results.
ISR in single HH neuron
Before presenting the simulation results, let us now discuss the bifurcation of the HH neuron. This is an important preliminary step, because the previous work has shown that the bifurcation structure plays an important role in the emergence of the ISR phenomenon. In the absence of noise, the HH neuron has a globally stable fixed point for I 0 < I s = 6.2 μA/cm2. In this situation, the membrane potential of the HH neuron undergoes a subthreshold response. At I 0 = I h = 9.8 μA/cm2, a Hopf bifurcation occurs and therefore the HH neuron has a stable limit cycle for I 0 > I h . In the case of I s < I 0 < I h , due to saddle-node bifurcation the HH neuron has a globally stable fixed point, a stable limit cycle and an unstable limit stable cycle. Thus, the membrane potential of the HH neuron undergoes either the subthreshold response or the regular spiking response depending on the initial parameter values in this situation.
As a starting point, we first investigate the inverse stochastic resonance in a single HH neuron driven by the colored noise. The corresponding results are plotted in Fig. 1a–c, respectively. All simulations are performed 6000 ms. We collect spikes from 1000 to 6000 ms for statistical analysis. The initial membrane potential is randomly chosen from a uniform distribution in (−10,70) mV, and the initial values of m, n, and h are randomly chosen from a uniform distribution in (0,1). Each data shown in Fig. 1a and b is based on 1000 independent simulations, and each data shown in Fig. 1c is based on 500 independent simulations.
Inverse stochastic resonance in a single HH neuron driven by the colored noise. a The firing rate f as a function of noise intensity D for different values of I 0, with τ c = 1 ms. b The firing rate f as a function of noise intensity D for different correlation times, with I 0 = 7.9 μA/cm2. c The dependence of inhibition measure K on the value of I 0 for different correlation times
Figure 1a shows the dependence of the firing rate f on the colored noise intensity D for several typical values of I 0. For I 0 < I s , an unstrict monotonic increasing and nonlinear relationship is found to exist between the noise intensity and firing rate. This implies that the colored noise plays a positive role and purely enhances the firing capability of the HH neuron in this case. If I 0 is slightly larger than the bifurcation value I s , the firing rate curve basically drops at first and then rises with the increase of D, suggesting that the colored noise indeed suppresses the spiking activity of the HH neuron for suitable value of I 0. It is shown that the minimal firing rate occurs at an optimal noise intensity, which clearly demonstrates that the ISR phenomenon can exist in the neural system driven by the colored noise. Furthermore, we find that the effect of the ISR is largely influenced by the value of I 0 even for the case of I 0 > I s . When I 0 is close to and just above the bifurcation value I s , the colored noise with an appropriate intensity shows a significant inhibition effect on the spiking activity of the HH neuron. For example, a proper noise can even completely suppress the firing behavior for I 0 = 6.6 μA/cm2 (see Fig. 1a). As I 0 grows, the inhibition effect of the colored noise becomes weaker and weaker. If I 0 is increased to a rather large value (see I 0 = 8.5 μA/cm2 in Fig. 1a), the inhibition effect is so weak that the ISR phenomenon almost disappears. Note that our results are consistent with the findings for the case of Gaussian white noise given by Gutkin et al. (2009) and Tuckwell et al. (2009).
In fact, the fundamental mechanism that the colored noise is able to suppress the spiking activity of the HH neuron near the saddle-node bifurcation point is similar to the case of Gaussian white noise, which has been discussed in detail in the previous work (Gutkin et al. 2009; Tuckwell et al. 2009). Besides the stable limit cycle, the HH neuron has another stable fixed point for I s < I 0 < I h . In this case, noise can drive the membrane potential of the HH neuron from one stable solution to the other, which provides the underlying mechanism to reduce its spiking activity. Based on the mathematical theory given by Tuckwell et al. (2009), the basin of attraction of the stable limit cycle (the region between the stable and unstable limit cycles) is smaller than that of the stable fixed point (the region between the unstable cycle and the stable fixed point) when I 0 is close to and above I s . Therefore, an appropriate small noise may move the membrane potential of the HH neuron from the limited cycle to its steady state but with a smaller probability moving back, thus leading to a spiking weakening phenomenon. For a relatively small I 0, once the membrane potential converges to the steady state after the initial several hundred milliseconds of the simulation due to the optimal noise, the HH neuron generally might need a stronger noise to make it escape from the steady state again. Therefore, for some ideal cases, the noise can completely suppress the spiking activity of the HH neuron (see I 0 = 6.5 μA/cm2 in Fig. 1a).
Figure 1b illustrates the firing rate f versus the noise intensity D for both the cases of Gaussian white noise (τ c = 0 ms) and colored noise (τ c = 1, 2.5, 5 ms). Note that here only small values of τ c are considered because the correlation time is short and typically less than several milliseconds in real biological neural systems (Collins et al. 1996). As we see in Fig. 1b, a pronounced minimal value exists at the corresponding optimal noise intensity for each firing rate curve, indicating that both types of noise are able to suppress the spiking activity successfully. It is obvious that the minimal value of the firing rate curve for each colored noise is smaller than that for the Gaussian white noise. This suggests that the colored noise shows a stronger inhibition effect than the Gaussian white noise. Furthermore, our results also reveal that the performance of ISR induced by the colored noise is also largely influenced by the correlation time. With the increase of τ c , we find that the inhibition effect of the colored noise becomes stronger and stronger. At the same time, the trough of firing rate curve also moves toward the right direction. This is because the correlation function of the colored noise considered in this work is determined by both the correlation time and the diffusion coefficient and increasing τ c results in decreasing the value of correlation function at corresponding time points.
To quantitatively characterize the inhibition effect of noise, we define an inhibition measure as K = (f 0 − f min)/f 0, where f min is the minimal firing rate at the corresponding optimal noise intensity and f 0 is the firing rate at zero noise intensity. By its definition, a larger positive K means a stronger inhibition effect of noise. In Fig. 1c, the inhibition measure K as a function of D for both the cases of Gaussian white noise (τ c = 0 ms) and colored noise (τ c = 2.5, 5, 8 ms) is plotted. For each considered case, we find the noise can almost completely suppress the spiking activity of the HH neuron after the initial 1000 ms of the simulation if I 0 < 7.3 μA/cm2. Although the K curves all decrease as the external constant current grows for I 0 ≥ 7.3 μA/cm2, it is clear that those curves for longer correlation times have slower decreasing speeds. Our results, on the one side, further demonstrate that the inhibition effect of the colored noise is stronger than that of the Gaussian white noise, and on the other side, suggest that the colored noise with longer correlation time has the tendency to maintain the ISR phenomenon for a larger I 0.
To understand why the colored noise shows a stronger inhibition effect than the Gaussian white noise, we compare the interspike interval histogram (ISIH) for both the Gaussian white noise and colored noise at corresponding optimal noise intensities, which are depicted in Fig. 2a and b, respectively. For each case, the interspike interval histogram is computed by using 8000 spikes obtained from an independent simulation with sufficiently long simulation time. As we see in Fig. 2a and b, there exists a main peak located between 15 and 20 ms, and several other nonzero values that lie in some long-time locations (from tens of milliseconds to tens of seconds) for both the colored noise and the Gaussian white noise. The results suggest that the HH neuron driven by appropriate noise intensity has the tendency to first fire several spikes in a short period of time, after that keep silent for a long period of time due to the inhibition effect of noise, and then continuously repeat the above process. However, the distribution of the ISIH of the colored noise is obviously wider than that of the Gaussian white noise. The above results indicate that the HH neuron driven by the colored noise sometimes might need longer time to escape its steady state. This strengthening effect might be mainly attributable to the exponential correlation characteristics of the colored noise in temporal scales. Due to the existence of correlation time, it is obvious that the fluctuations of colored noise are less dramatic than those of Gaussian white noise (i.e., the colored noise is much more smooth than the Gaussian white noise). Let us assume that the HH neuron driven by noise is operating around its saddle bifurcation point. For the case of colored noise, when the state of the neuron is at the left of bifurcation point, it tends to stay here for a short period of time; while for the case of Gaussian white noise, the HH neuron will visit both the left and right of the bifurcation point transiently. The above mechanism leading to the colored noise has the ability to speed up the HH neuron moving to the stable fixed point and make the neuron converge towards the steady state more deeply at the appropriate intensity, which means that the neuron may need much time to escape its steady state (see Fig. 2a and b). As a result, the inhibition effect of the colored noise on spiking activity is stronger than that of the Gaussian white noise.
The interspike interval histograms for both the Gaussian white noise (τ c = 0 ms) and colored noise (τ c = 5 ms) at corresponding optimal intensities, with I 0 = 7.9 μA/cm2. a Gaussian white noise (D = 0.35) and b colored noise (D = 0.95). Here parameter N denotes the number of spikes. The inserts embedding in (a) and (b) show the distribution of the ISIH for ISI larger than 50 ms
ISR induced synchronized bursts in a neuronal network motif
Such inhibition effect of the colored noise might have some physiological significance. As a nontrivial example, we show that this type of inhibition effect might play an important role in the generation of synchronized bursts in type-2 triple-neuron mixed-feed-forward-feedback loop neuronal network motif (MFFL2), which is one of the most significant building blocks of the real biological neuronal networks (Guo and Li 2009; Li 2008; Milo et al. 2002; Reigl et al. 2004). In the MFFL2 motif, neurons 1 and 2 reciprocally drive each other, and neurons 1 and 2 both drive neuron 3. Thus, neurons 1 and 2 can be considered as the input neurons, and neuron 3 can be regarded as the output neuron. Here we only study the MFFL2 motif consisting of three excitatory neurons, and use V i and I i (i = 1, 2, 3) to represent the membrane potential and the total external current of neuron i, respectively.
For the MFFL2 motif, the total external current is given by \(I_i=I_0+I_{i}^s+\mu_i(t), \) where I 0 is the external constant current and μ i (t) is the noise current. For the input neurons, we choose I 0 = 6.9 μA/cm2, and for the output neuron, we choose I 0 = 0 μA/cm2 The total synaptic current I s i onto neuron i is the linear sum of the currents of all incoming synapses, i.e., \(I_{i}^s=\Upsigma_j{gr_j[E_s-V_i(t)]},\) where parameter g is the coupling strength and E s = 85 mV is the reversal potential. The synapse variable r j is the fraction of postsynaptically bound neurotransmitter which obeys \(\dot{r}_j=F(v_j)(1 - r_j) - r_j/\tau_s, \) with the synaptic recovery function \(F(v_j)= 1/[1 + \exp(-v_j +60)]\) and the synaptic decay rate τ s .
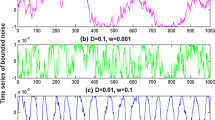
Figure 3 illustrates the membrane potentials V i as a function of time for the three HH neurons, with sufficiently strong synapses (g = 0.1 and τ s = 2 ms). When D is too small, there is almost no effect of noise on the spiking activities of the coupled neurons (see Fig. 3a). In this case, all neurons repeatedly fire spikes and then excite other neurons. Thus, the firing behaviors of these neurons almost display tonic firing activities and no quiescent state emerges. For an appropriate noise intensity, due to the inhibition effect of noise, the spiking activities of all neurons might be cut off (see Fig. 3b and c). The aforementioned cutting-off process is “almost” simultaneous, since in the MFFL2 motif neuron 3 is controlled by both neurons 1 and 2, and neurons 1 and 2 mutually excite each other. Note that here we call it “almost” simultaneous, because the firing patterns of these three neurons are the same on the whole but vary somewhat in detail. Typically, the spiking activities are cut off only when both input neurons are in the resting state within a small time interval. If only one input neuron is in the resting state, due to strong excitatory synaptic interaction, the other input neuron will kick this neuron back to the oscillating state immediately with a very high probability. Such recovery process is usually very quick, so that we can almost omit the recovery time and treat the corresponding firings as a whole burst group (see Fig. 3b). This provides a useful mechanism to generate and control the quiescent states of the burst firings. As a result, the phenomenon of synchronized bursts can be seen clearly in this situation. If D is increased to a large value, the inhibition effect of noise itself is less pronounced and at the same time the excitation among neurons tends to further reduce such inhibition, thus leading to the vanish of the synchronized bursts (see Fig. 3d). The above example suggests that neural systems might use the ISR mechanism to generate the synchronized bursts.
On the other hand, our above discussions also suggest that whether the MFFL2 motif can successfully generate the synchronized bursts depends not only on noise intensity but also on synaptic parameters, such as the coupling strength g and synaptic decay rate τ s . In fact, it is found that only sufficiently strong synapses (strong coupling strength and slow synaptic decay rate) can support the occurrence of synchronized bursts (see Fig. 4a–d). This phenomenon can be easy to understand by considering that, once only one input neuron is in the resting state, this neuron cannot immediately or even completely cannot recovery from its resiting state to the oscillating state due to weak excitatory synaptic interaction, which tends to destroy the synchronized bursts.
It should be noted that here we mainly focus on the case of colored noise, but the similar results can also be observed for the Gaussian white noise. However, similar with the case of signal HH neuron, it is also found that the inhibition effect of colored noise is stronger than that of Gaussian white noise for the case of MFFL2 motif (data not shown).
Conclusion and future work
In this paper, we have systemically investigated the inhibition effect of colored noise on the spiking activity of neural systems. Our simulation results showed that the inverse stochastic resonance phenomenon indeed occurs in the neural systems driven by colored noise. Moreover, we found that the inhibition effect of the colored noise is stronger than that of Gaussian white noise. With the increase of the correlation time, such inhibition effect can be largely enhanced. Finally, we also gave an example to elucidate the underlying physiological significance of the ISR phenomenon. Noise is ubiquitous in neural systems and plays important roles in the dynamics of neural ensembles. The inhibition effect of noise shown here tends to suppress the spiking activity of the neural systems, but it may not simply play a negative role in the real biological neural systems. In fact, the results presented in this work, to some extent, suggest that the neural systems might make full use of the ISR mechanism to enrich their inherently stochastic dynamics. Further work on this topic includes studying the inhibition effect of noise in complex neuronal networks, as well as exploring other possible relevant important biological implications.
References
Adey WR (1972) Organization of brain tissue: is the brain a noisy processor. Int J Neurosci 3(6):271–284
Chialvo DR, Longtin A, Muller-Gerking J (1997) Stochastic resonance in models of neuronal ensembles. Phys Rev E 55(2):1798–1808
Chik DTW, Wang Y, Wang ZD (2001) Stochastic resonance in a Hodgkin-Huxley neuron in the absence of external noise. Phys Rev E 64(2):021913
Collins JJ, Chow CC, Imhoff TT (1995) Stochastic resonance without tuning. Nature 376:236–238
Collins JJ, Imhoff TT, Grigg P (1996) Noise-enhanced information transmission in rat SA1 cutaneous mechanoreceptors via aperiodic stochastic resonance. J Neurophysiol 76(1):642–645
Deco G, Rolls ET, Romo R (2009) Stochastic dynamics as a principle of brain function. Prog Neurobiol 88(1):1–16
Destexhe A, Contreras D (2006) Neuronal computations with stochastic network states. Science 314(5796):85–90
Destexhe A, Rudolph M, Pare D (2003) The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci 4:739–751
Gailey PC, Neiman A, Collins JJ, Moss F (1997) Stochastic resonance in ensembles of nondynamical elements: the role of internal noise. Phys Rev Lett 79(23):4701–4704
Gerstner W, Kistler WM (2002) Spiking neuron models. Cambridge University Press, Cambridge
Guo D, Li C (2009) Stochastic and coherence resonance in feed-forward-loop neuronal network motifs. Phys Rev E 79(5):051921
Gutkin BS, Tuckwell HC, Jost J (2009) Inhibition of rhythmic neural spiking by noise: the occurrence of a minimum in activity with increasing noise. Naturwissenschaften 96(9):1091–1097
Hansel D, Sompolinsky H (1992) Synchronization and computation in a chaotic neural network. Phys Rev Lett 68(5):718–721
Hodgkin AL, Huxley AF (1952) A quantitative description of ion currents and its applications to conduction and excitation in nerve membranes. J Physiol 117:500–544
Kloeden PE, Platen E, Schurz H (1994) Numerical solution of SDE through computer experiments. Springer, Berlin
Lee SG, Kim S (1999) Parameter dependence of stochastic resonance in the stochastic Hodgkin-Huxley neuron. Phys Rev E 60(1):826–830
Li C (2008) Functions of neuronal network motifs. Phys Rev E 78(3):037101
Li C, Chen L, Aihara K (2006) Transient resetting: a novel mechanism for synchrony and its biological examples. PLoS Comput Biol 2(8):e103
Li Q, Gao Y (2008) Control of spiking regularity in a noisy complex neural network. Phys Rev E 77(3):036117
Lindner B, Schimansky-Geier L, Longtin A (2002) Maximizing spike train coherence and incoherence in the leaky integrate-and-fire model. Phys Rev E 66(3):031916
Milo R, Shen-Orr S, Itzkovitz S, Kashtan N, Chklovskii D, Alon U (2002) Network motifs: simple building blocks of complex networks. Science 298(5594):824–827
Neiman AB, Yakusheva TA, Russell DF (2007) Noise-induced transition to bursting in responses of paddlefish electroreceptor affrents. J Neurophysiol 98(5):2795–2806
Paydarfar D, Forger DB, Clay JR (2006) Noisy inputs and the induction of on-off switching behavior in a neuronal pacemaker. J Neurophysiol 96(6):3338–3348
Pikovsky AS, Kurths J (1997) Coherence resonance in a noise-driven excitable system. Phys Rev Lett 78(5):775–778
Reigl M, Alon U, Chklovskii DB (2004) Search for computational modules in the C. Elegans brain. BMC Biol 2(25):25
Sun X, Perc M, Lu Q, Kurths J (2008) Spatial coherence resonance on diffusive and small-world networks of Hodgkin-Huxley neurons. Chaos 18(2):023102
Tuckwell HC, Jost J (2010) The effects of various spatial distributions of weak noise on rhythmic spiking. J Comput Neurosci (in press). doi:10.1007/s10827-010-0260-5
Tuckwell HC, Jost J, Gutkin BS (2009) Inhibition and modulation of rhythmic neuronal spiking by noise. Phys Rev E 80(3):031907
Acknowledgements
We sincerely thank Prof. Chunguang Li and Feng Chen for valuable comments on an early version of this manuscript. This work is supposed by the award of the ongoing best PhD thesis support from the University of Electronic Science and Technology of China as well as the Chinese Universities Scientific Fund (Grant No. 2010QNA5031).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, D. Inhibition of rhythmic spiking by colored noise in neural systems. Cogn Neurodyn 5, 293–300 (2011). https://doi.org/10.1007/s11571-011-9160-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-011-9160-2