Abstract
Effects of protein isolate from bambara groundnut (BGPI) at different levels (0–6 %, w/w) in combination with microbial transglutaminase (MTGase) at different concentrations (0, 0.3 and 0.6 U g−1surimi) on gels properties of sardine (Sardinella albella) surimi were investigated. In the absence of MTGase, the increases in breaking force and deformation of gels were obtained when BGPI at levels of 1.5–3 % was incorporated (P < 0.05). The further increases in BGPI levels (4.5–6 %) resulted in the decrease in breaking force and deformation (P < 0.05). When MTGase (0.3 and 0.6 U g−1surimi) was added, the increase in breaking force and deformation were noticed, regardless of BGPI levels, and the strengthening effect was in dose-dependent manner. The increases in hardness, gumminess and chewiness were also observed when surimi gel was added with BGPI and MTGase (P < 0.05). Water-holding capacity of gels was improved with increasing level of BGPI, and MTGase incorporated (P < 0.05). Whiteness of gels slightly decreased with increasing BGPI levels, however the addition of MTGase had no impact on whiteness (P > 0.05). Based on electrophoretic study, myosin heavy chain decreased with addition of MTGase, indicating the formation of cross-links. More compact structure was observed in gel added with MTGase (0.6 U g−1surimi) and 6 % BGPI, and was accompanied by an increased gel strength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing consumer demand for low fat, high protein meat products has resulted in the development of non-meat protein additives. Due to relatively low cost and high nutrition value, the legume seed proteins have gained much attention for their use in surimi-based food products of which soy proteins are probably the most widely employed [1, 2]. Surimi is concentrated myofibrillar protein obtained from mechanically deboned fish flesh. It has unique functionalities including gel-forming ability and water holding capacity. Soy proteins, in the form of isolates or concentrates, have been used in processed meats as binders or gelling agents to improve the physical and textural properties upon heating [3]. They are also known as meat replacer to reduce the formulation costs [2]. Soy protein isolate was reported to inhibit proteolysis in surimi gel, termed ‘modori’ [4]. Recently, Oujifard et al. [5] and Kudre and Benjakul [6] reported that proteolysis of threadfin bream and sardine surimi caused by heat activated proteases could be partially inhibited by the addition of protein isolate from bambara groundnut, respectively. Apart from inhibitory action, protein isolate from bambara groundnut exhibits gelling property, high water holding capacity and bulking density for successful utilization in various food products [7]. However, the addition of protein isolate from bambara groundnut at levels higher than 2 % negatively affected the gel property of surimi from threadfin bream, mainly due to the ‘dilution effect’ [5]. Low interaction between legume seed protein and meat protein was also proposed [5, 8]. Proteins from soybean, glycinin and β-conglycinin are relatively resistant to denaturation. Limited unfolding of those proteins hampered the interaction with muscle proteins [8, 9].
Transglutaminase (E.C. 2.3.2.13), an enzyme that promotes polymerization of proteins through intermolecular ε (γ-glutamyl) lysine cross-links, has attracted much attention and has been considered as a potential agent to enhance the gelation of food proteins [10]. Ramíez-Suáez and Xiong [9] reported that a rigid mixed soy and muscle protein gel was produced with the aid of microbial transglutaminase. Recently, Sun and Arntfield [11] reported that the gel strength of myofibrillar protein/pea protein isolate mixture (3:1) was greatly increased by addition of MTGase, in which ε (γ-glutamyl) lysine (G-L) crosslink was formed between myofibrillar and pea proteins. Sardine (Sardinella albella) is a small coastal pelagic dark fleshed fish species, which can be used for surimi production in Thailand. Bambara groundnut is abundant in Thailand and other regions, which can be used as non-meat protein additive. Thus, the objective of this study was to investigate the impact of MTGase in combination of protein isolate from bambara groundnut on properties of gel from sardine (Sardinella albella).
Materials and Methods
Chemicals and Surimi
N-Carbobenzoxy (CBZ)-L-glutaminylglycine, N-α-Benzoyl-DL-arginine-p-nitroanilide (BAPNA), trypsin from bovine pancreas (BAEE 10,200 units/mg), bovine serum albumin, glutaraldehyde and wide range molecular weight protein markers were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Sodium chloride and sodium hydroxide were purchased from Merck (Darmstadt, Germany). Sodium dedocyl sulphate (SDS), Coomassie Blue R-250, N,N,N′,N′-tetramethyl ethylene diamine (TEMED) and all chemicals for electrophoresis were procured from Bio-Rad Laboratories (Hercules, CA, USA). Microbial transglutaminase (MTGase) from Streptoverticillium mobaraense (TG-K) was supplied by Ajinomoto (Thailand) Co., Ltd. (Bangkok, Thailand). Frozen surimi grade A from sardine (Sardinella albella) containing 5 % sucrose as a cryoprotectant was obtained from Pacific Fish Processing Co., Ltd. (Songkhla, Thailand) and kept at −20 °C until use, but not longer than 2 months.
Preparation of Protein Isolate from Bambara Groundnut
Bambara groundnut (Vigna subterranean) was purchased from a local market, Hat Yai, Songkhla, Thailand. The sample was dehulled and ground using a blender (Moulinex, Type AY46, Shenzhen, Guangdong, China) to obtain the fine powder. The powder was sieved using a mesh with an aperture size of 500 μm (No. 35, ASTM E11, serial number 5666533, FRITSCH GMBH, Idar-Oberstein, Germany).
Protein isolate from bambara groundnut was prepared according to the method of Kudre and Benjakul [6]. Bambara groundnut powder was suspended in 10 volumes of 0.2 % NaOH solution (pH 12). The mixture was stirred continuously for 2 h at room temperature (28–30 °C), followed by centrifugation at 8000 × g for 30 min at 25 °C (Avanti J-E centrifuge, Beckman Coulter, Inc., Fullerton, CA, USA). The supernatant was collected and pH was adjusted to 4.5 using 6 N HCl. The precipitate formed was recovered by centrifugation at 8000 × g for 30 min. The pellet was washed twice with 10 volumes of distilled water adjusted to pH 4.5 using 6 N HCl, followed by centrifugation at 8000 × g for 30 min. The resulting pellet was freeze-dried using a freeze dryer (ScanVac Model CoolSafe 55–4, Lynge, Denmark). Dried powder obtained was referred to as ‘bambara groundnut proteins isolate, BGPI’. BGPI was placed in polyethylene bag and stored at −40 °C until use. BGPI had the protein content of 86.2 % as determined by Kjeldahl method [12].
Trypsin Inhibitory Activity Assay
Trypsin inhibitory activity of BGPI was measured by the method of Benjakul et al. [13] with a slight modification using BAPNA as substrate. To extract trypsin inhibitors from BGPI, BGPI (0.5 g) was added with 100 ml of 0.2 M Tris–HCl, pH 8.5. The mixture was stirred continuously at a speed of 300 rpm at room temperature (28–30 °C) for 90 min using a magnetic stirrer (IKA® Model Colour Squid white, BEC THAI, Bangkok, Thailand). The mixture was then subjected to centrifugation at 12000 × g at 25 °C for 25 min. The supernatant was used for determination of trypsin inhibitor.
To assay trypsin inhibitor, 200 μl of supernatant, 200 μl of bovine pancreas trypsin (0.1 mg/ml) and 1,000 μl of 50 mM Tris–HCl, pH 8.5 containing 10 mM CaCl2 were mixed thoroughly and incubated at 37 °C for 15 min. Thereafter, 200 μl of BAPNA (0.4 mg/ml in DMSO, pre-warmed to 37 °C) were added and vortexed immediately to start the reaction. After incubating for 10 min, 200 μl of 30 % acetic acid (v/v) were added to terminate the reaction. For the control, distilled water was used instead of inhibitor solution. The reaction mixture was centrifuged at 8000 × g for 5 min at room temperature. The activity of trypsin in the absence and presence of inhibitor solution was determined by measuring the absorbance at 410 nm due to p-nitroaniline released. One unit of trypsin was defined as an increase of 0.01 absorbance unit min−1 under the assay condition. One unit of trypsin inhibitory activity (TIU) was defined as the amount of inhibitor, which reduced trypsin activity by one unit.
Preparation of Crude Microbial Transglutaminase (MTGase)
Crude MTGase was prepared according to the method of Visessanguan et al. [14] with a slight modification. Commercial enzyme powder (2 g) was dissolved with 20 ml of 20 mM Tris–HCl, pH 7.0, at 4 °C. The mixture was stirred gradually for 20 min, followed by centrifugation at 14, 000 × g at 4 °C for 30 min. Supernatant was filtered through a Whatman filter paper No.1 (Whatman Ltd, Maidstone, UK) and then through a 0.20 mm nylon syringe filter. The filtrate was dialyzed against 15 volumes of 20 mM Tris–HCl, pH 7.0, at 4 °C for 36 h, with four changes of dialysis buffer. The dialysate was centrifuged at 14, 500 × g for 30 min at 4 °C. The resulting supernatant was analyzed for MTGase activity.
Determination of MTGase Activity
MTGase activity was measured by the hydroxamate method [15]. Freshly prepared substrate mixture containing 350 μl of 0.1 M Tris-acetate (pH 6.0), 25 μl of 2.0 M hydroxylamine, 75 μl of 0.1 M N-carbobenzoxy (CBZ)-L-glutaminylglycine and 25 μl of deionized water was used. To initiate the reaction, 25 μl of crude extract were added and reaction was performed for 10 min at 37 °C. The reaction was terminated by adding 500 μl of 15 % trichloroacetic acid containing 5 % ferric chloride. The resulting suspension was centrifuged at 8000 × g for 5 min and the absorbance of supernatant was measured at 525 nm using a UV-160 spectrophotometer (Shimadzu, Kyoto, Japan). L-glutamic acid-γ-monohydroxamic acid (0–0.3 mM) was used as standard. One unit of MTGase was defined as the amount of enzyme required to catalyze the formation of 1 μmole hydroxamic acid min−1 at pH 6.0 and 37 °C.
Effect of MTGase and BGPI on Gel Properties of Sardine Surimi
Frozen sardine surimi was partially thawed at 4 °C for 2–3 h, cut into small pieces with an approximate thickness of 1 cm and then placed in the mixer (National Model MKK77, Tokyo, Japan). The mixture was chopped for 1 min, followed by addition of 2.5 % salt (w/w), MTGase (0, 0.3, and 0.6 U g−1surimi) and BGPI (0, 1.5, 3, 4.5 and 6 %, w/w). The final moisture content of mixture was adjusted to 80 % by adding iced water. Chopping was continued for additional 3 min. Temperature was maintained at below 7 °C during chopping. The sol was stuffed into a polyvinylidine chloride casing with a diameter of 2.5 cm and both ends were sealed tightly. Gels were prepared by incubating the sol at 40 °C for 30 min, followed by heating at 90 °C for 20 min. Subsequently, all gels were cooled in iced water for 30 min and stored at 4 °C overnight prior to analyses.
Analyses
Textural Analysis
Textural analysis of surimi gels was carried out using a Model TA-XT2 texture analyzer (Stable Micro Systems, Surrey, UK). Gels kept at 4 °C were equilibrated at room temperature (28–30 °C) before analysis. Cylindrical samples (2.5 cm in length) were prepared and subjected to determination. Breaking force (gel strength) and deformation (elasticity/ cohesiveness) were measured using the texture analyzer equipped with a spherical plunger (diameter 5 mm; depression speed 60 mm min−1).
Textural Profile Analysis (TPA)
TPA was performed using a TA-XT2i texture analyzer (Stable Micro Systems, Surrey,England) with a cylindrical aluminum probe (50 mm diameter). The samples were cut into cylinders (2.5 cm height × 2.5 cm diameter) and placed on the instrument’s base. The tests were performed with two compression cycles. TPA textural parameters were measured at room temperature with the following testing conditions: cross head speed 5.0 mm/s, 50 % strain, surface sensing force 99.0 g, threshold 30.0 g, and time interval between the first and the second compressions was 1 s. The Texture Expert version 1.0 software (Stable Micro Systems, Surrey, England) was used to collect and process the data. Hardness, springiness, cohesiveness, gumminess and chewiness were calculated from the force-time curves generated for each sample.
Determination of Whiteness
All gels were subjected to whiteness measurement using a HunterLab (ColorFlex, Hunter Associates Laboratory, Reston, VA, USA). Illuminant C was used as the light source of measurement. L*, a* and b* values were measured and whiteness was calculated using the following equation [16]:
Determination of Expressible Moisture Content
Expressible moisture content was measured according to the method of Rawdkuen and Benjakul [17]. Cylindrical gel samples were cut to obtain a thickness of 5 mm, weighed (X) and placed between three pieces of Whatman paper No.1 at the bottom and two pieces of paper on the top. A standard weight (5 kg) was placed on the top of the sample for 2 min, and then the sample was removed from the papers and weighed again (Y). Expressible moisture content was calculated with the following equation and expressed as percentage of sample weight:
SDS-Polyacrylamide Gel Electrophoresis
Protein patterns of surimi and gels were analyzed by SDS-PAGE according to the method of Laemmli [18]. To prepare the protein sample, 27 ml of 5 % (w/v) hot SDS (85 °C) solution were added to surimi or gel samples (3 g). The mixture was then homogenized at a speed of 11,000 rpm for 2 min using an IKA homogenizer (Labortechnik, Selangor, Malaysia). The homogenate was incubated at 85 °C for 1 h to dissolve proteins. The samples were then centrifuged at 8,000 × g for 20 min to remove undissolved debris. Protein concentration in the supernatants was determined as per the method of Lowry et al. [19]. Solubilized samples were mixed at 1:1 (v/v) ratio with the sample buffer (0.5 M Tris–HCl, pH 6.8, containing 4 % SDS, 20 % glycerol and 10 % βME) and boiled for 3 min. The samples (15 μg protein) were loaded onto the polyacrylamide gel made of 10 % running gel and 4 % stacking gel and subjected to electrophoresis at a constant current of 15 mA per gel using a Mini Protean III unit (Bio-Rad Laboratories, Inc., Richmond, CA, USA). After separation, the proteins were stained with 0.02 % (w/v) Coomassie brilliant blue R-250 in 50 % (v/v) methanol and 7.5 % (v/v) acetic acid and destained with 50 % methanol (v/v) and 7.5 % (v/v) acetic acid, followed by 5 % methanol (v/v) and 7.5 % (v/v) acetic acid.
Scanning Electron Microscopy (SEM)
Microstructure of surimi gels including the control gels (without BGPI and MTGase), gels added with MTGase at levels of 0.3 and 0.6 U MTGase g−1surimi in the presence and absence of 6 % BGPI as well as gel containing 6 % BGPI (without MTGase) were fixed with 2.5 % (v/v) glutaraldehyde in 0.2 M phosphate buffer (pH 7.2). The samples were then rinsed for 1 h in distilled water before being dehydrated in ethanol with a serial concentration of 50, 70, 80, 90 and 100 % (v/v). Dried samples were mounted on a bronze stub and sputter-coated with gold (Sputter coater SPI-Module, PA, USA). The specimens were observed with a scanning electron microscope (JEOL JSM-5800 LV, Tokyo, Japan) at an acceleration voltage of 20 kV.
Sensory Analysis
Sensory evaluation was performed by 30 untrained panelists, who were the graduate students in Food Science and Technology program with the age of 25–33 years and were familiar with surimi products. Panelists were asked to evaluate for color, taste, texture, odor, flavor and overall likeness of the control gel (without MTGase and BGPI ) and gel added with MTGase (0.6 U g−1surimi) and 6 % BGPI. A nine-point hedonic scale, in which a score of 1, dislike extremely; 2, dislike very much; 3, dislike moderately; 4, dislike slightly; 5, neither like nor dislike; 6, like slightly; 7, like moderately; 8, like very much; 9, like extremely, was used for sensory evaluation [20]. The samples were served on a white paper plate at room temperature. Panelists were instructed to rinse their mouths with water before starting and between sample evaluation.
Statistical Analysis
All experiments were run in triplicate and a completely randomized design (CRD) was used. Data were subjected to analysis of variance (ANOVA). Comparison of means was carried out by Duncan’s multiple range tests [21]. Analysis was performed using a SPSS package (SPSS 17.0 for Windows, SPSS Inc., Chicago, IL, USA).
Results and Discussion
Effect of MTGase and BGPI at Different Levels on Textural Properties of Sardine Surimi Gels
Breaking Force and Deformation
Breaking force and deformation of gels from sardine surimi added with various levels of BGPI in the presence and absence of MTGase (0.3 and 0.6 U g−1surimi) are depicted in Fig. 1(a and b). In the absence of MTGase, the highest breaking force and deformation of gels were found when 1.5 or 3 % BGPI was incorporated (P < 0.05). Breaking force and deformation of gels added with 3 % BGPI increased by 20.6 %, and 10.6 %, respectively, compared with that of control gel (without BGPI and MTGase). The decreases in breaking force and deformation were observed when BGPI at levels of 4.5–6 % was added without MTGase (P < 0.05). With addition of 6 % BGPI, the breaking force and deformation decreased by 22.7 % and 12.9 %, respectively, compared with that of control gel containing no BGPI and MTGase. Rawdkuen et al. [22] reported that the addition of non-muscle protein additives such as porcine plasma protein, egg white, chicken plasma protein and soy protein isolate at an excessive amount generally led to the decreases in both breaking force and deformation of sardine (Sardenella gibbosa) surimi gels. Lowered strength of surimi gel with non-muscle protein additives could be caused by “dilution” of myofibrillar proteins [23]. Myofibrillar proteins are known as the major contributor for gelation of surimi [24]. The increases in breaking force and deformation of surimi gel with addition of 1.5–3 % BGPI were more likely associated with the filler or binder effect of BGPI with myofibrillar proteins in conjunction with protease inhibitory effect. BGPI with protease inhibitory activity (1198.3 ± 16.2 U g−1) retarded the degradation of surimi muscle proteins caused by endogenous heat activated proteases [6].
Breaking force (a) and deformation (b) of sardine surimi gels added with MTGase and BGPI at different levels. Bars represent the standard deviation (n = 3). Different lowercase letters on the bars within the same level of MTGase indicate significant differences (P < 0.05). Different uppercase letters on the bars within the same BGPI level indicate significant difference (P < 0.05). MTGase: microbial transglutaminase; BGPI: bambara groundnut protein isolates
The addition of MTGase at a level of 0.3 U g−1surimi increased both breaking force and deformation of gels containing BGPI at all levels, compared with those without MTGase (P < 0.05). MTGase more likely induced cross-linking between myofibrillar proteins and bambara groundnut proteins via non-disulfide covalent bond. The result suggested that the addition of MTGase could conquer the gel strength lowering effect caused by BGPI. It was noted that MTGase at a higher level (0.6 U g−1surimi) exhibited the lower ability in gel improvement for the control, compared with the lower level (0.3 U MTGase g−1surimi). Excessive and rapid polymerization of myofibrillar proteins induced by MTGase at higher level might yield the gel matrix with lower strength and less elasticity. Similar result was reported by Seguro et al. [25] who found the decrease in breaking force and deformation of Alaska pollock surimi added with MTGase at level higher than 0.03 %. Nevertheless, no differences in both breaking force and deformation were noticeable between gels added with 0.3 and 0.6 U MTGase g−1surimi when BGPI at a level of 1.5 % was present (P > 0.5). Gels containing BGPI at levels of 3–6 % had higher breaking force and deformation (P < 0.05) when MTGase at 0.6 U g−1surimi was added in comparison with 0.3 U g−1surimi (P < 0.5). Breaking force and deformation of gels containing 6 % BGPI added with MTGase at levels of 0.3 and 0.6 U g−1surimi increased by 68.9 % and 38.1 %, and 75.1 % and 43.3 %, respectively, compared to those of the gel containing 6 % BGPI (without MTGase). Both gels showed the higher breaking force and deformation than the control gel (without BGPI and MTGase) (P < 0.05). In the presence of non-muscle proteins, MTGase might induce the formation of non-disulfide covalent bond between myofibrillar proteins and bambara groundnut proteins to a greater extent. As a result, the strength of gel matrix was enhanced. Addition of MTGase to tackle the negative effect of non-muscle proteins on surimi or muscle protein gel can be varied, depending on the composition and configuration of those proteins added. Our result was in agreement with Sun and Arntfield [11] who reported that gel strength of soy or pea/myofibrillar protein isolate mixture (1:3) was greatly increased by addition of MTGase (10 Unit/g protein). In the absence of MTGase, the gel strength of myofibrillar protein isolate markedly decreased by addition of soy or pea protein isolates, especially at higher levels (25 %, w/w). The results indicated that the distribution of bambara groundnut proteins in surimi might prevent the cross-linking of myofibrillar protein. As a consequence, MTGase added might induce the gradual polymerization of those mixed proteins. Thus, the stronger gel could be developed.
Texture Profile Analysis
Texture profile analysis (TPA) of sardine surimi gels added with BGPI (0–6 %, w/w) in the absence and presence of MTGase (0.3 and 0.6 U g−1surimi) is shown in Table 1. In the absence of MTGase, hardness, gumminess, cohesiveness and chewiness of surimi gels increased with increasing BGPI level up to 3 % (P < 0.05). However, further increase in BGPI levels (4.5–6 %) lowered hardness, gumminess and chewiness of resulting gels (P < 0.05). BGPI at higher levels hindered the gel formation by preventing the cross-linking of myofibrillar proteins [22]. This result was in accordance with the decreases in breaking force and deformation of gels added with 4.5–6 % BGPI (Fig. 1). BGPI addition had no marked impact on springiness (P > 0.05). Conversely, with addition of MTGase at levels of 0.3 and 0.6 U g−1 surimi, hardness, gumminess, cohesiveness and chewiness of surimi gels increased, particularly when BGPI levels increased (P < 0.05). This indicated that MTGase positively induced interactive effect between myofibrillar proteins and BGPI. In the presence of 6 % BGPI, gel added with MTGase at level of 0.6 U g−1surimi showed the higher values of hardness, gumminess, cohesiveness and chewiness than that added with lower level of MTGase (0.3 U g−1surimi) (P < 0.05). Therefore, increasing BGPI and MTGase plausibly resulted in greater cross-links between myofibrillar and bambara groundnut proteins in the gel matrix. In the absence of BGPI, gel added with MTGase at a level of 0.3 U g−1surimi showed higher values of hardness, gumminess and chewiness than that added with 0.6 U MTGase g−1surimi (P < 0.05). The result was in accordance with the higher breaking force and deformation found in surimi gel added with MTGase at lower level (0.3 U g−1surimi) (Fig. 1). Surimi gels containing BGPI at high levels (4.5 and 6 %) had lower springiness values than those without BGPI or with lower BGPI when MTGase was added (P < 0.05). Thus, both BGPI and MTGase levels affected the textural properties of sardine surimi gel differently.
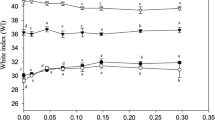
Effect of MTGase and BGPI at Different Levels on Whiteness and Expressible Moisture of Sardine Surimi Gels
Whiteness of surimi gels added with BGPI at different levels in the absence and presence of MTGase at levels of 0.3 and 0.6 U g−1surimi is shown in Table 2. The slight decreases in whiteness were found in surimi gel as BGPI levels increased, regardless of MTGase additions (P < 0.05). This result was in agreement with Oujifard et al. [5] who reported a linear decrease in whiteness of surimi gels from threadfin bream when BGPI levels added increased. Decrease in whiteness was most probably caused by indigenous pigments in BGPI. It was noted that slight increase in whiteness was found in surimi gels without BGPI and with 1.5 % BGPI when MTGase at a level of 0.6 U g−1surimi was incorporated, compared with those containing MTGase at 0 and 0.3 U g−1surimi (P < 0.05). Gel added with 6 % BGPI and MTGase (0.6 U g−1surimi) had the whiteness of 67.79, while the control gel (without BGPI and MTGase) had whiteness of 68.89. Thus, BGPI in combination with MTGase could be used as an additive in surimi to improve the gel strength without causing the marked changes in whiteness. The expressible moisture content of surimi gels added with BGPI at different levels in the absence and presence of MTGase at levels of 0.3 U and 0.6 U g−1surimi is shown in Table 2. The expressible moisture content of surimi gel decreased as the level of BGPI increased up to 3 % (w/w) (P < 0.05). Further increase in BGPI level decreased water holding capacity of gels, as indicated by increased expressible moisture content. BGPI at higher levels could impede the development of strong gel network, resulting in poor water-holding capacity of the gels. This result was coincidental with the decreases in breaking force and deformation of gels added with BGPI at levels of 4.5–6 % (Fig. 1). The results indicated that water holding capacity of surimi gel could be improved with addition of BGPI at the level of 1.5–3 % (w/w). High water holding capacity of protein additives causes them to swell and augment elasticity by reducing the moisture content of the mixtures and increasing the density of surrounding protein matrix [26]. When MTGase at both levels (0.3 and 0.6 U g−1surimi) was incorporated, water holding capacity of gels increased at all levels of BGPI used as evidenced by the lower expressible moisture content. This indicated that MTGase most likely improved gel forming ability via the formation of non-disulfide covalent bonds. As a consequence, a stronger gel network formed could imbibe more water. This result was in agreement with higher breaking force and deformation of gels (Fig. 1) as well as higher hardness, gumminess and cohesiveness values (Table 1) when MTGase was added. Gels added with MTGase at a level of 0.6 U g−1surimi containing 4.5 and 6 % BGPI showed the lower expressible moisture content than others (P < 0.05). Therefore, both MTGase and BGPI at appropriate levels could improve water holding capacity of surimi gel.
Effect of MTGase and BGPI on Protein Patterns of Sardine Surimi Gel
Protein patterns of surimi gels added with various levels of BGPI in the absence and presence of MTGase at levels of 0.3 and 0.6 U g−1surimi is depicted in Fig. 2. Surimi paste contained MHC and actin as the major proteins. In the absence of MTGase, the lowest band intensity of MHC was found in gel containing no BGPI, indicating the highest polymerization of MHC caused by endogenous transglutaminase during setting [24]. The intensity of MHC band increased as the level of BGPI increased, indicating that BGPI could inhibit the degradation of MHC in a concentration dependent manner. Rawdkuen et al. [22] reported the proteolysis of MHC from Sardinella gibbosa stilled occurred to some extent during setting (40 °C). The interfering effect of BGPI on polymerization of MHC was also postulated. Proteins with MW of 58 and 61 kDa were observed in gels added with BGPI, and the intensity increased with increasing levels of BGPI. The proteins having MW of 58 and 61 kDa more likely represented subunits of 7S protein from bambara groundnut. No MHC band was found in gels added with MTGase, both 0.3 and 0.6 U g−1surimi (without BGPI). The result suggested the formation of cross-linking stabilized by non-disulfide covalent bond mediated by MTGase as well as indigenous transglutaminase during setting [24, 27]. With addition of MTGase at both levels, the complete disappearance of MHC bands in gel samples with all BGPI level was observed. This result suggested that pronounced cross-linking of MHC took place via non-disulfide covalent bond induced by MTGase [9, 27]. Moreover, the intensity of actin and tropomyosin band slightly decreased as BGPI at 3–6 % was incorporated, irrespective of MTGase addition. Ramíez-Suáez and Xiong [9] reported that the mixture of chicken myofibrillar protein isolates and soy protein isolates had an almost reduction of myosin heavy chain and disappearance of actin when MTGase was present. Gel added with 4.5 and 6 % BGPI showed a lower band intensity of proteins with MW of 58 and 61 kDa, when MTGase at 0.6 U g−1surimi was added. This suggested that cross-linking of those proteins in BGPI was induced by MTGase. Thus, the increase in gel strength of sardine surimi added with BGPI at higher levels in the presence of MTGase might be due to the interactive effect between myofibrillar proteins and bambara groundnut proteins induced by MTGase. Recently, Sun and Arntfield [11] reported that the gel strength of myofibrillar/pea proteins isolate mixture (3:1) was greatly increased by addition of MTGase as indicated by formation of ε (γ-glutamyl) lysine (G-L) crosslinking between muscle and pea proteins. On the other hand, the addition of BGPI at higher levels might impede cross-linking of MHC during setting mediated by indigenous transglutaminase. Interfering effect of BGPI on MHC aggregation during gelation process was also presumed. As a result, the lower cross-linking was obtained when BGPI at higher levels was added. Nevertheless, the gel weakening effect caused by BGPI addition could be overcome by MTGase, especially at a level of 0.6 U g−1surimi.
SDS-PAGE pattern of proteins of sardine surimi gel added with BGPI at different levels without and with MTGase at levels of 0.3 U and 0.6 U g−1surimi. M: marker; BGPI: bambara groundnut protein isolate; S: surimi paste; MHC: myosin heavy chain; AC: actin; TM; tropomyosin. Numbers designate the level of BGPI added (%, w/w)
Effect of MTGase and BGPI at Different Levels on Microstructure of Sardine Surimi Gels
Microstructures of control surimi gel and gels added with MTGase at both levels (0.3 U and 0.6 U g−1surimi) in the absence and presence of 6 % BGPI are illustrated in Fig. 3. The surimi gel without MTGase and BGPI (control) had a coarse and loose structure with a large number of voids. The gels added with MTGase at levels of 0.3 and 0.6 U g−1surimi (without BGPI) showed the finer and denser structures, compared with the control gel. However, some cracks were observed in gel with the addition of MTGase at 0.6 U g−1surimi. This might be associated with the lower breaking force and deformation (Fig. 1). Myofibrillar proteins could generally undergo the aggregation more effectively in the presence of MTGase, which induced the protein cross-linking, to yield the more compact and denser gel network. The gel added with 6 % BGPI (without MTGase) exhibited coarser and irregular structure with large cavities and the separated clumps of bambara groundnut proteins appeared. Irregular structure with larger cavities was in agreement with poorer gel properties of surimi gel added with a higher amount of BGPI. When gels containing 6 % BGPI were added with MTGase (0.3 U and 0.6 U g−1surimi), more compact structure with smaller clusters of aggregated proteins without large cavity was obtained. These observations suggested that MTGase induced interaction between myofibrillar protein and bambara groundnut proteins via non-disulfide covalent bonds, resulting in the higher intermolecular aggregation between adjacent molecules. The finer and more ordered structure of gels added with MTGase and higher level of BGPI correlated with higher breaking force and deformation (Fig. 1) as well as the lower expressible moisture content (Table 2). Thus, the addition of higher level of BGPI in combination with MTGase resulted in the formation of fine gel network with improved gel strength and water holding capacity.
Electron microscopic image of sardine surimi gels added with BGPI and MTGase at different levels. a: surimi gel without MTGase and BGPI; b: surimi gel added with 6 % BGPI (without MTGase); c: surimi gel added with 0.3 U MTGase g−1surimi (without BGPI); d: surimi gel added with 6 % BGPI and 0.3 U MTGase g−1surimi; e: surimi gel added with 0.6 U MTGase g−1 surimi (without BGPI); f: surimi gel added with 6 % BGPI and 0.6 U MTGase g−1surimi MTGase: microbial transglutaminase; BGPI: bambara groundnut protein isolate. Magnification: ×10,000
Sensorial Properties of Surimi Gel
No differences in likeness scores for color (7.10 ± 0.19), taste (7.21 ± 0.09), odor (6.90 ± 0.25) and appearance (7.15 ± 0.12) were noticeable between control gel and gel containing 6 % BGPI and MTGase (0.6 U g−1surimi) (data not shown). For texture likeness, gels containing 6 % BGPI and MTGase (0.6 U g−1surimi) had the higher score (8.57 ± 0.36) than the control (4.34 ± 0.29) (P < 0.05). This was coincidental with the higher breaking force and deformation (Fig. 1), and hardness, gumminess and chewiness (Table 1) of the former gels. However, the gel added with 6 % BGPI and MTGase had the lower flavor likeness score (5.3), compared with the control (7.4). This was more likely due to the beany or grassy flavor caused by BGPI added. Li [28] stated that off-flavors (e.g., beany, green, grassy, bitter) can be found in legume seed proteins, especially oil seed protein.
Conclusions
The addition of MTGase in combination with BGPI in sardine surimi could improve the property of surimi gel. BGPI at levels of 4.5 or 6 % (w/w) was recommended to add in surimi in conjunction with 0.6 U MTGase g−1surimi. MTGase could effectively induce the interaction between surimi proteins and bambara groudnut proteins, thereby increasing breaking force and deformation. Thus, MTGase could conquer interfering effect of BGPI in surimi gel. BGPI at an appropriate level could improve gel properties of surimi from sardine in conjunction with MTGase.
References
Z. Pietrasik, A. Jarmoluk, P.J. Shand, LWT-Food Sci. Technol. 40, 915–920 (2007)
K.B. Chin, J.T. Keeton, M.T. Longnecker, J.W. Lamkey, Meat Sci. 53, 45–57 (1999)
J.M.S. Renkema, T. van Vliet, J. Agric. Food Chem. 50, 1569–1573 (2002)
Y.K. Luo, R. Kuwahara, M. Kaneniwa, Y. Murata, M. Yokoyama, J. Sci. Food Agric. 84, 663–671 (2004)
A. Oujifard, S. Benjakul, M. Ahmad, J. Seyfabadi, LWT-Food Sci. Technol. 47, 261–266 (2012)
T. Kudre, S. Benjakul, J. Food Process. Preserv. (2012). doi:10.1111/j.1745-4549.2012.00733.x
A.R.S.M. Eltayeb, A.O. Ali, A.A. Abou-Arab, F.M. Abu-Salem, Afr. J. Food Sci. 5, 82–90 (2011)
J. Feng, Y.L. Xiong, J. Food Sci. 67, 2851–2856 (2002)
J.C. Ramíez-Suáez, Y.L. Xiong, Meat Sci. 65, 899–907 (2003)
J.E. Folk, Annu. Rev. Biochem. 49, 517–531 (1980)
X.D. Sun, S.D. Arntfield, Food Hydrocolloids 27, 394–400 (2012)
AOAC, Official Methods of Analysis, 17th edn. (Gaithersberg, 2000)
S. Benjakul, W. Visessanguan, P. Thummaratwasik, J. Food Biochem. 24, 107–127 (2000)
W. Visessanguan, S. Benjakul, M. Tanaka, J. Sci. Food Agric. 83, 105–112 (2003)
J. E. Folk, in Methods in Enzymology, ed. By H. Tabor, C.W. Tabor (Academic Press, 1970), p. 889–894
National Fisheries Institute, A manual of standard methods for measuring and specifying the properties of surimi. (Washington, DC, 1991), p. 6–27
S. Rawdkuen, S. Benjakul, Food Chem. 106, 1077–1084 (2008)
U.K. Laemmli, Nature 227, 680–685 (1970)
O.H. Lowry, N.J. Rosebrough, L.A. Farr, R.J. Randall, J. Biol. Chem. 193, 265–275 (1951)
M. Meilgaard, G.V. Civille, B.T. Carr, Sensory evaluation techniques, 4th edn. (CRS Press, Boca Raton, 1999)
R.G.D. Steel, J.H. Torrie, Principles and procedures of statistics: A biometrical approach, 2nd edn. (McGraw-Hill, New York, 1980)
S. Rawdkuen, S. Benjakul, W. Visessanguan, T.C. Lanier, J. Food Process Preserv. 31, 492–516 (2007)
J.C. Burgarella, T.C. Lanier, D.D. Hamann, M.C. Wu, J. Food Sci. 50, 1595–1597 (1985)
S. Benjakul, W. Visessanguan, Food Res. Int. 36, 253–266 (2003)
K. Seguro, Y. Kumazawa, T. Ohtsuka, S. Toiguchi, M. Motoki, Microbial transglutaminase and ε-(γ-glutamyl) lysine crosslink effects on elastic properties of kamaboko gels. J. Food Sci. 60, 305–311 (1995)
E. Niwa, T.T. Wang, S. Kanoh, T. Nakayama, Nippon Suisan Gakk. 94, 989–992 (1988)
S. Chanarat, S. Benjakul, A. H-Kittikun, J. Sci. Food Agric. 92, 844–852 (2012)
R. Li, in Soy Applications in Food, ed. by M.N. Riaz (CRC Press, New York, 2006), pp. 227–248
Acknowledgments
The authors would like to express their sincere thanks to Graduate School, Prince of Songkla University, National Research Council of Thailand and the Thailand Research Fund (TRF) senior research scholar program for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kudre, T.G., Benjakul, S. Combining Effect of Microbial Transglutaminase and Bambara Groundnut Protein Isolate on Gel Properties of Surimi from Sardine (Sardinella albella). Food Biophysics 8, 240–249 (2013). https://doi.org/10.1007/s11483-013-9292-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-013-9292-5