Abstract
Transglutaminase (TGase) of microbial origin catalyzes the transamide reaction with a glutamine residue in proteins, which correlates with the surimi setting (suwari) process and plays a vital role in manufacturing fish surimi products. This study investigates the effect of TGase-assisted agglutination at different incubation temperatures (20, 30, 40, and 50 °C) in setting the stage to characterize its microstructural influence on agglutination strength and tilapia surimi. Results showed that the preparation of combined heat and TGase treatments significantly increased the hardness, elasticity, gel strength, and water-holding capacity of tilapia surimi gels (P < 0.05). G′ and G″ values were increased, whereas Tan δ was decreased, with significant differences among conditions (P < 0.05). In addition, SDS–polyacrylamide gel electrophoresis showed that myosin heavy chain (MHC) was cross-linked into aggregated macromolecules by TGase. In general, the good performance of tilapia surimi was obtained by heating with minimum addition TGase (0.147 U/g surimi) at 40–50 °C for 1 h. Hence, this study could provide a theoretical basis for developing surimi products with good structure, quality, and high nutritional value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tilapia Oreochromis mossambicus is native to Africa with over 100 species of tropical fish, including subspecies (Magbanua and Ragaza 2022). It is the third largest freshwater cultured species worldwide. In addition, tilapia is produced in more than 170 countries (Debnath et al. 2022); it has delicious meat, few spines, no small intermuscular spines, and high protein content, making it suitable for fillets. It is a species recommended by the Food and Agriculture Organization of the United Nations for sustainability (Zhang et al. 2023). Recently, it has emerged as a hot spot for breeding, processing, and export. Thus, the traditional processing of tilapia products is monotonous. In addition, the depletion of marine resources by overfishing and the fluctuating effects of climate change have become pronounced, which results in the increasing scarcity of fish species suitable for surimi production. However, these risks and challenges indicate unmanageable revenue for enterprises involved. To our knowledge, high-quality surimi is typically produced only from lean white meat fish such as Alaska cod; however, considerable effort has been exerted on making good surrey from the flesh of other fish species (Kristinsson et al. 2007; Xiong 2018).
Surimi products are important in the marine processed-food industry. Therefore, research on the mechanism of surimi gelation (including process improvement and quality enhancement) has been conducted, and consumers increasingly prefer its beneficial properties (Fang et al. 2021). Consequently, the superior texture (e.g., softness and chewiness) and water holding capacity (WHC) of surimi products are considered fundamental characteristics (Picard et al. 2017). The muscle proteins in fish form a compact three-dimensional gel network structure that contributes to the above-mentioned characteristics. The thermal gelation of fish protein is the most crucial phenomenon in processing surimi products. In general, the heat treatment of surimi is divided into one-step heating and two-step heating. One-step heating naturally heats directly at a high-temperature (90 °C) or boiling water, whereas two-step heating heats at a low-temperature (40–50 °C) for some time and then imposes high-temperature treatment such as one-step heating. By contrast, two-step heating has an enhanced effect on gelatin formation, known as setting or suwari (Kristinsson et al. 2007; Benjakul and Visessanguan 2003). Watabe et al. (2020) have been reporting the production of surimi by Japanese codling in 0.5 M NaCl with a two-step process of heating which produced thermal gels with a breaking strength of more than 10N. The occurrence of setting may be associated with endogenous transglutaminase (TGase) in fish, which induces the formation of ε-(γ-glutamyl)-lysine covalent bond (GL bond) in fish proteins to strengthen the structure of surimi gel (Wang et al. 2022c; Cao et al. 2019; Kumazawa et al. 1993; Ebitani et al. 2015). It is widely agreed that the freshness and species of the fish affect the quality of the surimi (Wang et al. 2022a; Nozawa and Ezou 2009), which has been reported that endogenous TGase remains significantly unstable in vivo (10.7–2.6) and in vitro (27.3–4.1 U) (Ebitani et al. 2015). However, TGase has been widely used in the surimi industry because the cross-linking of GL bonds in food facilitates protein polymerization and changes in protein conformation and three-dimensional structure, thereby affecting the functional and nutritional properties (Fang et al. 2021; Wang et al. 2022a). In addition, the conditions of the additives cost and the tilapia surimi setting (suwari) have yet to be thoroughly investigated and optimized. Thus, this study aimed to investigate the effect with a minimum quantity of TGase-assisted agglutination at different incubation temperatures (20, 30, 40, and 50 °C) in setting the stage to characterize its microstructural influence on agglutination strength and tilapia surimi. It also aimed to understand the implication of a minimum quantity of TGase-induced gelation to improve low-temperature gelling of freshwater surimi.
Materials and methods
Materials
Freshwater cultured tilapia Oreochromis mossambicus was purchased from a local market (Taichung, Taiwan). The specifications for each fish were as follows: weight of approximately 500–600 g, length of 25–32 cm, and width of 11–15 cm. The sample was packed in PVC boxes with an ice-to-water ratio of 1:2 (w/w) and transported to the laboratory within 1 h. TGase (44.25 U/g) was purchased from Ajinomoto Co., Inc. (Chūō, Japan). The chemicals and reagents used in this study were purchased from Merck (Merck KGaA, Burlington, MA, USA) and of analytical grades unless otherwise specified. Furthermore, they were used as received without further purification.
Preparation of surimi gel
The surimi was prepared according to Long et al. (2022) and Lou et al. (2005) descriptions with slight modifications. The fish were washed with tap water; the ordinary muscle was cut using a blade and ground using a 4-mm meat grinder. Next, minced fish (moisture content up to 80%) was mixed for 1 min using a mixing and kneading machine, followed by 2.5% NaCl salt for 3 min. Finally, TGase (0–0.295 U/g of surimi) was mixed for 5 min to obtain surimi. During operation, the room temperature was below 18 °C, whereas the temperature of surimi mixing was kept below 10 °C. The following physicochemical properties were determined and compared by incubation at different temperatures (20–50 °C) for 1 h to allow the TGase-catalyzed reaction.
Measurement of TGase activity
The TGase activity was determined using the colorimetric hydroxamate method described by Zhong et al. (2019) with some modifications. In this study, 700 μl of 0.1 M Tris–acetate buffer (pH 6.0), 50 μl of 2 M hydroxylamine, 150 μl of 0.1 M N-α-carbobenzoxy-glutaminyl-glycine, and 100 μl of enzyme solution were collected and mixed. The reaction was incubated in a 37 °C water bath for 10 min. Finally, 1 ml of 15% trichloroacetic acid- 5% FeCl3 solution was added to abort the reaction. The above-mentioned solutions were centrifuged at 4 °C for 5 min at 3000 × g using a centrifuge (SCR 20B, Hitachi, Ltd., Tokyo, Japan), and absorbance at a wavelength of 525 nm was determined by spectrophotometry (U-2000, Hitachi). The amount of hydroxamate formed (μmole) was calculated using the standard curve of the equation obtained for l-glutamine acid- and γ-mono hydroxamic acid (0.5–5 μmole/ml). The formation of 1 μmole hydroxamate per min indicated 1 unit of enzymatic activity.
Color analysis
Color analysis was performed by the method of Huang et al. (2022a) with modifications. L, a, and b values of surimi colors were measured using a colorimeter (Σ80, Nippon Denshoku Co., Ltd., Osaka, Japan). The correction was performed using a standard whiteboard (Y = 94.01, X = 92.02, Z = 110.59), and the measurement was performed by reflection, with six points randomly measured for each sample. The white index (WI) was calculated as follows:
where L indicates brightness, a indicates redness/greenness, b indicates yellowness/blueness.
Determining the surimi gel properties
The gel strength of surimi was determined by the protocols of Hou et al. (2022) and Watabe et al. (2020) with slight modifications. In brief, the surimi samples were cut into cubes of 15 mm3 size and then measured by using a Sun Rheo meter (Model CR-200D, Sun Scientific Co., Ltd., Tokyo, Japan) at room temperature (approximately 25 °C) concerning breaking force (N), breaking strain (mm), and gelatin strength (breaking force × breaking strain; g × mm) as indices of textural properties were calculated by Rheo data analyzer pro program. The measurement condition was MODEL1; the adapter used a 5-mm diameter spherical plunger; the elevated speed of the carrier was 150 mm/min, whereas the compression rate was 75% of the sample.
Rheological analysis
The measurements were performed by Haake Rotavisco (Haake Mess Technik GmbHu Co., Karlsruhe, Germany) using the method of Huang et al. (2022b). G′, G″, and Tan δ values of the samples were measured at room temperature (approximately 25 °C) at the forced oscillation mode. The measured conditions were as follows: using a flat plate (PP35 Ti), gap of 2 mm, shear stress of 80 Pa, and a scanning frequency range of 0.1–100 Hz.
Determination of protein solubility
The solubility of enzyme-treated surimi was determined using the method described in Zhou et al. (2019) with minor modifications. 0.8 g of sample in a 15-ml solution of a sample containing 20 mM of Tris–HCl, pH 8.0, 1% (w/v) SDS, 8 M of urea, and 2% β-ME was collected and heated at 100 °C for 5 min while stirring at room temperature for 24 h. Next, the supernatant was centrifuged at 5000 × g for 30 min, and the TCA concentration was modified to 10% by adding 50% TCA to the supernatant and then centrifuged again under the same conditions. The precipitate was collected, washed once with 10% TCA, and centrifuged. The final precipitate was dissolved in 10 ml of 0.5 M NaOH. The BIO-RAD protein assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA) measured the protein concentration using bovine serum albumin as a standard (Wu et al. 2007).
WHC
WHC was determined using the method of Liu et al. (2021). The sample was weighed, wrapped with three Whatman No. 2 filter papers (Membrane Solutions, LLC. Auburn, WA, USA), placed in a centrifuge tube, and centrifuged at 3000 × g for 20 min. Then, the following equation absorbed extra water by weighing and calculating the WHC.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
The method was performed as described by Bradford (1976). The sample was dissolved in 15 ml of 20 mM Tris–HCl under the following conditions: pH 8.0, 1% (w/v) SDS, 8 M of urea, and 2% β-ME. In addition, four folds sample buffer (0.25 M Tris–HCl, pH 6.8, 10% glycerol, 2% SDS, 5% β-ME, and 0.125% bromophenol blue tracking dye) was used for SDS-PAGE protein electrophoresis analysis. The analysis was performed with the preparation of 10% separating gel (0.375 M Tris, pH 8.8) and 3% stacking gel (0.125 M Tris- pH 6.8), while loading a 20-µl sample per lane. Electrophoretic conditions were as follows 30 M (~ 100 V) for 2–3 h at room temperature (approximately 25 °C). Next, the gels were stained with protein (0.1% Coomassie blue, 40% methanol, 10% acetic acid, 50% deionized water) for 30 min and decolorized (40% methanol, 10% acetic acid, 50% water deionized water) for 1–3 h. Finally, the gels were dehydrated and preserved with a sandwich method.
Observation by scanning electron microscopy (SEM)
SEM was performed as described by Li et al. (2022a). The samples were freeze-dried and coated with gold under a vacuum (ion sputter coater, JBS-ES 150 model, Topon Co., Ltd., Tokyo, Japan) for 90 s. The cross-sectional crosslinking network of tilapia surimi was observed at 100× with an SEM (SU 8010 Hitachi, Tokyo, Japan) and photographed and recorded.
Statistical analysis
In this study, all measurements were performed in triplicate, and the results were expressed as mean ± standard deviation. The data were analyzed by Statistical Analysis System (9.0 SAS, Cary, NC, USA), ANOVA, and Duncan’s multiple range test with a significance level of α = 0.05 to compare the differences.
Results and discussion
White index
The color of the surimi is a significant factor influencing the quality of surimi products, which have unique colors and flavors. However, the color of the surimi formed by heat treatment differs with regard to fish species, rinsing conditions, additives, freshness, temperature, and heat treatment time (Reppond et al. 1995; Chen et al. 1997; Shie and Park 1999; Suvanich et al. 2000). In general, a whiter color of surimi products not only enhances consumers’ preference for the appearance of the products but also promotes the widespread use of surimi in the processing of different products, such as coloration. The changes in WI of tilapia surimi treated with TGase (0–0.295 U/g of surimi) at 20–50 °C are shown in Fig. 1, which showed that surimi treated with different concentrations of TGase had no significant effect on the WI. In addition, the WI tends to increase with the increase of temperature, as the values from high to low range from 50 °C (40.0–41.5), 40 °C (36.0–36.5), 30 °C (30–31.8), and 20 °C (29.3–31.5). In this case, it is attributed to the increased turbidity of chyme by thermal denaturation of TGase (Techaratanakrai et al. 2012). At the same time, it catalyzes the cross-linking reaction of myosin to form intra- and intermolecular covalent bonds (Matsuoka et al. 2013). In addition, significant differences were observed (P < 0.05). Notably, no significant differences were found among different doses of TGase at the same temperature. TGase had no significant effect on the chromatic properties (L, a, and b values) of the chicken doner kebab (Kilic 2003). Tseng et al. (2000) observed the effect of TGase on low-salt chicken meatballs and showed that TGase had no significant effect on color. All of the above findings are consistent with the results of this study.
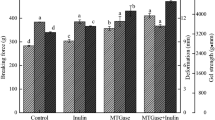
Effects of TGase-treated surimi on the breaking force, breaking strain, and gel strength
In general, the breaking force, breaking strain, and surimi gel strength have been used as indicators to determine surimi properties. The breaking force serves as a factor determining the gel strength, which provides the maximum strength for the breakdown of the tissue (expressed as “force”), also known as the breaking point of the tissue (Hamann and Webb 1979). Figure 2A shows the TGase treatment's effect on tilapia surimi's breaking force in this study. The breaking force tended to increase with TGase concentration, which showed that TGase promoted the increase of surimi hardness. In particular, the breaking force increases significantly when treated at 40–50 °C. However, at 50 °C, the TGase addition was approximately 0.074 U/g of surimi. In addition, the breaking force had reached the maximum value, and increased TGase concentration could not significantly increase the breaking strength of surimi. Moreover, the effect of temperature on breaking force at various temperatures without TGase is shown as follows: 20 °C = 30 °C < 40 °C < 50 °C. Thus, a temperature treatment over 40 °C has caused changes in surimi. Treatment at 50 °C was significantly higher than the others, which was primarily due to structural changes in proteins, as a positive correlation was found between the degree of exposure to the hydrophobicity of proteins and the hardness of gel formation (Wicker et al. 1986), where the most critical protein in fish was myosin. Furthermore, tilapia myosin denaturation was found to occur at approximately 48 °C by DSC analysis (Ko et al. 2004). Therefore, the denaturation of myosin occurs slowly at 50 °C, which exposes the hydrophobic and hydrogen sulfide groups, followed by inter- and intra-molecular formation of hydrophobic, hydrogen, and disulfide bonds (Lee and Lanier 1995). Hence, the breaking force of the surimi was increased, and the breakdown strength of the surimi treated at 50 °C was significantly higher than that treated at 20, 30, and 40 °C. In addition, the breaking force of surimi was increased with TGase treatment. The increased breaking strength of surimi with TGase concentration indicated that enzymatic treatment induced the covalent bonding of ε-(γ-Glu)-Lys cross-links among surimi molecules (Lee et al. 1997; Kumazawa et al. 1993; Wang et al. 2022a); whereas the bond was formed by catalyzing an acyl-transfer reaction between the ε-amino group of the lysine residue and the γ-amino group of the glutamine residue (Wang et al. 2022c; Fang et al. 2019; Cao et al. 2019).
In addition, the breaking strain indicates the maximum strength required for tissue breakdown (expressed in mm). Figure 2B shows the effect of TGase treatment on the breaking strain of tilapia surimi, where a higher breaking strain value indicates higher elasticity. As the TGase concentrations increased, the breaking strain value of surimi rose. However, at 40 and 50 °C, TGase addition caused a decrease in breaking strain value because of the inhibition of the homogeneous development of the protein network structure by excessive GL bonding.
Moreover, gel strength was an index of the quality of surimi products, which was the product of breaking force and strain values. The stronger the gel strength, the better the quality of the surimi product. Figure 2C shows the change in gel strength of tilapia surimi treated with TGase. The gel strength of tilapia surimi tended to increase when treated at 20–40 °C. In addition, the gel strength was significantly lower at 20 and 30 °C than at 40 and 50 °C, whereas no increase in gel strength was observed at 40 °C with enzymatic concentrations higher than 0.044 unit/g of surimi. The gel strength of surimi treated at 50 °C showed a decreasing trend with enzyme addition higher than 0.074 U/g of surimi, which was also attributed to the decrease in the overall quality of surimi caused by the decrease in breaking stress and the inhibition of the homogeneous development of the protein network structure by excessive GL bonding. Yang et al. (2020) reported that preheating frozen longtail cod (Patagonotothen ramsayi) at 30 °C for up to 6 h did not improve the gelation efficiency of the surimi. By contrast, the addition of TGase (300 U/kg surimi) at 35 °C for 2 h increased the gelation strength from 1000 g × mm to 1800–2200 g × mm, which was similar to the trend shown in this study. However, compared with the conditions of this study, the above-mentioned incubation time was longer; the added amount of TGase was greater, and different fish species were used.
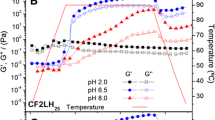
Rheological properties
The rheological property is a dynamic and non-destructive test (Wang et al. 2022c), commonly used in forced oscillatory mode to measure the sample, in which the principle involves the application of sinusoidal stress or strain to the sample and measurement of response stress or strain for obtained storage modulus (G′), loss modulus (G″), and the calculation of the phase angle difference between them to accept the loss tangent (Tan δ) as viscoelastic indicators. In addition, the change in G′ was equivalent to the evolution of hardness and elasticity, which showed the change in G′ of TGase-treated tilapia surimi (Fig. 3A). G′ showed a tendency to increase with TGase concentrations, which indicates that TGase treatment will promote the rising preference for hardening and elasticity in surimi. However, the enzymatic concentration reaches high levels, similar to the above-mentioned gel strength results, indicating deterioration. Significant differences were observed among different TGase treatments (P < 0.05). Further heating led to a sustained increase in G′, whereas gelation at 35–45 °C resulted in the aggregation of HMM (Wang et al. 2017). Moreover, as the temperature rises, the peak occurs at 35–55 °C; molecules are unfolded; reactive groups are exposed, and proteins begin to aggregate, precipitate, and cross-link (Shan et al. 2020); finally, a highly elastic gel network structure is formed (Wang et al. 2022b).
With regard to loss modulus (G″), which represents the change in viscosity, the results of this study showed that the effect of TGase on G″ of tilapia surimi also revealed the same trend, which initially increased and then decreased, indicating that TGase treatment would increase the viscosity of surimi. On the contrary, the TGase effect was remarkable, which caused a decrease in G″ (Fig. 3B). The changes in G′ and G′′ of tilapia surimi gel during heating indicated the opening and unfolding of the protein structure. However, G′′ of the sample reaches a maximum value, indicating that a sufficient number of bonds have been broken. The sample transitions from a viscoelastic solid to a viscoelastic fluid. Therefore, the liquidity of the sample has increased (Wang et al. 2019). In addition, it indirectly reflected protein denaturation, polymerization, and spatial network formation (Wang et al. 2022b). However, Wang et al. (2022c) confirmed that protein particles within the surimi gel were initially free of dispersion in continuous phases until a network structure was formed, leading to a successive movement restriction. Subsequently, the rheological value decreased, which indicated an increase in elasticity. Eventually, the particles escaped from the network with a characteristic macroscopic viscosity.
Furthermore, the loss tangent (Tan δ) was the ratio of G″ divided by G′, which was commonly used to determine the physical properties of the food system, either toward a perfectly elastomeric (tan δ = ∞) or a viscous fluid (tan δ = 0). In this study, the variation of the tan δ value of TGase in tilapia surimi (Fig. 3C) was observed; that is, increasing the concentration of TGase, may initially decrease and then increase the tan δ value. In this study, the difference between G′ and G′′ values with high concentrations of TGase showed that the gel strength was gradually increased, which was consistent with the findings of Wang et al. (2022b). However, the TGase concentration at 0.111 U/g of surimi showed the weakest performance. This concentration was closest to the elastomer, which might be related to the optimum amount of TGase bonding. Siu et al. (2002) reported the effect of TGase on the rheological properties of oat globulins, which increased the G′ and G″ values with decreasing tan δ of oat globulins. This result was consistent with the findings of this study.
WHC
The WHC of surimi products has also been identified as an essential quality indicator because poor WHC not only causes moisture loss but also leads to changes in the taste and tissue properties of the products (Yang et al. 2020; Wang et al. 2022c). In general, surimi products could retain moisture with high WHC. However, the increased water loss might be due to extensive protein aggregation caused by oxidation (Wang et al. 2022c). The effect of different concentrations of TGase on the WHC of surimi is shown in Fig. 4A. The WHC was affected by heat in the non-TGase-treated sections, where the values increased significantly at higher temperatures, from 50 °C > 40 °C > 60 °C > 30 °C, respectively, which showed a significant difference among the groups (P < 0.05). This result was attributed to the denaturation of the protein, thereby forming hydrogen, hydrophobic, and disulfide bonds and stabilizing the water encapsulated in the surimi. Nevertheless, tilapia is a species that readily degum at high temperatures. By contrast, it was less susceptible to gelation. Hence, the enzymatic delamination of surimi at 60 °C decreased WHC.
Meanwhile, TGase treatments could significantly improve the WHC of the surimi; thus, the WHC trend increased with the content of enzymes, and all the treatments showed significant differences (P < 0.05). In addition, no significant differences concerning WHC values with TGase concentrations of 0.147–0.295 U/g of surimi were observed at 30 °C and 40 °C, which were close to each other, and both showed an increasing trend. By contrast, 50 and 60 °C showed opposite results in the same conditions. In this case, this phenomenon correlates to the action of TGase, which leads to the formation of valence GL bonds in surimi. The intra-molecular and inter-molecular bridging of the structural proteins forms a network structure of surimi, effectively encapsulating water in tissues while improving the WHC of surimi. Moreover, Wang et al. (2022c) reported that treatment with TGase showed a strengthened WHC, which was attributed to the formation of a compact gel network with more interconnections induced by TGase preventing water from being extruded. Simultaneously, it has been reported that microwave treatment results in a larger area of pore space within the tissue, allowing more water molecules to be absorbed (Li et al. 2022b).
Protein solubility
The effect of tilapia surimi treated with different concentrations of TGase on the solubility of surimi protein (Fig. 4B) showed that protein solubility decreased continuously with TGase concentrations. However, Wang et al. (2022a) reported that the salt-soluble protein content of marine fish golden pompano and skipjack tuna was higher than that of freshwater tilapia. In particular, the protein solubility of 40–60 °C treatments showed a significant decrease, from 3.2 to 0.6 mg/ml, with a significant difference (P < 0.05). However, the decrease in protein solubility in the 30 °C treatment group was smaller, from 3.3 to 2.3, probably because the GL covalence bonding that formed at 30 °C was less than that formed at 40–60 °C, which led to higher protein solubility. The results were similar to the observation by Benjakul and Visessanguan (2003), which showed that protein solubilization decreased with time during setting. In general, TGase processing promotes the formation of GL covalent bonds among proteins, which tend not to be solubilized by chemical solvents, thereby decreasing protein solubility.
SDS–polyacrylamide gel electrophoresis
The changes in the protein composition of surimi were due to different concentrations of TGase (Fig. 5). All the protein components consisted of a myosin heavy chain (MHC; 205 kDa), actin (44 kDa), and myosin light chain (MLC; 20 kDa). The contents of MHC protein bands decreased with the increase of TGase (0.037–0.221 U/g of surimi), which was due to the effect of TGase on the MHC in surimi, thereby aggregating the MHC into large molecules and decreasing the MHC contents (Yang et al. 2020; Li and Xiong 2015). However, the bands above 205 kDa remained consistent, indicating that the aggregated proteins were resistant to TGase, similar to the results reported by Fang et al. (2019). Meanwhile, the polymerization of MHC occurred during cross-link formation (Wang et al. 2022a). Nevertheless, TGase actions on the MHC reduce protein concentration while promoting elevated myosin cross-linking (Yang et al. 2020), which is consistent with the results of this study. In addition, no evident decrease in MHC without TGase addition was observed, which indicates that tilapia has weak active endogenous TGase. TGase catalyzes the γ-carboxy amide group of glutamines within the protein structure with monoamine (e.g., lysine) in a 1:1 equivalent ratio for acyl-transfer reaction. Compared with other myofibrils, TGase serves preferentially on MHC to form GL covalent bonds, thereby promoting the strength of surimi gel (Fang et al. 2021; Yang et al. 2020). Many studies have pointed out that TGase acts preferentially on the rod site of MHC compared with the subfragment-1 site (Yang et al. 2020; Wang et al. 2022c; Fang et al. 2021). In addition, Wang et al. (2022c) reported that treatment with TGase revealed a significantly extensive loss of myosin rather than actin, confirming that myosin was the preferred target for TGase and actin oxidation.
SDS–polyacrylamide gel electrophoretic patterns of tilapia surimi gels were treated with various TGase concentrations (0, 0.037, 0.075, 0.147, and 0.295 U/g of surimi) at 40 °C for 1 h. The main protein bands contain a myosin heavy chain (MHC; 205 kDa), actin (44 kDa), and a myosin light chain (MLC; 20 kDa)
Scanning electron microscopic photographs
Microstructural observation by 100× SEM significantly revealed that surimi treated with TGase (0.147, 0.295 U/g of surimi) at 40 °C for 60 min had larger pores than that without TGase treatment (Fig. 6A–C). The pores between the cross-links of the TGase-treated surimi were larger and slightly loosened, which were consistent with those reported by Fang et al. (2019). In particular, the pores formed by added TGase concentration of 0.295 (U/g of surimi) were the largest. In addition, compared with heat-treated surimi gels, TGase treatment promotes the formation of more regular gel structures with more strands and clusters, which leads to larger particles in the gel (Siu et al. 2002). TGase had no evident effect on actin, an important protein in muscle, because of the absence of the TGase action substrate, which has also been found in the study of Fang et al. (2019). Compared with other meat myofibrillar protein gels, fish could observe a large porosity, a rough surface, and a loose protein network structure (Wang et al. 2022b), which was consistent with the rheological results.
Conclusions
In this study, TGase has been shown to catalyze the formation of GL covalent bonds in protein molecules, enhancing the tilapia surimi's properties, such as hardness, elasticity, gel strength, WHC, G′ and G″ values. In addition, it has no significant effect on the color of the surimi gel. Therefore, supplementing exogenous TGase in tilapia surimi may improve surimi gelation efficacy, whereas the progression of the cross-linking reaction catalyzed by exogenous TGase depends on the temperature. The best performance was achieved at 40–50 °C for 1 h when heated with TGase (0.147 U/g of surimi). The involved TGase-assisted in determining the optimum conditions for gelling during the setting period, whereas the effect of myosin molecules on gelling efficacy and microstructure was a valuable future direction for freshwater fish surimi research.
References
Benjakul S, Visessanguan W (2003) Transglutaminase-mediated setting in bigeye snapper Surimi. Food Res Int 36:253–266
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao H, Jiao X, Fan D, Huang J, Zhao J, Yan B, Zhou W, Zhang W, Ye W, Zhang H (2019) Catalytic effect of transglutaminase mediated by myofibrillar protein crosslinking under microwave irradiation. Food Chem 284:45–52
Chen HH, Chiu EM, Huang JR (1997) Color and gel-forming properties of horse mackerel (Trachurus japonicus) as related to washing conditions. J Food Sci 62:985–991
Debnath PP, Jansen MD, Delamare-Deboutteville J, Mohan CV, Dong HT, Rodkhum C (2022) Is tilapia mortality a latent concern for the aquaculture sector of Bangladesh? An epidemiology and health economic impact study. Aquaculture 560:738607
Ebitani K, Sugawara A, Konno K (2015) A quick loss of myosin cross-linking ability of Arabesque greenling dorsal muscle during its refrigerated storage. Fish Sci 81:1169–1176
Fang M, Xiong S, Hu Y, Yin T, You J (2019) In vitro pepsin digestion of silver carp (Hypophthalmichthys molitrix) surimi gels after cross-linking by Microbial Transglutaminase (MTGase). Food Hydrocolloids 95:152–160
Fang M, Luo X, Xiong S, Yin T, Hu Y, Liu R, Du H, Liu Y, You J (2021) In vitro trypsin digestion and identification of possible cross-linking sites induced by transglutaminase (TGase) of silver carp (Hypophthalmichthys molitrix) surimi gels with different degrees of cross-linking. Food Chem 364:130443
Hamann DD, Webb NB (1979) Sensory and instrumentally evaluated material properties of fish gels. J Texture Stud 10:117–130
Hou C-Y, Lin C-M, Patel AK, Dong C, Shih M-K, Hsieh C-W, Hung Y-L, Huang P-H (2022) Development of novel green methods for preparation of lead-free preserved pidan (duck egg). J Food Sci Technol 60:966–974
Huang P-H, Cheng Y-T, Chan Y-J, Lu W-C, Li P-H (2022a) Effect of heat treatment on nutritional and chromatic properties of mung bean (Vigna radiata L.). Agronomy 12:1365
Huang P-H, Chiu C-S, Lu W-C, Li P-H (2022b) Effect of compositions on physicochemical properties and rheological behavior of gelatinized adzuki-bean cake (Yokan). LWT 168:113870
Kilic B (2003) Effect of microbial transglutaminase and sodium caseinate on quality of chicken döner kebab. Meat Sci 63:417–421
Ko W-C, Hwang J-S, Jao C-L, Hsu K-C (2004) Denaturation of tilapia myosin fragments by high hydrostatic pressure. J Food Sci 69:C604–C607
Kristinsson HG, Theodore AE, Ingadottir B (2007) 7—Chemical processing methods for protein recovery from marine by-products and underutilized fish species. In: Shahidi F (ed) Maximising the value of marine by-products. Woodhead Publishing, Sawston, pp 144–168
Kumazawa Y, Seguro K, Takamura M, Motoki M (1993) Formation of ε-(γ-glutamyl) lysine cross-link in cured horse mackerel meat induced by drying. J Food Sci 58:1062–1064
Lee HG, Lanier TC (1995) The role of covalent cross-linking in the texturizing of muscle protein sols. J Muscle Foods 6:125–138
Lee HG, Lanier TC, Hamann DD, Knopp JA (1997) Transglutaminase effects on low temperature gelation of fish protein sols. J Food Sci 62:20–24
Li C, Xiong YL (2015) Disruption of secondary structure by oxidative stress alters the cross-linking pattern of myosin by microbial transglutaminase. Meat Sci 108:97–105
Li Q, Li H-T, Bai Y-P, Zhu K-R, Huang P-H (2022a) Effect of thermal treatment on the physicochemical, ultrastructural, and antioxidant characteristics of Euryale ferox seeds and flour. Foods 11:2404
Li Q, Yi S, Wang W, Xu Y, Mi H, Li X, Li J (2022b) Different thermal treatment methods and TGase addition affect gel quality and flavour characteristics of Decapterus maruadsi surimi products. Foods 11:66
Liu C, Li W, Lin B, Yi S, Ye B, Mi H, Li J, Wang J, Li X (2021) Comprehensive analysis of ozone water rinsing on the water-holding capacity of grass carp surimi gel. LWT 150:111919
Long K, Zhang T, Park JW, Park J, Yin T (2022) Effect of modified washing process on water usage, composition and gelling properties of grass carp surimi. J Sci Food Agric 102:7136–7143
Lou S-N, Chen H-H, Hsu P-Y, Chang D-H (2005) Changes in purine content of tilapia surimi products during processing. Fish Sci 71:889–895
Magbanua TO, Ragaza JA (2022) Selected dietary plant-based proteins for growth and health response of Nile tilapia Oreochromis niloticus. Aquac Fish. https://doi.org/10.1016/j.aaf.2022.04.001
Matsuoka Y, Wan J, Ushio H, Watabe S (2013) Thermal gelation properties of white croaker, walleye pollack and deepsea bonefish surimi after suwari treatment at various temperatures. Fish Sci 79:715–724
Nozawa H, Ezou M (2009) Identification of the glutamine residue that may be involved in the transglutaminase-mediated intramolecular crosslinking of carp and walleye pollack myosin. Fish Sci 75:1445–1452
Picard B, Gagaoua M, Hollung K (2017) Chapter 12—Gene and protein expression as a tool to explain/predict meat (and fish) quality. In: Purslow PP (ed) New aspects of meat quality. Woodhead Publishing, Sawston, pp 321–354
Reppond KD, Babbitt JK, Berntsen S, Tsuruta M (1995) Gel properties of surimi from pacific herring. J Food Sci 60:707–710
Shan L, Li Y, Wang Q, Wang B, Guo L, Sun J, Xiao J, Zhu Y, Zhang X, Huang M, Xu X, Yu J, Ho H, Kang D (2020) Profiles of gelling characteristics of myofibrillar proteins extracted from chicken breast: effects of temperatures and phosphates. LWT 129:109525
Shie JS, Park JW (1999) Physical characteristics of surimi seafood as affected by thermal processing conditions. J Food Sci 64:287–290
Siu N-C, Ma C-Y, Mock W-Y, Mine Y (2002) Functional properties of oat globulin modified by a calcium-independent microbial transglutaminase. J Agric Food Chem 50:2666–2672
Suvanich V, Marshall DL, Jahncke ML (2000) Microbiological and color quality changes of channel catfish frame mince during chilled and frozen storage. J Food Sci 65:151–154
Techaratanakrai B, Okazaki E, Osako K (2012) Effect of organic salts on setting gels and their corresponding acids on kamaboko gels prepared from squid Todarodes pacificus mantle muscle. Fish Sci 78:707–715
Tseng T-F, Liu D-C, Chen M-T (2000) Evaluation of transglutaminase on the quality of low-salt chicken meat-balls. Meat Sci 55:427–431
Wang X, Xiong YL, Sato H (2017) Rheological enhancement of pork myofibrillar protein-lipid emulsion composite gels via glucose oxidase oxidation/transglutaminase cross-linking pathway. J Agric Food Chem 65:8451–8458
Wang Y, Eastwood B, Yang Z, De Campo L, Knott R, Prosser C, Carpenter E, Hemar Y (2019) Rheological and structural characterization of acidified skim milks and infant formulae made from cow and goat milk. Food Hydrocolloids 96:161–170
Wang H, Pei Z, Xue C, Cao J, Shen X, Li C (2022a) Comparative study on the characterization of myofibrillar proteins from Tilapia, Golden Pompano and Skipjack Tuna. Foods 11:1705
Wang H, Yang Z, Yang H, Xue J, Li Y, Wang S, Ge L, Shen Q, Zhang M (2022b) Comparative study on the rheological properties of myofibrillar proteins from different kinds of meat. LWT 153:112458
Wang Q, Geng X, Zhao H, Yu D, Shao J, Li C (2022c) Tetrasodium pyrophosphate ameliorates oxidative damage to the TGase-catalyzed gelation of actomyosins. Food Chem 378:132128
Watabe S, Ikeda D, Mashiro T, Kagetakubo Y, Takahashi Y, Uemura M, Mizusawa N, Koyama H, Yasumoto K, Jimbo M, Kan-No N, Ueda T, Matsuoka Y, Ueki N, Wan J (2020) Suitability of Japanese codling as a raw material for surimi-based products revealed by primary sequence analysis of myosin heavy chain and thermal gel properties. Fish Sci 86:711–719
Wicker L, Lanier TC, Hamann DD, Alahane T (1986) Thermal transitions in myosin-ANS fluorescence and gel rigidity. J Food Sci 51:1540–1543
Wu M-C, Jiang C-M, Huang P-H, Wu M-Y, Wang YT (2007) Separation and utilization of pectin lyase from commercial pectic enzyme via highly methoxylated cross-linked alcohol-insoluble solid chromatography for wine methanol reduction. J Agric Food Chem 55:1557–1562
Xiong YL (2018) 5—Muscle proteins. In: Yada RY (ed) Proteins in food processing, 2nd edn. Woodhead Publishing, Sawston, pp 127–148
Yang N, Fan X, Yu W, Huang Y, Yu C, Konno K, Dong X (2020) Effects of microbial transglutaminase on gel formation of frozen-stored longtail southern cod (Patagonotothen ramsayi) mince. LWT 128:109444
Zhang D, Ayed C, Fisk ID, Liu Y (2023) Effect of cooking processes on tilapia aroma and potential umami perception. Food Sci Hum Wellness 12:35–44
Zhong M, Wang Y, Hou K, Shu S, Sun J, Guo S (2019) TGase positively regulates photosynthesis via activation of Calvin cycle enzymes in tomato. Hortic Res 6:92
Zhou X, Chen T, Lin H, Chen H, Liu J, Lyu F, Ding Y (2019) Physicochemical properties and microstructure of surimi treated with egg white modified by tea polyphenols. Food Hydrocolloids 90:82–89
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, PH., Cheng, YT., Chan, YJ. et al. Minimal addition of transglutaminase on the preparation and characteristics of tilapia (Oreochromis mossambicus) surimi. Fish Sci 89, 699–708 (2023). https://doi.org/10.1007/s12562-023-01699-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-023-01699-1