Abstract
The poor solubility and functionalities of soy protein concentrate (SPC) limits its utilization in meat products. Phosphate-assisted hydrothermal cooking (HTC) was applied to refunctionalize SPC in this work. The resultant soy protein isolate (SPI) was used as an ingredient for improving the gelation of porcine myofibrillar protein (MP) which was induced by microbial transglutaminase (MTGase). The addition of sodium tripolyphosphate (STPP) enhanced the refunctionalization efficiency of HTC and increased the phosphorus content in the obtained SPI. Dynamic rheological analysis indicated that this SPI itself could not form a gel with the help of MTGase. However, the SEM observation revealed that the presence of this SPI strengthened the gel network of MP and made it become denser and more ordered. As a result, the mechanical properties and water holding capacity of the MP gel were significantly improved by this SPI prepared by phosphate-assisted HTC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meat products are important components in our daily diet. Microbial transglutaminase (MTGase, EC 2.3.2.13), which is able to induce the formation of covalent cross-links among the protein molecules, has been widely used to improve the gelation characteristics in processed meat products such as sausage [1]. Although the texture-related properties were improved, it had been reported that MTGase treatments tended to reduce the water-holding capacity (WHC) of the myofibrillar protein (MP) gels [2]. Therefore, food hydrocolloids including vegetable proteins and polysaccharide were often used to improve the mechanical properties and water retention ability of the MP gels induced by MTGase [3, 4].
Non-muscle proteins can participate in the MTGase-induced cross-linking reaction and increase the protein content in the meat products, while polysaccharide additives cannot. Soy protein, an economical source of non-muscle protein, is by far the most utilized vegetable protein in formulated foods including meat products for their functional properties, such as gelation, water-binding and fat-binding ability, and enhancement of emulsion stability [5]. However, there are still two problems to be solved in the application of soy protein in meat products. One is the lack of interaction between soy protein and MP under normal meat processing conditions, which limits their ability to improve the texture-related properties of the MP gel. The other is the off-flavor derived from the lipids in soybean, reducing the customer acceptance of the meat products with soy protein [6, 7]. Various modification strategies for soy protein which aim at increasing the interaction between soy protein and MP have been reported, such as pre-heating, enzyme-hydrolyzed, oxidation, and extreme pH treatment [8–11]. However, how to minimize the impact of the beany flavor from soy protein products on the muscle food has received little attention.
Soy protein concentrate (SPC) prepared by aqueous ethanol leaching has desirable light color and bland flavor [12]. This feature allows it to be incorporated into the meat products without covering up the flavor of the meat. But the ethanol-induced denaturation greatly reduced the solubility and functionalities of SPC, which made it difficult to interact with MP. Hydrothermal cooking (HTC), where steam and protein slurry were injected into a holding tube through a restriction orifice, had been proven to be an efficient method for SPC refunctionalization [13, 14]. After processing at temperatures higher than the usual boiling point of water and under certain steam pressure, the extractability, solubility, emulsification capacity, and foaming properties of the soy protein were improved [13]. As HTC provided a high-temperature and high-pressure environment for the material in the aqueous phase, it can be used as a continuous reaction device. When soy protein was treated by HTC with other components such as saccharides and phosphates, some changes might take place in the groups on the side chain of the protein molecules. Thus, HTC could be applied to SPC together with different additives, so as to alter the functionality of soy protein according to the purpose of the product.
In this study, sodium tripolyphosphate (STPP)-assisted HTC was used to refunctionalize SPC into an effective functional ingredient which was able to improve the mechanical properties and water-holding capacity of the porcine MP gel. STPP, a commonly used phosphate additive for quality improvement of meat products [15], was employed to introduce more charge groups to the resultant SPI and increase its solubility and functionality. This SPI was incorporated into MP dispersion, and the mixed gels were prepared with MTGase. The gelation kinetics, mechanical properties, WHC and microstructure of the gels were investigated.
Materials and Methods
Materials
Porcine M. longissimus dorsi was purchased from a local retailer. All visible fat and connective tissue were trimmed off, and the muscle was cut into cubes (~2 cm3). The meat cubes were vacuum-packed, and placed in a freezer (−40 °C) prior to use (within 4 weeks). SPC was obtained from Qinhuangdao Goldensea Grain & Oil (Hebei, China), and its protein content was 65.16 ± 0.89 % (n × 6.25, dry basis), determined by the Dumas method. Food-grade STPP was obtained from Lianyungang YouJin Food Additives Technology Development (Jiangsu, China). MTGase was purchased from Ajinomoto (Tokyo, Japan). The activity of MTGase was 100 U/g as determined according to the method proposed by Folk and Cole [16]. All chemicals used in this work were of analytical or better grade without further purification.
Preparation of Myofibrillar Protein (MP)
MP was prepared according to the method described by Xiong [17] with a slight modification. After being thawed at 4 °C for 12 h, the meat was washed three times with fourfold volumes (w/v) of 0.1 M NaCl. 50 mM Na2HPO4 buffer (pH 7.0), followed by washing with eightfold volume (w/v) of 0.1 M NaCl (pH 6.0). All procedures were carried out at 4 °C. Centrifugation was conducted at 2000g for 15 min at 4 °C, using a high-speed refrigerated centrifuge (Himac CR 22 G; Hitachi, Tokyo, Japan) between each washing step. The final MP pellet was kept in a refrigerator at 4 °C and used within 3 days. Protein concentration of MP was determined by the Biuret method described by Gornall et al. [18], using bovine serum albumin as a standard.
Preparation of Soy Protein Isolate by Phosphate-Assisted Hydrothermal Cooking
SPC flour was dispersed in deionized water at a solid–liquid ratio of 1:15 (w/v). Certain amounts of STPP were added to the SPC suspension. The mixture was then stirred at room temperature for 2 h, and the pH was maintained at 9.0 with 2 N NaOH. Then, the slurry was subjected to an HTC system (Nan-Liang Food Machine, Guangzhou, China) at 130 °C for 90 s. Steam was directly infused into the slurry to control the processing temperature. The cooked slurry was immediately cooled to 25 °C, and centrifuged at 5000g for 30 min (25 °C). The precipitate was discarded and the pH of the supernatant was adjusted to 3.5. The precipitate was collected by centrifugation (5000g, 30 min, 25 °C). It was washed twice with deionized water, suspended in a minimum amount of deionized water, and adjusted to pH 7.5 with 2 N NaOH, then dialyzed and lyophilized. The resultant SPI was denominated as SPI-0, SPI-1, SPI-3, SPI-5 and SPI-7 with the STPP addition of 0, 0.1, 0.3, 0.5 and 0.7 g/100 g SPC, respectively.
Protein content of these products was determined by Dumas method (n × 6.25, dry basis) with a Rapid N Cube analyzer (Elementar Analysensysteme, Hanau, Germany). Protein yield was calculated as
where W SPI and C SPI were the weight and protein content of lyophilized SPI obtained after HTC treatment, respectively, and W SPC and C SPC were the weight and protein content of SPC used in HTC treatment, respectively.
The phosphorus content of the protein samples was determined according to the method described by Chen et al. [19] and Enomoto et al. [20]. Protein samples were digested in perchloric acid. The phosphorus content in these digest was regarded as the total phosphorus of protein. For the inorganic phosphorus, the protein samples were dispersed in 100 g/L trichloroacetic acid. The dispersions were centrifuged at 3000g for 20 min (25 °C). The phosphorus content in the supernatant was regarded as the inorganic phosphorus. The amount of phosphorus which was bound to the soy protein was estimated by the difference between the total phosphorus and the inorganic phosphorus.
For the determination of protein solubility, protein samples were first dispersed in deionized water with a concentration of 10 g/L. The pH of the dispersions was adjusted to 2.0–10.0 with either 0.2 N HCl or 0.2 N NaOH. The samples were then centrifuged at 10,000g for 10 min (25 °C). Protein solubility was the protein content in the supernatant relative to the total dry and protein matter. Protein content was estimated according to the method described by Lowry et al. [21], using bovine serum albumin as a standard.
Gel Preparation
Prior to gelation, SPI was dispersed in deionized water with a concentration of 100 g/L. NaCl, and various amounts of SPI were added to the MP pellet. If necessary, STPP was also added. The pH value was adjusted to 7.0 for each sample. The mixtures were stirred at room temperature for 2 h. For the gelation step, an MTGase concentration of 20 U/g of protein was used. Then, the mixtures were incubated at 45 °C in a water bath for 4 h. The protein in the gels reached a final concentration of 60 g/L. The concentration of NaCl was 500 mmol/L.
Dynamic Rheological Measurements
The dynamic rheological analysis was conducted using a HAAKE RheoStress 600 rheometer (Thermo Electron, Karlsruhe, Germany) equipped with parallel plate geometry (plate sensor PP 35 Ti, 35 mm diameter, 1 mm gap) and a circulating system for temperature control. Time-sweep analysis at a frequency of 1 Hz and a strain of 0.5 %, which was used to monitor the gelation kinetics of MP and SPI, was performed. The measurements were conducted within the linear viscoelastic range. A cover for solvent trap was used to prevent dehydrating during analysis.
Mechanical Strength Measurements
Uniaxial compression analysis was used for the measurement of gel mechanical properties. After consolidation at 4 °C for 12 h, gel samples were cut into cubes with an edge length of 10 mm. The samples were subjected to the measurements using a TA.TX2 texture analyzer (Stable Micro System, Surrey, UK) with a cylinder measuring probe (P/20a) which had a diameter of 20.0 mm. The measurement of the recoverable energy was conducted according to the method described by Çakır et al. [22] with a few modifications. The gel samples were compressed at a constant probe speed of 1.0 mm/s to 30 % of their initial height and removed at the same speed. With this compression–decompression cycle, a force–deformation curve was recorded. The recoverable energy was calculated as the ratio of the area under the curve during decompression to the area under the curve during compression. In order to avoid the fracture of the gel sample, 30 % deformation was used.
The gels were compressed at a constant speed of 0.2 mm/s to 80 % of their original height at room temperature so as to obtain the fracture pattern of the gel samples. The Hencky strain (ε h) and stress (σ) were calculated according to Eqs. (2) and (3), respectively [23].
where h 0 and A 0 are the original height and area of the test gel, respectivel, and h(t) and F(t) are the height and force of the gel at time t.
The strain (ε f) and stress (σ f) at fracture were obtained from the maximum point of the stress–strain curve. The Young’s modulus (E) was obtained from the slope of the initial linear region in the stress–strain curve by applying linear regression (up to 5 % strain).
Water-Holding Capacity
Water-holding capacity (WHC) of the gel samples was determined according to the method described by Wu et al. [24] with a few modifications. Gel samples were centrifuged at 3000g for 5 min (25 °C). The water that was released from the gels was carefully removed. The weights of the tubes with the gel samples before and after the centrifugation were accurately recorded. WHC was calculated as
where W gel was the weight of water in the gel before centrifugation, and W release was the weight of moisture lost from the gel during centrifugation.
Scanning Electron Microscopy (SEM) Observations
The gels were immersed in liquid nitrogen for 5 min and then freeze-dried. Lyophilized samples were sliced and attached to the SEM aluminum sample plate through the double-sided conductive carbon tabs. Samples were coated with gold in an E-1010 ion sputter (Hitachi High-Technologies, Tokyo, Japan). SEM observation was performed using a TM-3000 tabletop microscope (Hitachi High-Technologies) at an accelerating voltage of 15 kV.
Statistical Analysis
All measurements were conducted at least in triplicate. An analysis of variance (ANOVA) of the data was performed, and a least significant difference (LSD) with a confidence interval of 95 % was used to compare the means.
Results and Discussion
Preparation of Soy Protein Isolate
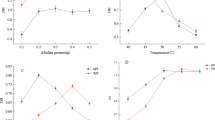
Phosphate-assisted HTC was used to refunctionalize SPC, and the resultant SPI with different characteristics was prepared by different additions of STPP. As shown in Table 1, SPI with higher protein yield and phosphorus content was prepared when more STPP was added. Protein content of the SPI with the addition of STPP was around 90 %, which was higher than the one without STPP. Figure 1 indicated the solubility profiles of SPC and SPI. The solubility of the untreated SPC did not exceed 9 % over the pH range of 2–10. After HTC treatment at pH 9.0, the solubility of the SPI was considerably improved, which was in agreement with the previous reports [13, 14]. It was further increased when more STPP was used during preparation. Compared with SPC and SPI-0, the isoelectric point (minimum solubility) of the SPI containing phosphorus shifted from pH 4.0 to 3.5.
Water acts as the heat- and mass-transferring medium in HTC treatment. During HTC, it is under pressure at a temperature higher than the usual boiling point, which is regarded as the “superheated” state. As the driving forces for the formation of the insoluble protein aggregates such as hydrogen bonds are disrupted in this circumstance [25], disaggregation and unfolding of the protein molecules might occur. After the pressure of the water is released and the slurry is cooled, protein molecules tend to refold and reaggregate. Since soy protein is treated in alkaline dispersion which makes it away from its isoelectric point, the extent of reaggregation is restricted in this case. Therefore, more protein could be extracted from SPC [26]. As STPP was used in refunctionalizing SPC, protein yield, protein content and solubility were further increased (Fig. 1; Table 1). In particular, protein yield was increased from 48.3 % (SPI-0) to over 70 % when the addition of STPP was more than 0.5 g/100 g SPC. It is worth noting that the phosphorus content of the SPI increased with the increase of STPP addition (Table 1). Since no phosphate was added during preparation, phosphorus in SPI-0 was not detected. This indicated that the phosphorus in the SPI came from STPP rather than SPC. As the procedure of dialysis was conducted after HTC, little STPP was left in the obtained SPI. This implied that some phosphate groups might be bound to the protein molecules. These charge groups provided additional electrostatic repulsion to the protein molecules, and the aggregation of soy protein that occurred after HTC was effectively suppressed. Consequently, the protein extractability and solubility were further improved.
Fabrication of Myofibrillar Protein/Soy Protein Isolate Gel
Untreated SPC, SPI-0 and SPI-5 were used as ingredients to replace part of MP, and the mixed gels were fabricated with the help of MTGase. The ratio of MP:SPI was 60:40 in this section. In addition, STPP was a commonly used additive for improving the gelation properties of meat products [15]. The effects of the free STPP and the phosphorus groups bound to the soy protein molecules on the gelation characteristics of MP also needed to be examined. Therefore, two gels (MP/SPC and MP/SPI-0) were prepared with additional STPP. The phosphorus content of these two gels was in agreement with that of the MP/SPI-5 gel.
Time-sweep rheological profiles of the MP/SPI mixtures are shown in Fig. 2. For samples containing only MP, the storage modulus (G′) and lost modulus (G″) both increased as the reaction induced by MTGase proceeded. Gelation of this sample occurred rapidly, and the gel network was gradually formed. As SPC or SPI was added to substitute for part of the MP, the moduli of the samples shared a similar trend with those of the MP sample. And G′ of these samples, which was related to the firmness of the gel, was significantly higher than that of the MP sample. This indicated that partial substitution of SPI for MP improved the firmness of the resulted gels. Although G′ of SPI-5 kept increasing during the measurement, it remained lower than 10 Pa at the end of the observation. This implied that the gelation of SPI-5 induced by MTGase did not occur during the measurement. Soy proteins ought to be suitable substrates for MTGase [27]. However, the phosphorus groups bound to SPI-5 made it difficult for the protein molecules to get close to each other. Fewer covalent linkages among the protein molecules were formed, leading to the failure in the formation of the protein network. The formation of a network containing only SPI-5 was not detected, but the MP/SPI-5 mixture had the highest value of G′ among all the samples at the end of the measurement.
The stress–strain curves of the gel samples obtained from the uniaxial compression are shown in Fig. 3, and the mechanical parameters calculated from the curves are listed in Table 2. The four parameters of fracture strain, fracture stress, Young’s modulus and recoverable energy were associated with the deformation capacity, toughness, firmness and resilience of the gel, respectively. As shown in Table 2, the parameters shared a similar trend. This suggested that the gel firmness was consistent with gel deformation capacity, gel toughness and gel resilience. All the parameters of the MP gel were lower than those of other gel samples. This gel sample was easy to fracture, and lacked resilience. Since part of MP was substituted by SPC or SPI, the gel properties were significantly improved. The four parameters of the MP/SPI-0 gel were all apparently higher than the MP/SPC gel. And the MP/SPI-5 gel possessed the highest parameters among all the gels. The parameters of the MP/SPC and MP/SPI-0 gels could be slightly increased by STPP which was added during the preparation of the gels, but they did not exceed those of the MP/SPI-5 gel.
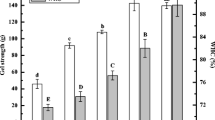
In addition to texture, water-holding capacity (WHC) is also regarded as an important quality characteristic of meat products. The WHC of the MP/SPI gels are shown in Fig. 4. As expected, the WHC of the MP gel (25.3 %) was considerably lower than that of other gel samples. When MP was partial substituted by SPC and SPI-0, the WHC of the form gels was significantly increased. A greater increase of the WHC was observed in the gel with SPI-0 which had better solubility than SPC. The addition of STPP during gel preparation further increased the WHC of the gel with SPC, but this effect was not observed in the gel with SPI-0. WHC of the gel with SPI-5 (69.5 %) was apparently higher than that of other gels.
Water-holding capacity of MP and MP/soy protein gels. SPI-0 and SPI-5 correspond to the SPI with the STPP addition of 0 and 0.5 g/100 g SPC, respectively. The ratio of MP and soy protein was 60:40. Different lowercase letters indicate significant differences at the p < 0.05 level among all MP and MP/soy protein gels
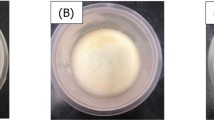
As part of the MP was replaced by SPC or SPI, the parameters of the resulted gels including mechanical properties and WHC were increased to different extents. The MP/SPI-5 gel possessed the highest parameters among these gels. This indicated that SPI-5 prepared by phosphate-assisted HTC was more capable of improving the gelation properties of MP than SPC and SPI-0. And the phosphorous groups bound to the soy protein molecules were more effective than the phosphate added during gel preparation in improving the gel properties. The characteristics of the gel were related to its microstructure. SEM images of the MP/SPI gels were presented in Fig. 5. The improvement of the gel properties was due to the modification that SPI brought to the MP gel network. Fibrous network was found in the gel that consisted of only MP. The strands that constructed the gel network were thin, and holes were not observed. This structure was not favorable for retaining water in the gel [28]. The microstructure of the gels that contained SPC, SPI-0 and SPI-5 showed another case. Soy protein adhered to the MP gel network, resulting in the appearance of thicker strands and the formation of holes. This structure improved the mechanical attributes of the gels. On the other hand, the interaction between soy protein and water was stronger when compared with to the interaction between MP and water [29]. Due to this interaction and the modified gel structure, more water could be retained in the mixed gels. As shown in Fig. 5, thick strands and relatively homogeneous holes were found in the microstructure of the MP/SPI-5 gel. This ordered structure which was assembled with the help of the phosphorous groups gave the gel better gelation properties.
Effect of MP/SPI Ratio on the Gelation Properties of MP
SPI-5 was used for substitution of MP with different percentages, so as to investigate the effect of MP/SPI ratio on the properties of the mixed gel. The mechanical properties and microstructure of the resulted gels are shown in Table 3 and Fig. 6 (the corresponding parameters and image of the gel with a MP:SPI-5 ratio of 60:40 are shown in Table 2 and Fig. 5). As more MP was replaced by SPI-5, all four mechanical parameters of the gels first increased and then decreased. The fracture stress, Young’s modulus and recoverable energy of the mixed gel with a MP/SPI-5 ratio of 60:40 reached the maximum values. And the fracture strain of the gel with the ratio of 70:30 was higher than that of other gels. Further increase in the proportion of the SPI-5 resulted in the decrease of the four parameters. SPI-5 changed the microstructure morphology of MP gel. When more SPI-5 was used to prepare the mixed gel, the strands of the network became denser and more homogeneous holes appeared. This brought about the enhancement of the gel strength. The gel network containing only SPI-5 could not be obtained by the cross-linking reaction induced by MTGase (Fig. 2). As more MP was replaced by SPI-5, the skeleton of the gel network constructed by MP was weakened. Therefore, the decline of the mechanical parameters was observed as excess SPI-5 was used to prepare the mixed gel.
Effect of SPI with Various Phosphorus Contents on the Gelation Properties of MP
SPI with various phosphorus contents, which was prepared by adding different amounts of STPP, was employed to fabricate MP/SPI mixed gel. The ratio of MP:SPI was 60:40. The mechanical properties and SEM images of these gels are presented in Table 4 and Fig. 7 (the corresponding parameters and image of the gel with SPI-5 are shown in Table 2 and Fig. 5). The mechanical parameters of the formed gels increased with the increase of the phosphorus content in the SPI. The fracture strain, fracture stress and recoverable energy of the gel with SPI-7 were higher than those of other gels. Compared with other gel samples, smaller holes, denser and more ordered network was detected in this gel. This structure greatly enhanced the strength of the mixed gel. It suggested that the deformation capacity and toughness of the mixed gel could be improved by adding the SPI with higher phosphorus content. The gel with SPI-5 had the highest Young’s modulus among the gels. Further increase of the phosphorus content in SPI resulted in the improvement of the gel toughness rather than the firmness.
Conclusions
STPP-assisted HTC treatment was employed to process SPC into an effective functional ingredient for improving the gelation properties of MP gel. The addition of STPP dramatically increased the efficiency of HTC and phosphorus content of the resulting SPI. This SPI was incorporated into the dispersion of MP with MTGase to form gels. Although this SPI could not form a gel with the help of MTGase, it was able to modify the gel network of MP. As part of MP was replaced by this SPI, a denser and more ordered structure was observed in the resultant mixed gel. This led to significant improvements in the mechanical properties and WHC of the gel. SPI prepared by phosphate-assisted HTC can be used as a highly functional ingredient to improve the gelation properties of MP.
References
Sun XD, Holley RA (2011) Factors influencing gel formation by myofibrillar proteins in muscle foods. Compr Rev Food Sci Food Saf 10:33–51
Hong GP, Chin KB (2010) Effects of microbial transglutaminase and sodium alginate on cold-set gelation of porcine myofibrillar protein with various salt levels. Food Hydrocoll 24:444–451
Ramírez-Suárez JC, Barrera M, Morales OG, Vazquez M (2002) Effect of xanthan and locust bean gums on the gelling of myofibrillar protein. Food Hydrocoll 16:11–16
Sun XD, Arntfield SD (2012) Gelation properties of myofibrillar/pea protein mixtures induced by transglutaminase crosslinking. Food Hydrocoll 27:394–400
Day L (2013) Proteins from land plants-potential resources for human nutrition and food security. Trends Food Sci Tech 32:25–42
Wolf WJ (1975) Lipoxygenase and flavor of soybean protein products. J Agric Food Chem 23:136–141
Das AK, Anjaneyulu ASR, Gadekar YP, Singh RP, Pragati H (2008) Effect of full-fat soy paste and textured soy granules on quality and shelf-life of goat meat nuggets in frozen storage. Meat Sci 80:607–614
Feng J, Xiong YL (2002) Interaction of myofibrillar and preheated soy proteins. J Food Sci 67:2851–2856
Feng J, Xiong YL (2003) Interaction and functionality of mixed myofibrillar and enzyme-hydrolyzed soy proteins. J Food Sci 68:803–809
Liu G, Xiong YL, Butterfield DA (2000) Chemical, physical, and gel-forming properties of oxidized myofibrils and whey- and soy-protein isolates. J Food Sci 65:811–818
Jiang J, Xiong YL (2013) Extreme pH treatments enhance the structure-reinforcement role of soy protein isolate and its emulsions in pork myofibrillar protein gels in the presence of microbial transglutaminase. Meat Sci 93:469–476
Wu N, Wang L, Yang X, Yin S, Teng Z, Zheng E (2011) Comparison of flavor volatiles and some functional properties of different soy protein products. J Am Oil Chem Soc 88:1621–1631
Wang C, Johnson LA (2001) Functional properties of hydrothermally cooked soy protein product. J Am Oil Chem Soc 78:189–195
Zheng HG, Yang XQ, Tang CH, Li L, Ahmad I (2008) Preparation of soluble soybean protein aggregates (SSPA) from insoluble soybean protein concentrates (SPC) and its functional properties. Food Res Int 41:154–164
Detienne NA, Wicker L (1999) Sodium chloride and tripolyphosphate effects on physical and quality characteristics of injected pork loins. J Food Sci 64:1042–1047
Folk JE, Cole PW (1965) Structural requirements of specific substrates for guinea pig liver transglutaminase. J Biol Chem 240:2951–2960
Xiong YL (1993) A comparison of the rheological characteristics of different fractions of chicken myofibrillar proteins. J Food Biochem 16:217–227
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177:751–766
Chen PS, Toribara TY Jr, Warner H (1956) Microdetermination of phosphorus. Anal Chem 28:1756–1758
Enomoto H, Li CP, Morizane K, Ibrahim HR, Sugimoto Y, Ohki S, Ohtomo H, Aoki T (2007) Glycation and phosphorylation of β-lactoglobulin by dry-heating: effect on protein structure and some properties. J Agric Food Chem 55:2392–2398
Lowry OH, Rosebrough NJ, Farr AL, Randall OJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Çakır E, Daubert CR, Drake MA, Vinyard CJ, Essick G, Foegeding EA (2012) The effect of microstructure on the sensory perception and textural characteristics of whey protein/κ-carrageenan mixed gels. Food Hydrocoll 26:33–43
Truong VD, Daubert CR (2000) Comparative study of large strain methods for assessing failure characteristics of selected food gels. J Texture Stud 31:335–353
Wu M, Xiong YL, Chen J, Tang X, Zhou G (2009) Rheological and microstructural properties of porcine myofibrillar protein-lipid emulsion composite gels. J Food Sci 74:207–217
Rastogi S, Terry AE, Vinken E (2004) Dissolution of hydrogen-bonded polymers in water: a study of nylon-4,6. Macromolecules 37:8825–8828
Wang H, Wang T, Johnson LA (2006) Mechanism for refunctionalizing heat-denatured soy protein by alkaline hydrothermal cooking. J Am Oil Chem Soc 83:39–45
Guo J, Zhang Y, Yang XQ (2012) A novel enzyme cross-linked gelation method for preparing food globular protein-based transparent hydrogel. Food Hydrocolloid 26:277–285
Sun J, Li X, Xu X, Zhou G (2011) Influence of various levels of flaxseed gum addition on the water-holding capacities of heat-induced porcine myofibrillar protein. J Food Sci 76:C472–C478
Pietrasik Z, Jarmoluk A, Shand PJ (2003) Effect of non-meat proteins on hydration and textural properties of pork meat gels enhanced with microbial transglutaminase. LWT-Food Sci Technol 40:915–920
Acknowledgments
This work was supported by the China Postdoctoral Science Foundation (No. 2013M530367), the National Natural Science Fund of China (Nos. 31371744 and 31130042) and the Project of National Key Technology Research and Development Program for the 12th Five-year Plan (No. 2012BAD34B04).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Guo, J., Hu, L., Yang, XQ. et al. Influence of Soy Protein Isolate Prepared by Phosphate-Assisted Hydrothermal Cooking on the Gelation of Myofibrillar Protein. J Am Oil Chem Soc 92, 523–531 (2015). https://doi.org/10.1007/s11746-015-2617-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-015-2617-4