Abstract
Soybean protein isolates (SPI) represent an important source of proteins that are used to prepare oil-in-water (o/w) emulsions. The influence of an innovative treatment (high hydrostatic pressure, HHP) combined with calcium addition at different pH levels and protein concentrations on the formation and stability of o/w SPI emulsions was evaluated in this work. When applied separately, calcium addition or HHP treatment produced different effect at pHs 5.9 and 7.0. Calcium addition led to stable emulsions with decreased flocculation index (FI) at pH 5.9 and low protein concentration (5 g L−1), whereas at pH 7.0, this effect was observed at high protein concentration (10 g L−1). In these conditions, calcium would favor the arrival of big aggregates to interface, which would be modified and adsorbed during homogenization. Treatment with HHP decreased FI and stabilized emulsions during storage at pH 7.0 (but not at pH 5.9) when prepared from 10 g L−1 protein dispersions. In these conditions, protein unfolding due to HHP-induced denaturation, and high ζ-potential would be responsible for emulsion improvement. Combination of calcium addition and HHP treatment impaired both formation and stabilization abilities of SPI at both pHs. Bridging flocculation was enhanced in these samples while interfacial protein concentration and percentage of adsorbed protein were increased. Thus, soybean proteins that were subjected to combined calcium addition and HHP treatment exhibited a great ability to associate each other, what can be useful to improve other functional properties such as gelation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calcium addition to plant-based foodstuff represents an important topic regarding human nutrition because calcium is an essential nutrient and specific populations such as vegetarians, lactose intolerants, or allergic do not consume its main natural sources. On the other hand, incorporation of plant proteins fulfilling nutritional and functional requirements interests food industries. Particularly, soybean protein isolates (SPI) are widely used due to their high functionality and nutritional value (Delbyshire et al. 1976; Utsumi et al. 1997; Jones 2016). The interactions between calcium and soybean proteins (and also between calcium and phytic acid that appears as component of SPI) may translate into effects on protein functionality and also in calcium bioaccessibility (Galán and Drago 2014). Emulsions are involved in a variety of natural and processed foods. Globular proteins are used as emulsifier because they have lipophilic and hydrophilic groups that interact with oil and water and form a stable interfacial film (McClements 2005). Electrostatic repulsion plays a major role in preventing droplet flocculation, and so in the overall stability of protein-stabilized emulsions. Thus, emulsion stability is particularly sensitive to pH, electrolyte concentration, and ion type (Keowmaneechai and McClements 2002). In this regard, the presence of calcium may impair the emulsifying properties of proteins by acting as counter ion in negatively charged droplets, thus leading to a decrease of the Debye’s layer. Calcium induces the formation of aggregates that affect the adsorption characteristics of milk proteins and so the stability of oil-in-water emulsions (Agboola and Dalgleish 1995; Dickinson and Davies 1999; Ye and Singh 2001). Nevertheless, in other system such as water-in-oil emulsions prepared with polyglycerol polyricinoleate, Márquez et al. (2010) found that calcium chloride improved the stability of emulsions, probably due to the decrease of the droplets size, the decrease of the attractive force between droplets, and the increment of adsorption density of the emulsifier.
High hydrostatic pressure (HHP) is an emerging food processing technology applied to eliminate harmful pathogens and inactivate spoilage microbiota and enzymes with minimal effect on nutritional and sensory quality (Swientek 1992; Palou et al. 2000; Wang et al. 2016). HHP also induces changes in functional properties of soybean proteins. Li et al. (2011) reported that HHP treatment (up to 300 MPa during 5–15 min) improved solubility, water holding capacity, emulsification activity index, and foam capacity of SPI dispersions. Puppo et al. (2005) reported that HHP treatment (from 200 to 600 MPa for 10 min at 20 °C) of SPI dispersions improved their emulsifying properties by increasing the percentage of adsorbed proteins, decreasing bridging flocculation and demonstrated that the magnitudes of these effect were dependent on pH (pH 3.0 or 8.0).
The interaction between SPI and calcium leads to decrease in protein solubility and colloidal stability. HHP can reverse this effect by splitting aggregates while denaturing proteins, so protein structure is substantially modified after combination of calcium addition and HHP treatment (Speroni et al. 2010a; Añón et al. 2012; Manassero et al. 2018). Glycinin aggregation due to high-pressure homogenization was reported as responsible for increase in flocculation (Floury et al. 2002). On the other hand, Castellani et al. (2005) stated that aggregation may improve or impair emulsifying properties of protein depending on type of protein and environmental conditions. According to Dickinson (2010), a mechanistic linkage exists among physicochemical factors that affect flocculation (and other interactions between protein-coated emulsions droplets) and factors that control aggregation of the same proteins in bulk aqueous solution. Denaturation, changes in solubility, and aggregation affect functional properties of proteins, and this could be the case for interfacial activity of calcium-added SPI dispersions. As far as we know, there is no information about the effect of HHP on emulsifying properties of calcium-enriched SPI dispersions. These emulsions, if stable, could be useful as substitute of dairy creams for desserts or other foodstuff as dressings.
In the first part of this work, we found that HHP improved colloidal stability of calcium-enriched SPI at pHs 5.9 and 7.0 by different mechanisms that involved decrease in aggregates size. A fraction of these aggregates was soluble whereas another fraction was insoluble but remained dispersed even after centrifuging at 10,000g. These insoluble aggregates seemed to impair interfacial activity. In the same way, calcium addition decreases ζ-potential of SPI, which could get worse emulsion stability, favoring droplet flocculation. Treatment with HHP decreased the magnitude of ζ-potential at pH 5.9, but not at pH 7.0 (Manassero et al. 2018) so the effect of HHP on emulsifying properties may be different at each pH.
Given the variety of species formed by combination of different factors (protein and calcium concentration, HHP level, pH values), with different characteristics (ζ-potentials and molecular sizes), it would be interesting to evaluate their emulsifying properties. For this reason, the aim of the second part of this work was to analyze the formation and stability of emulsions prepared from calcium-added SPI treated with HHP.
Materials and Methods
Experimental Design and Statistical Analysis
Emulsions were characterized by evaluating the effect of pH (5.9 or 7.0), protein concentration (5 or 10 g L−1), calcium concentration (0, 1.25, or 2.5 mmol CaCl2 L−1 for pH 5.9 and 0, 2.5, or 5.0 mmol CaCl2 L−1 for pH 7.0) and HHP level (0.1 or 600 MPa) (Table 1) on particle size distribution, microstructure, flocculation index, and surface protein concentration and composition. Control samples for each protein concentration and pH corresponded to non-added (0 mmol CaCl2 L−1) and unpressurized (0.1 MPa) ones. Each treatment was performed in triplicate. Analyses of variance (one-way ANOVA) were conducted. Differences among sample means were analyzed by Tukey’s test at a α level of 0.05. The statistical analysis was completed using the Origin software (OriginLab Corporation, Northampton, MA, USA).
Sample Preparation
Preparation of Soybean Protein Isolate
SPI was prepared from defatted soybean flour manufactured by The Solae Company (Brazil). Alkaline extraction (pH 8.0, 90 min, 20 °C) was followed by isoelectric precipitation (pH 4.5, 15 min), as described by Speroni et al. (2010b). The isoelectric precipitate was dispersed in distilled water and its pH was adjusted to 7.0 with 2 mol L−1 NaOH. Then dispersion was freeze-dried. The same batch of SPI was used for the whole study.
Preparation of Calcium-Added SPI Dispersions
Dispersions of SPI were prepared at 5 or 10 g protein L−1 in 30 mmol L−1 BIS-TRIS pH 5.9 (Sigma, St Louis, USA) or 50 mmol L−1 TRIS-HCl pH 7.0 (Merck, Germany). Calcium was added at different concentrations from stock solution at 1 mol L−1 (CaCl2)—1.25 and 2.5 mmol L−1 or 2.5 and 5.0 mmol L−1 for dispersions at pH 5.9 or 7.0, respectively. Stock solution of CaCl2 was prepared from CaCl2 dihydrate (Sigma, St Louis, USA). The conditions were chosen according to Manassero et al. (2015) who reported substantial effects of HHP on protein solubility in those conditions. At each pH, the ratio calcium:protein was kept constant.
High Hydrostatic Pressure Treatment
Prior to HHP treatment, SPI dispersions were vacuum packed in polyethylene bags (La Bovida, France). Then, they were treated at 600 ± 5 MPa for 5 min in a HHP system ACB Pressure Systems, Nantes, France, equipped with temperature (Julabo, Seelbach, Germany) and pressure regulator device (vessel capacity, 3.0 L; maximum working pressure, 600 MPa). The compression fluid used was water. The working pressure was reached at 3.4 MPa s−1 and released almost instantaneously. Temperature during treatment was controlled to avoid overheating of dispersions. Conditioning temperature of vessel and initial temperature of samples were 20 °C. An increase in sample temperature up to 25 °C was verified during treatment due to compression heating. The temperature of one sample was registered with a thermocouple type K placed in the sample-containing bag (sealed with a stuffing box).
Emulsion Preparation
Oil-in-water emulsions were prepared in triplicate with 25-g sunflower seed oil and 70-g protein dispersion with an oil volume fraction (Φ) of 0.28. The two phases were premixed for 1 min at 20,000 rpm with a Polytron type Silent Crusher M (Heidolph Instruments, Germany) equipped with a 12-mm diameter head. Homogenization of emulsions were performed with a high pressure valve homogenizer Stansted TC5W (Stansted Fluid Power Ltd., UK) at 12 MPa with a recirculation of 5 min, which corresponds to ten passes.
Sample Analysis
Particle Size Distribution
Immediately after emulsion preparation and after 3 or 8 days of storage, 0.25 mL of emulsion was diluted in 5 mL of buffer with or without 10 g L−1 SDS. A Malvern MasterSizer 3600 (Malvern Instruments Ltd., Malvern, U.K.) was used to determine the droplet size distribution using the presentation code 3 NHD. The refractive index of emulsion particle and oil were 1.46 and 1.33, respectively. The absorbance value of emulsion particle was 0.1. The mean diameter of emulsion droplet (or flocs), weighted in volume (d4,3) and in surface (d3,2), expressed in micrometer, were determined in triplicate.
Flocculation Index
The flocculation index (FI) at 0, 3, and 8 days of refrigerated storage (4 °C) was calculated by the ratio between the floc size (d4,3 in the buffer without SDS) and the droplet size (d4,3 in the 10 g L−1 SDS buffer):
Both diameters were determined at the same time of storage.
Optical Microscopy
The destabilization processes of emulsions, such as coalescence and flocculation, were analyzed by optical microscopy. Immediately after emulsion preparation, 200 μL of each sample was diluted in 5 mL of buffer without 10 g L−1 SDS. One droplet of diluted emulsions was placed onto a microscopy glass slide, covered with a glass cover slip and immediately observed. A Zeiss Axioskop 2 (Carl Zeiss, Oberkochen, Germany) microscope was used.
Surface Dilatational Rheology
Surface dilatational parameters—such as surface dilatational modulus (E), elastic (E´), and viscous (E´´)—were obtained using a drop tensiometer from IT Concept (Longessaigne, France). An axisymmetric air drop was formed at the tip of the needle of a syringe whose verticality was controlled by a computer. Drop surface was subjected to small periodic sinusoidal compressions and expansions at a given frequency (ω) and amplitude (ΔA/A) and the response of the surface pressure (π) was monitored. The drop profile was digitized and analyzed through a CCD camera coupled to a video image profile digitizer board connected to a computer. The image was continuously visualized on a video monitor. Drop profiles were processed according to the Laplace equation as was described by Castellani et al. (2009).
Protein dispersions in which surface pressure isotherms showed differences (Manassero et al. 2018) were used for surface dilatational rheology. All the experiments were carried out, at least in triplicate, in an optical glass cuvette (8 mL), containing the corresponding protein dispersion. Protein dispersions were diluted to a concentration of 0.001 g L−1 and thermally equilibrated at 20.0 ± 0.1 °C for 30 min just before measurements. A freshly air drop was formed and interfacial tension measurements started once its final volume (8 μL) was accomplished. The area of the drop was sinusoidally fluctuated when interfacial tension value reached 62.5 mN m−1 (equivalent to a surface pressure of 10 mN m−1) at a relative amplitude of 0.005 (ΔA/A) for an oscillation frequency (ω) of 0.005 and 0.01 Hz or a relative amplitude of 0.05 for 0.02 and 0.05 Hz. According to Rodríguez Patino et al. (2003a) a surface pressure of 10 mN m-1 may be associated to a soy monolayer structure at the air-water interface.
Interfacial Protein Concentration
Non-adsorbed proteins were washed from the oil droplets following a method adapted from the procedure described by Patton and Huston (1986). Fresh emulsion (2 mL) was diluted in 2-mL sucrose solution (500 g L−1, at the same pH value (5.9 or 7.0) as the aqueous phase of the emulsion). The diluted sample (4 mL) was then carefully deposited at the bottom of a centrifuge tube containing 10 mL of a sucrose solution (50 g L−1) prepared in the same buffer as the respective emulsion. The tubes were then centrifuged at 3000g for 2 h at 10 °C. After centrifugation, two phases were observed in the tubes: the creamed oil droplets at the top and the aqueous phase of the emulsion deprived of oil droplets at the bottom. The tubes were frozen (− 20 °C) and cut so as to recover the phases. Adsorbed proteins at the creamed phase were desorbed by adding buffer with 10 g L−1 SDS and the dispersion was then centrifuged at 10,000g for 20 min at 10 °C.
Adsorbed (Ad) and non-adsorbed (NAd) (bottom aqueous phase) protein concentrations were determined in triplicate by the method used by Markwell et al. (1978). Interfacial protein concentration was calculated as:
where Sv is the specific interfacial area. The Sv was calculated according to Walstra (1983):
where Φ is the oil volume fraction and d3,2 is the volume-surface average diameter of the particles suspended in SDS buffer. Adsorbed protein percentage (AP %) was calculated as the adsorbed protein respect to initial protein concentration.
Interfacial Protein Composition
The composition of the whole (W) dispersion, adsorbed (Ad), and non-adsorbed (NAd) proteins at the interface of droplets was determined by SDS-PAGE under non-reducing conditions. Running and stacking gels of 90 g L−1 and 35 g L−1 of acrylamide, respectively, were prepared. A buffer system containing 0.05 mol L−1 Tris, 0.38 mol L−1 glycine, and 10 g L−1 SDS, pH 8.8 was used for the running buffer. Low molecular weight markers (GE Healthcare, Buckinghamshire, UK) included phosphorylase b (97 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (30 kDa), soybean trypsin inhibitor (20.1 kDa), and α-lactalbumin (14.4 kDa). Gels were fixed with water/ethanol/phosphoric acid solution (5:5:0.2) and stained using a Coomassie blue G-250 solution (17%v/v ethanol, 15%v/v ammonium sulfate, 2%v/v phosphoric acid), and distained with 20%v/v ethanol.
Results and Discussion
Characterization of Emulsions: Particle Size Distribution and Microstructure
Particle size distributions and volume-average droplet sizes (d4,3) are shown in Figs. 1, 2, 3, and 4 and Table 2. In the presence of SDS, all emulsions showed droplet size distribution with two populations. The presence of two populations in fresh emulsions may be due to coalescence of small droplets during the emulsification process due to a low amount of emulsifier. If a droplet is not protected by a strong enough interfacial film, it tends to coalesce with another one during a collision (Walstra 1993). Measured in the absence of the deflocculating agent (SDS), all emulsions showed high level of bridging flocculation (panels c and d of Figs. 1, 2, 3, and 4), probably due to a low amount of emulsifier (McClements 2005).
Emulsions Prepared with 5 g L−1 Protein Dispersions at pH 5.9
In these conditions (5 g L−1 protein dispersions at pH 5.9), calcium addition or HHP treatment separately did not affect the mean oil droplet size or size distribution: the most abundant population was that of smallest droplets (mode was 2.1 μm), followed by another of 7.3 μm (Table 2 and Fig. 1a, b). On the other hand, the combination of calcium addition and HHP treatment resulted in an increase in the abundance of the largest droplets (mode was 6.6 μm) with the highest d4,3 value (p < 0.05, Table 2). This fact indicates that at 5 g L−1 and pH 5.9, the combination of calcium and HHP led to an important coalescence among droplets (Fig. 1b and Table 2). Through the analysis of surface dilatational parameters at air-water interface, we found that supernatant of these calcium-added and HHP-treated SPI dispersions showed the lowest elastic modulus value (40.2 mN m−1, p < 0.05, Table 2), which could be related to an interface more prone to deformation and rupture. The high susceptibility of soybean proteins to interact with one another or aggregate (Tang 2017) could have been increased by changes in their structure due to calcium-HHP combination. This fact could lead to an excessive protein-protein interaction, thus avoiding the formation of an efficient interfacial film. In absence of SDS, the emulsions exhibited wide and heterogeneous particle size distribution profiles, ranging from 0.2 to 250 μm (unpressurized − 0.1-MPa samples) and from 0.2 to 170 μm (pressurized ones). Calcium single addition contributed to smaller flocs sizes and flocculation index (Fig. 1c and Table 2). Emulsions obtained from calcium-added and HHP-treated and from HHP-treated (without calcium addition) SPI dispersions showed similar floc sizes to those obtained from control (no calcium-added nor HHP-treated) (Fig. 1c, d and Table 2).
Through the analysis of flocs by optical microscopy, we found that the structure of the flocs in all samples was compact and with irregular shapes and that the largest flocs appeared in emulsions prepared from dispersions that were subjected to both calcium addition and HHP treatment (Supplementary Fig. 1).
Emulsions Prepared with 5 g L−1 Protein Dispersions at pH 7.0
In these conditions (5 g L−1 protein dispersions at pH 7.0), in the presence of SDS, emulsions obtained from unpressurized dispersions (with or without calcium addition) (Fig. 2a) showed similar droplet size distributions to those obtained for unpressurized samples at pH 5.9 (Fig. 1a). On the other hand, pressurized samples (with or without calcium addition) showed lower levels of coalescence (relative increase on droplet population with smaller size) than emulsions obtained from unpressurized dispersions (Fig. 2a, b). These data suggest that at pH 7.0, HHP treatment improve the ability of these proteins to form an interfacial membrane more resistant in these conditions, which can be supported from the high elastic modulus value (44.4 mN m−1, p < 0.05, Table 2) shown by the supernatant of 5-0Ca-pH7.0-600 at the air-water interface. Moreover, it was observed that supernatants of pH 7.0 HHP-treated SPI dispersions were more effective to decrease interfacial tension than unpressurized ones (Manassero et al. 2018). Calcium addition provoked no changes in droplet size distribution neither in emulsion prepared from unpressurised (0.1 MPa) nor pressurized (600 MPa) SPI dispersions (Fig. 2a, b).
In the absence of SDS, the emulsions exhibited wide and heterogeneous particle size distribution profiles, ranging from 0.2 to 84 μm (unpressurized samples) and 0.2 to 180 μm (HHP-treated samples) (Fig. 2c, d). For emulsions prepared from unpressurized (0.1 MPa) SPI dispersions, calcium addition produced a decrease in flocs size as compared with samples without calcium (modes were 19 μm and 32 μm, respectively; Fig. 2c). Emulsions prepared from HHP-treated SPI dispersions (without calcium) presented smaller flocs than those from unpressurized ones, but FI remained constant because droplets size also decreased (Fig. 2c, and Table 2). Nonetheless, calcium addition combined with HHP-treatment increased FI (Fig. 2c, d and Table 2).
The microstructure analysis showed that smallest flocs were present in emulsions obtained from 5-0Ca-pH7.0-600 (Supplementary Fig. 2, panel d).
Emulsions Prepared with 10 g L−1 Protein Dispersions at pH 5.9
Coalescence phenomenon at 10 g protein L−1 was less intense than at 5 g protein L−1. The emulsifier was not effective in preventing coalescence, but the higher protein concentration helped to decrease it. Thus, d4,3 values for each condition (calcium and/or HHP) were smaller than those corresponding to 5 g protein L−1 (Fig. 3a, b and Table 2). This effect of increasing protein concentration was less noticed for emulsions obtained from calcium-added and HHP-treated SPI dispersions, in which droplet size was maximum (Table 2).
In the absence of SDS, all emulsions exhibited high levels of bridging flocculation, with wide and heterogeneous particle size distribution profiles, ranging from 0.2 to 50 μm (unpressurized samples) and from 0.2 to 220 μm (HHP-treated samples) (Fig. 3c, d). Separately, calcium addition and HHP treatment provoked no change in flocculation, but their combination led to the highest FI value and the biggest flocs size (up to 200 μm) (Fig. 3d and Table 2).
The microstructure of flocs was affected by the presence of calcium both at 0.1 and 600 MPa (Supplementary Fig. 3, panels b and d). In these cases, the flocs presented more irregular shapes than emulsions obtained from SPI dispersions at pH 5.9 without calcium (Supplementary Fig. 3, panels a and c).
Emulsions Prepared with 10 g L−1 Protein Dispersions at pH 7.0
The increase in protein concentration, as observed for pH 5.9, caused decrease in d4,3 values of droplets, compared to those of 5 g L−1 (Table 2). At 10 g L−1, the greatest level of coalescence and therefore the highest value of d4,3 was observed in emulsions prepared with calcium-added and HHP-treated SPI dispersions (Fig. 4a, b and Table 2). The combination of calcium and HHP treatment, as it was detected also for pH 5.9, would decrease interfacial activity of soybean proteins.
In the absence of deflocculating agent (SDS), all emulsions showed high level of bridging flocculation. The emulsions exhibited wide and heterogeneous particle size distribution profiles, ranging from 0.2 to 40 μm (unpressurized − 0.1-MPa samples) and 0.2 to 500 μm (HHP-treated samples) (Fig. 4c, d). It is noteworthy that separately, calcium and HHP treatment decreased flocculation index, but their combination led to the highest value of this parameter (Table 2).
The microstructure analysis showed that bigger flocs were found in emulsions prepared with calcium-added and HHP-treated SPI dispersions (Supplementary Fig. 4, panel d). Similar behavior was found in emulsions obtained from 5 g L−1 SPI dispersion at pH 7.0.
Discussion on Particle Size Distribution
All emulsions exhibited coalescence that had less intensity with the highest protein concentration. Increasing protein concentration led to a decrease in droplet size for almost every condition. The decrease in droplet size due to increase in protein concentration was less pronounced for combined calcium-added and HHP-treated samples at pH 5.9 and was null at pH 7.0 (Table 2). Our data suggest that the combination of calcium addition and HHP treatment reduced emulsifying capacity of soybean proteins. Moreover, all emulsions showed bridging flocculation. The lowest FI values were detected in emulsions obtained at pH 7.0 and at 10 g L−1 for separately HHP-treated or calcium-added samples. On the other hand, calcium addition combined with HHP treatment produced the greatest values of FI.
Coalescence and bridging flocculation could be related to insufficient protein covering. However, increasing protein concentration in emulsions obtained from calcium-added dispersion at 600 MPa did not reduce flocculation behavior. These data indicate that not only protein concentration governed coalescence and flocculation processes. Ye and Singh (2001) proposed that presence of calcium in casein-stabilized emulsions may cause more efficient packing of adsorbed caseins, which could allow more protein to be adsorbed, favoring interface coverage. This mechanism could occur in emulsions obtained from calcium-added SPI dispersion at 0.1 MPa, in which protein was involved in aggregates bigger than 1 μm (Manassero et al. 2018). It is noteworthy that although the ζ-potential and solubility of proteins was lower in the presence of calcium (Manassero et al. 2018), the droplets flocculated less in calcium-added samples. Notably, this fact suggests that, under certain conditions, big and insoluble aggregates present in calcium-added SPI dispersions may generate fine emulsions; Wang et al. (2018) assayed calcium addition to emulsion prepared from soybean proteins (60 g L−1); they dispersed the emulsions in distilled water and found no change in droplet size (d4,3) as calcium concentration increased up to 7.5 mmol L−1. Conversely, Ye and Singh (2000) reported that presence of calcium induced an increment in flocculation in emulsions stabilized by whey proteins. These authors also found a calcium-induced increase in interfacial protein concentration and stated that calcium ion acted as bridge between protein-coated droplets; the magnitude of these effects was modulated by calcium:protein ratio. It is possible that in our unpressurized samples, the calcium:protein ratio and the structure of protein at the interface were matched in a condition that avoid bridging flocculation. Otherwise, in emulsions obtained from pressurized calcium-added SPI dispersions, in which the protein species were “small” and soluble (Manassero et al. 2018), another mechanism could occur, in which these species may interact through hydrogen bonding or hydrophobic interactions (SDS was able to disassemble these flocs) with a resulting enhanced flocculation. These interactions could be established during homogenization, whose mechanical force may produce changes in aggregates structure. In this sense, Speroni et al. (2010a) analyzed thermal gelation of soybean proteins treated with HHP and/or calcium addition. They found that HHP treatment without calcium addition induced the formation of weaker heat-induced gels, whereas HHP treatment in the presence of calcium induced the formation of stronger heat-induced gels. These results suggest that the diverse HHP-induced aggregates (in presence or absence of calcium) have different abilities to interact among themselves. In our samples, HHP treatment in presence of calcium could favor interactions among polypeptides (increasing bridging flocculation) whereas in absence of calcium HHP treatment would disfavor interactions (decreasing bridging flocculation). Optical microscopy also suggests the occurrence of this phenomenon since more compact flocs (green arrows in Supplementary Figures) were observed in emulsions obtained from HHP-treated dispersions without calcium addition. On the other hand, flocs with open packing (yellow arrows in Supplementary Figures) and irregular shapes were observed in emulsions obtained from HHP-treated calcium-added dispersions. McClements (2005) postulated that when the attraction between droplets is relatively strong compared to the thermal energy, the flocs formed have open structures. On the other hand, when the attraction between droplets is relatively weak compared to the thermal energy, the droplets are able to roll around each other forming flocs with close packing.
Regarding to the ability to decrease surface tension, surface pressure isotherms were obtained with the soluble protein fraction and showed relatively small differences among conditions (Manassero et al. 2018). Nevertheless, the emulsions, obtained with the whole dispersions, exhibited greater differences in terms of droplet size. Thus, we hypothesize that insoluble aggregates are responsible for differences in coalescence and flocculation behavior.
Emulsion Stability
Stability of emulsions stored at 4 °C for 8 days was studied through the analysis of size distribution of droplets and FI. No changes in coalescence were detected in any emulsion analyzed throughout the storage time evaluated (data not shown). This fact could be related to the presence of a structured and elastic interfacial film. Proteins from supernatants of centrifugation (10.000g at 4 °C for 20 min) of our dispersions formed interfacial films whose elastic moduli were higher than viscous ones (Table 2). Although the amount of emulsifier seemed to be insufficient, which promoted an initial coalescence, the interfacial film was structured enough to avoid an increase in coalescence during storage despite the high level of flocculation. This phenomenon was verified in all samples, which would reflect the impact of soybean proteins and homogenization process on the film characteristics, beyond the presence of calcium and/or HHP-induced denaturation. Rodríguez Patino et al. (2003b) reported that soybean globulin films adsorbed at the air-water interface were practically elastic.
Destabilization phenomena observed in the present work were associated with bridging flocculation. FI of emulsions obtained from unpressurized SPI dispersions remained constant for 8 days at both pHs and both protein concentrations (Table 3). The only exception to this behavior was 10-2.5Ca-pH5.9-0.1 sample (Table 3), in which FI increased with time. This sample exhibited the biggest insoluble aggregates and low ζ-potential (Manassero et al. 2018) that could be involved in flocculation during storage. HHP treatment without calcium addition provoked no change in FI in samples at pH 7.0, whereas it provoked an increase in FI in samples at pH 5.9 (Table 3). The combination of HHP treatment and calcium addition induced notorious increases in FI at both pHs and both protein concentrations. These increases in FI exhibited greatest magnitude for 10 g protein L−1concentration, where flocs up to 183 (pH 5.9) and 124 (pH 7.0) μm were formed during storage.
Even though bridging flocculation is usually favored by low emulsifier concentration, in our case, the highest FI were detected during storage of emulsions obtained with the highest protein concentration, in pressurized calcium-added samples. This fact suggests that HHP treatment promoted the formation of calcium-protein species that could establish bridges between droplets. The bridges would be given by polypeptides with good ability to establish interactions with each other. The low magnitude of ζ-potential detected in these samples (− 14.7 ± 0.7 (pH 5.9) and − 16.8 ± 0.9 (pH 7.0) mV, Manassero et al. 2018) could also contribute to promote association between adsorbed polypeptides and, consequently, between droplets. Nevertheless, unpressurized calcium-added samples also exhibited low values of ζ-potential (Manassero et al. 2018) but had not the highest FI.
Quantification and Molecular Characterization of Adsorbed and Non-adsorbed Proteins
Adsorbed Protein Percentage
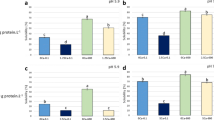
Percentages of adsorbed protein were high in all samples, ranged between 65.6 and 91.0% (Fig. 5a, b). Noticeably, dispersions in which protein solubility was low and the size of aggregates was large (10-2.5Ca-pH5.9-0.1 and 10-2.5Ca-pH5.9-600) reached percentages of adsorbed protein similar to dispersions with higher solubility and lower size of aggregates (10-0Ca-pH7.0-600) (Manassero et al. 2018). By comparing the size of “large aggregates” (bigger than 2.5 μm) with the size of droplets (ca. 1.5 μm), and taking into account the mechanical force exerted during homogenization, it seems evident that modifications of aggregates must have occurred. In this sense, in dynamic conditions “large aggregates” could reach the interface due to turbulent flow (Walstra 1983), once at the interface these aggregates would disassemble, and their components would rearrange to form an interfacial film. In this process, the large initial aggregate size could favor interface coverage with a great amount of protein (Ye and Singh 2001). Nevertheless, it cannot be ruled out that “large aggregates” have been split during homogenization, prior to arrival to interface, as was described by Bouaouina et al. (2006) for whey proteins and higher level of homogenization pressure than that applied in our work. On the other hand, in SPI dispersions without calcium addition that contained “small aggregates” denatured by HHP (Puppo et al. 2005), the adsorption could be favored due to flexibility and superficial hydrophobicity increases due to denaturation (Wang et al. 2012).
Percentages of adsorbed protein (a and b) and interfacial protein concentration (Γ, c and d) of emulsions obtained from 5 g protein L-1 (a and c) or 10 g protein L-1 (b and d) SPI dispersions at pH 5.9 or 7.0. Values are expressed as means ± standard deviation. Different letters within a same panel indicate significant differences (p < 0.05)
At pH 5.9, the highest percentages of adsorbed protein were found in emulsions obtained from HHP-treated dispersions, with or without calcium addition, at both protein concentrations (Fig. 5a, b). At pH 7.0, calcium addition and/or HHP treatment increased percentage of adsorbed protein, particularly in emulsions obtained from 10 g L−1 protein dispersion, where produced the greatest increase (p < 0.05) (Fig. 5a, b). Calcium effect could be explained based on the statements of Ye and Singh (2001), who found that the presence of calcium may cause more compact packing of adsorbed protein due to the reduction of repulsion among polypeptides.
Interfacial Protein Concentration
Interfacial protein concentration (Г) was sensitive to every factor assayed. Thus, the increase in protein concentration increased Г while emulsions at pH 5.9 exhibited higher values of Г than those at pH 7.0. Separately, calcium addition or HHP treatment also promoted increases in Г (Fig. 5c, d). In emulsions prepared with 10 g L−1 protein dispersions, the combination of calcium and HHP treatment led to the highest values of Г for both pH values (7.2 and 5.0 mg m−2 for 10-2.5Ca-pH5.9-600 and 10-5.0Ca-pH7.0-600, respectively, Fig. 5c, d). Values of Г close to or higher than 5 mg m−2 are associated with multilayer interfacial films (Graham and Phillips 1979; Dickinson and Semenova 1992). The higher values of Г at pH 5.9 than at pH 7.0 may be explained by the proximity to isoelectric point (ca. 4.5 for SPI). In the same way, the presence of calcium, as it decrease ζ-potential, decrease electrostatic repulsion and allows protein association. Cui et al. (2014) analyzed the adsorption of heated soybean proteins at oil-water interface and reported that aggregated proteins exhibited higher percentages of adsorption and Г than non-aggregated proteins. These authors also reported the formation of a multilayer film and found that the higher the fraction of aggregated protein, the higher the Г. It is possible that in samples such as 10-2.5Ca-pH5.9-0.1 and 10-2.5Ca-pH5.9-600 (in which protein solubility was as low as ca. 12%), the first adsorbed layer worked as a non-aqueous substrate for deposition of insoluble protein.
Noteworthy, emulsions obtained from 10 to 2.5Ca-pH5.9-600 exhibited the greatest Г and FI values; this fact suggests that the thick interfacial film was cohesive. It is also possible that some aggregates have been associated to the film and would work as adherent projections (without being part of a structured film) and enhanced bridging flocculation. Moreover, high values of Г seem contradictory with coalescence observed in Figs. 1, 2, 3, and 4. This behavior suggests that protein heterogeneously accumulated at the interface, with poorer zones and more concentrated ones. Speroni et al. (2010a) stated that the combination of calcium and HHP-favored protein-protein interactions when SPI was subsequently treated by heat. In our system, homogenization energy would work as the second treatment that allowed association between these modified polypeptides, this phenomenon would explain the high values of Г and FI.
Interfacial Protein Composition
The nature of the adsorbed and non-adsorbed proteins at the interface was analyzed by SDS-PAGE under non-reducing conditions (Figs. 6 and 7).
SDS-PAGE profile of adsorbed (Ad) and non-adsorbed (NAd) proteins at the o/w interface of emulsions obtained from 5 g protein L-1 SPI dispersions at pH 5.9 (a and b) or pH 7.0 (c and d) unpressurized (a and c) or treated at 600 MPa (b and d). W whole sample. MW molecular weight standards. Blue solid arrows: basic polypeptide (B) of glycinin subunit (20 kDa) (a–d). Green short dashed arrows: AB subunit of glycinin (a and c)
SDS-PAGE profile of adsorbed (Ad) and non-adsorbed (NAd) proteins at the o/w interface of emulsions obtained from 10 g protein L-1 SPI dispersions at pH 5.9 (a and b) or pH 7.0 (c and d) unpressurized (a and c) or treated at 600 MPa (b and d). W whole sample. MW molecular weight standard. Green short dashed arrows: AB subunit of glycinin (a and c). Blue solid arrows: B polypeptide of glycinin subunit (b and d)
Emulsions Obtained from 5 g Protein L−1 Dispersions
Electrophoretic profiles of unpressurized (0.1 MPa) samples, with or without calcium addition, at both pHs, showed that almost all species present in whole dispersion (W) also appeared in the adsorbed fraction (Ad), even those which by their large size did not enter the resolving gel (Fig. 6a, c). The relative intensities of bands were similar in W and Ad with exception of those corresponding to basic polypeptide of glycinin (B, 20 kDa) which exhibited lower relative intensity in Ad lanes than in W ones (blue solid arrows, Fig. 6a, c). Chapleau and de Lamballerie-Anton (2003) also reported that polypeptides of ca. 21–22 kDa from lupin 11S globulins poorly adsorbed at the oil interface. In non-adsorbed (NAd) lanes, polypeptides corresponding to β-conglycinin (α´, α, and β) and to glycinin (acidic (A) and basic (B)) were observed, while aggregates and the band corresponding to AB subunit were absent (green short dashed arrows, Fig. 6a, c). Taken together, these results indicate that high molecular weight aggregates, possibly stabilized by disulfide bonds, had a preferential adsorption. On the other hand, protein species that were not adsorbed (NAd) corresponded to aggregates that were disassembled by SDS from electrophoresis buffer or were already dissociated in emulsion.
HHP treatment mainly modified electrophoretic profiles of NAd fractions, decreasing relative intensities of almost all bands except that corresponding to B polypeptide of glycinin. This effect was greater at pH 5.9 (blue solid arrows, Fig. 6b, d).
Emulsions Obtained from 10 g Protein L−1 Dispersions
Electrophoretic profiles were similar to those obtained at 5 g protein L−1 except for NAd fraction, where the increase of protein concentration promoted the presence of aggregates (molecular weight between ca. 100 and 150 kDa) and AB subunit (green short-dashed arrows, Fig. 7a, c). It could be hypothesized that the interface presented a preferential affinity for AB subunit and large aggregates, retaining almost all of these species when protein concentration was 5 g L−1. At 10 g L−1, a fraction of these species exceeded and remained in aqueous phase (NAd fraction).
HHP treatment decreased relative intensities of all bands present in NAd fraction except that corresponding to B polypeptide (blue solid arrows, Fig. 7b, d). Similar behavior was observed at 5 g protein L−1. Moreover, this effect was more important in samples at pH 5.9. The levels of B polypeptide detected in the aqueous phase of all samples are in agree with data of Yuan et al. (2009), who reported that B polypeptide had the lowest emulsifying activity than A polypeptide or glycinin at pHs between 5.0 and 7.0.
Polypeptides and aggregates distribution showed that interface had affinity to disulfide bond-stabilized large aggregates, while smaller or SDS-disassembled aggregates were found both at interface and aqueous phase.
Concluding Remarks
When applied separately, addition of calcium or HHP treatment produced different effects at pH 5.9 or 7.0. At pH 5.9, calcium addition led to stable emulsions with low FI when prepared from 5 g L−1 protein dispersions, whereas at pH 7.0, calcium addition led to stable emulsions with low FI when prepared from 10 g L−1 ones. Treatment with HHP decreased droplet size and led to stable emulsions with low FI when prepared from 10 g L−1 dispersion at pH 7.0, but induced no improvements at pH 5.9.
The better emulsions (lowest levels of coalescence and bridging flocculation, stable during storage) were obtained from dispersions at 10 g L−1 and at pH 7.0 either with calcium or with HPP treatment. Interestingly, under those conditions (10-0Ca-pH7.0-600 and 10-5Ca-pH7.0-0.1) proteins exhibited very different properties; this fact reflects the complexity of interactions of factors. Proteins subjected to HHP treatment were denatured (what led to enhanced hydrophobicity and flexibility), their solubility was high and formed small aggregates with a ζ-potential of − 20.4 ± 1.2 mV (Manassero et al. 2018). On the other hand, unpressurized proteins subjected to calcium addition presented a poor solubility and formed macroaggregates with a ζ-potential of − 16.1 ± 0.7 mV (Manassero et al. 2018). Probably, calcium favored the arrival of more protein to the interface, but then these aggregates were substantially modified by mechanical stress during homogenization.
The combination of calcium addition and HHP treatment impaired both formation and stabilization abilities of SPI since it resulted in increases of droplet size and FI at both pHs. This effect of calcium and HHP combination was paradoxical because it also increased the percentage of adsorbed protein and the interfacial protein concentration and this behavior was more intense at the highest protein concentration. Our results suggest that the thick film was made up from protein aggregates that were prone to sticking other proteins from bulk or from other droplets. This mechanism would explain high percentages of adsorbed protein and high FI.
Conclusions
Even though the combination of calcium and HHP led to improvement in protein solubility and colloidal stability of insoluble aggregates, it was detrimental to the emulsifying ability of SPI. These protein species would have an increase protein-protein association upon homogenization, which in turn resulted in flocculation. However, separately, calcium and HHP improved emulsion features. Our data suggest that combination of calcium and HHP may be more useful to improve functional properties in which protein-protein association is needed, such as gelation.
References
Agboola, S. O., & Dalgleish, D. G. (1995). Calcium-induced destabilization of oil-in-water emulsions stabilized by caseinate or by β-lactoglobulin. Journal of Food Science, 60(2), 399–404.
Añón, M. C., de Lamballerie, M., & Speroni, F. (2012). Effect of high pressure on solubility and aggregability of calcium-added soybean proteins. Innovative Food Science and Emerging Technologies, 16, 155–162.
Bouaouina, H., Desrumaux, A., Liosel, C., & Legrand, J. (2006). Functional properties of whey proteins as affected by dynamic high-pressure treatment. International Dairy Journal, 16(4), 275–284.
Castellani, O., David-Briand, E., Guérin-Dubiard, C., & Anton, M. (2005). Effect of aggregation and sodium salt on emulsifying properties of egg yolk phosvitin. Food Hydrocolloids, 19(4), 769–776.
Castellani, O., Al-Assaf, S., Axelos, M., Phillips, G. O., & Anton, M. (2009). Hydrocolloids with emulsifying capacity. Part 2- adsorption properties at the n-hexadecane – water interface. Food Hydrocolloids, 24, 161–174.
Chapleau, N., & de Lamballerie-Anton, M. (2003). Improvement of emulsifying properties of lupin proteins by high pressure induced aggregation. Food Hydrocolloids, 17(3), 273–280.
Cui, Z., Chen, Y., Kong, X., & Hua, Y. (2014). Emulsifying properties and oil/water (o/w) interface adsorption behavior of heat soy proteins: effects of heating, concentration, homogenizer rotating speed, and salt addition level. Journal of Agricultural and Food Chemistry, 62, 1634–1642.
Delbyshire, E., Wright, D. J., & Boulter, D. (1976). Legumin and vicilin storage protein of legume seeds. Phytochemistry, 15(1), 3–24.
Dickinson, E. (2010). Flocculation of protein-stabilized oil-in-water emulsions. Colloids Surf B Biointerfaces, 81, 130–140.
Dickinson, E., & Davies, E. (1999). Influence of ionic calcium on stability of sodium caseinate emulsions. Colloids and Surfaces B: Biointerfaces, 12(3–6), 203–212.
Dickinson, E., & Semenova, M. G. (1992). Emulsifying behaviour of protein in the presence of polysaccharide under conditions of thermodynamic incompatibility. Journal of the Chemical Society, Faraday Transactions, 88(6), 849–854.
Floury, J., Desrumaux, A., & Legrand, J. (2002). Effect of ultra-high-pressure homogenization on structure and on rheological properties of soy protein-stabilized emulsions. Journal of Food Science, 67(9), 3388–3395.
Galán, M. G., & Drago, S. R. (2014). Effects of soy protein and calcium levels on mineral bioaccessibility and protein digestibility from enteral formulas. Plants Foods for Human Nutrition, 69(3), 283–289.
Graham, D. E., & Phillips, M. C. (1979). Proteins at liquid interfaces: I. Kinetics of adsorption and surface denaturation. Journal of Colloids and Interface Science, 70, 403–414.
Jones, O. G. (2016). Recent advances in the functionality of non-animal-sourced proteins contributing to their use in meat analogues. Current Opinion in Food Science, 7, 7–13.
Keowmaneechai, E., & McClements, D. J. (2002). Influence of EDTA and citrate on physicochemical properties of whey protein-stabilized oil-in-water emulsions containing CaCl2. Journal of Agricultural and Food Chemistry, 50(24), 7145–7153.
Li, H., Zhu, K., Zhou, H., & Peng, W. (2011). Effects of high hydrostatic pressure on some functional and nutritional properties of soy protein isolate for infant formula. Journal of Agricultural and Food Chemistry, 59(22), 12028–12036.
Manassero, C. A., Vaudagna, S. R., Añón, M. C., & Speroni, F. (2015). High hydrostatic pressure improves protein solubility and dispersion stability of mineral-added soybean protein isolate. Food Hydrocolloids, 43, 629–635.
Manassero, C. A., David-Briand, E., Vaudagna, S. R., Anton, M., & Speroni, F. (2018). Calcium addition, pH and high hydrostatic pressure effects on soybean protein isolates – Part 1: colloidal stability improvement. Food and Bioprocess Technology. Article in press, 11(6), 1125–1138. https://doi.org/10.1007/s11947-018-2084-7.
Markwell, M. A., Haas, S. M., Bieber, L. L., & Tolbert, N. E. (1978). A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Analytical Biochemistry, 87(1), 206–210.
Márquez, A. L., Medrano, A., Panizzolo, L. A., & Wagner, J. R. (2010). Effect of calcium salts and surfactant concentration on the stability of water-in-oil (w/o) emulsions prepared with polyglycerol polyricinoleate. Journal of Colloid and Interface Science, 341(1), 101–108.
McClements, D. J. (2005). Food emulsions. Principles, practices, and techniques. Second edition. Boca Raton: CRC Press.
Palou, E., López-Malo, A., Barbosa-Cánovas, G. V. & Welti-Chanes, J. (2000). High hydrostatic pressure and minimal processing. In: S. M. Alzamora, M. S. Tapia, A. López-Malo (Ed.), Minimally Processed Fruits and Vegetables. Fundamentals and applications (pp. 205–222). Gaithersburg: Aspen Publishers.
Patton, S., & Huston, G. H. (1986). A method for isolation of milk fat globules. Lipids, 21(2), 170–174.
Puppo, M. C., Speroni, F., Chapleau, N., de Lamballerie, M., Añón, M. C., & Anton, M. (2005). Effect of high-pressure treatment on emulsifying properties of soybean proteins. Food Hydrocolloids, 19(2), 289–296.
Rodríguez Patino, J. M., Molina Ortiz, S. E., Carrera Sánchez, C., Rodríguez Niño, M. R., & Añón, M. C. (2003a). Behavior of soy globulin films at the air-water interface. Structural and dilatational properties of spread films. Industrial & Engineering Chemistry Research, 42, 5011–5017.
Rodríguez Patino, J. M., Molina Ortiz, S. E., Carrera Sánchez, C., Rodríguez Niño, M. R., & Añón, M. C. (2003b). Dynamic properties of soy globulin adsorbed films at the air-water interface. Journal of Colloid and Interface Science, 268(1), 50–57.
Speroni, F., Jung, S., & de Lamballerie, M. (2010a). Effects of calcium and pressure treatment on thermal gelation of soybean protein. Journal of Food Science, 75, 30–38.
Speroni, F., Milesi, V., & Añón, M. C. (2010b). Interactions between isoflavones and soybeans proteins: Applications in soybean-protein-isolate production. LWT-Food Science and Technology, 43(8), 1265–1270.
Swientek, R. J. (1992). High hydrostatic pressure for food preservation. Food Processing, 53, 90–91.
Tang, C.-H. (2017). Emulsifying properties of soy proteins: a critical review with emphasis on the role of conformational flexibility. Critical Reviews in Food Science and Nutrition, 57(12), 2636–2679.
Utsumi, S., Matsumura, Y. & Mori, T. (1997). Structure-function relationships of soy proteins. In: S. P. A. Damodaran (Ed.), Food Proteins and their applications (pp. 257–291). First edition. New York: Marcel Dekker.
Walstra, P. (1983). Formation of emulsion. In: P.Becher (Ed.), Encyclopedia of Emulsion Technology, Volume 1 Basic Theory (pp. 57–127). New York: Marcel Dekker.
Walstra, P. (1993). Principles of emulsion formation. Chemical Engineering Science, 48(2), 333–349.
Wang, J.-M., Xia, N., Yang, X.-Q., Yin, S.-W., Qi, J.-R., He, X.-T., Yuan, D.-B., & Wang, L.-J. (2012). Adsorption and dilatational rheology of heat-treated soy protein at the oil−water interface: relationship to structural properties. Journal of Agricultural and Food Chemistry, 60(12), 3302–3310.
Wang, C.-Y., Huang, H.-W., Hsu, C.-P., & Yang, B. B. (2016). Recent advances in food processing using high hydrostatic pressure technology. Critical Reviews in Food Science and Nutrition, 56(4), 527–540.
Wang, X., Zeng, M., Qin, F., Adhikari, B., He, Z., & Chen, J. (2018). Enhanced CaSO4-induced gelation properties of soy protein isolate emulsion by pre-aggregation. Food Chemistry, 242, 459–465.
Ye, A., & Singh, H. (2000). Influence of calcium chloride addition on the properties of emulsions stabilized by whey protein concentrate. Food Hydrocolloids, 14(4), 337–346.
Ye, A., & Singh, H. (2001). Interfacial composition and stability of sodium caseinate emulsions as influenced by calcium ions. Food Hydrocolloids, 15(2), 195–207.
Yuan, D. B., Yang, X. Q., Tang, C. H., Zheng, Z. X., Wei-Min, Ahmad, I., & Yin, S. W. (2009). Physicochemical and functional properties of acidic and basic polypeptides of soy glycinin. Food Research International, 42(5–6), 700–706.
Acknowledgements
The authors thank Marie de Lamballerie for facilitating the use of HHP equipment installed in ONIRIS. The stay of Carlos A. Manassero in Biopolymères Interactions Assemblages-INRA was funded by Bec. Ar program of Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manassero, C.A., Beaumal, V., Vaudagna, S.R. et al. Calcium Addition, pH and High Hydrostatic Pressure Effects on Soybean Protein Isolates—Part 2: Emulsifying Properties. Food Bioprocess Technol 11, 2079–2093 (2018). https://doi.org/10.1007/s11947-018-2164-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2164-8