Abstract
Background
Radiosurgical treatment of brain metastases is well established in daily clinical routine. Utilization of flattening-filter-free beams (FFF) may allow for more rapid delivery of treatment doses and improve clinical comfort. Hence, we compared plan quality and efficiency of radiosurgery in FFF mode to FF techniques.
Materials and methods
Between November 2014 and June 2015, 21 consecutive patients with 25 brain metastases were treated with stereotactic radiosurgery (SRS) in FFF mode. Brain metastases received dose-fractionation schedules of 1 × 20 Gy or 1 × 18 Gy, delivered to the conformally enclosing 80 % isodose. Three patients with critically localized or large (>3 cm) brain metastases were treated with 6 × 5 Gy. Plan quality and efficiency were evaluated by analyzing conformity, dose gradients, dose to healthy brain tissue, treatment delivery time, and number of monitor units. FFF plans were compared to those using the FF method, and early clinical outcome and toxicity were assessed.
Results
FFF mode resulted in significant reductions in beam-on time (p < 0.001) and mean brain dose (p = 0.001) relative to FF-mode comparison plans. Furthermore, significant improvements in dose gradients and sharper dose falloffs were found for SRS in FFF mode (−1.1 %, −29.6 %; p ≤ 0.003), but conformity was slightly superior in SRS in FF mode (−1.3 %; p = 0.001). With a median follow-up time of 5.1 months, 6‑month overall survival was 63.3 %. Local control was observed in 24 of 25 brain metastases (96 %).
Conclusion
SRS in FFF mode is time efficient and provides similar plan quality with the opportunity of slightly reduced dose exposure to healthy brain tissue when compared to SRS in FF mode. Clinical outcomes appear promising and show only modest treatment-related toxicity.
Zusammenfassung
Hintergrund
Die radiochirurgische Behandlung (SRS) von Hirnmetastasen wird vielfach in der klinischen Routine durchgeführt. Die zusätzliche Anwendung von ausgleichsfilterfreien Bestrahlungstechniken (FFF) kann die Bestrahlungszeit verkürzen und den Patientenkomfort erhöhen. Daher führten wir einen Plan- und Effizienzvergleich zwischen der Radiochirurgie in FFF-Technik und FF-Technik durch.
Material und Methode
Zwischen November 2014 und Juni 2015 wurden 21 Patienten mit 25 Hirnmetastasen mit SRS in FFF-Technik behandelt. Die Hirnmetastasen wurden mit 1 × 20 Gy oder 1 × 18 Gy auf die konformal umschließende 80 %-Isodose bestrahlt. Drei Patienten mit kritisch lokalisierten oder großen Metastasen (>3 cm) erhielten eine Bestrahlung mit 6 × 5 Gy. Konformität, Dosisgradienten, Behandlungszeiten, Normalgewebsdosis (Gehirn) sowie Anzahl an Monitoreinheiten wurden zur Evaluation der Planqualität herangezogen. Des Weiteren wurden Überleben und Toxizität analysiert.
Ergebnisse
Sowohl die Bestrahlungszeit sank signifikant um 57,9 % (p ≤ 0,001) für die SRS in FFF-Technik im Vergleich zur FF-Technik. als auch die durchschnittliche Bestrahlungsdosis des Gehirns (p = 0,001). Des Weiteren wurden signifikant verbesserte Dosisgradienten und folglich ein steilerer Dosisabfall für die SRS in FFF-Technik (−1,1 %, −29,6 %; p ≤ 0,003) festgestellt. Dagegen zeigte die SRS in FF-Technik einen leicht verbesserten Konformitätsindex (−1,3 %; p = 0,001). Bei einer medianen Nachbeobachtungzeit von 5,1 Monaten betrug das 6‑Monats-Gesamtüberleben 63,3 %. Bei den behandelten 25 Hirnmetastasen (96 %) waren 24 ohne lokalen Progress.
Schlussfolgerung
SRS in FFF-Technik ist zeiteffizient und ermöglicht gleiche Planqualität sowie eine leicht reduzierte Dosisbelastung des gesunden Hirngewebes im Vergleich zur SRS in FF-Technik. Entsprechend vielversprechend sind die ersten klinischen Ergebnisse bei moderater Toxizität.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the 1980s, stereotactic radiosurgery (SRS) has evolved into a precise and effective treatment option with minimal morbidity for patients with oligometastatic cranial disease [1–5]. SRS is particularly useful for the treatment of single brain metastases less than 3 cm in diameter in patients with a life expectancy of more than 3 months [6, 7]. For patients with 2–4 brain metastases, SRS is preferred over whole-brain radiotherapy (WBRT) given SRS minimizes neurocognitive side effects and yields improved quality of life [8]. Furthermore, adjuvant WBRT following SRS has not been shown to improve survival when compared to SRS with salvage WBRT [6, 9].
SRS is a noninvasive, highly conformal technique that allows for high single doses of radiation to be delivered in an effort to effectively ablate metastatic disease [10]. However, long duration of radiotherapy fractions with single doses up to 20 Gy has been a worry for many patients who are commonly positioned within rigid and uncomfortable masks. In an effort to reduce treatment time, the flattening filter (FF) of a linac can be removed leading to a higher dose–rate delivery and thereby substantially shortening beam-on time. By utilizing these steep dose gradients surrounding normal tissue is spared to a greater extent than could be achieved with conventional radiotherapy [11]. In addition, these flattening-filter-free (FFF) techniques have certain dosimetric advantages with lower out-of-field dose due to reduced head scatter, leaf transmission, and lower dose outside the field edge [12, 13].
In our institution, this method has been clinically available for over a year. Here we report the dosimetric and clinical outcomes of 21 patients with 25 brain metastases treated with SRS in FFF mode relative to standard FF mode.
Materials and methods
Patients and brain metastases
Patient characteristics are summarized in Table 1. Between November 2014 and June 2015, 21 patients with 25 brain metastases were treated using SRS in the Department of Radiooncology of our institution. Three patients had multiple brain metastases (2, 2, and 3). Graded Prognostic Assessment scores (GPA) were calculated for all patients [14]. The analysis was approved by the ethics committee of our institution (S-140/2016).
Planning and treatment features
Patients were immobilized using custom Aquaplast masks (IT V, Innsbruck, iCAST micro perforation). For treatment planning, the gross tumor volume (GTV) of each brain metastasis was delineated on both contrast-enhanced computer tomography (CT) and magnetic resonance imaging (MRI). As the geometric accuracy due to intrafractional movement was expected to be in the range of 2–3 mm, a safety margin of 3 mm was expanded from GTV to create the planning target volume (PTV). The PTV was prescribed to receive 1 × 20 Gy for metastases <2 cm or 1 × 18 Gy for metastases <3 cm, delivered to the conformally enclosing 80 % isodose, yielding a central dose maximum of 25 or 22.5 Gy, respectively. Three patients with critically localized (those near the brain stem) or large (>3 cm) brain metastases were treated with 6 × 5 Gy. Prior to irradiation, image guidance was performed by means of KV cone beam CT (CBCT).
Concordant plans in FF mode were created for each treated lesions. Plans were designed and calculated using Oncentra (version 4.5). Delivery techniques comprised 3‑dimensional (3D) conventional (n = 21), step-and-shoot intensity-modulated radiotherapy (step-and-shoot IMRT; n = 3) and volumetric modulated arc therapy (VMAT; n = 1) radiotherapy in FFF and FF mode, respectively. The same number of fields or arcs was used for the corresponding plans in FF mode. For 3D conventional radiotherapy 10 fields (n = 4) and 11 fields (n = 17) were applied, while 8 fields (n = 1) and 11 fields (n = 2) were used for step-and-shoot IMRT. The VMAT plan comprised 4 arcs. Step-and-shoot IMRT and VMAT plans were applied whenever dose coverage was inadequate with 3D conventional plans. A collapsed cone (CC) algorithm was used for dose calculation. All patients were treated with 6 MV flattening filter-free plans using the Elekta Versa HD with a maximum dose rate of 1400 MU/min. The multileaf collimator (MLC) agility with 5 mm leafs at the isocenter was used for radiation delivery.
Plan evaluation and comparison
For comparative plan evaluation, 6 MV flattening filter plans were calculated. The modified Paddick Conformity Index was applied for comparison [15, 16]:
where V ptv,pi is the partial volume of the PTV covered by the prescribed isodose, V ptv is the planning target volume, and V pi is the body volume of the patient covered by the prescribed isodose [15]. Hence, a score of 1.0 indicates perfect conformity, while a score of less than 1 shows inferior conformity. In addition, two gradient indices as described by Paddick et al. and modified by Stieler et al. were used [17, 18]: GIHigh = V 50%Presc.Dose /V 90%Prescr.Dose and GILow = V 25%Presc.Dose /V 50%Prescr.Dose. As a steep dose falloff outside the target is intended, the ideal value for the gradient indices is supposed to be the lowest achievable. No homogeneity index was calculated as all lesions except for three were treated with dose prescriptions to the conformally enclosing 80% isodose. The other three fractionated plans were normalized such that the prescription dose was the median dose to the PTV.
No detailed analysis of organs at risk (OAR) as chiasma or optical nerves was conducted as their distance from the PTV was too long to result in clinically meaningful dose exposure. However, we compared the respective brain volume receiving 10, 5, 2, 1, 0.5, and 0.25 Gy (V10Gy, V5Gy, V2Gy, V10Gy, V1Gy, V0.5Gy, V0.25Gy) between radiosurgery in FFF mode and FF technique. Beam-on time, as well as total treatment time was registered for all patients during each day of treatment. Total treatment time comprised beam-on time as well as time for daily image guidance and was registered for each patient separately. Furthermore, beam-on and total treatment times were also calculated for FF plans for comparison.
Outcome evaluation
All patients were seen for follow-up visits at the University Hospital and underwent a clinical examination. When patients presented with neurological deteriotration or if new neurological symptoms were recorded, cranial MRI scan was performed. Overall survival (OS) and progression-free survival (PFS) as well as local and extracerebral PFS was calculated from the end of radiosurgery treatment. Local control was defined as no progression of the metastasis within the treated area. Newly diagnosed brain metastases were not classified as local failure but as intracerebral progression. Statistical comparisons were performed with SPSS (version 20.0) using the nonparametric Wilcoxon signed-rank test. Significance was noted for two-tailed p-values of ≤0.05. Outcome was calculated using the Kaplan–Meier method and treatment-related toxicity was classified according to CTCAE v4.0.
Results
Planning procedure and technical administration
In total, 21 patients with up to 3 brain metastases were treated using SRS in FFF mode. Mean PTV for all lesions was 4.5 ml (range 0.9–23.9 ml). Patient characteristics are shown in Table 1.
To show comparability between FFF and FF techniques, we generated both FFF and FF plans for all lesions with the respective technique. Average conformity was noted to be slightly better for radiosurgery plans in FF mode (−1.3 %; p = 0.001; Table 2). On the contrary, both low and high dose gradients were significantly superior for radiosurgery in FFF technique (−1.1 %, −29.6 % mean; p = 0.003; p ≤ 0.001, respectively; Table 3). Concordantly, mean brain dose was significantly lower with radiosurgery in FFF mode (−3.9 % mean; p = 0.001). Radiation dose to healthy brain tissue was particularly reduced in low-dose regions. Brain volume receiving 0.25 or 0.5 Gy or more was decreased by 4.2 % or 17.9 % on average, with FFF mode relative to FF technique, respectively (p = 0.001; p = 0.039; Table 3).
Using FFF techniques, beam-on time was significantly reduced by 57.9 % on average compared to FF techniques, and this reduction also led to a significant decrease in total treatment time of 27.8 % on average (p ≤ 0.001; p ≤ 0.001). Total number of monitor units was slightly reduced for SRS using FFF mode (−1.6 % mean; p = 0.013; Table 2).
Survival and local control
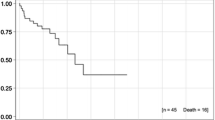
Median follow-up time was 5.1 months (range 0–12.4 months) with 6‑month OS of 63.3 % (Fig. 1a). In detail, 7 patients died due to nonneurological tumor progression during the follow-up period. The 6‑month LPFS was 100 %; however, one patient developed local progression after 10.6 months (Fig. 1b). Eight patients developed additional brain metastases and required salvage treatment (38.1 %). Major failure pattern was extracerebral progression with a 6‑month extracerebral PFS of 36.1 %. Furthermore, OS, LPFS, and extracerebral PFS were not significantly influenced by Karnofsky performance score, lesion size or time to metastasis (synchronous vs. metachronous). Treatment planning MRI scans and follow-up examinations for 2 patients are illustrated in Fig. 2.
Treatment planning and follow-up magnetic resonance imaging (MRI) scans after radiosurgery in FFF technique. a After 12 months (mo), both irradiated brain metastases still show increased diameter, while contrast enhancement is profoundly reduced demonstrating a good treatment response. b After 6 months the initially treated left temporal brain metastasis delineates complete treatment response, however a further temporopolar brain metastasis is newly diagnosed and also treated with radiosurgery. After another 3 months, the patient is diagnosed with clinically asymptomatic temporopolar, intralesional hemorrhage. Metastasis progression was excluded with blood-suppressed MRI imagining (data not shown)
Toxicity
Early toxicity was very low with only mild medically managed nausea and headaches reported in only 2 patients (CTCAE 1°). Late toxicity was only available for 20 patients (80 %). One patient developed asymptomatic intracerebral hemorrhage (Fig. 2b). No radionecrosis events were detected.
Discussion
In the present study, we report preliminary clinical results for our first patients treated with SRS in FFF mode. Furthermore, we performed plan comparisons and time efficiency analysis of SRS with FFF technique to treatment in FF mode for all patients.
In recent years, SRS has been increasingly utilized for treatment of brain metastases as it is known to be safe and highly efficient [7, 9]. A current report of the DEGRO Working Group on Stereotactic Radiotherapy recommends considering SRS as treatment for patients with 1–4 brain metastases (<2.5 cm) and a life expectancy of more than 3 months [6]. According to the Graded Prognostic Assessment scores defined by Sperduto et al. [14], all patients in this study showed a life expectancy of more than 3 months (median life expectancy 9.4 months [range 3.1–15.1 months]) and hence were candidates for SRS. However, a recent meta-analysis compared three prospective trials investigating SRS with or without WBRT for patients with 1–4 brain metastases and showed that patients >55 years of age had a significantly increased risk of intracranial failure following SRS alone [19]. The patient cohort in this study had a median age of 62.6 years and therefore an increased risk of distant brain failure. However, the above mentioned meta-analysis failed to show that SRS had a statistically significant negative impact on survival in this cohort [19]. In line with these results, the EORTC 22952-26001 study reported that WBRT after SRS or surgery did not improve the duration of functional independence and OS [20]. Hence, the DEGRO guideline recommends to withhold WBRT for as long as possible for patients with 1–4 small (<2.5 cm) brain metastases, as WBRT carries the risk of causing neurocognitive decline [20].
Two recent trials even questioned whether primary treatment with SRS could also be extended to patients with larger numbers of brain metastases [21, 22]. In a multi-institutional prospective trial Yamamoto et al. [22] reported that SRS alone as initial treatment for patients with five to ten brain metastases demonstrated noninferior survival compared to patients with two to four brain metastases. However, radiotherapy with SRS using conventional linacs is known to last up to 45–60 min. Hence, treatment of multiple brain metastases would force patients to spend hours in rigid and uncomfortable scotch cast masks. In this study, we illustrated that beam-on time as well as total treatment was significantly reduced by 57.9 % and 27.8 %, respectively (p ≤ 0.001; p ≤ 0.001), when using FFF techniques compared to conventional SRS in FF mode. Furthermore, due to accelerated radiotherapy treatment and improved image guidance, we were able to use more convenient Aquaplast masks instead of scotch cast masks, thus, further increasing patient comfort. Interestingly, the time advantage of FFF beams was recently shown to be dose dependent [23]. Analyzing extracranial stereotactic radiotherapy (SBRT) of lung and abdominal tumors (26 cases), Lang et al. [23] detected a significant treatment time reduction starting at 4 Gy for 6 MV FFF and 10 Gy for 10 MV FFF beams compared to SBRT in FF mode. Hence, radiosurgery using high doses per fraction is an ideal treatment for utilization of the FFF technique.
One further advantage of FFF beams is believed to be their different physical characteristics when compared to conventional unflattened photon beams. Removing the flattening filter leads to a reduction of out-of-field dose due to reduced head scatter, leaf transmission, and lower dose outside the field edge [11, 12, 24]. Correspondently, we detected significantly reduced mean brain dose when comparing SRS in FFF mode to that in FF mode (p = 0.001). Mean reduction was rather small (−3.9 %), as reduction was mainly found in low-dose regions. In detail, the brain volume receiving at least 0.25 or 0.5 Gy was on average decreased by 4.2 % or 17.9 %, respectively (p = 0.001; p = 0.039) when comparing radiosurgery in FFF mode to FF technique. In summary, we detected a slight reduction in mean brain dose which may be of only minor clinical relevance.
We also showed improved dose gradients and sharper dose falloff for SRS in FFF mode compared to SRS in FF mode. In particular, the high dose gradient index was reduced by a mean 29.6 %in when using FFF mode (p ≤ 0.001). Hence, dose spillage to healthy brain tissue is significantly reduced in SRS when applying the FFF technique. Of note, we detected a slight but significant reduction of conformity in FFF plans when compared to respective FF plans (p = 0.001). Absolute mean reduction was small with only 1.3 %. In line with our results, Reggiori et al. [25] showed slightly reduced conformity indices and PTV coverage for smaller targets like brain metastases when compared to treatment in FF mode. To our knowledge, there is only one study analyzing SRS in FFF mode for the treatment of brain metastases: Stieler et al. [18] performed plan comparison of 15 theoretical patients treated with SRS in FFF mode and FF technique. Compared to our results, Stieler et al. detected slightly better conformity and PTV coverage when using volumetric modulated arc therapy (VMAT) relative to 3D conventional SRS. However, similar to our results, they detected marginally better conformity for plans in FF mode [18]. In general, using highly variable treatment techniques (3D conformal radiotherapy, volumetric modulated arc therapy [VMAT] and step-and-shoot intensity-modulated radiotherapy [step-and-shoot IMRT]) and analyzing radiotherapy for different tumor locations and sizes, several previous studies described similar plan quality and OAR sparing for radiotherapy in FFF mode compared to FF technique [26–32].
Regarding other radiosurgery devices, there is evidence that plan quality is probably improved when performing SRS with Cyber- or Gammaknife compared to SRS with classical linacs [33, 34]. However, when applying VMAT techniques, plan quality was shown to be equivalent to Cyberknife and Gammaknife plans [35, 36]. The further usage of FFF mode with VMAT techniques might provide optimal plan quality for metastases at critical locations combined with faster treatment time compared to Cyber- or Gammaknife irradiation.
Although FFF beams have been increasingly applied in patient treatment, only few clinical data regarding their safety and clinical efficacy have been reported. Clinical data with respect to toxicity and outcome for FFF treatment have mainly been reported by an Italian group from IRCCS Instituto Clinico Humanitas in Milan [26, 37–39]. Analyzing 25 oligometastatic patients with isolated abdominal or pelvic lymph nodes treated with VMAT using FFF beams, they detected no local progression or toxicity ≥CTCAE 3° after 6 months [38]. Furthermore, they recently showed preliminary results of a phase II study investigating the clinical potential of hypofractionated radiotherapy in prostate cancer and reported only minimal acute toxicity [37]. A previous study by Stieb et al. [40] reported minimal toxicity and excellent 1‑year local control using SBRT in FFF technique for various tumors of 84 patients. Similar results were also shown by Prendergast and Wang et al. investigating feasibility of SBRT for patients with lung malignancies and hepatocellular carcinoma, respectively [41, 42]. To our knowledge, we are the first to evaluate early clinical outcomes and toxicity for radiosurgery of brain metastases with FFF techniques. Analyzing SRS for 21 patients with 25 brain metastases, we detected only mild acute and late side-effects with no toxicity ≥CTCAE 2°. Preliminary local control was good with only one local failure identified during follow-up. Furthermore, our study is one of the few studies which did not only perform comparative plan and time efficiency analysis but also provided preliminary clinical data for the analyzed patients.
One limitation to the study was the relatively short follow-up time which was caused by the fact that radiosurgery treatment in FFF mode has only been available for 18 months at our institution. Furthermore, one third of the patients died due to extracranial tumor progression during follow-up time further reducing follow-up time. In addition, all clinical analyses were performed retrospectively on the basis of medical records which may have led to an underestimation of side effects.
Conclusion
Patient treatment with SRS in FFF mode was time efficient and safe. In general, plan quality was comparable between SRS in FF mode and FFF technique; however SRS in FFF technique provided slightly reduced dose spillage to normal brain parenchyma.
References
Leksell DG (1987) Stereotactic radiosurgery. Present status and future trends. Neurol Res 9(2):60–68
Lutz W, Winston KR, Maleki N (1988) A system for stereotactic radiosurgery with a linear accelerator. Int J Radiat Oncol Biol Phys 14(2):373–381
Sturm V, Kober B, Hover KH et al (1987) Stereotactic percutaneous single dose irradiation of brain metastases with a linear accelerator. Int J Radiat Oncol Biol Phys 13(2):279–282
Rades D, Hornung D, Blanck O et al (2014) Stereotactic radiosurgery for newly diagnosed brain metastases: comparison of three dose levels. Strahlenther Onkol 190(9):786–791. doi:10.1007/s00066-014-0625-1
Treuer H, Hoevels M, Luyken K et al (2014) Intracranial stereotactic radiosurgery with an adapted linear accelerator vs. robotic radiosurgery. Strahlenther Onkol 191(6):470–476. doi:10.1007/s00066-014-0786-y
Kocher M, Wittig A, Piroth MD et al (2014) Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol 190(6):521–532. doi:10.1007/s00066-014-0648-7
Lin X, DeAngelis LM (2015) Treatment of brain metastases. J Clin Oncol 33(30):3475–3484. doi:10.1200/jco.2015.60.9503
Soffietti R, Kocher M, Abacioglu UM et al (2013) A European Organisation for Research and Treatment of Cancer phase III trial of Adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life result. J Clin Oncol 31(1):65–72. doi:10.1200/jco.2011.41.0639
Nieder C, Grosu AL, Gaspar LE (2014) Stereotactic radiosurgery (SRS) for brain metastases: a systematic review. Radiat Oncol 9:155. doi:10.1186/1748-717X-9-155
Hartmann GH, Schlegel W, Sturm V et al (1985) Cerebral radiation surgery using moving field irradiation at a linear accelerator facility. Int J Radiat Oncol Biol Phys 11(6):1185–1192
Kragl G, af Wetterstedt S, Knäusl B et al (2009) Dosimetric characteristics of 6 and 10 MV unflattened photon beams. Radiother Oncol 93(1):141–146. doi:10.1016/j.radonc.2009.06.008
Cashmore J (2008) The characterization of unflattened photon beams from a 6 MV linear accelerator. Phys Med Biol 53(7):1933–1946. doi:10.1088/0031-9155/53/7/009
Vassiliev ON, Titt U, Ponisch F et al (2006) Dosimetric properties of photon beams from a flattening filter free clinical accelerator. Phys Med Biol 51(7):1907–1917. doi:10.1088/0031-9155/51/7/019
Sperduto PW, Kased N, Roberge D et al (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30(4):419–425. doi:10.1200/JCO.2011.38.0527
Audet C, Poffenbarger BA, Chang P et al (2011) Evaluation of volumetric modulated arc therapy for cranial radiosurgery using multiple noncoplanar arcs. Med Phys 38(11):5863–5872. doi:10.1118/1.3641874
Paddick I (2000) A simple scoring ratio to index the conformity of radiosurgical treatment plans. J Neurosurg 93(supplement 3):219–222. doi:10.3171/jns.2000.93.supplement 3.0219
Paddick I, Lippitz B (2006) A simple dose gradient measurement tool to complement the conformity index. J Neurosurg 105(Suppl):194–201. doi:10.3171/sup.2006.105.7.194
Stieler F, Fleckenstein J, Simeonova A et al (2013) Intensity modulated radiosurgery of brain metastases with flattening filter-free beams. Radiotherapy and oncology. J Eur Soc Ther Radiol Oncol 109(3):448–451. doi:10.1016/j.radonc.2013.10.017
Sahgal A, Aoyama H, Kocher M et al (2015) Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol 91(4):710–717. doi:10.1016/j.ijrobp.2014.10.024
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29(2):134–141. doi:10.1200/JCO.2010.30.1655
Bhatnagar AK, Flickinger JC, Kondziolka D et al (2006) Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol 64(3):898–903. doi:10.1016/j.ijrobp.2005.08.035
Yamamoto M, Serizawa T, Shuto T et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15(4):387–395. doi:10.1016/S1470-2045(14)70061-0
Lang S, Shrestha B, Graydon S et al (2013) Clinical application of flattening filter free beams for extracranial stereotactic radiotherapy. Radiother Oncol 106(2):255–259. doi:10.1016/j.radonc.2012.12.012
Kragl G, Baier F, Lutz S et al (2011) Flattening filter free beams in SBRT and IMRT: Dosimetric assessment of peripheral doses. Z Med Phys 21(2):91–101. doi:10.1016/j.zemedi.2010.07.003
Reggiori G, Mancosu P, Castiglioni S et al (2012) Can volumetric modulated arc therapy with flattening filter free beams play a role in stereotactic body radiotherapy for liver lesions? A volume-based analysis. Med Phys 39(2):1112–1118. doi:10.1118/1.3679858
Navarria P, Ascolese AM, Mancosu P et al (2013) Volumetric modulated arc therapy with flattening filter free (FFF) beams for stereotactic body radiation therapy (SBRT) in patients with medically inoperable early stage non small cell lung cancer (NSCLC). Radiotherapy and oncology. J Eur Soc Ther Radiol Oncol 107(3):414–418. doi:10.1016/j.radonc.2013.04.016
Nicolini G, Ghosh-Laskar S, Shrivastava SK et al (2012) Volumetric modulation arc radiotherapy with flattening filter-free beams compared with static gantry IMRT and 3D Conformal radiotherapy for advanced esophageal cancer: A feasibility study. Int J Radiat Oncol 84(2):553–560. doi:10.1016/j.ijrobp.2011.12.041
Zwahlen DR, Lang S, Hrbacek J et al (2012) The use of photon beams of a flattening filter-free linear accelerator for Hypofractionated volumetric modulated arc therapy in localized prostate cancer. Int J Radiat Oncol 83(5):1655–1660. doi:10.1016/j.ijrobp.2011.10.019
Lu JY, Zheng J, Zhang WZ et al (2016) Flattening filter-free beams in intensity-modulated radiotherapy and volumetric modulated arc therapy for Sinonasal cancer. PLoS ONE 11(1):e0146604. doi:10.1371/journal.pone.0146604
Spruijt KH, Dahele M, Cuijpers JP et al (2013) Flattening filter free vs flattened beams for breast irradiation. Int J Radiat Oncol 85(2):506–513. doi:10.1016/j.ijrobp.2012.03.040
Gasic D, Ohlhues L, Brodin NP et al (2014) A treatment planning and delivery comparison of volumetric modulated arc therapy with or without flattening filter for gliomas, brain metastases, prostate, head/neck and early stage lung cancer. Acta Oncol 53(8):1005–1011. doi:10.3109/0284186X.2014.925578
Lechner W, Kragl G, Georg D (2013) Evaluation of treatment plan quality of IMRT and VMAT with and without flattening filter using Pareto optimal fronts. Radiother Oncol 109(3):437–441. doi:10.1016/j.radonc.2013.09.020
Kaul D, Badakhshi H, Gevaert T et al (2015) Dosimetric comparison of different treatment modalities for stereotactic radiosurgery of meningioma. Acta Neurochir (Wien) 157(4):559–563 (discussion 563–554) doi:10.1007/s00701-014-2272-9
Treuer H, Hoevels M, Luyken K et al (2014) Intracranial stereotactic radiosurgery with an adapted linear accelerator vs. robotic radiosurgery : Comparison of dosimetric treatment plan quality. Strahlenther Onkol. doi:10.1007/s00066-014-0786-y
Kim H, Potrebko P, Rivera A et al (2015) Tumor volume threshold for achieving improved conformity in VMAT and Gamma Knife stereotactic radiosurgery for vestibular schwannoma. Radiother Oncol 115(2):229–234. doi:10.1016/j.radonc.2015.03.031
Mayo CS, Ding L, Addesa A et al (2010) Initial experience with volumetric IMRT (RapidArc) for intracranial stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 78(5):1457–1466. doi:10.1016/j.ijrobp.2009.10.005
Alongi F, Cozzi L, Arcangeli S et al (2013) Linac based SBRT for prostate cancer in 5 fractions with VMAT and flattening filter free beams: preliminary report of a phase II study. Radiat Oncol 8(1):1–8. doi:10.1186/1748-717x-8-171
Alongi F, Fogliata A, Clerici E et al (2012) Volumetric modulated arc therapy with flattening filter free beams for isolated abdominal/pelvic lymph nodes: report of dosimetric and early clinical results in oligometastatic patients. Radiat Oncol 7(1):1–9. doi:10.1186/1748-717x-7-204
Scorsetti M, Alongi F, Castiglioni S et al (2011) Feasibility and early clinical assessment of flattening filter free (FFF) based stereotactic body radiotherapy (SBRT) treatments. Radiat Oncol 6:113. doi:10.1186/1748-717X-6-113
Stieb S, Lang S, Linsenmeier C et al (2015) Safety of high-dose-rate stereotactic body radiotherapy. Radiat Oncol 10(1):27. doi:10.1186/s13014-014-0317-0
Prendergast BM, Dobelbower MC, Bonner JA et al (2013) Stereotactic body radiation therapy (SBRT) for lung malignancies: preliminary toxicity results using a flattening filter-free linear accelerator operating at 2400 monitor units per minute. Radiat Oncol 8:273. doi:10.1186/1748-717X-8-273
Wang P‑M, Hsu W‑C, Chung N‑N et al (2014) Feasibility of stereotactic body radiation therapy with volumetric modulated arc therapy and high intensity photon beams for hepatocellular carcinoma patients. Radiat Oncol 9(1):1–9. doi:10.1186/1748-717x-9-18
Acknowledgements
This work was supported by the Medical Faculty providing a research grant for JR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Rieber, E. Tonndorf-Martini, O. Schramm, B. Rhein, S. Stefanowicz, J. Kappes, H. Hoffmann, K. Lindel, J. Debus and S. Rieken declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Ethical approval was obtained from the local Ethics Committee (S-140/2016).
Rights and permissions
About this article
Cite this article
Rieber, J., Tonndorf-Martini, E., Schramm, O. et al. Radiosurgery with flattening-filter-free techniques in the treatment of brain metastases. Strahlenther Onkol 192, 789–796 (2016). https://doi.org/10.1007/s00066-016-1012-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-016-1012-x