Abstract
The effects of thermal treatment (TT) and high hydrostatic pressure treatment (HHPT) on calcium-added soybean protein 1% (w/w) aqueous dispersions at pH 7.0 were compared. High hydrostatic pressure, but not thermal treatment, improved protein solubility and colloidal stability. Despite the fact that the glycinin solubility is more affected by calcium than that of β-conglycinin, glycinin could remain in dispersion in the presence of calcium when denatured by HHPT (calcium added before or after treatment), but not when denatured by TT or without denaturing treatment. Thus, polypeptide composition of soluble aggregates depended on type of treatment. Colloidal stability and molecular weight of soluble aggregates depended on the order of application of calcium and denaturing treatment: when calcium was present during either HHPT or TT, the dispersions had higher stability and higher proportion of soluble aggregates with high molecular weight than when calcium was added after treatments. After freeze drying and re-dispersing at higher protein content (10% w/w) calcium-added dispersions subjected to HHPT formed cold-set gels that were transparent and exhibited excellent water holding capacity. Our results provide the basis for the development of ready-to-use functional ingredients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incorporation of calcium to vegetable foodstuff represents an interesting topic due to several conditions such as allergies, veganism and/or intolerance to lactose that limit the consumption of foods naturally rich in calcium. Soybean is an inexpensive source of protein used to prepare many products. The addition of calcium to soybean proteins induces changes in structural and functional properties. Food processing involves treatments that can denature proteins. Thermal treatment (TT) and treatment with high hydrostatic pressure (HHPT) represent a conventional and an emerging technology, respectively, which differently affect protein structure. HHPT provides certain advantages over conventional thermal processing being rapid and providing uniform distribution of pressure throughout the sample [1].
Driving forces and mechanisms for denaturation are different in these treatments. For example, TT increases Brownian motion and breaks hydrogen bonds, while HHPT increases the order (principle of microscopic ordering) and favors the formation of hydrogen bonds [2]. Thus, it is expected that protein structure, physicochemical properties and consequently functional properties change differently when calcium-added SPI is subjected to TT or HHPT. Since HHPT favors phenomena that are accompanied by decrease in volume, electrostriction is favored. Thus, the mechanism of HHPT-induced denaturation may involve reversible or irreversible dissociation of cations from negatively charged amino acid residues.
Many studies exist about the effect of HHPT and TT on functional properties of soybean proteins [3,4,5,6]. Moreover, our group has studied the effect of calcium addition to SPI combined with TT or combined with HHPT [7, 8]. Comparison between those effects from bibliography is not easy because assays were carried out on samples with different protein and calcium concentrations, ionic strength, proportions of glycinin and β-conglycinin present in SPI, etc. Moreover, the order of application of calcium and denaturing treatment (presence or absence of calcium during treatment) also may affect the degree of denaturation achieved and/or the structure of protein species [7, 8]. Protein solubility and colloidal stability are crucial properties for liquid foodstuff, transparency or cloudiness must be controlled for consumer acceptance. Protein solubility and physical stability of calcium-added soybean protein dispersions and soymilk was improved by HHPT [9, 10].
The aim of this work was to compare the effects of TT and HHPT on solubility and colloidal stability on samples with the same composition and find some conditions in which soybean protein and calcium make an interesting product like a cold gel.
Materials and Methods
Materials
Defatted soybean flour was provided by The Solae Company (Brazil). Calcium was incorporated as a CaCl2 solution, prepared from CaCl2 dihydrate (Sigma, Saint Louis, USA). All other chemicals were reagent grade.
Preparation of Soybean Protein Isolate (SPI)
Protein isolate was obtained according to Speroni, Milesi, and Añón [11]. Briefly, alkaline extraction (pH 8.0, agitation during 90 min at 20 °C) was followed by isoelectric precipitation (pH 4.5, agitation during 15 min at 20 °C). The isoelectric precipitate was dispersed in distilled water, pH was adjusted to 7.0 with 2 M NaOH, and the dispersion was freeze-dried. SPI was maintained at 4 °C until use.
Determination of Protein Content of SPI
Protein content of SPI was determined by the Kjeldahl method [12]. Digester and distillation unit were from BÜCHI (Flawil, Switzerland). Conversion factor used was 5.8.
Protein Dispersions
Aqueous dispersions of soybean protein isolate with a protein content of 1.0% (w/w) were prepared at pH 7.0 and were stirred for 60 min at room temperature. CaCl2 was added (1.8 or 5.0 mM) from a stock solution (1000 mM) prepared with CaCl2.2H2O. Calcium was added before or after denaturing treatments. The pH was adjusted before and after denaturing treatments with 1 M NaOH. Protein concentration was chosen according to Añón, de Lamballerie and Speroni [8], at this concentration some aggregation phenomena occur, but not gelation or coagulation during treatments, for which higher protein content is required.
Thermal Treatment
Protein dispersions (1.0% (w/w)) were packed in plastic bottles with screw cap and subjected to thermal treatment at 95 ± 1 °C for 15 min in a thermostatic bath (Vicking, Buenos Aires, Argentina). Conditions were chosen to completely denaturate β-conglycinin and glycinin [13].
High Hydrostatic Treatment
Protein dispersions (1.0% (w/w)) were vacuum packed in polyethylene bags (Cryovac BB2800, Sealed Air, Buenos Aires, Argentina) before treatments. Samples were pressurized at 600 ± 5 MPa for 5 min in a high pressure system Stansted Fluid Power Ltd. model FPG 9400:922 (Stansted, United Kingdom). The compression fluid consisted in a mixture of propylene glycol and water (30:70). The working pressure was reached at 5 MPa/s and released at 20 MPa/s. Conditioning temperature of vessel and initial temperature of samples were 20 °C. The compression heating led to an increase in temperature (maximal temperature was 33.5 °C at the end of compression step). Conditions were chosen according to Manassero, Vaudagna, Añón, and Speroni [9] in order to reach the solubilizing effect of high hydrostatic treatment.
Molecular Characterization of Soluble Proteins
Supernatants of centrifugation (10,000 g, 15 min at 4 °C) were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), using a separating gel (12% w/v polyacrylamide) and a stacking gel (4% w/v polyacrylamide). Gels were prepared in a 0.375 M Tris-HCl, 1.0 g L−1 SDS buffer, pH 8.8, in a mini slabs system (BioRad Mini-Protean II Model). Low molecular weight markers in the range 14.4 to 94 kDa (Pharmacia, Amersham, England) were used. Sample buffer contained 0.125 M Tris-HCl, 10.0 g L−1 SDS, 200.0 g L−1 glycerol and its pH was 6.8. Samples to be run under reducing conditions were boiled, before centrifugation, for 1 min in the same buffer that also contained 5% v/v 2-mercaptoethanol. Samples were loaded at a protein concentration between 0.15 and 0.50% w/v, depending on their protein solubility. Electrophoresis was performed at a constant current of 30 mA for ca. 60 min. Gels were fixed and stained with R250 Coomasie blue (0.2% w/v) in water/methanol/acetic acid (5:5:2) overnight and distained with 25% v/v methanol and 10% v/v acetic acid.
Determination of Protein Solubility
Samples were centrifuged at 10,000 x g for 15 min at 4 °C in an aircooled benchtop centrifuge Universal 320R (Hettich, Tuttlingen, Germany). Protein concentration was determined in the supernatants (PS). Bicinchoninic Acid Protein Assay (BCA Sigma Kit, Sigma Chemical Co., St. Louis, MO, USA) was used for quantification of PS. Bovine serum albumin was used as standard (Sigma Chemical Co., St. Louis, MO, USA). Absorbance was measured at 562 nm in an Epoch™ Multi-mode Microplate Reader (BIOTEK Instruments, Winooski, VT, USA). Protein solubility was determined in triplicate for each sample. Results were expressed as percentage of solubility, %S:
Calcium Ion Concentration
Concentration of free Ca2+ was determined with a calcium ion selective electrode HANNA Instruments model HI4104 (Woonsocket, R.I., USA) attached to an Ion 510 series benchtop meter OAKTON Instruments (Vernon Hills, IL, USA). Ionic strength was adjusted by adding a solution provided by HANNA Instruments and pH was then adjusted with 100 mM NaOH. A calibration curve for Ca2+ was made within the range 0.025–2.5 mM with a stock solution provided by HANNA instruments.
Colloidal Stability - Turbidimetry
Turbidity was evaluated as absorbance at 600 nm, which was measured in whole samples or supernatants from centrifugation at 1000, 5000 or 10,000 g for 15 min at 4 °C in an Air-cooled Microlitre Centrifuge Z 233 MK-2 Hermle (Gosheim, Germany). Absorbance was measured in a Synergy HT™ Multi-mode Microplate Reader (BIO TEK Instruments, Winooski, VT, USA). Results were expressed as absorbance units (AU).
Colloidal Stability - Transmittance and Backscattering Measures
The sedimentation of insoluble aggregates was analyzed through the use of a vertical dynamic light scan analyzer Quick Scan (Beckman–Coulter inc., USA). Dispersions were poured into a cylindrical glass tube and transmittance of light (λ = 850 nm) was determined along as a function of time, every 5 min during 24 h.
Gel Formation
Calcium-added protein dispersions (1% w/w) were freeze-dried and then re-dispersed at higher protein concentration (10% w/w). In re-dispersed samples, calcium concentration was also 10-fold increased. The reconstituted dispersion was poured into cylindrical tubes and stored at 4 °C for 12 h until characterization.
Gel Characterization
Water Holding Capacity
Portions of gels of ca. 1 g were centrifuged at 2000 g for 10 min at 4 °C. Supernatant was separated and the residue was weighted. Water loss was determined by weighing before and after centrifugation. Water holding capacity (WHC) was expressed as:
where Wo is the amount of water in the gel before centrifugation and Wr is the amount of water released upon centrifugation.
Texture Profile Analysis
Texture profile analysis (TPA) was carried out on gels using a TA.XT2 Texture analyzer (Stable Micro Systems Ltd., Surrey, United Kingdom) in compression mode. Compression was exerted by a cylindrical probe with a flat section (diameter 75 mm) at a displacement speed of 0.5 mm/s. The force at 20% compression was registered (hardness). Gel adhesivity was calculated as the absolute value of the integral of force vs. time obtained after the first compression cycle, representing the work necessary to pull the compressing probe away from the sample.
Small Deformation Rheology
Dynamic rheological analysis (elastic modulus (G´) and viscous (G´´) vs. frequency) was carried out at 20 °C using a serrated plate-and-plate geometry (35 mm diameter, 1 mm gap) in a Controlled Stress Rheometer Haake RS 600 (Thermoelectron, Karlsruhe, Germany). Frequency of oscillations belonged to the range 0.01–10 Hz. The linear viscoelasticity region was determined through stress sweep tests at a fixed frequency of 1 Hz, all samples were analyzed at 2 Pa, which was within the range of linear viscoelasticity. Tan δ was calculated as G´´/G´. Before starting the measures, samples were allowed to rest for 10 min after positioning the sample on the sensor system.
Sample Denomination
In order to facilitate reading, a nomenclature was built to indicate the order of application of calcium addition and denaturing treatment, and the type of denaturing treatment. For gelling assays, names also indicates calcium concentration (Table 1).
Statistical Analysis
Assays were performed at least by triplicate. One way analyses of variance were conducted. Differences between means were analyzed by Tukey’s test at α level of 0.05. Statistical analysis was carried out using the Origin software (OriginLab Corporation, Northampton, MA, USA).
Results and Discussion
Protein Content and Solubility
Protein content of soybean protein isolate was 83.7 ± 1.2% d.b. (conversion factor 5.8). This datum was used for preparation of protein dispersions and for %S calculus.
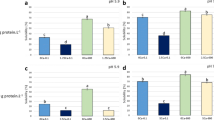
Protein solubility (%S) of SPI was 83.7 ± 0.5%. This result is in accord with those of Renkema, Gruppen, and Van Vliet [14] and Nishinari, Fang, Guo, and Phillips [5] and represents a high value of solubility among vegetable storage proteins. The %S was decreased by calcium addition (Fig. 1); this fact was due to protein aggregation induced by calcium bridges between polypeptides and charge neutralization [15, 16]. Without calcium addition, TT provoked no change in %S, protein concentration could be low enough to avoid the formation of big insoluble aggregates, despite the heat-induced denaturation. On the other hand, without calcium addition, HHPT induced an increase in %S, dissociation of aggregates present in untreated SPI could account for this effect [8, 17]. The combination of calcium addition and TT (whatever the order of application of calcium and TT) induced no change when compared to calcium addition only (Fig. 1). Scilingo and Añón [7] found that thermal-treated calcium-added dispersion exhibited lower %S than calcium-added ones, the difference with the present result might be due to the higher protein concentration (5% w/v) used in the previous study, that favored protein aggregation. In the case of CaHHPT (calcium was present during treatment) %S was increased to 92.3 ± 2.6%, a result that is in agreement with that of Añón, de Lamballerie, and Speroni [8]. In the case of calcium addition after pressurization (HHPTCa) no solubilizing effect was observed (Fig. 1). Manassero, David-Briand, Vaudagna, Anton, and Speroni [17] reported that the effect of sequence of application of calcium and HHPT depended on pH, at pH 5.9 HHPT after calcium addition led to a smaller increase in %S than at pH 7.0. HHPT induce a transient decrease in pH whose magnitude depend on composition of samples [18]. In the work of Manassero, David-Briand, Vaudagna, Anton, and Speroni [17] protein dispersions at pH 7.0 were prepared in buffer Tris-HCl, which exhibit a nearly zero ionization volume and is used to keep the pH constant over a large pressure range [19] then, the transient HHPT-induced decrease in pH was probably smaller than in the samples of the present work that were prepared in bi-distilled water. Thus, the transient decrease in pH could have a magnitude big enough to reach a value in which the order of application was a determinant factor for %S (e.g. pH shift close to isoelectric point would disfavor dissociation of aggregates since a decrease in electric charge could minimize the electrostriction effect). This fact would explain the difference between results and confirms that the composition of the sample modulates the solubilizing effect of HHPT, among other mechanisms, by influencing pH during treatment. Thus, in our experimental conditions the mechanisms and/or the protein species formed were different when proteins were HHPT-denatured in presence or absence of calcium.
Our results indicate that aqueous dispersions of denatured soybean proteins with high %S can be obtained by TT and HHPT, in the case of HHPT, calcium-added dispersion can exhibit high %S if calcium is incorporated before treatment (CaHHPT).
Free Calcium Concentration
Calcium binds to negatively charged amino acid residues and to histidine from soybean polypeptides [15]. In a previous work [9] our group reported that Ca2+ allowed a higher solubilizing effect of high hydrostatic pressure than Mg2+ or Fe2+ on soybean proteins, thus a mechanism in which Ca2+-protein complexes are affected could occur. Denaturation may involve conformational changes that modify the exposition of binding sites for calcium. If that was the case, free calcium concentration could also change. It can not be ruled out that the solubilizing effect of HHPT on calcium-added proteins was due to a reduction in the number or affinity of sites for calcium. In order to check that hypothesis, free calcium concentration was evaluated in protein dispersions after different treatments.
The average free calcium concentration in samples without calcium addition was 0.04 ± 0.01 mM, and represented calcium coming from soybean. In calcium-added samples the average free calcium concentration was 1.95 ± 0.08 mM and represented 40% of calcium added. No differences among samples untreated or subjected to TT or HHPT (regardless the order of application) were detected. Thus, the denaturing treatments applied to proteins provoked no irreversible changes in the degree of binding to calcium. Thus, if dissociation induced by electrostriction occurred during HHPT, it was reversible and the solubilizing effect was stabilized by other mechanisms than irreversible calcium-protein dissociation.
Colloidal Stability – Turbidimetry
Non-added Samples
The samples SPI, TT y HHPT exhibited similar turbidity values before centrifuging. Turbidity decreased slightly but significantly after centrifugation (Fig. 2). The effect of each velocity was similar in magnitude for the three non-added samples. Thus, we conclude that in the conditions of this work (pH, protein concentration, ionic strength, centrifugation speeds) HHPT and TT produced no change in turbidity (whole samples) or sedimentation behavior (centrifuged samples).
Turbidity of soybean protein dispersions determined by absorbance at 600 nm. Without centrifugation (black bars) or the supernatant of samples centrifuged at different accelerations (1000 g, red-diagonal lines bars; 5000 g blue-horizontal lines bars; 10,000 g green-vertical lines bars). Values shown are mean and standard error
Calcium-Added Samples
Calcium addition induced an important increase in turbidity in whole samples (Fig. 2, black bars). The highest values were found in Ca, CaTT, TTCa and HHPTCa, which was expected, since these samples had low values of %S. In the case of CaHHPT turbidity was much lower than other calcium added samples, this fact seemed to be due to its high %S, and also to a change in the structure and size of insoluble aggregates. Manassero, David-Briand, Vaudagna, Anton, and Speroni [17] reported that HHPT reduced the size of aggregates in calcium-added soybean protein isolate dispersions, from 1862 nm (unpressurized) to 200 nm (pressurized in calcium presence). In consequence, the interaction of light with those aggregates was different, the smaller the size, the lower the turbidity.
After centrifugation at 1000 g turbidity decreased significantly (p < 0.05), but it remained higher than that of SPI (Fig. 2, red-diagonal lines bars). This fact suggests that a fraction of insoluble aggregates were colloidal stable in this condition of acceleration. Turbidity values of Ca, CaTT and CaHHPT were higher than those of TTCa and HHPTCa. This behavior indicates that colloidal stability of aggregates depended on the order of application of denaturing treatment and calcium addition. Colloidal stability was higher when calcium was present during denaturation.
After centrifugation at 5000 g (Fig. 2, blue-horizontal lines bars) turbidity values were lower than those of samples centrifuged at 1000 g. Nevertheless, the colloidal stability of dispersions denatured in the presence of calcium was higher than that corresponding to calcium addition after denaturation, as it was seen for 1000 g.
After centrifugation at 10,000 g (Fig. 2, green-vertical lines bars) turbidity values were low and indistinguishable among different conditions, with the exception of CaHHPT that have a higher value (p < 0.05). This fact indicates that aggregates formed by HHPT in the presence of calcium exhibited the highest colloidal stability.
Our data indicate that the moment of calcium addition influenced colloidal stability of aggregate induced by both TT and HHPT. In the cases of CaTT and TTCa %S was similar (10,000 g) but colloidal stability was different when lower accelerations were applied (1000 and 5000 g). On the other hand, CaHHPT and HHPTCa had differences in both %S and colloidal stability. Calcium presence revealed differences between TT and HHPT. In CaHHPT, the high value of %S (defined as protein in supernatant after centrifugation at 10,000 g) and the relative high value of turbidity (after centrifugation at 10,000 g) indicate that a fraction of the HHPT-stabilized aggregates were insoluble, but remained in the supernatant, which is in accord with the statement of Manassero, David-Briand, Vaudagna, Anton, and Speroni [17].
Colloidal Stability - Transmittance and Backscattering Measures
Ca and CaTT exhibited a similar behavior at zero time; dispersions were turbid enough to exhibit a null transmittance. Clarification in the top of the tube began to be evident with time. Sedimentation occurred faster in CaTT than in Ca. Clarification in CaTT was detected in about 20 h in the lower region of the tube (15 mm) and the transmittance in the upper region of the tube reached 100% (Fig. 3). Clarification in Ca did not occur in the lower region of the tube, and after 24 h transmittance was 90% in the top of the tube (Fig. 3A). On the other hand, CaHHPT was less turbid at zero time, transmittance was 68%. In CaHHPT a slight drop in transmittance was detected in the whole length of the tube, except for the upper 5 mm were a slight clarification was verified (Fig. 3E).
Backscattering behavior in Ca and CaTT (Fig. 3B and D) consisted in an increase this parameter in the bottom of the tube, which coincided with the accumulation of insoluble protein. This accumulation was not noticed as a decrease in transmittance due to its initial null value. Changes observed in CaTT were faster than in Ca, which indicates that insoluble aggregates formed by thermal treatment were more unstable than those induced by calcium at room temperature. This difference in stability seemed to be of small magnitude because it was detected when acceleration was 1 g (gravitational separation) but not in centrifugation assays (when acceleration was at least 1000 g (Fig. 2). The increase in backscattering in the top of the tubes could represent an artifact: the increase in transmittance allow that a fraction of light was scattered by reflection in the wall of tube. In CaHHPT, backscattering showed a slight decrease over time along the entire tube, except in its top 5 mm (Fig. 3F). No sedimentation was detected in the bottom of the tube by visual inspection or by increase in backscattering. These results confirm that CaHHPT were more stable than Ca and CaTT.
Molecular Characterization of Soluble Proteins
Calcium addition promoted the decrease of intensity of almost all bands; this effect was more conspicuous for AB subunit (glycinin) and polypeptides β (β-conglycinin) and A (glycinin) (Fig. 4A). The intensity of the band corresponding to polypeptide B (glycinin) exhibited no change; it is possible that its basic character [20] was responsible for low degree of insolubilization in the presence of calcium.
The denaturation by TT or HHPT promoted the formation of large aggregates that did not enter the stacking gel. In the case of TT, a considerable decrease in intensity of band AB was observed, which may be related with the formation of B-β aggregates [21]. On the other hand, in HHPT samples, the decrease in intensity of all bands of resolving gel seemed to have similar magnitude. These data indicate that aggregates had different polypeptide composition depending on treatment applied; the TT seemed to be more specific to promote the formation of B-β aggregates, whereas HHPT seemed to promote nonspecific aggregation.
CaTT and TTCa exhibited different profiles: when calcium was present during treatment aggregates big enough to not be able to enter the stacking gel were formed and the polypeptides that entered the resolving gel showed bands with low intensity, especially AB subunit and A polypeptide (glycinin). On the other hand, when calcium was added after treatment, the profile of TTCa was similar to that of Ca, the intensities of all bands were higher than those of CaTT. Despite showing similar %S, protein species had different sizes and compositions. Presence of calcium during thermal treatment allowed the formation of soluble and very big aggregates, whereas when calcium was added after thermal treatment, the soluble aggregates had smaller sizes.
CaHHPT and HHPTCa showed similar profiles in the resolving gel, but they were different in the entrance to stacking gel: in CaHHPT a more intense band appeared corresponding to very large aggregates. Thus, in both CaTT and CaHHPT there was a higher proportion of large soluble aggregates than in TTCa and HHPTCa (Fig. 4A).
In presence of β-mercaptoethanol (Fig. 4B), the most of aggregates were dissociated in every lane. The intensity of bands corresponding to polypeptides A and B increased as consequence of reduction of disulfide bonds in the most of lanes but did not in the lanes of Ca, CaTT and TTCa. Taking into account that SDS-PAGE was carried out on supernatants of 10,000 g, that fact indicates that glycinin was involved in insoluble aggregates in Ca, CaTT and TTCa, whereas in CaHHPT and HHPTCa glycinin was involved in soluble aggregates.
The partial conclusions of this work up to this point allow us to affirm that for both heat and high hydrostatic pressure, the colloidal stability and the proportion of soluble aggregates with high molecular weight was higher when calcium was present during denaturing treatment than when calcium was added after treatment. Calcium-induced insolubilization mainly affected glycinin, HHPT reversed (when calcium was added before treatment) or partially prevented (when calcium was added after treatment) that insolubilization. In the case of HHPTCa the %S was indistinguishable of Ca, but soluble species included glycinin. The highest %S and colloidal stability were obtained by HHPT when calcium was present during treatment.
Stable turbid dispersions obtained from CaHHPT may be an alternative as clouding agents for beverages, with advantages (comparing with emulsions) because they have a lower caloric index and no lipid oxidation occurs.
Potential and Innovative Practical Use
In order to evaluate a potential practical use of these modified proteins and taking into account that Manassero, David-Briand, Vaudagna, Anton, and Speroni [17] stated that calcium added soybean proteins subjected to HHPT exhibited an enhanced capacity to interact with themselves, the idea of essaying cold-set gelation came up. The samples chosen were CaTT and CaHHPT and the strategy consisted of freeze-drying and re-dispersing at a ten-fold higher concentration.
Cold-set gelation of soybean proteins has been studied by adding calcium to dispersions of denatured proteins [22, 23]. These researchers obtained gels with calcium/protein ratios in the range 0.11 to 0.24 mmol calcium/g protein. Since the ratio of our samples was higher (0.50 mmol calcium/g protein), we also prepared protein dispersions with 1.8 mM CaCl2 to obtain a ratio of 0.18 mmol calcium/g protein.
In the case of CaTT-50 mM no gel was formed, but a turbid dispersion that was destabilized in a few minutes by sedimentation. Protein-protein and calcium-protein interactions predominated at the expense of water-protein ones. This result indicate that the low protein solubility was a limiting factor for gel forming ability, as it was stated by Brito-Oliveira, Bispo, Moraes, Campanella, and Pinho [24]. On the other hand CaTT-18 mM was a viscous and semitransparent dispersion. Even though decrease in calcium concentration improved homogeneity and induced the formation of a matrix that could be interesting as a thickening ingredient, no self-standing gel was formed in this condition.
When CaHHPT was assessed, self-standing gels were formed at both calcium concentrations. Differences between gels obtained at 18 and 50 mM were detected in terms of visual aspect, WHC and rheological properties (Table 2 and Fig. 5). Properties of CaHHPT-50 mM corresponded to those of aggregated gels: low WHC, opaque and hard [25]. On the other hand, CaHHPT-18 mM gels had an excellent WHC, were translucent and weaker than CaHHPT-50 mM. These properties suggest that the CaHHPT-18 mM gels were fine-stranded and formed by soluble proteins [25]. Besides, the amber-like color coincided with that of soluble soybean protein isolate dispersions such as those prepared at pH 7 or 8. The solubilization of glycinin induced by high hydrostatic pressure could be in part responsible for the good gelation ability of CaHHPT, especially for CaHHPT-18 mM. The higher adhesivity of CaHHPT-50 mM may be due to the higher ionic strength [26]. The elastic modulus was higher than the viscous modulus throughout the frequency range for both calcium concentrations. The values of tan δ found in the frequency range analyzed corresponded to weak gels [27]. Clearly, calcium/protein ratio governed the features of these gels.
Conclusions
Under the conditions of the present work, TT induced no change in %S of calcium-added aqueous dispersions, but induced a slight decrease in colloidal stability. On the other hand, HHPT increased both %S and colloidal stability of insoluble aggregates when calcium was added prior to treatment. Differences would be due to different interactions affected by each treatment, which would led to different conditions during treatment (i.e. reversible dissociation of hydrogen and calcium ions during HHPT) or to specific associations (i.e. preferential insolubilization of certain polypeptides upon TT). Glycinin fraction behaved differentially in calcium-added dispersions when heated or pressurized and remained involved in insoluble aggregates when subjected to TT, whereas remained involved in soluble and large aggregates when subjected to HHPT (whatever the order of application of calcium and pressurization).
The order or application of calcium and denaturing treatment significantly influenced the size of soluble aggregates (larger when calcium was present during both denaturing treatments than when it was added after treatments) and colloidal stability (enhanced stability when calcium was present during both denaturing treatments).
After freeze drying and re-dispersing, pressurized and heated samples also behaved differently. CaTT formed no gel under the conditions assayed, fact that merits a study at other calcium and protein concentration. On the other hand, CaHHPT formed gels at both calcium concentrations, with very promising properties at the lowest one. Thus, proteins in CaHHPT exhibited interesting ability to interact simultaneously with water and with other proteins.
Our data represent an important input toward the development of new functional food ingredients, either clouding agents for liquid foodstuff (enhanced colloidal stability of insoluble aggregates) or texturing agent (good cold-gelling ability).
References
N.K. Rastogi, K.S.M.S. Raghavarao, V.M. Balasubramaniam, K. Niranjan, D. Knorr, Crit. Rev. Food Sci. Nutr. 47, 69 (2007)
B.B. Boonyaratanakornkit, C.B. Park, D.S. Clark, Biochim. Biophys. Acta 1595, 235 (2002)
E. Molina, A. Papadopoulou, D.A. Ledward, Food Hydrocoll. 15, 263 (2001)
R. Lakshmanan, M. de Lamballerie, S. Jung, Food Eng Phys Prop 71, 384 (2006)
K. Nishinari, Y. Fang, S. Guo, G.O. Phillips, Food Hydrocoll. 39, 301 (2014)
N. Chen, M. Zhao, F. Niepceron, T. Nicolai, C. Chassenieux, Food Hydrocoll. 66, 27 (2017)
A. Scilingo, M.C. Añón, J. Am. Oil Chem. Soc. 81, 63 (2004)
M.C. Añón, M. de Lamballerie, F. Speroni, Innov. Food Sci. Emerg. Technol. 16, 155 (2012)
C.A. Manassero, S. Vaudagna, M.C. Añón, F. Speroni, Food Hydrocoll. 43, 629 (2015)
C.A. Manassero, S. Vaudagna, A.M. Sancho, M.C. Añón, F. Speroni, Innov. Food Sci. Emerg. Technol. 35, 86 (2016)
F. Speroni, V. Milesi, M.C. Añón, LWT-Food SciTech 43, 1265 (2010)
AOAC, Official Methods of Analysis, 15th Edn (Association of Official Analytical Chemists, Washington, 1990)
M.C. Añón, M. de Lamballerie, F. Speroni, Innov. Food Sci. Emerg. Technol. 12, 443 (2011)
J.M.S. Renkema, H. Gruppen, T. Van Vliet, J. Agric. Food Chem. 50, 6064 (2002)
A.G. Appu Rao, M.S. Narasinga Rao, J. Agric. Food Chem. 24, 490 (1976)
R.D. Kroll, Cereal Chem. 61, 490 (1984)
C.A. Manassero, E. David-Briand, S.R. Vaudagna, M. Anton, F. Speroni, Food Bioprocess Tech 11, 1125 (2018)
C.P. Samaranayake, S.K. Sastry, Innov. Food Sci. Emerg. Technol. 17, 22 (2013)
V.V. Mozhaev, K. Heremans, J. Frank, P. Masson, C. Balny, Protein Struct Funct Genet 24, 81 (1996)
D.B. Yuan, X.-Q. Yang, C.-H. Tang, W. Z-X Zheng, I. Min, S.-W. Ahmad, Yin Food Res Int 42, 700 (2009)
S. Petruccelli, M.C. Añón, J. Agric. Food Chem. 43, 3035 (1995)
A. Maltais, G.E. Remondetto, R. Gonzalez, M. Subirade, J. Food Sci. 70, 67 (2005)
F. Speroni, M.C. Añón, Food Hydrocoll. 33, 85 (2013)
T.C. Brito-Oliveira, M. Bispo, I.C.F. Moraes, O.H. Campanella, S.C. Pinho, Food Biophys 13, 226 (2018)
A.M. Hermansson, J. Am. Oil Chem. Soc. 63, 658 (1986)
A.J. Pastorino, C.L. Hansen, D.J. McMahon, J. Dairy Sci. 86, 60 (2003)
A.H. Clark, S.B. Ross-Murphy, Gels. Adv. Polym. Sci. 83, 60 (1987)
Acknowledgments
The authors wish to thank Claudio Sanow from the Instituto de Tecnología de Alimentos, INTA, for his kind assistance during the use of the HHPT equipment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piccini, L., Scilingo, A. & Speroni, F. Thermal Versus High Hydrostatic Pressure Treatments on Calcium-added Soybean Proteins. Protein Solubility, Colloidal Stability and Cold-set Gelation. Food Biophysics 14, 69–79 (2019). https://doi.org/10.1007/s11483-018-9558-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-018-9558-z