Abstract
Calcium addition to soybean protein dispersions increases nutritional value but harms functional properties, such as protein solubility and colloidal stability. The high hydrostatic pressure (HHP) treatment can reverse those effects. The aims of this work were to evaluate the influence of pH and protein and calcium concentration on HHP solubilizing/stabilizing effect and to characterize the physicochemical properties of HHP-stabilized species. Proteins without calcium addition were stabilized by HHP at both pHs. However, calcium-added proteins behaved differentially: at pH 5.9, the effect was verified only at low protein concentration, whereas at pH 7.0, the effect was verified under both assayed protein concentrations (5 and 10 g L−1) and with a higher magnitude in calcium-added samples. Moreover, at pH 7.0, the effect was independent of the order of calcium addition and HHP treatment, whereas at pH 5.9, the effect was smaller when calcium was added after HHP treatment. At both pHs, the solubilizing/stabilizing effect of HHP on soybean proteins seemed to be largely dependent on the decrease in the size of protein species. The smaller the size, the greater the amount of protein that remained in dispersion after intense centrifugation (10,000g, 20 min, 4 °C). Although the effect of HHP consisted, at least in part, of stabilizing insoluble protein, turbidity decreased in all samples after HHP treatment. By combining different levels of pH, calcium, and protein concentrations, translucent or turbid colloidal-stable dispersions can be obtained by HHP treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calcium is an essential nutrient that plays critical roles in a wide variety of physiological processes (Sacco and L’abbé 2016). The main sources of calcium are foods of animal origin. Some conditions such as intolerances, allergies, and/or vegetarian diets prevent this way of incorporation. In this regard, the development of formulated liquid foodstuff with added calcium is a topic of current importance. Soybean is a non-expensive source of proteins with high biological value (compared with other vegetable proteins), interesting functional properties, and health-improving compounds (Nishinari et al. 2014). Nevertheless, calcium addition to soybean proteins negatively affects some functional properties such as solubility, colloidal stability, and surface activity, limiting their utilization to soy-based drinks (Ono et al. 1993).
Novel food-processing technologies have been studied and applied to provide safer and fresher-tasting nutritive foods. In this regard, HHP is a non-thermal technology that has been applied in the processing of soy-based foods (Lakshmanan et al. 2006; Molina et al. 2001; Puppo et al. 2004). HHP technology improves proteins’ functional properties such as solubility and colloidal stability (Añón et al. 2012; Manassero et al. 2015; Baier et al. 2015).
In previous works by our group, it was found that HHP induced an increment in protein solubility and improvement in colloidal stability in calcium-added soybean protein isolate (SPI) dispersions and in more complex foodstuff such as soymilk, which also contains fiber and emulsified oil. The increase in protein solubility and colloidal stability of calcium-added SPI depended on pH and calcium concentration. The increase of pH extended the calcium concentration range in which the solubilizing effect was observed (Manassero et al. 2015; Manassero et al. 2016). The effect seems to be related to aggregation and dissociation. Zhang et al. 2005 proposed that soybean proteins were dissociated by HHP into subunits, some of which associated to aggregate and became insoluble. Nevertheless, the mechanisms involved have not been elucidated yet. We hypothesized that electrostatic interactions during pressurization could be implicated in these effects. The role of calcium during HHP treatment and the discrimination between soluble and insoluble but non-settling protein have not been understood yet.
Gelation of calcium-added SPI was also improved by HHP treatment under certain conditions. Thus, functional properties that depend on the balance of protein-protein and protein-water interactions can be improved by combination of calcium and HHP (Speroni et al. 2010a; Speroni and Añón 2013). Effects of this combination on other functional properties that depend on interfacial activity (emulsification, foaming) have not been reported either.
The aims of this work were to deepen the knowledge about mechanisms involved in the stabilizing and solubilizing effects of HHP on calcium-added soybean protein and to evaluate the emulsifying properties of these modified proteins. This work will be presented in two parts. In the first part, whole and supernatant dispersions will be characterized in terms of particle size distribution, ζ-potential, turbidity, and interfacial pressure, in order to better understand solubility, colloidal stability, and interfacial behaviors. In the second part, the formation and stability of emulsions prepared with calcium-added SPI treated with HHP will be analyzed. The whole study can be a contribution for the utilization of calcium-enriched SPI dispersions to conceive and manufacture soybean-based food.
Materials and Methods
Experimental Design
Protein dispersions were characterized by evaluating the effect of pH (5.9 or 7.0), protein concentration (5 or 10 g L−1), calcium concentration (1.25 or 2.5 mmol CaCl2 L−1 for pH 5.9 and 2.5 or 5.0 mmol CaCl2 L−1 for pH 7.0), and HHP level (0.1 or 600 MPa) (Table 1) on protein solubility, colloidal stability, particle size distribution, ζ-potential, and interfacial behavior of adsorbed protein films. Each treatment was performed in triplicate. These conditions were chosen because they involve pH values compatible with foodstuff and, according to previous work performed in our laboratory (Manassero et al. 2015), in which the effect of HHP on protein solubility was substantial. Analyses of variance (one-way ANOVA) were conducted. Differences between sample means were analyzed by Tukey’s test at an α level of 0.05. The statistical analysis was completed using the Origin software (OriginLab Corporation, Northampton, MA, USA).
Sample Preparation
Preparation of Soybean Protein Isolate (SPI)
SPI was prepared from defatted soybean flour manufactured by The Solae Company (Brazil). Alkaline extraction (pH 8.0—90 min—20 °C) was followed by isoelectric precipitation (pH 4.5—15 min), as described by Speroni et al. (2010b). The isoelectric precipitate was dispersed in distilled water and its pH was adjusted to 7.0 with 2 mol L−1 NaOH. Then, dispersion was freeze-dried. The same batch of SPI was used for the whole study.
Preparation of Calcium-Added SPI Dispersions
Dispersions of SPI were prepared at 5 or 10 g protein L−1 in 30 mmol L−1 BIS-TRIS pH 5.9 (Sigma, St Louis, USA) or 50 mmol L−1 TRIS-HCl pH 7.0 (Merck, Germany). Calcium was added from a stock solution at 1 mol L−1 (CaCl2): 1.25 or 2.5 mmol L−1 for dispersions at pH 5.9 and 2.5 or 5.0 mmol L−1 for dispersions at pH 7.0. Stock solution of CaCl2 was prepared from CaCl2.2H2O (Sigma, St Louis, USA). Calcium concentrations were higher at pH 7.0 than at pH 5.9 in order to take advantage of pH influence on the solubilizing effect of HHP and then maximize the content of soluble protein (Manassero et al. 2015). For each pH, calcium and protein concentrations were at a constant ratio (2.5 mmol CaCl2/g protein for pH 5.9 and 5.0 mmol CaCl2/g protein for pH 7.0). Furthermore, calcium was added after HHP treatments in samples prepared with 5 g protein L−1 at both pH values.
High Hydrostatic Pressure Treatment
Prior to HHP treatment, SPI dispersions were vacuum packed in a polyethylene bag (La Bovida, France). Then, they were treated at 600 ± 5 MPa for 5 min in a high-pressure system (ACB Pressure Systems, Nantes, France) equipped with temperature (Julabo, Seelbach, Germany) and pressure regulator devices (vessel capacity, 3.0 L; maximum working pressure, 600 MPa). The compression fluid used was water. The working pressure was reached at 3.4 MPa s−1 and released instantaneously. Temperature during treatment was controlled to avoid overheating of dispersions. Initial temperature of compression fluid and samples and conditioning temperature of vessel were 20 °C. An increase in sample temperature up to 25 °C was verified during the HHP treatment due to compression heating. The temperature of one sample was registered with a thermocouple type K placed in the sample-containing bag (sealed with a stuffing box). Conditions of HHP treatment were selected in accordance to previous experiments (Manassero et al. 2015).
Sample Analysis
Determination of Protein Content of SPI
The protein content of SPI was determined by Kjeldahl method (AOAC 1990). The digester and distillation unit were from BÜCHI (Flawil, Switzerland). The conversion factor was 5.8.
Determination of Protein Solubility
Samples were centrifuged at 10,000g for 20 min at 4 °C in an air-cooled benchtop centrifuge Universal 320R (Hettich, Tuttlingen, Germany). Protein concentration was determined in the supernatants. The Bicinchoninic Acid Protein Assay (BCA Sigma Kit) (Sigma Chemical Co., St. Louis, MO, USA) was used for the quantification of soluble proteins. Bovine serum albumin was used as a standard (Sigma Chemical Co., St. Louis, MO, USA). Absorbance was measured at 562 nm in an Epoch™ Multi-mode Microplate Reader (BIOTEK Instruments, Winooski, VT, USA). Protein solubility was determined in triplicate for each sample. Results were expressed as:
Colloidal Stability
Absorbance at 600 nm was used as an indicator of turbidity. Absorbance was measured in whole samples or supernatants obtained at 3000 or 10,000g for 20 min at 4 °C in a centrifuge Jouan GR 20-22 (Thermo Scientific, Waltham, USA). Absorbance was measured using a UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Absorbance was determined in triplicate for each sample. Results were expressed as AU (absorbance units).
Particle Size Distribution
Particle size distributions were determined by dynamic light scattering (DLS) experiments using a Zetasizer Nano Zs (Malvern Instruments Ltd., Malvern, UK). This technique measures the time-dependent fluctuations in the intensity of scattered light due to Brownian motion. DLS measures were carried out in triplicate for each sample both in whole samples or supernatants obtained at 3000 or 10,000g. The particle size distribution of the samples was based on intensity measurements. Data analysis was performed with Zetasizer Nano software according to the Stokes-Einstein equation:
where d (nm) is the hydrodynamic diameter of the particle, k (J K−1) is the Boltzmann’s constant, T (K) is the absolute temperature, η (Pa s) is sample viscosity, and D (m2 s−1) is the diffusion coefficient.
ζ-Potential
ζ-potential was evaluated in triplicate for each sample in a dynamic light scattering instrument (Zetasizer Nano Zs, Malvern Instruments Ltd., Malvern, UK). The ζ-potential was evaluated from the electrophoretic mobility of the particles present in whole samples or supernatants obtained after centrifugation in the same conditions described for turbidity and particle size distribution experiments. The conversion of the measured electrophoretic mobility data into ζ-potential was obtained using Henry’s equation:
where U e (m2 V−1 s−1) is the electrophoretic mobility, ε (C2 N−1 m−2) is the dielectric constant, ζ (mV) is the ζ-potential, η (Pa.s) is sample viscosity, and f(K a ) (dimensionless) is Henry’s function.
Surface Pressure Isotherms
The surface pressure (π) vs. trough area (A) measures were performed on a fully automated Wilhelmy-type balance (NIMA 601 BAM, NIMA Technology Ltd., Coventry, UK), as described elsewhere (Baeza et al. 2004; Murray and Nelson 1996). The balance consisted of two movable barriers that surrounded the Wilhelmy plate (Whatman’s chromatography paper). The area of the vessel, initially of 230 cm2, was reduced to 55 cm2 at the end of compression. The barrier speed was fixed at 40 cm2 min−1. The vessel was filled with each one of the buffers used for SPI dispersion preparation. Temperature was kept at 20 °C by circulating water from a thermostat. Aliquots (16 μg protein) of supernatants from SPI dispersions (5 g L−1) obtained at 10,000g were spread on the interface. The deposition of the drops was performed with a micrometric syringe to avoid thick smears, and located on the surface. In order to allow the processes of spreading, adsorption, and rearrangements of proteins, samples were left to stand for 30 min before compression. At least three isotherms were performed for each sample.
Results and Discussion
Protein Content
The SPI protein content determined by Kjeldahl method was 81.6 ± 0.2% w.b. (the conversion factor was 5.8). This value was used for protein dispersion preparation at different concentrations and for protein solubility calculation.
Protein Solubility
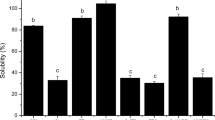
Solubility was higher at pH 7.0 than at pH 5.9 for each condition assayed. This fact was verified even for calcium-added samples, although calcium concentrations were higher at pH 7.0 (Fig. 1). This behavior was due to a greater protein aggregation near the isoelectric point (pI): about 4.5–4.9 for β-conglycinin and 5.0 for glycinin (Kella et al. 1986; Koshiyama 1968). The addition of CaCl2 decreased protein solubility at both pHs in unpressurized samples (Fig. 1). The relative effect was higher at pH 7.0 than at 5.9 probably due to the higher concentration of CaCl2 added and to the higher initial solubility value observed at pH 7.0.
Protein solubility of SPI dispersions subjected to different conditions: protein concentration (a, b, 5 g L−1; c, d, 10 g L−1), pH values, and calcium-added concentration (mmol CaCl2 L−1) before (0.1 MPa) or after HHP treatment (600 MPa). Values are expressed as means ± standard deviation. Different letters within a same protein concentration and pH indicate significant differences (p < 0.05)
In 5 g L−1 protein dispersions, HHP treatment increased protein solubility in samples with and without calcium addition at both pHs (Fig. 1a, b). More precisely, in calcium-added samples, HHP produced a relative increase in solubility of 162% at pH 5.9 and 109% at pH 7.0. Noticeably, pressurized calcium-added dispersions exhibited higher protein solubility than unpressurized samples without calcium at both pHs.
We changed the sequence of CaCl2-HHP treatments by incorporating calcium after HHP. In this way, 5 g L−1 protein dispersions were treated at 600 MPa for 5 min and then 1.25 or 2.5 mmol L−1 CaCl2 was added at pH 5.9 or 7.0, respectively. At pH 5.9, protein solubility of these samples was 36.9 ± 0.4%, i.e., higher than those of unpressurized dispersions (19.5 ± 0.4%; 5-1.25 Ca-pH 5.9-0.1), but lower than those of dispersions pressurized after calcium addition (51.1 ± 1.2%; 5-1.25 Ca-pH 5.9-600). However, at pH 7.0, no significant difference (p > 0.05) in protein solubility was found between pressurized calcium-added dispersions, regardless of the moment of calcium incorporation (solubility was 74.8%, in average). These data indicate that the magnitude of the effect of HHP treatment on protein solubility at pH 5.9 depends on the presence of calcium during HHP-treatment. This differential effect of calcium suggests that HHP-induced aggregation/dissociation mechanisms were different between pHs 5.9 and 7.0 for soybean proteins.
In 10 g L−1 protein dispersions, HHP-treatment significantly increased (p < 0.05) protein solubility in both non-added and calcium-added SPI dispersions at pH 7.0, but only in non-added samples at pH 5.9 (Fig. 1c, d). Moreover, in calcium-added samples, HHP produced a relative increase in solubility of 175% at pH 7.0, which was higher than that obtained at 5 g L−1 (109%). Since calcium/protein ratio was constant, this difference in solubility seems to be due to protein and calcium concentrations.
At pH 7.0, the increases in solubility induced by HHP on calcium-added samples were higher than those induced on non-added samples at both protein concentrations. On the other hand, at pH 5.9, the magnitude of the effect of HHP on solubility was independent of calcium addition at 5 g L−1, and no effect was detected at 10 g L−1 in calcium-added samples (10-2.5 Ca-pH 5.9-0.1 vs. 10-2.5 Ca-pH 5.9-600). These behaviors indicate that the mechanisms are different at each pH and the magnitude of HHP effects also depend on protein concentration.
In previous work, we found that at pH 5.9, a treatment at 600 MPa during 5 min increased protein solubility in samples with the same calcium and protein concentrations (2.5 mmol L−1, 10 g L−1) (Manassero et al. 2015), but prepared in bi-distilled water. This difference with the results of the current work may be due to the presence of BIS-TRIS buffer. Binding of buffers to proteins may result in charge screening, which can lead to poorer colloidal stability (Zbacnik et al. 2017). Moreover, transient pH decreases occur during HHP treatments and can affect protein denaturation process. The magnitude of pH-decrease depends on medium composition and pressure level (Samaranayake and Sastry 2013). Thus, the presence of BIS-TRIS could modulate this change in pH and, consequently, affect the denaturation process and the structure of protein aggregates.
Colloidal Stability
The colloidal stability of protein dispersions was evaluated by analyzing the turbidity of whole samples and supernatants of centrifugation (Fig. 2).
Absorbance at 600 nm of SPI dispersions as function of speed centrifugation, for different protein concentrations (a, b, 5 g L−1; c, d, 10 g L−1), pH values, and calcium-added concentration (mmol CaCl2 L−1) before (0.1 MPa) or after HHP treatment (600 MPa). Values are expressed as means ± standard deviation. Different letters within a same protein concentration, pH, and speed centrifugation indicate significant differences (p < 0.05)
Colloidal Stability of Whole Samples
The values of absorbance of whole samples (1g, non-centrifuged) ranged between 0.04 AU (5-0 Ca-pH 7.0-600; Fig. 2b) and 2.65 AU (10-2.5 Ca-pH 5.9–0.1; Fig. 2c). These values inversely correlated to those of protein solubility: 82.6% (5-0 Ca-pH 7.0-600) and 11.8% (10-2.5 Ca-pH 5.9-0.1). Moreover, samples with solubility values equal or lower than 51.1% exhibited absorbance values higher than 1.24 AU, whereas samples with solubility values equal or higher than 55.8% exhibited absorbance values lower than 0.42 AU. The addition of calcium increased dispersion turbidity at both pHs and at both protein concentrations in unpressurized whole samples by increasing the content of insoluble species. The effect was higher in 10 g L−1 dispersions. These results agree with those of Ferreira et al. (1999) who found an increment in turbidity for calcium-added Lupinus albus storage proteins and for (calcium + magnesium)-added soybean globulins. Dispersion turbidity strongly depends on size, concentration, and the refraction index of colloids (Dezelić et al. 1963).
HHP treatment decreased turbidity in most of the calcium-added and non-added samples at both pHs assayed. The only sample in which HHP treatment promoted no change in turbidity was the one with calcium added after HHP treatment at pH 5.9 and at 5 g L−1 (2.12 AU), indistinguishable from the value of 5-1.25 Ca-pH 5.9-0.1. In most samples, the decrease in turbidity was accompanied by an increase in protein solubility. For example, in 5 g L−1 protein dispersion with 1.25 mmol L−1 calcium-added at pH 5.9, HHP increased protein solubility (from 19.5 to 51.1%) and decreased turbidity (from 2.24 to 1.24 AU). Nevertheless, it is noteworthy that the only sample in which HHP had no effect on protein solubility (10-2.5 Ca-pH 5.9-600) exhibited a lower turbidity after HHP treatment (absorbance decreased from 2.65 to 1.55 AU). Taken together, these data indicate that in some conditions, calcium and protein concentrations can modulate the effect of HHP on aggregates’ structure, decreasing turbidity of dispersions, but keeping protein solubility constant. HHP treatment decreased turbidity of milk protein dispersions by disruption of casein micelles due to colloidal calcium phosphate solubilization (Needs et al. 2000). In our system, the amount of calcium bound to soybean proteins was not irreversibly affected by HHP (Manassero et al. 2015), but reversible dissociation could occur during pressurization (electrostriction is favored by HHP) allowing rearrangements of aggregates.
Colloidal Stability of Centrifuged Samples
Supernatants of centrifugation showed low values of absorbance because of insoluble particle sedimentation (Fig. 2). As it was expected, the turbidity reduction induced by centrifugation was higher in samples with low solubility and high initial absorbance values. Nevertheless, 5-1.25 Ca-pH 5.9-600 with solubility of 51.1% had a high absorbance value (0.43 AU, Fig. 2a). This result indicates that insoluble aggregates were present in this supernatant. Since our operational definition of solubility involves a centrifugation step at 10,000g, these data also indicate that part of what was reported as “soluble protein” is represented by kinetically stable insoluble aggregates. Turbidity of supernatant of 10-2.5 Ca-pH 5.9-600 was low (0.16 AU), but higher than that of 10-2.5 Ca-pH 5.9-0.1 (0.046 AU, Fig. 2c). Thus, protein and calcium concentrations affected sedimentation behavior: at pH 5.9, pressurized calcium-added SPI dispersions exhibited a higher colloidal stability than unpressurized ones, and this effect had a higher magnitude at low concentrations. Ye and Harte (2014) reported that casein-hydroxypropyl cellulose aqueous systems homogenized by high pressure at 300 MPa showed exceptional stability during 2 weeks of storage, indicating the formation of very small aggregates. Thus, the stabilizing effect of HHP in our system may also be due to the breakdown of aggregates.
At pH 7.0, sedimentation behavior of calcium-added dispersions was also a function of protein concentration. At 5 g L−1, HHP-treated samples were less stable than unpressurized ones (the highest value of absorbance was found for 5-2.5 Ca-pH 7.0-0.1 (0.182 AU)), whereas at 10 g L−1, HHP-treated samples were more stable than unpressurized ones (the highest value of absorbance was found for 10-5 Ca-pH 7.0-600 (0.127 AU, Fig. 2b, d).
Regarding the order of application of calcium and HHP, at 5 g L−1, aggregates were more stable when HHP was applied in the presence of calcium than when calcium was added after HHP treatment at pH 5.9. On the other hand, at pH 7.0, the change in order of application promoted no change in colloidal stability (data not shown).
Centrifugation at 3000g generated behaviors similar to those generated by centrifugation at 10,000g in most samples (data not shown). A remarkable behavior was that of the sample in which calcium was added after HHP treatment at pH 5.9, since after a 3000g centrifugation, the absorbance was 1.3 ± 0.2 AU, suggesting a high content of particles that resisted that acceleration.
ζ-Potential
The ζ-potential describes the magnitude of electrical charges present on colloidal particles and strongly influences their stability. The effective charge on a protein particle is due to ionization of various amino acid residues and is affected by pH, ionic strength, and the accumulation of ligands or surfactants at the interface. The ζ-potentials of 5 g L−1 protein dispersions are shown in Fig. 3. Light scattering studies can only be performed in optically clear suspensions, so it was necessary to make proper dilutions before measures.
ζ-potential of 5 g L−1 soybean proteins dispersions for different pH values and calcium-added concentrations (mmol CaCl2 L−1) before (0.1 MPa) or after HHP treatment (600 MPa). Values are expressed as means ± standard deviation. Different letters within a same pH and speed centrifugation indicate significant differences (p < 0.05)
The highest absolute value of ζ-potential was observed in samples without calcium at pH 7.0. These results agree with those by Manassero et al. (2015). who determined the ζ-potential of SPI dispersions in bi-distilled water. Nevertheless, absolute values of ζ-potential of SPI dispersed in buffer were lower than those obtained in bi-distilled water. This decrease in magnitude of ζ-potential seems to be related to an association between protonated-buffer and negative charges of proteins (Zbacnik et al. 2017). The addition of CaCl2 decreased (p < 0.05) the magnitude of ζ-potential at both pHs due to partial neutralization of negative charges on protein surface. Liu et al. (2011) found that CaCl2 affected the ζ-potential-pH profiles of glycinin-rich fractions, with lower ζ-potential magnitudes at the higher CaCl2 concentrations.
HHP treatment decreased the absolute value of ζ-potential in samples with and without calcium at pH 5.9 (p < 0.05). On the other hand, at pH 7.0, there was a tendency in samples without calcium to decrease ζ-potential after HHP treatment in non-centrifuged samples (Fig. 3); this effect was significant (p < 0.05) in samples centrifuged at 10,000g (data not shown). Signally, the HHP effect at pH 5.9 was a decrease in the magnitude of ζ-potential and an increase in protein solubility. These contradictory results (a reduction of ζ-potential accompanied by an increase in protein solubility) would suggest a complex mechanism, e.g., an increase in the association between protonated-buffer and protein, which would hinder protein-protein interaction by a steric factor, avoiding aggregation and insolubilization. In our previous work, HHP-treatment caused no change in ζ-potential in SPI dispersions prepared in bidistilled water at pH 5.9 (Manassero et al. 2015); thus, the presence of BIS-TRIS seems to account for some specific behaviors.
Centrifugation exerted minor effect in samples at both pHs (data not shown). This fact seems to be related to the greater colloidal stability of charged species than that of uncharged ones (Tang and Ma 2009). However, pressurized calcium-added dispersion at pH 5.9 showed the lower ζ-potential and the greater colloidal stability (Fig. 2a and Fig. 3). These data suggest that colloidal stability is not only governed by ζ-potential and that the effect of HHP on colloidal stability and solubility was not associated with an increase in surface charge of proteins.
Particle Size Distribution
The samples presented different distributions in a wide range of sizes (28 to 5560 nm). We classified them arbitrarily into 3 groups: “small aggregates” (size ranged between 20 and 300 nm), “medium aggregates” (size between 300 and 2500 nm), and “large aggregates” to species with sizes larger than 2500 nm.
Figure 4 shows the typical particle size distributions obtained for 5 g L−1 SPI dispersions at pH 5.9. Whole 5-0 Ca-pH 5.9-0.1 samples (1g, non-centrifuged) presented a polydisperse distribution, with three populations at 188 (the most abundant), 1401, and 4532 nm (Fig. 4a). Calcium addition promoted the formation of larger aggregates (246 and 1862 nm) and induced an increase in the abundance of larger aggregates (Fig. 4a). Liu et al. (2011) found that the addition of calcium to soybean protein dispersions at pH 5.8 promoted the aggregation of glycinin and β-conglycinin. The authors suggested that this effect could be partially attributed to a decrease in the absolute value of ζ-potential, leading to a weakening of the repulsive forces between globulins.
Treatment with HHP decreased the size of the aggregates and homogenized particle size distribution through the reduction or elimination of “medium” and “large” aggregates, in both calcium added and non-added samples (Fig. 4b). Tang and Ma (2009) reported that HHP treatment (600 MPa, 15 min, 25 °C) on SPI dispersions at pH 7.4 led to a transformation of insoluble aggregates into soluble ones. Moreover, the authors found that the soluble aggregates formed during HHP treatments were much more homogenous in terms of molecular weight distributions. Besides, Añón et al. (2012) analyzed the effect of HHP on aggregation of calcium-added soybean proteins present in SPI and in protein fractions enriched in β-conglycinin or glycinin. The authors found that HHP treatment split insoluble large aggregates into smaller ones, whose sizes were a function of pressure level, calcium concentration, and protein composition of samples. In the present study, when calcium was added after HHP treatment, particle size distribution showed three populations at 213, 766, and 5121 nm (Fig. 4b); that is, aggregates were larger than those obtained with calcium incorporated before HHP treatment. This result indicates that at pH 5.9, in our experimental conditions, the structure of aggregates depended on the order of HHP application and calcium incorporation. Possibly, the aggregates formed by HHP in the absence of calcium (mode 119 nm) were bridged by calcium when the ion was added later and accounted for aggregates of 213 and 766 nm (that seemed to be dimers and tetramers, respectively, Fig. 4b). On the other hand, when calcium was present during HHP-induced denaturation, dissociation of “medium aggregates” (1862 nm) seemed to be the main process, leading to the formation of species of 200 nm (Fig. 4a, b).
At pH 5.9, centrifugation either decreased (3000g) or eliminated (10,000g) the largest aggregates leaving a single predominant population (Fig. 4c, d, e, f). Nevertheless, centrifugation (3000g) of 5-600-1.25 Ca-pH 5.9 left in supernatant two overlapping populations, whose modes were 164 and 504 nm (Fig. 4d). Probably, those aggregates accounted for the high turbidity exhibited by this sample (1.3 AU). After centrifugation at 10,000g, the distribution was monomodal for the five samples, the peaks were narrower than those corresponding to 1 and 3000g, and the largest aggregates (mode 232 nm) were detected when calcium was incorporated after HHP treatment (Fig. 4e, f).
Figure 5 shows the typical particle size distributions obtained for 5 g L−1 soybean protein dispersions at pH 7.0. At pH 7.0, the sizes of aggregates were lower than at pH 5.9 (in both unpressurized and HHP-treated samples). This fact reflects the effect of pH on electrostatic interactions and its consequence on protein aggregation beyond other factors, such as HHP-induced denaturation or calcium presence. The 5-0 Ca-pH 7.0-0.1 samples (1g, non-centrifuged) presented a polydisperse distribution, with three populations at 27 (corresponding to non-aggregated globulins), 158, and 4829 nm. Calcium addition promoted the formation of “medium aggregates” (324 and 2041 nm, Fig. 5a).
The HHP treatment applied on samples without calcium addition promoted the dissociation of the initial aggregates (158 nm) and the aggregation of globulins molecules (27 nm), leading to the appearance of a major peak of 69 nm (Fig. 5b). These findings agree with those of Añón et al. (2012) who worked with SPI dispersions at pH 8.0 and reported simultaneous dissociation and aggregation after 400 and 600 MPa treatments.
The particle size distributions of 5-600-2.5 Ca-pH 7.0 and 5-2.5 Ca-pH 7.0-600 were indistinguishable (Fig. 5b). The difference between calcium-added dispersions at pH 5.9 and 7.0 was remarkable: at pH 5.9, calcium addition after HHP treatment produced “medium” and “large” aggregates, whereas at pH 7.0, calcium addition after HHP treatment produced “small” aggregates. These behaviors could explain the differences in solubility: at pH 5.9, the relative increase in solubility was lower when calcium was added after HHP treatment (89%) than when calcium was added before HHP (162%), whereas at pH 7.0, both samples (calcium addition before and after HHP) had a relative increase in solubility of 105%).
At pH 7.0, centrifugation either decreased (3000g) or eliminated (10,000g) the populations of “medium” and “large” aggregates. In 5-2.5 Ca-pH 7.0-0.1, after centrifugation at 10,000g, a narrow population with a mode of 245 nm remained and would account for the higher turbidity exhibited by this sample (Fig. 2b and Fig. 5e). Noticeably, pressurized calcium-added dispersions at pH 7.0, regardless of the order of calcium incorporation, showed similar particle size distributions before and after centrifugation (Fig. 5b, d, f). The resistance of aggregates towards centrifugation-induced sedimentation indicates that the aggregates formed by the combination of calcium and HHP at pH 7.0 show high colloidal stability. These results agree with those of solubility (Fig. 1) and turbidity (Fig. 2).
Whole 10 g L−1 SPI dispersions were also characterized (Fig. 6). At pH 5.9, unpressurized dispersions showed lower polydispersity than 5 g L−1 ones. On the other hand, at pH 7.0 unpressurized samples showed similar particle size distributions at both protein concentrations in terms of polydispersity. At both pHs, the modal values were greater at 10 g L−1 than at 5 g L−1.
Calcium addition promoted the formation of “medium” and “large” aggregates, larger than those obtained at 5 g L−1. In the case of calcium-added dispersions at pH 5.9 (with or without HHP treatment), measuring of particle size distribution was performed after separating large particles by decantation and sample diluting, due to equipment requirements. In consequence, in particle size distribution shown in Fig. 6a, c, “large” aggregates that were also part of the dispersion do not appear. Treatment with HHP decreased the size of the aggregates and homogenized particle size distribution in samples with and without calcium added at both pHs (Fig. 6). This effect was similar to that obtained at 5 g L−1. However, at pH 7.0, calcium-added dispersion treated with HHP showed two overlapping populations, whose modes were 68 and 226 nm (Fig. 6d), whereas at 5 g L−1, only a major population at 137 nm was observed. The increase in protein concentration from 5 to 10 g L−1 promoted the formation of larger aggregates.
The relationship between the size of aggregates and the protein content of supernatants (“Solubility”) was depicted in Fig. 7. HHP-treated samples “solubility” (calcium-added and non-added) seemed to be a function of the size distribution modes in protein species that resisted centrifugation at 10,000g. Aggregate size was an important factor in colloidal stability of HHP-treated soybean proteins, the smaller the size, the greater the amount of protein that remained in dispersion (r2 was 0.88 for a linear regression; Fig. 7). HHP stabilizing effect on soybean proteins would be largely explained by the decrease in aggregate size. In a different way, in unpressurized samples, other factors substantially contributed to solubility and no linear (or other) fit was observed (r2 was 0.42; Fig. 7).
Surface Pressure Isotherms
Compression was applied and registered by using the Langmuir trough method. These experiments allow the analysis of proteins present in supernatants of 10,000g centrifugation at a planar air-water surface.
Surface Pressure Isotherms at pH 5.9
Figure 8 shows the surface pressure (π) isotherms obtained at pH 5.9. The supernatant of 5-0 Ca-pH 5.9-600 showed the greatest efficiency to increase π. This sample began to increase π at higher area values than the others. Samples 5-0 Ca-pH 5.9-0.1 and 5-0 Ca-pH 5.9-600 reached practically the same π at collapse (30.6 and 31.6 mN m−1, respectively, Fig. 8 and Table 2), but 5-0 Ca-pH 5.9-600 was more efficient at the beginning of the compression. This behavior may be due to changes in adsorption mechanisms or rearrangements at the interface of aggregates with different sizes and structures (McClements 2005). The aggregates present in supernatants were smaller in 5-0 Ca-pH 5.9-600 than in 5-0 Ca-pH 5.9-0.1 (Fig. 4e, f). The smaller absolute value of ζ-potential of 5-0 Ca-pH 5.9-600 (compared with 5-0 Ca-pH 5.9-0.1, Fig. 3) could also improve its interfacial activity.
Calcium addition decreased interfacial activity, achieving lower π values at the end of the compression cycle, than those obtained in samples without calcium (Table 2). Probably, calcium-bridges limited protein flexibility and decreased packaging efficiency (Camejo et al. 1968; Ye and Singh 2001).
Surface Pressure Isotherms at pH 7.0
Figure 9 shows the π isotherms obtained at pH 7.0. The supernatants from 5-0 Ca-pH 7.0-0.1 and 5-2.5 Ca-pH 7.0-0.1 reached the lowest values of π at the collapse (25.4 and 25.3 mN m−1, respectively, Fig. 9 and Table 2). HHP-treatment significantly increased (p < 0.05) interfacial activity in samples with and without calcium, reaching a greater π than unpressurized samples (Table 2). At pH 7.0, HHP-treatment produced “small aggregates,” mostly soluble, fully or partially denatured, able to spread and get faster at the interface. This could explain the improvement observed in the interfacial activity. The greatest value of π was found for samples in which calcium was added after HHP-treatment (Table 2).
Comparing the isotherms obtained from calcium-added samples, we found an inverse relationship between π and turbidity: samples with higher turbidity showed the lowest π. Possibly, relative abundance of insoluble aggregates impaired the interfacial activity of these samples (Figs. 2, 8, and 9).
Our results suggest that interfacial activity can be improved by HHP treatment under certain conditions, e.g., at pH 5.9 without calcium addition, pH 7.0 with and without calcium addition. The increase in protein solubility and the decrease in size of aggregates seemed to be involved in this effect.
Conclusions
Calcium-enriched soybean-based products present a deficit in stability due to a decrease in protein solubility. HHP and pH combination can solve this problem. Treatment with HHP increased the amount of protein that remained in dispersion after centrifugation (“soluble protein”) in calcium-added and non-added samples at pHs 5.9 and 7.0. Treatment with HHP split aggregates and decreased turbidity in all conditions assayed. A fraction of the aggregates formed by HHP was soluble (low turbidity, small size of aggregates, especially at pH 7.0 without calcium addition), whereas another fraction was insoluble but remained dispersed despite centrifuging at 10,000g (higher turbidity, higher size of aggregates, especially at pH 7.0 with calcium addition, and at pH 5.9 with or without calcium addition). The size of the aggregates appeared to be the most important factor determining the protein content in the supernatants of pressurized samples. These effects were modulated by protein concentration, with the highest sizes of aggregates at the highest protein concentration. The dissociation of aggregates induced by HHP was detected at both pHs, whereas aggregation induced by HHP was only evident at pH 7.0. The order of application of calcium and HHP treatment had an effect on protein solubility and size distribution at pH 5.9, but not at pH 7.0. Besides, for protein solubility interaction between calcium and HHP was null or negative at pH 5.9, but positive at pH 7.0. Thus, we conclude that mechanisms involved in structural changes induced by HHP were pH-dependent, which suggests a substantial role of electrostatic interactions. The mechanisms of dissociation and aggregation depended also on the presence of buffer, which could bind proteins, modulate the effect of HHP, and influence colloidal stability.
Since many foods have acidic pHs, the HHP-induced increase in colloidal stability of calcium-added insoluble aggregates observed at pH 5.9 may be useful in the development of beverages with an increased nutritional value. The presence of insoluble aggregates seemed to impair interfacial activity of these protein dispersions. Thus, further studies should be carried out in order to determine which matrix would work best to exploit the potential of these modified proteins. In particular, given the variety of species formed by combination of SPI, calcium, and HHP (with different ζ-potentials and molecular sizes), it would be interesting to evaluate their emulsifying properties.
References
Añón, M. C., de Lamballerie, M., & Speroni, F. (2012). Effect of high pressure on solubility and aggregability of calcium-added soybean proteins. Innovative Food Science and Emerging Technologies, 16, 155–162.
AOAC. (1990). In A. O. A. C. International (Ed.), Official methods of analysis of the AOAC (15th ed.). Arlington, VA., USA: Association of Official Analytical Chemists.
Baeza, R. I., Carrera Sánchez, C., Pilosof, A. M. R., & Rodríguez Patino, J. M. (2004). Interactions of polysaccharides with β-lactoglobulin spread monolayers at the air-water interface. Food Hydrocolloids, 18(6), 959–966.
Baier, D., Schmitt, C., & Knorr, D. (2015). Effect of high pressure-low temperature processing on composition and colloidal stability of casein micelles and whey proteins. International Dairy Journal, 43, 51–60.
Camejo, G., Colacicco, G., & Rapport, M. M. (1968). Lipid monolayers: interactions with the apoprotein of high density plasma lipoprotein. Journal of Lipid Research, 9(5), 562–569.
Dezelić, G., Dezelić, N., & Tezak, B. (1963). A simple method for particle size determination by turbidity measurement. Journal of Colloid Science, 18(9), 888–892.
Ferreira, R. B., Franco, E., & Teixeira, A. R. (1999). Calcium- and magnesium-dependent aggregation of legume seed storage proteins. Journal of Agricultural and Food Chemistry, 47(8), 3009–3015.
Kella, N. K. D., Barbeau, W. E., & Kinsella, J. E. (1986). Effect of oxidative sulfitolysis of disulfide bonds of glycinin on solubility, surface hydrophobicity, and in vitro digestibility. Journal of Agricultural and Food Chemistry, 11, 251–256.
Koshiyama, I. (1968). Chromatographic and sedimentation behavior of a purified 7S protein in soybean globulins. Cereal Chemistry, 45, 405–412.
Lakshmanan, R., de Lamballerie, M., & Jung, S. (2006). Effect of soybean-to-water ratio and pH on pressurized soymilk properties. Journal of Food Science, 71(9), 1–8.
Liu, C., Teng, Z., Lu, Q. Y., Zhao, R. Y., Yang, X. Q., Tang, C. H., & Liao, J. M. (2011). Aggregation kinetics and ζ-potential of soy protein during fractionation. Food Research International, 44(5), 1392–1400.
Manassero, C. A., Vaudagna, S. R., Añón, M. C., & Speroni, F. (2015). High hydrostatic pressure improves protein solubility and dispersion stability of mineral-added soybean protein isolate. Food Hydrocolloids, 43, 629–635.
Manassero, C. A., Vaudagna, S. R., Sancho, A. M., Añón, M. C., & Speroni, F. (2016). Combined high hydrostatic pressure and thermal treatments fully inactivate trypsin inhibitors and lipoxygenase and improve protein solubility and physical stability of calcium-added soymilk. Innovative Food Science and Emerging Technologies, 35, 86–95.
McClements, D. J. (2005). Colloidal interactions. In: Food Emulsions. Principles, Practices, and Techniques. Second edition, CRC Press.
Molina, E., Papadopoulou, A., & Ledward, D. A. (2001). Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocolloids, 15(3), 263–269.
Murray, B. S., & Nelson, P. V. (1996). A novel Langmuir trough for equilibrium and dynamic measurements on air-water and oil-water monolayers. Langmuir, 12(25), 5973–5976.
Needs, E. C., Stenning, R. A., Gill, A. L., Ferragut, V., & Rich, G. T. (2000). High-pressure treatment of milk: effects on casein micelle structure and on enzymic coagulation. Journal of Dairy Research, 67(1), 31–42.
Nishinari, K., Fang, Y., Guo, S., & Phillips, G. O. (2014). Soy proteins: a review on composition, aggregation and emulsification. Food Hydrocolloids, 39, 301–318.
Ono, T., Katho, S., & Mothizuki, K. (1993). Influences of calcium and pH on protein solubility in soybean milk. Bioscencie, Biotechnology and Biochemistry, 57(1), 24–28.
Puppo, C., Chapleau, N., Speroni, F., de Lamballerie-Anton, M., Michel, F., Añón, C., & Anton, M. (2004). Physicochemical modifications of high-pressure-treated soybean protein isolates. Journal of Agricultural and Food Chemistry, 52(6), 1564–1571.
Sacco, S. M. & L’abbé, M. R. (2016). Calcium: physiology. In: Encyclopedia of Food and Health. Academic Press, volume 1, 583.
Samaranayake, C. P., & Sastry, S. K. (2013). In-situ pH measurement of selected liquid foods under high pressure. Innovative Food Science and Emerging Technologies, 17, 22–26.
Speroni, F., & Añón, M. C. (2013). Cold-set gelation of high pressure-treated soybeans proteins. Food Hydrocolloids, 33(1), 85–91.
Speroni, F., Jung, S., & de Lamballerie, M. (2010a). Effects of calcium and pressure treatment on thermal gelation of soybean protein. Journal of Food Science, 75, 30–38.
Speroni, F., Milesi, V., & Añón, M. C. (2010b). Interactions between isoflavones and soybeans proteins: applications in soybean-protein-isolate production. LWT-Food Science and Technology, 43(8), 1265–1270.
Tang, C. H., & Ma, C. Y. (2009). Effect of high pressure treatment on aggregation and structural properties of soy protein isolate. LWT - Food Science and Technology, 42(2), 606–611.
Ye, R., & Harte, F. (2014). High pressure homogenization to improve the stability of casein-hydroxypropyl cellulose aqueous systems. Food Hydrocolloids, 35, 670–677.
Ye, A., & Singh, H. (2001). Interfacial composition and stability of sodium caseinate emulsions as influenced by calcium ions. Food Hydrocolloids, 15(2), 195–207.
Zbacnik, T. J., Holcomb, R. E., Katayama, D. S., Murphy, B. M., Payne, R. W., Coccaro, R. C., Evans, G. J., Matsuura, J. E., Henry, C. S., & Manning, M. C. (2017). Role of buffers in protein formulations. Journal of Pharmaceutical Sciences, 106(3), 713–733.
Zhang, H., Li, L., Tatsumi, E., & Isobe, S. (2005). High-pressure treatment effects on protein in soy milk. LWT-Food Science and Technology, 38(1), 7–14.
Acknowledgments
The authors thank Marie de Lamballerie for facilitating the use of HHP equipment installed in ONIRIS. The stay of Carlos A. Manassero in Biopolymères Interactions Assemblages-INRA was funded by BEC.AR program from Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manassero, C.A., David-Briand, E., Vaudagna, S.R. et al. Calcium Addition, pH, and High Hydrostatic Pressure Effects on Soybean Protein Isolates—Part 1: Colloidal Stability Improvement. Food Bioprocess Technol 11, 1125–1138 (2018). https://doi.org/10.1007/s11947-018-2084-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2084-7