Abstract
Aim
For selected patients with brain metastases (BMs), the role of stereotactic radiosurgery (SRS) or fractionated stereotactic radiotherapy (SFRT) is well recognized. The recent introduction of flattening filter free (FFF) delivery during linac-based SRS or SFRT allows shorter beam-on-time, improving patients’ comfort and facility workflow. Nevertheless, limited experiences evaluated the impact of FFF linac-based SRS and SFRT in BMs treatment. Aim of the current study was to analyze SRS/SFRT linac-based FFF delivery for BMs in terms of dosimetric and early clinical results.
Materials and methods
Patients with life expectancy >3 months, number of BMs <5, diameter <3 cm, and controlled or synchronous primary tumor received SRS/SFRT. The prescribed total dose and fractionation, based on BMs size and proximity to organs at risk, ranged from 15 Gy in 1 fraction to 30 Gy in 5 fractions. A FFF volumetric modulated arc therapy (VMAT) plan was generated with one or two coplanar partial arcs. Toxicity was assessed according to CTCAE v4.0.
Results
From April 2014 to February 2016, 45 patients (89 BMs) were treated with SRS/SFRT linac-based FFF delivery. The mean beam-on-time was 140 s for each lesion (range 90–290 s) and the average brain Dmean was 1 Gy (range 0.1–4.8 Gy). At the time of analysis, local control was reported in 93.2% (83/89 BMs). With a median follow-up time of 12 months (range 1–27 months), the median overall survival was 14 months and the 6-month overall survival was 77%. Finally, the median intracranial disease control was 11 months. Acute and late toxicities were acceptable without severe events (no adverse events ≥G2 were recorded).
Conclusions
These preliminary results highlighted the feasibility and safety of linac-based SRS/SFRT with FFF mode for BMs patients. A longer follow-up is necessary to confirm the efficacy of this treatment modality in BM patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases (BMs) are the most common intracranial tumors in adults; in fact, about 20–40% of patients affected by cancer will develop BMs during oncological history [1]. Moreover, in the last decades, the probability to develop BMs increased up to five times due to the improving the efficacy of anti-cancer therapies [1–3].
Most of BMs patients are defined as oligometastatic (i.e., patients with a limited number of metastases), and in this setting, the role of stereotactic radiosurgery (SRS) or fractionation stereotactic radiotherapy (SFRT) is well recognized [4]. In particular, SRS/SFRT approaches can be recommended in the treatment of 1–4 BMs and in patients with a life expectancy of more than 3–6 months [3, 5]. Recently, SRS is preferred over whole-brain radiotherapy (WBRT) in order to minimize the probability of developing neurocognitive dysfunctions [6, 7], even though innovative hippocampal avoidance techniques were recently developed [8]. Moreover, analyzing clinical outcomes, WBRT did not show an improvement in overall survival (OS) when compared to SRS [5, 9].
Historically, the definition of intracranial SRS was introduced by Leksell as ‘a single high dose fraction of radiation, stereotactically directed to an intracranial region of interest’ [10]. In 1968, Leksell designed the first Gamma Knife® (GK), a device with 60Co sources for irradiating a brain tumor volume [11]. To date, due to the technological advancement of linear accelerator (Linac), there was a continuous increasing interest in SRS Linac-based applications, and recent data reported no substantial advantages in terms of clinical outcome of any specific SRS system (i.e., GK vs. Linac-based SRS) [12–14].
One of the most relevant advantages in Linac-based SRS/SFRT approach is the treatment delivery time. In fact, beam/time ratio is usually limited to few minutes in single lesion setting, even though several parameters, such as prescription dose and target shape, could affect treatment delivery time [15, 16]. In order to further reduce beam-on-time (BOT), the removal of Linac flattening filter (FF) allowed to produce a higher dose rate delivery with a shorter BOT. Nevertheless, the FF-free (FFF) technique has several dosimetric advantages and hypothetical radiobiological effect, including a steep dose gradients, lower out-of-field dose, leaf transmission, lower dose outside the field edge, and cell killing [17–20]. Despite FFF mode is increasing, few reports on dosimetric data and feasibility are published [19, 21, 22]. The aim of the present study was to analyze BMs patients treated by SRS/SFRT with FFF technique in terms of dosimetric and preliminary clinical outcomes.
Materials and methods
Patients
All cases have been discussed in the multidisciplinary team, which included a radiation oncologist, a neurosurgeon, a medical oncologist, and a neuroradiologist.
SRS or SFRT was performed according to the following criteria: (a) critical anatomic position, (b) the absence of acute neurological symptoms, (c) life expectancy >3 months, (d) number of brain metastases < 5, (e) diameter <30 mm, and (f) controlled primary tumor or metachronous diagnosis.
Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Target definition and treatment
Computed tomography (CT) with contrast enhancement was requested in all patients to define the extracranial disease status, while magnetic resonance imaging (MRI) with contrast enhancement was performed to evaluate the anatomical presentation and number of brain metastases. Patients underwent CT simulation with a 1-mm slice thickness for radiation therapy planning in a thermoplastic mask (BrainLAB®, Feldkirchen, Germany). A co-registration of volumetric CT and MRI-T1 sequences, typically a 3-dimensional spoiled gradient series with 1-mm slice thickness, was used to define target and organs at risk (OARs). Gross tumor volume (GTV) was defined as macroscopic contrast enhancing lesion on T1-MRI and it was equal to clinical target volume. Planning target volume (PTV) was obtained adding an isotropic margin of 2 mm in all directions.
OARs were brain (normal brain minus PTV), eyes, lens, optic chiasm, optic nerves, brainstem, and spinal cord.
Dose prescription (ranged between 15 and 30 Gy) and fractionation (ranged between 1 and 5 fractions) were based on different clinical and radiological parameters including BM size, the presence of subacute or acute neurological symptoms, proximity to OARs, or critical anatomical position. [23, 24]. SFRT was conventionally preferred to SRS in patients with BMs size ≥30 mm, significant perilesional edema, neurological symptoms or proximity to OAR/critical anatomical structures. For each BM, a FFF SRS/SFRT plan was generated with one or two partial coplanar or non-coplanar arcs, according to the anatomical position. Plans were optimized aiming to achieve a PTV coverage of D95% > 95% with a homogeneous dose distribution. All patients were treated with the volumetric modulated arc technique RapidArc (Varian Medical System, Palo Alto, USA) on True Beam™ equipped with a 6D couch and a micro multi-leaf collimator. Exactrac (BrainLAB®, Feldkirchen Germany) and Cone Beam CT imaging were performed daily for patient setup and positioning verification.
In all patients, a prophylactic corticosteroid therapy (dexamethasone 8 mg per day) was prescribed and progressively reduced after radiotherapy according to clinical conditions and internal Institutional protocol.
Evaluation of tumor response and radiological toxicity evaluation
Clinical evaluation and MRI were requested after 45–60 days from the end of the radiation treatment, then every 3 months for the first 2 years, and finally every 6 months or as appropriate after 3 years. Radiological response was assessed according to RECIST criteria [25]. In patients with diagnostic suspect of radionecrosis, a histological proof was requested. If a neurosurgical approach was excluded, an expert neuroradiologist evaluated MRI changes. MRI included diffusion, perfusion-weighted imaging, and spectroscopy in order to differentiate a local metastatic progression or a necrotic process. More specifically, the diagnosis of radionecrosis was defined by means of the following radiological features: white matter high signal edema and mass effect early loss of volume later at T2/FLAIR, white or grey matter single or multiple nodular or curvilinear “soap-bubble” enhancement at T1 with contrast enhancement, MR spectroscopy: typically low choline, creatine, and N-acetylaspartate at spectroscopy [26].
Statistical analysis
In order to summarize the most relevant features of the clinical variables, descriptive statistics were performed. Local control (LC), OS, and intracranial progression-free survival (PFS) rates were estimated using the Kaplan–Meier method. LC was defined from the beginning of the treatment to the local relapse date. Local recurrence was defined as any relapse inside radiation field. Intracranial progression was considered from the beginning of the radiation treatment to the time of any new central nervous system progression. OS was calculated from the date of diagnosis to the death or last follow-up date. Statistical analyses were carried out using R-software 3.1.2 version. Clinical outcomes and toxicity data according to the Common Terminology Criteria for Adverse Events (CTCAE version 4.0) were collected prospectively.
Results
Patients and dosimetric results
From April 2014 to February 2016, 45 consecutive patients (89 intracranial lesions) with a median age of 67 years (range 23–83) were treated with SRS/SFRT for BMs in our Cancer Care Center. Twenty out of 45 (44.4%) were female, and median KPS and GPA were 90 and 2.5, respectively. All patients’ characteristics are shown in Table 1.
In SRS and SFRT patients, median dose prescriptions were 25 Gy (range 15–25 Gy) and 24 Gy (range 21–30 Gy), respectively, while median number of fractions delivered in SFRT patients was 3 (range 3–5). Median BOT was 140 s (range 80–290 s) in SRS and 130 s (range 80–270 s) in SFRT, respectively. Finally, the interpatient average brain Dmean was 0.71 Gy (range 0.11–1.56) in SRS and 1.6 Gy (0.48–4.81) in SFRT, and the V12 Gy for brain was 0.82% (0–4.11) and 2.68% (0.59–9.64) in SFRT. All the other dosimetric parameters evaluated are reported in Table 2.
Survival, local control, intracranial progression-free survival, and toxicity
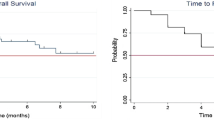
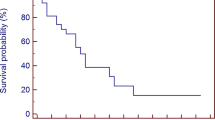
With a median follow-up time of 12 months (range 1–27 months), median OS was 14 months, while 6 months and 1 year OS were 77 and 63%, respectively (Fig. 1). At the time of the analysis, 12 patients out of 45 were dead and all of them died for extracranial disease. No differences were observed among the groups in terms of OS: primary tumor (p = 0.5) and extra cranial diseases status (p = 0.1).
Six out of 89 lesions (6.8%) showed a disease progression during follow-up, with a median time of progression of 9 months and a 6-month LC probability of 76.4%. LC was not influenced significantly by GPA score (p = 0.2) and GTV dimension (p = 0.2). The median Intracranial PFS was 11 months, and at 6 months, 65% of patients did not report an intracranial progression.
Sixteen patients (36%) developed subsequently new brain metastases and required salvage treatments: nine received WBRT (56.25%) and seven received SRS (43.75%).
Acute toxicity was low: Grade 1 nausea and headaches were reported in only two patients (4.4%). In terms of late toxicity, one patient (2.22%) developed asymptomatic intracerebral hemorrhage and another one asymptomatic radionecrosis. In the first case, it was not requested any specific medication. In the second case, a corticosteroid therapy was prescribed for 50 days with a resolution to radionecrotic process.
Discussion
BMs are the most common intracranial tumors in adults and generally, central nervous system metastatic involvement is characterized by a poor prognosis (3–6 months) [1]. Historically, WBRT with or without surgical resection or SRS have been considered the standard treatment for solitary or oligometastatic BMs patients, while WBRT alone was performed in multiple BMs setting [27–29].
Currently, SRS/SFRT alone is proposed to patients with 1–4 BMs (with diameter inferior to 2.5–3 cm) and a life expectancy of more than 3 months [5, 30].
In a prospective study, Yamamoto and colleagues reported about the use of SRS in 1194 patients with one to ten BMs; authors suggested that SRS alone in patients with five to ten BMs is non-inferior to that in patients with two to four BMs [31]. Nevertheless, these results caused several criticisms concerning the heterogeneity of population of study analyzed.
A recent report from the Working Group on Stereotactic Radiotherapy of the German Society of Radiation Oncology (DEGRO) provides recommendations for the use of SRS in BMs, suggesting to choose the dose prescription according to the dimension and number of the lesions and the proximity to OARs [5]. In fact as reported in DEGRO guidelines, stereotactic radiation treatment can be offered in patients with a single brain metastases (<30 mm diameter). Additionally, in patients with multiple (2–4) metastases (all with a diameter <25 mm), local treatment should be offered than WBRT. Analyzing dose prescription, a single dose of 20 Gy is considered acceptable, while higher doses (22–25 Gy) should be limited in small lesions (<1 cm). In patients with BMs diameter greater than 25–30 mm, a dose reduction to 18 Gy is recommended, despite RTOG 90-05 considered adequate a dose prescription of 15 Gy [23]. Moreover, recent publications have started to propose hypofractionated radiation treatment (i.e., SFRT) in order to further decrease the risk of late side effects (radionecrosis). In fact, different dose prescription schedules have been published as 27 Gy in 3 fractions [32], 25 Gy or 30 Gy in 5 fractions [33], demonstrating good results in terms of clinical outcomes and tolerability. Thus, from these multiple and various experiences, a standardized dose seems to be not well defined and a heterogeneous prescription still continues to be commonly used in a case of SRS and/or SFRT.
In the here reported experience, we decided to treat with SRS/SFRT up to five BMs [31] and the choice of the dose and/or fractionation was made based on DEGRO guidelines and international clinical experiences [5, 32, 33].
To our knowledge, this is the second analysis published combining: clinical outcomes, toxicity, and dosimetric results for BMs treated with SRS/SFRT Linac-based and FFF mode. Preclinical studies investigated the role of FFF delivery in terms of radiobiological potential influences on tumor cell killing related to higher dose rate compared to FF mode. [20, 34] In contrast to this assumption, available data remain inconclusive.
Several authors reported the dosimetric characteristics of FFF modality in different anatomical sites, including prostate, lungs, or liver [35–41], but despite the utilization of FFF modality is increasing, few clinical data regarding its safety and clinical efficacy have been reported, especially for BMs [19].
Focusing in brain metastases, two experiences have been recently published on the impact of FFF in the treatment of brain metastases [21, 22].
Stieler et al. analyzed the combination of the FFF delivery for radiosurgery of brain metastases in comparison to delivery with FF intensity-modulated radiotherapy (IMRT) and volumetric modulated radiotherapy (VMAT). Authors reported that FFF treatment plans had similar quality when compared to FF plans. In FFF approach, a BOT reduction was observed, even though an increased number of monitor unit was requested. [22].
Lai et al. published similar results. Authors evaluated the dosimetric superiority of FFF—volumetric modulated arc therapy when compared to FF—volumetric modulated arc therapy in the setting of single brain metastasis in 68 patients with a dose prescription of 20 Gy in single fraction. Authors performed a dosimetric comparison analyzing target coverage, dose gradients, BOT, gantry speed, and number of monitor units (MU). Authors reported an advantage in the use of FFF in terms of BOT (5.45 min), treatment delivery time, and mean dose rate (p < 0.001) when compared to FF. Additionally, normal brain sparing was higher in FFF approach with reduction in brain irradiation of about 2% reductions in low-dose regions (about 5–10 Gy). Authors reported a Dmean brain irradiation of 0.935 ± 0.46 in the setting of SRS treatment [22]. In our experience, we obtained a lower BOT (mean SRS 140 s) and a lower brain irradiation (Dmean brain: 0.71 Gy) in patients receiving a SRS treatment.
In terms of clinical outcomes, the first analysis was reported by Rieber et al. [19]. Authors analyzed the dosimetric and clinical outcomes of 21 consecutive patients (with a total of 25 BMs). In regards to the dosimetric parameters, the Authors showed a significant reduction of the average BOT with FFF modality respect to the standard FF, reporting a median value of BOT was 128 s (range 45–278 s). Moreover, they showed a median brain Dmean of 0.38 Gy (range 0.13–0.89) and brain V10 Gy of 31 ml (5–136). In terms of outcomes, with a median follow-up of 5.1 months (range 0–12.4 months), the Authors reported a 6-month OS, local PFS, and extracerebral PFS of 63.3, 100, and 36.1%, respectively. The acute and late toxicities were mild: nausea and headaches in acute setting, and for late side effects, only one case of asymptomatic intracerebral hemorrhage [19]. In terms of dosimetric and clinical outcomes, the present results were similar to those reported by Rieber et al., despite in the present population, the median number of lesions for patients was two respect to one in the Rieber’s analysis.
An important limitation of the present study is related to the absence of basal neurocognitive state evaluation of the patients before and after radiotherapy. This information can be in fact considered useful in follow-up clinical assessment. However, detailed basal neurocognitive state definition will be object of prospective future evaluation.
Moreover, in the here discussed data, correlation between dosimetric parameters and toxicity/outcomes was not analyzed because the number of events (radionecrosis/bleeding) was very limited (only two cases) and follow-up was too short for any further consideration.
Conclusion
Despite the limitations of the study (retrospective evaluation, relatively short follow-up time, and limited sample size), and considering the few clinical data published, the present results add clinical information about safety and efficacy of the SRS/SFRT in BMs oligometastatic patients. Moreover, when compared to other dosimetric experiences, we observed a lower BOT and Dmean brain irradiation in SRS setting. Further studies and a longer follow-up are mandatory to confirm our preliminary results in the treatment of BMs.
References
Mehta MP, Tsao MN, Whelan TJ et al (2005) The American society for therapeutic radiology and oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastasis. Int J Radiat Oncol Biol Phys 63:37–46. doi:10.1016/j.ijrobp.2005.05.023
Patchell RA (2003) The management of brain metastases. Cancer Treat Rev 29:533–540. doi:10.1016/S0305-7372(03)00105-1
Lin X, DeAngelis LM (2015) Treatment of brain metastases. J Clin Oncol 33:3475–3484. doi:10.1200/JCO.2015.60.9503
NCCN guide lines (2016) https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 30 Oct 2016
Kocher M, Wittig A, Piroth MD et al (2014) Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol 190:521–532. doi:10.1007/s00066-014-0648-7
Soffietti R, Kocher M, Abacioglu UM et al (2013) A European organisation for research and treatment of cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life result. J Clin Oncol 31:65–72. doi:10.1200/JCO.2011.41.0639
Brown PD, Jaeckle K, Ballman KV et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316:401–409. doi:10.1001/jama.2016.9839
Giaj Levra N, Sicignagno G, Fiorentino A et al (2016) Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for brain metastases: a dosimetric volumetric-modulated arc therapy study. Radiol Med 121:60–69. doi:10.1007/s11547-015-0563-8
Nieder C, Grosu AL, Gaspar LE (2014) Stereotactic radiosurgery (SRS) for brain metastases: a systematic review. Radiat Oncol 9:155. doi:10.1186/1748-717X-9-155
Leksell L (1951) The stereotactic method and radiosurgery of the brain. Acta Chir Scand 102:316–319
Wu A, Lindner G, Maitz AH et al (1990) Physics of Gamma Knife approach on convergent beams in stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 18:941–949
Alongi F, Fiorentino A, Mancosu P et al (2016) Stereotactic radiosurgery for intracranial metastases: linac-based and gamma-dedicated unit approach. Expert Rev Anticancer Ther 16:731–740. doi:10.1080/14737140.2016.1190648
Fiorentino A, Levra NG, Mazzola R et al (2015) Letter: volumetric arc therapy (RapidArc) vs Gamma Knife radiosurgery for multiple brain metastases: not only a dosimetric issue. Neurosurgery 77:E310. doi:10.1227/NEU.0000000000000796
Fiorentino A, Giaj-Levra N, Mazzola R et al (2015) Dosimetrics of intracranial stereotactic radiosurgery: only “an exercise of style”? Strahlenther Onkol 191:810–811. doi:10.1007/s00066-015-0879-2
Abacioglu U, Ozen Z, Yilmaz M et al (2014) Critical appraisal of RapidArc radiosurgery with flattening filter free photon beams for benign brain lesions in comparison to GammaKnife: a treatment planning study. Radiat Oncol 9:119. doi:10.1186/1748-717X-9-119
Kragl G, Sigamani A, Nambiraj A et al (2009) Dosimetric characteristics of 6 and 10 MV unflattened photon beams. Radiother Oncol 93:141–146. doi:10.1016/j.radonc.2009.06.008
Cashmore J (2008) The characterization of unflattened photon beams from a 6 MV linear accelerator. Phys Med Biol 53:1933–1946. doi:10.1088/0031-9155/53/7/009
Vassiliev ON, Titt U, Pönisch F et al (2006) Dosimetric properties of photon beams from a flattening filter free clinical accelerator. Phys Med Biol 51:1907–1917
Rieber J, Tonndorf-Martini E, Schramm O et al (2016) Radiosurgery with flattening-filter-free techniques in the treatment of brain metastases: plan comparison and early clinical evaluation. Strahlenther Onkol 192:789–796
Lohse I, Lang S, Hrbacek J et al (2011) Effect of high dose per pulse flattening filter-free beams on cancer cell survival. Radiother Oncol 101:226–232. doi:10.1016/j.radonc.2011.05.072
Lai Y, Chen S, Xu C et al (2016) Dosimetric superiority of flattening filter free beams for single-fraction stereotactic radiosurgery in single brain metastasis. Oncotarget 3. doi:10.18632/oncotarget.13085 (Epub ahead of print)
Stieler F, Fleckenstein J, Simeonova A et al (2013) Intensity modulated radiosurgery of brain metastases with flattening filter-free beams. Radiother Oncol 109:448–451. doi:10.1016/j.radonc.2013.10.017
Shaw E, Scott C, Souhami L et al (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 47:291–298. doi:10.1016/S0360-3016(99)00507-6
Lischalk JW, Oermann E, Collins SP et al (2015) Five-fraction stereotactic radiosurgery (SRS) for single inoperable high-risk non-small cell lung cancer (NSCLC) brain metastases. Radiat Oncol. 10:216. doi:10.1186/s13014-015-0525-2
van Persijn van Meerten EL, Gelderblom H, Bloem JL (2010) RECIST revised: implications for the radiologist. A review article on the modified RECIST guideline. Eur Radiol 20:1456–1467. doi:10.1007/s00330-009-1685-y
Kumar AJ, Leeds NE, Fuller GN et al (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384
Koay E, Sulman EP (2012) Management of brain metastasis: past lessons, modern management, and future considerations. Curr Oncol Rep. 14:70–78. doi:10.1007/s11912-011-0205-9
Maclean J, Fersht N, Singhera M et al (2013) Multi-disciplinary management for patients with oligometastases to the brain: results of a 5 year cohort study. Radiat Oncol 8:156. doi:10.1186/1748-717X-8-156
D’Agostino GR, Autorino R, Pompucci A et al (2011) Whole-brain radiotherapy combined with surgery or stereotactic radiotherapy in patients with brain oligometastases: long-term analysis. Strahlenther Onkol 187:421–425. doi:10.1007/s00066-011-2228-4
Balducci M, Autorino R, Chiesa S et al (2015) Radiosurgery or fractionated stereotactic radiotherapy plus whole-brain radioherapy in brain oligometastases: a long-term analysis. Anticancer Res 35:3055–3059
Yamamoto M, Serizawa T, Shuto T et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15:387–395. doi:10.1016/S1470-2045(14)70061-0
Minniti G, Scaringi C, Paolini S et al (2016) Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 95:1142–1148. doi:10.1016/j.ijrobp.2016.03.013
Croker J, Chua B, Bernard A et al (2016) Treatment of brain oligometastases with hypofractionated stereotactic radiotherapy utilising volumetric modulated arc therapy. Clin Exp Metastasis 33:125–132. doi:10.1007/s10585-015-9762-x
King RB, Hyland WB, Cole AJ et al (2013) An in vitro study of the radiobiological effects of flattening filter free radiotherapy treatments. Phys Med Biol 58(5):N83–N94. doi:10.1088/0031-9155/58/5/N83
Franco P, De Bari B, Ciammella P et al (2014) The role of stereotactic ablative radiotherapy in oncological and non-oncological clinical settings: highlights from the 7th meeting of AIRO–Young Members Working Group (AIRO Giovani). Tumori 100:e214–e219. doi:10.1700/1778.19280
Navarria P, Ascolese AM, Mancosu P et al (2013) Volumetric modulated arc therapy with flattening filter free (FFF) beams for stereotactic body radiation therapy (SBRT) in patients with medically inoperable early stage non small cell lung cancer (NSCLC). Radiother Oncol 107:414–418. doi:10.1016/j.radonc.2013.04.016
Reggiori G, Mancosu P, Castiglioni S et al (2012) Can volumetric modulated arc therapy with flattening filter free beams play a role in stereotactic body radiotherapy for liver lesions? A volume-based analysis. Med Phys 39:1112–1118. doi:10.1118/1.3679858
Zwahlen DR, Lang S, Hrbacek J et al (2012) The use of photon beams of a flattening filter-free linear accelerator for hypofractionated volumetric modulated arc therapy in localized prostate cancer. Int J Radiat Oncol Biol Phys 83:1655–1660. doi:10.1016/j.ijrobp.2011.10.019
Zhuang M, Zhang T, Chen Z et al (2012) Volumetric modulation arc radiotherapy with flattening filter-free beams compared with conventional beams for nasopharyngeal carcinoma: a feasibility study. Chin J Cancer 32:397–402. doi:10.5732/cjc.012.10182
Lechner W, Kragl G, Georg D (2012) Evaluation of treatment plan quality of IMRT and VMAT with and without flattening filter using Pareto optimal fronts. Radiother Oncol 103:97. doi:10.1016/j.radonc.2013.09.020
Lang S, Shrestha B, Graydon S et al (2013) Clinical application of flattening filter free beams for extracranial stereotactic radiotherapy. Radiother Oncol 106:255–259. doi:10.1016/j.radonc.2012.12.012
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that they have no conflict of interest. Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Fiorentino, A., Giaj-Levra, N., Tebano, U. et al. Stereotactic ablative radiation therapy for brain metastases with volumetric modulated arc therapy and flattening filter free delivery: feasibility and early clinical results. Radiol med 122, 676–682 (2017). https://doi.org/10.1007/s11547-017-0768-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-017-0768-0