Abstract
Objective
To develop a feasible volumetric modulated arc therapy (VMAT) treatment in whole brain radiotherapy (WBRT) with a simultaneous integrated boost (SIB) and hippocampal (HP) sparing in 1–5 brain metastases (BMs).
Methods and materials
Ten patients with 20 BMs received a WBRT prescription of 20 Gy, SIB dose on BMs of 40 Gy/5 fractions. PTVWBRT was generated from brain minus BMs-PTVs (PTVSIB) and planning organ at risk volume to HP. All plans were evaluated in: homogeneity index (HI), target coverage (TC), maximum dose to prescription dose ratio (MDPD), prescription isodose to target volume ratio (PITV) and paddick conformity index (CI). We also evaluate D100 %, mean and maximum doses to HP. Planning objectives were for PTVWBRT, D2 % = 25 Gy with acceptable deviation of 26.7 Gy and D98 % ≥ 16.7 Gy; for PTVSIB D95 % ≥ 38 Gy; for HP, D100 % = 6 Gy with acceptable deviation of 6.7 Gy, Dmax = 10.7 Gy with acceptable deviation of 11.3 Gy, a mean dose of 8 Gy.
Results
Mean number of BMs was 2 (range 1–5). Mean values for BMs were volume of PTVSIB = 5.1 ± 4.9 cc, dose to 95 % of PTVSIB 39.3 ± 0.9 Gy, HI 0.083 ± 0.03, TC 0.96 ± 0.24, CI 0.78 ± 0.17. Mean MDPD was 1.06 ± 0.02 and PITV 0.96 ± 0.24. For WBRT, mean target volume was (13.46 ± 2)*102 cc, mean dose to 90 % of PTVWBRT 19.8 ± 0.2 Gy, mean HI 0.42 ± 0.12 and TC 0.78 ± 0.11. Mean and maximum HP doses were 7.7 ± 0.3 Gy and 10.5 ± 0.5 Gy. Mean dose to 100 % of HP volume (D100 %) was 6.7 ± 0.3 Gy.
Conclusions
WBRT plus SIB with HP avoidance with VMAT was feasible. All dosimetric parameters were satisfied for PTVWBRT and PTVSIB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases (BMs) are the most common intracranial tumors in adults. About 20–40 % of patients with a diagnosis of cancer will develop brain metastases during oncological history of the disease [1] and whole brain radiotherapy (WBRT) is considered historically the standard treatment in these patients. Local control (LC) probability is considered between 0 and 71 % and median overall survival (OS) is estimated to be between 4 and 6 months [2–4]. In the last decades, the implementation in neurosurgical techniques and radiosurgery (SRS) has allowed to use more aggressive local treatment with the goal to increase LC probability and potentially OS. The literature reports a statistical advantage on OS probability in patients with a single brain metastasis (BM) treated with a combination of WBRT and SRS compared with WBRT alone. Similarly, in patients with more than 1 BM, an advantage in the use of WBRT and SRS was confirmed in terms of LC probability, intracranial time to progression, performance status improvement and decrease in corticosteroid use [5].
Nowadays, improvement in technology represented by volumetric modulated arc therapy (VMAT) technique and other rotational intensity modulated radiotherapy (IMRT) associated with the introduction of image guided radiotherapy (IGRT) allows the possibility to integrate WBRT and a simultaneous integrated boost (SIB) to the macroscopic BMs [6, 7]. The rationale is to mimic the disruptive potential of high dose per fraction prescribed in radiosurgery and stereotactic hypofractionated radiotherapy in a maximum of 5 macroscopical sites, delivering in the same time a WBRT to sterilize microscopical disease. This approach overcrosses the traditional SRS techniques and potentially can guarantee a radiobiological advantage to reduce tumor cell proliferation and cellular repopulation that are associated with treatment failure and decrease of treatment tolerability. In a recent dosimetric study, Lagerwaard et al. [8] explored the feasibility of a new hypofractionated schedule in WBRT (20 Gy in 5 fractions) and SIB (40 Gy in 5 fractions). This schedule offers the advantage of shorter treatment time, which could be very useful in oligometastatic patients that need systemic therapy. The use of 20 Gy in 5 fractions to the WBRT can be considered an acceptable fractionations as reported by RTOG determined that 30 Gy in 2 weeks or 20 Gy in 1 week were associated with a comparable median survival (15–18 weeks) and overall response rates probability (75–80 percent for symptom palliation) [9].
To date, the disadvantage in the use of WBRT could be caused by the risk of neurocognitive decline related to radiation. Recent analysis showed that hippocampal-dependent functions, including learning, memory and spatial information processing, are preferentially affected by radiotherapy [10, 11].
The use of WBRT with hippocampal sparing to preserve neurocognitive functions is a novel concept [12–14]. The analysis of WBRT induced neurocognitive decline can be misinterpreted for intracranial disease itself that, at the time of diagnosis or during progression, could affect neurocognitive function. Some clinical studies hypothesized that radiation-induced damage to neuronal progenitor cells in the subgranular zone of the hippocampi may increase cognitive decline in BMs patients [15, 16].
Hippocampal sparing is considered safe as reported by Ghia et al. [12] because the incidence of metastases within 5 mm of hippocampi was very low, estimated to be 3.3 %. They analyzed 272 BMs suggesting that the use of hippocampal sparing was not associated with a decrease in central nervous system control probability.
Currently, the Radiation Therapy Oncology Group (RTOG) is investigating whether WBRT with hippocampal sparing preserves neurocognitive function using IMRT techniques including VMAT (available at: http://www.rtog.org).
The feasibility of delivering WBRT with hippocampal avoidance and SIB for BMs has been reported using different rotation IMRT techniques [14, 16, 17]. Aim of this study is to evaluate the feasibility of planning WBRT with simultaneous integrated boost (SIB) and hippocampal sparing with a short schedule of 5 fractions proposed by Lagerwaard et al. in patients with a limited number of BMs.
Materials and methods
Target definition and treatment planning
Ten cases of patients with diagnosis of BMs were planned for a VMAT hypofractionated hippocampal sparing SIB treatment. Inclusions criteria were: 1–5 BMs and maximum volume of BMs ≤50 cc. All patients presented a minimal distance between BMs and hippocampus PRV inferior than 5 mm. The patients underwent computed tomography (CT) simulation with a 1-mm slice thickness for radiation therapy planning in a thermoplastic mask (BrainLAB®, Feldkirchen, Germany). A co-registration of volumetric T1 sequences of diagnostic magnetic resonance imaging (MRI), typically a 3-dimensional spoiled gradient series with 1-mm slice thickness was used to define target and organs at risk (OARs). Gross Tumour Volume of Simultaneous Integrated Boost (GTVSIB) was defined as macroscopic contrast enhancing lesion on T1-MRI, SIB Clinical Tumour Volume (CTVSIB) was equal to GTVSIB. A SIB Planning Target Volume (PTVSIB) was defined as GTVSIB plus 3 mm to correct for possible residual positional inaccuracies using an online cone beam CT setup protocol. CTVWBRT was defined as the entire brain. PTVWBRT was defined as equal to CTVWBRT.
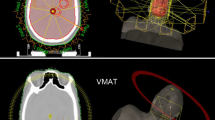
OARs defined were: eyes, optic nerves, optic chiasm, brainstem and hippocampus. The hippocampus was contoured manually by a single radiation oncologist (NGL) and reviewed by a second senior radiation oncologist (FA) and in accordance with RTOG atlas definition (available at: http://www.rtog.org), as shown in Fig. 1.
A hippocampal Planning Risk Volume (PRV) was generated using a computer-automated 5 mm 3D margin expansion of the contoured hippocampus. The PTVWBRT for optimization was generated by contouring the whole brain and excluding the PTVSIB for each metastasis and the hippocampal avoidance structure.
A linear accelerator-based radiosurgery system equipped with a multileaf collimator (True Beam™, Varian Medical Systems, Palo Alto, CA, and BrainLAB®, Feldkirchen, Germany) was adopted for all treatments’ delivery.
For patient positioning, a frameless system with an optical tracking system and a cone beam computed tomography (CBCT) were utilized.
The prescription to the PTVWBRT was 20 Gy with an Equivalent Dose in 2 Gy fractions (EQD2) of 23.33 Gy and a/ß ratio of 10 Gy and PTVSIB of 40 Gy (EQD2 = 60 Gy-a/ß ratio of 10 Gy), respectively, in 5 fractions.
Normal tissue sparing was quantified using the mean normalized total dose, which is the total dose given in 2-Gy fractions that would give a biologically equivalent effect as the actual fractionation schedule. Constraints values were derived from RTOG 0933 and from Timmerman et al. [18]. An a/ß ratio of 2 Gy was assumed for the hippocampus and 3 Gy for the eyes.
VMAT plan
CT planning images and contours were transferred to the RapidArc® optimization planning system environment from the Eclipse™ (Varian Medical Systems, Palo Alto, CA) treatment planning system using the digital imaging and communications in medicine format (DICOM).
All plans were optimized in Eclipse™ version 11 for 6MV photons for linear accelerator with a Millennium 120-leaf multileaf collimator (Varian Medical Systems, Palo Alto, CA). The RapidArc® planning was based on three coplanar full arcs: two arcs rotated clockwise and one rotated counter-clockwise. Collimators were rotated of ±10° to ensure that possible tongue and grove underdosage did not add up along the same lines.
To reduce the inter-variability planner, an initial set of constraints, including target coverage and sparing of OARs, according to previously described constraints (Table 1b), was defined and utilized for all treatment plans.
The main planning objective was to reduce the mean dose (Dmean) to the hippocampus without compromising coverage of the PTVSIB and PTVWBRT. It was possible due to a region of dose gradient resulted by hippocampus PRV subtracted from hippocampus structure. A second planning objective was to optimize the PTV dose conformity while maintaining target coverage (TC) for the BMs. The same template set of constraints was used in inverse planning to PTVSIB and PTVWBRT, hippocampus, eyes, optical nerves and brainstem for each plan.
Volume dose was calculated using the Anisotropic Analytical Algorithm (AAA 10.0.28) with a 2.5-mm dose grid. After a first optimization and dose calculation, “Continue Previous Optimization” (CPO) was performed. CPO uses the AAA calculated plan as a starting point to compensate for differences between the simplified dose calculation algorithm used during optimization (Progressive Resolution Optimizer PRO 10.0.28) and final dose calculation with AAA.
To verify the delivery feasibility of RapidArc® plan, Quality Assurance (QA) procedures were performed for each treatment plan. Each RapidArc® treatment plan was delivered to a cylindrical solid water phantom to simulate treatment. Plans were delivered using 6MV photons with a maximum dose rate of 600MU/min.
Quantitative and qualitative evaluation of the RapidArc® plans was performed using the cumulative dose–volume histogram.
Within each plan and for each PTVSIB, the quality of RapidArc® plans was assessed according to the following index: homogeneity index (HI), target coverage (TC), maximum dose to prescription dose ratio (MDPD), VMAT prescription isodose to target volume ratio (PITV) and a paddick conformity index (CI). For PTVWBRT each plan was evaluated by HI and TC.
Homogeneity index quantifies dose homogeneity, as recommended by the International Commission on Radiation Units and measurements [19] and as used by Gutierrez et al. [14]. The HI was defined as the maximum dose delivered to 2 % of the target volume (D2%) minus the dose delivered to 98 % of the target volume (D98%) divided by the median dose of the target volume (Dmedian):
Smaller values for the HI correspond to more homogeneous dose across the target volume, and values close to 0 are optimal.
TC for the target metastases and whole brain volume was measured as the volume within the target receiving a dose greater than or equal to the prescription dose (VTpres) divided by the target volume (TV) [14]:
for TC, values can range from 0 to 1, where a value of 1.0 represents a complete coverage.
For the target metastases, we also quantified dose homogeneity using MDPD, defined as the maximum dose (MD) divided by the prescription dose (PD):
in the RTOG guidelines [20], MDPD should be <1.25. An MDPD between 1.25 and 1.40 constitutes a minor variation, and an MDPD >1.40 is a major deviation.
Dose conformity for the target metastases was quantified using PITV, defined as the prescription isodose volume (PI) divided by the target volume (TV):
in the RTOG guidelines, PITV should be kept as close to 1.0 as possible while maintaining TC and target homogeneity criteria [20].
A PITV between 1.0 and 2.0 is optimal. A PITV between 2.0 and 2.5 is a minor variation, and a PITV >2.5 is a major deviation.
Dose conformity was also evaluated in terms of Paddick Conformity Index CI [21]. In 2000, Paddick proposed an alternative conformity index with the goal of providing an objective method for comparing plan quality and eliminating “false scores” provided by the RTOG index (CIRTOG = Vpres/TV) [22]. The proposed index builds on the criticism of the RTOG index that the overlap of the volume receiving the prescription isodose and the target volume is not accounted for.
CI is defined as
where VTpres is volume within the target receiving a dose ≥ the prescription dose, Vpres is volume receiving a dose ≥ the prescription dose.
The CI gives a measure of conformity for the prescription isodose volume around the target volume and also accounts for spatial deviations by measuring the normal tissue being irradiated to the prescription dose. Values can range from 0 to 1.0, and values closer to 1.0 are optimal.
Results
The 10 patients evaluated for the study presented a total of 20 BMs. Patients’ characteristics were resumed in Table 2. The mean PTVSIB volume was 5.1 ± 4.9 cc (range, 0.7–19.4 cc), PTVWBRT was (13.46 ± 2)*102 cc (range, 992–1650 cc) and mean hippocampal volume 1.92 ± 0.7 cc (range, 0.9–2.94 cc).
Table 2 also reports the delivery time and number of monitor units (MU) for each RapidArc® treatment plan. The mean values to deliver a single treatment to a solid water phantom were 3.8 ± 0.2 min and 1680 ± 276 MU.
The mean dose to 95 % of PTVSIB was 39.3 ± 0.9 Gy (range, 37.4–40 Gy) and to 90 % of PTVWBRT was 19.8 ± 0.2 Gy (range, 19.3–20 Gy).
Table 3 shows the mean quality measures for PTVs achieved with VMAT.
For target BMs, the mean HI was 0.083 ± 0.03 (range, 0.03–0.16), the mean TC was 0.96 ± 0.24 (range, 0.41–1.51) with a mean CI of 0.78 ± 0.17 (range, 0.41–0.98). The mean PITV was 0.96 ± 0.24 (range, 0.41–1.51), and MDPD was 1.06 ± 0.02 (range, 1.03–1.09).
All values for PITV and MDPD were within the RTOG QA guidelines for SRS, with no minor variations or major deviations.
For the WBRT, the mean HI was 0.42 ± 0.12 (range, 0.27–0.66) and the TC was 0.78 ± 0.11 (range, 0.51–0.88).
Table 4 summarizes dosimetric values for hippocampus, chiasm, optical nerves and brainstem.
Hippocampal mean dose was 7.7 ± 0.3 Gy (range, 7.4–8.5 Gy), mean Dmax dose 10.5 ± 0.5 Gy (range, 9.4–11.3 Gy), mean D100% dose 6.7 ± 0.3 Gy (range, 6.3–7.3 Gy). Apparently patients with brain metastases closer to hippocampal structure presented more complex radiation planning. In these cases, we pursued an acceptable compromise between target coverage and hippocampal sparing. Nevertheless, a minimal distance of 5 mm was mandatory between BMs and hippocampus.
For chiasm, D0.2 cc was 20.8 ± 1.1 Gy (range, 19–23 Gy), for right optical nerve D0.2 cc was 16.5 ± 2.4 Gy (range, 12–20 Gy), for left optical nerve D0.2 cc was 15.6 ± 1.5 Gy (range, 14–18.8 Gy) and for brainstem D0.1 cc was 23.1 ± 2.6 Gy (range, 20.8–28.3 Gy) and D1 cc was 22.36 ± 1.9 Gy (range, 20.5–26 Gy).
Figures 2 and 3 show an example of an isodose distribution and the corresponding cumulative normalized dose–volume histogram for WBRT with hippocampal avoidance and SIB for two BMs using VMAT technique.
The QA procedures were performed for all 10 treatment plans. To numerical and graphical evaluation and to compare dose matrices measured with a PTW detector arrays (OCTAVIUS DETECTOR 729) and corresponding calculated matrices (from treatment planning system), a PTW software was used (VeriSoft 4.2.2.0).
Dose profiles on coronal planes showed good correlation between the calculated and measured doses for the PTVWBRT, PTVSIB and hippocampal avoidance region. An example of the dose distributions and dose profiles in the coronal plane, for a sample treatment plan, is shown in Fig. 4.
a Calculated dose distribution for a sample treatment plan in coronal plane. b Dose distribution for the same treatment plan delivered to a solid water phantom measured with a PTW array. c Superior–inferior dose profile through the right hippocampus. d Transverse profile through both hippocampi. The measured (discrete points) and calculated dose profiles (continued line) are in good agreement (a blue point means a underdosage and a red point means overdosage)
Figure 5 shows the results of a gamma index distribution map (acceptance criterion, 3 % and 3-mm distance to agreement), as described by Low et al. [23]. The values of the gamma index >1 corresponded to locations in which the calculation did not meet the acceptance criterion. Comparison is acceptable if points with a gamma index ≈1 were ≥90 %. In this study, mean pass rate was (97.7 % ± 0.5).
a Compare pop-up window with parameters for the Gamma Index method used in current study and institute: 3 mm for accepted spatial deviation and 3 % for accepted dose deviation for doses at the corresponding local position of reference matrix. Below 0.3 Gy, we use a higher tolerance threshold (5 %). Gamma calculation is not performed if the dose values of reference matrix are below 10 % of maximum dose. b representation of color gradient gamma index: from green (gamma index = 0) via yellow to red (gamma index ≥1.5)
Discussion
WBRT is considered the standard treatment in patients with a diagnosis of multiple BMs [1]. Introduction of focal treatment as stereotactic radiosurgery (SRS) associated with WBRT has been considered an innovative approach with the goal to increase local control and time to central nervous system progression 1 in case of limited number of lesions, not suitable for surgery only [24]. In the past decades, the association of SRS and WBRT has been usually performed sequentially [8, 25]. This approach in two times could deserve several radiobiological and dosimetrical considerations: (1) time interval between WBRT and SRS could influence the tumor cell repopulation; (2) use of a single stereotactic fraction does not guarantee the radiobiological advantages of multiple fractionated treatment, including reoxygenation and reassortment of tumor cell in the target; (3) dosimetric uncertainty concerning WBRT and SRS could combine in separated phases of planning.
To date, advances in IMRT, including VMAT technique, permit to obtain optimal target dose coverage and OARs sparing by the delivery of different doses to different volumes in the same time. The adoption of SIB during WBRT could overcross the previous described radiobiological concerns, improving dosimetric accuracy and confirming the clinical feasibility of the procedure, as reported in the literature [26].
In the last decades, different studies established the feasibility of SIB and WBRT in multiple BMs [27, 28].
Recently, Lagerwaard et al. purposed a new hypofractionated WBRT–SIB schedule with a prescription of 20 Gy to the WBRT and 40 Gy to the macroscopic BMs, in 5 fractions. The authors concluded that WBRT–SIB to multiple BMs (range, 1–5) for 8 patients is a rapid and accurate technique with a higher conformity index than conventional sequential WBRT and radiosurgery boost [8].
To date, the disadvantage in the use of WBRT is determined by the risk of neurocognitive decline related to radiation and recently, several analyses showed that hippocampal-dependent functions are preferentially affected by radiotherapy [29, 30]. Thus, to reduce this potential risk, some experiences evaluated hippocampal sparing during WBRT–SIB, using different arc therapies [14, 17, 31–33].
Gutierrez et al., in a dosimetric study, delivered hippocampal avoidance WBRT–SIB using helical Tomotherapy® (HT) and changing some plan parameters (field width, pitch). They found, in 10 cases with a number of BMs between 1 and 5, that HT could guarantee good results in terms of WBRT coverage, homogeneous dose distribution on BMs and conformal hippocampal sparing [12].
To our knowledge, only 3 experiences with small sample size and an overall treatment time of about 2–3 weeks have been published on the use of VMAT technique and hippocampal sparing WBRT–SIB: of these, only a single report is a clinical study, 32 while the others are only dosimetric analyses [14, 17, 31].
Recently, Kim et al. reported a clinical study of 11 patients with 70 BMs (range 2–15) treated with hippocampal sparing WBRT–SIB. Median brain metastases volume was 0.235 cc (range 0.020–10.140 cc). The dose prescription was 25–28 Gy on WBRT (EQD2 = 26.04 Gy–28 Gy) and 30–42 Gy on BMs (EQD2 = 32.5 Gy–45.5 Gy) in 10–14 fractions. On whole brain, HI was 0.52 ± 0.16, TC 0.89 ± 0.05; while on BMs, HI was 0.17 ± 0.04, TC 0.99 ± 0.02 and CI 0.48 ± 0.16. Mean dose on hippocampus was 13.65 Gy. After a median follow-up of 14 months, a complete remission was observed in 33 % of lesions and a partial response in 45 % with a 65 % reduction of tumor volume. The paper did not report any data about neurocognitive functions [32].
Prokic et al. compared the use of WBRT–SIB and WBRT sequential SRS associated with hippocampal sparing with VMAT technique. The study enrolled 10 cases with 57 BMs (range 2-8). The dose prescription was: 30 Gy (EQD2 = 31.25 Gy) on WBRT and 51 Gy (EQD2 = 60.56 Gy) on BM–SIB in 12 fractions. The results reported a HI on WBRT: 0.54 ± 0.04 and TC 0.96 ± 0.01. HI on brain metastases was 0.11 ± 0.02 and TC 0.95 ± 0.01. Mean dose to hippocampus was 7.55 ± 0.62 Gy. The use of SIB achieved better sparing of the hippocampus compared with sequential approach [31].
Hsu et al. evaluated in 10 cases with 18 BMs (range 1–3) the dosimetric feasibility of hippocampal avoidance WBRT–SIB with a dose prescription of PTVWBRT 32.25 Gy (EQD2 = 32.65 Gy) in 15 fractions and PTVSIB of 63 Gy (EQD2 = 74.55 Gy) for lesions with a diameter ≥2.0 cm and 70.8 Gy (EQD2: 86.85 Gy) for lesions with a diameter <2.0 cm. The study reported HI 0.39 ± 0.06 and TC 0.960 ± 0.002 on WBRT, and HI 0.07 ± 0.02, TC 0.98 ± 0.01 and CI 0.73 ± 0.1 on BMs. The mean dose to hippocampal structure was 5.23 ± 0.3 Gy. The previous 2 analysis reported similar results in terms of dosimetric and treatment time delivery (about 4 min), using different fractionations and EQD2 dose. Moreover, they showed the equivalence in terms of excellent dose distribution of hippocampal avoidance WBRT–SIB with VMAT technique compared to HT, and furthermore that VMAT treatments can drastically reduced the time of delivery (about one-third) [17].
Considering the lack of literature data, this is the first dosimetric study facing the issue of SIB and hippocampal sparing during extreme hypofractionationed WBRT in 5 fractions, using VMAT. The study was designed to evaluate the feasibility of the WBRT and SIB approach, very convenient in terms of overall treatment time duration for the patient and very appealing in terms of radiobiological potential advantages. The rationale was to mimic the disruptive potential of high dose per fraction prescribed in radiosurgery and stereotactic hypofractionated radiotherapy in a maximum of 5 macroscopical sites, delivering in the same time a WBRT to sterilize microscopical disease while sparing hippocampal regions.
Despite the limitations of the study (sample size, dosimetric results, limited BMs), the results, here presented, showed that all dosimetric parameters were satisfied in terms of target coverage and homogeneity index for PTVWBRT and PTVSIB. When compared to the literature experiences, our analysis showed that the increasing BM’s number could compromise the feasibility of planning, in particular regard to the HI for WBRT. In fact, comparing HI, our results were similar to the Hsu’s data (WBRT HI Hsu = 0.39 vs HI our study = 0.42) and superior to Kim’s results (for WBRT HI Kim = 0.52 vs HI our study = 0.42). Probably, this difference between Kim’s finding and us could be related to the number of BMs: in fact Kim et al. enrolled patients up to 15 metastases. All the studies reported similar results in terms of TC for WBRT. Furthermore, higher BM’s number reduced the possibility of spearing hippocampal structures, in fact Dmean. to hippocampus was 5.23 Gy in Hsu experience vs 7.7 Gy in the present analysis, while Kim et al. reported a Dmean of 13.65 Gy.
Conclusions
The present study investigated, in patients with limited number of BMs (until 5) and with a dose prescription of 20 Gy to WBRT and 40 Gy to BMs in 5 fractions, the use of WBRT–SIB associated with hippocampal sparing. Our study confirms the feasibility in achieving planning objectives and efficacy treatment delivery time using VMAT technique. Despite the correlation between hippocampal sparing and neurocognitive preservation has not been demonstrated, and considering the complexity of WBRT–SIB in terms of planning and potential time consuming, patient selection to this approach remains crucial [34, 35]. Moreover, the here presented schedule, with an EQD2 dose approximately of 60 Gy similar to the reported literature data, offers the advantage of shorter treatment time (only 5 fractions), which could be very useful in oligometastatic patients that need systemic therapy.
Considering the dosimetric validation of the study here reported the next step will be its application in a clinical Phase II prospective trial correlating with the use of neurocognitive test to establish the impact of WBRT, SIB and hippocampal sparing in good prognosis and/or oligometastatic patients with BMs. Furthermore, other controlled prospective, also observational, studies assessing neurocognitive aspects, within daily practice, to verify the difference in neurocognitive outcome in patients submitted to WBRT or other radiotherapy regimen with or without hippocampal sparing are advocated.
References
Mehta MP, Tsao MN, Whelan TJ, Morris DE, Hayman JA, Flickinger JC, Mills M, Rogers CL, Souhami L (2005) The American society for therapeutic radiology and oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastasis. Int J Radiat Oncol Biol Phys 63:37–46
Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC (1999) Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 45:427–434
Andrews DW, Scott CB, Sperduto PW et al (2004) Whole-brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG-9508 randomized trial. Lancet 363:1665–1672
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R (1997) Recursive partitioning analysis of prognostic factors in three radiation oncology group (RTOG) brain metastasis trials. Int J Rad Oncol Biol Phys 37:745–751
Aoyama H, Shirato H, Tago M et al (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491
Rodrigues G, Yartsev S, Yaremko B et al (2011) Phase I trial of simultaneous in-field boost with helical tomotherapy for patients with one to three brain metastases. Int J Radiation Oncology Biol Phys 80:1128–1133. doi:10.1016/j.ijrobp.2010.03.047
Rodrigues G, Eppinga W, Lagerwaard F et al (2012) A pooled analysis of arc-based image-guided simultaneous integrated boost radiation therapy for oligometastatic brain metastases. Radiother Oncol 102:180–186. doi:10.1016/j.radonc.2011.05.032
Lagerwaard FJ, van der Hoorn EA, Verbakel WF, Haasbeek CJ, Slotman BJ, Senan S (2009) Whole-brain radiotherapy with simultaneous integrated boost to multiple brain metastases using volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys 75:253–259. doi:10.1016/j.ijrobp.2009.03.029
Berk L (1995) An overview of radiotherapy trials for the treatment of brain metastases. Oncology 9(11):1205–1212
Laack NN, Brown PD (2004) Cognitive sequelae of brain radiation in adults. Semin Oncol 31:702–713
Shi L, Molina DP, Molina DP, Robbins ME, Wheeler KT, Brunso-Bechtold JK (2008) Hippocampal neuron number is unchanged 1-year after fractionated whole-brain irradiation at middle age. Int J Radiat Oncol Biol Phys 71:526–532. doi:10.1016/j.ijrobp.2008.02.015
Ghia A, Tomé WA, Thomas S, Cannon G, Khuntia D, Kuo JS, Mehta MP (2007) Distribution of brain metastases in relation to the hippocampus: implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys 68:971–977
Jack CR Jr (1994) MRI-based hippocampal volume measurements in epilepsy. Epilepsia 35(Suppl. 6):S21–S29
Gutiérrez AN, Westerly DC, Tomé WA et al (2007) Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: a planning study. Int J Radiat Oncol Biol Phys 69:589–597
Abayomi OK (1996) Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol 35:659–663
Roman DDSP (1995) Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys 31:983–998
Hsu F, Carolan H, Nichol A et al (2010) Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for 1-3 brain metastases: a feasibility study using Volumetric Modulated Arch Therapy. Int J Radiat Oncol Biol Phys 76:1480–1485. doi:10.1016/j.ijrobp.2009.03.032
Timmerman RD (2008) An overview of hypofractionation and introduction to the issue of seminars in radiation oncology. Semin Radiat Oncol 18:215–222. doi:10.1016/j.semradonc.2008.04.001
International commission on radiation units and measurements, ICRU report 62 (1999) Prescribing, recording, and reporting photon beam therapy (supplement to ICRU report 50) Bethesda: ICRU
Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, Martin L (1993) Radiation therapy oncology group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys 27:1231–1239
van’t Riet A, Mak AC, Moerland MA, Elders LH, van der Zee W (1997) A conformation number to quantify the degree of conformity in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys 37:731–736
Paddick I (2000) A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 93(Suppl 3):219–222
Low DA, Harms WB, Mutic S, Purdy JA (1998) A technique for the quantitative evaluation of dose distributions. Med Phys 25:656–661
Alongi F, Fiorentino A, Navarria P, Bello L, Scorsetti M (2014) Stereotactic radiosurgery for patients with brain metastases. Lancet Oncol 15:e246–e247. doi:10.1016/S1470-2045(14)70151-2
D’Agostino GR, Autorino R, Pompucci A et al (2011) Whole-brain radiotherapy combined with surgery or stereotactic radiotherapy in patients with brain oligometastases: long-term analysis. Strahlenther Onkol 187:421–425
Levegrün S, Pöttgen C, Wittig A, Lübcke W, Abu Jawad J, Stuschke M (2013) Helical tomotherapy for whole-brain irradiation with integrated boost to multiple brain metastases: evaluation of dose distribution characteristics and comparison with alternative techniques. Int J Radiat Oncol Biol Phys 86:734–742. doi:10.1016/j.ijrobp.2013.03.031
Gupta T, Basu A, Master Z, Jalali R, Munshi A, Sarin R (2009) Planning and delivery of whole brain radiation therapy with simultaneous integrated boost to brain metastases and synchronous limited-filed thoracic radiotherapy using helical tomotherapy: a preliminary experience. Technol Cancer Res Treat 8:15–22
Sterzing F, Welzel T, Srok-Perez G, Schubert K, Debus J, Herfarth KK (2009) Reirradiation of multiple brain metastases with helical tomotherapy. A multifocal simultaneous integrated boost for eight or more lesions. Strahlenther Onkol 185:89–93. doi:10.1007/s00066-009-1971-2
Laack NN, Brown PD (2004) Cognitive sequelae of brain radiation in adults. Semin Oncol 31:702–713
Shi L, Molina DP, Molina DP, Robbins ME, Wheeler KT, Brunso-Bechtold JK (2008) Hippocampal neuron number is unchanged 1-year after fractionated Whole-Brain Irradiation at Middle Age. Int J Radiat Oncol Biol Phys 71:526–532. doi:10.1016/j.ijrobp.2008.02.015
Prokic V, Wiedenmann N, Fels F, Schmucker M, Nieder C, Grosu AL (2013) Whole brain irradiation with hippocampal sparing and dose escalation on multiple brain metastases: a planning study on treatment concepts. Int J Radiat Oncol Biol Phys 85:264–270. doi:10.1016/j.ijrobp.2012.02.036
Kim KH, Cho BC, Lee CG, Kim HR, Suh YG, Kim JM, et al. (2015) Hippocampus-sparing Whole-Brain Radiotherapy and Simultaneous Integrated Boost for Multiple Brain Metastases From Lung Adenocarcinoma: Early Response and Dosimetric Evaluation. Technol Cancer Res Treat (Epub ahead of print)
Gondi V, Tolakanahalli R, Mehta M et al (2010) Hippocampal-sparing Whole-Brain radiotherapy: a “How-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 78:1244–1252. doi:10.1016/j.ijrobp.2010.01.039
Fiorentino A, Ricchetti F, Mazzola R, Fersino S, Giaj Levra N, Alongi F (2015) Regarding Ening et al. Charlson comorbidity index: an additional prognostic parameter for preoperative glioblastoma patient stratification. J Cancer Res Clin Oncol (Epub ahead of print)
Fiorentino A, Caivano R, Chiumento C, Cozzolino M, Clemente S, Pedicini P, Fusco V (2012) Comorbidity assessment and adjuvant radiochemotherapy in elderly affected by glioblastoma. Med Oncol 29:3467–3471. doi:10.1007/s12032-012-0246-4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Giaj Levra, N., Sicignano, G., Fiorentino, A. et al. Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for brain metastases: a dosimetric volumetric-modulated arc therapy study. Radiol med 121, 60–69 (2016). https://doi.org/10.1007/s11547-015-0563-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-015-0563-8