Abstract
Gas cell stability during bread making is controlled by both surface and bulk properties. This paper is focused on studying the surface properties of the water-soluble phase of the dough, the dough liquor (with and without lipids), as well as the composition of the air/water interface. Using infrared reflection measurements, we showed that in lipid-poor liquor, proteins are the dominant species present at the air/water interface. With complete liquor (including the lipids), a mixed interface of protein and lipids is obtained. However, the presence of lipids in the surface layer did not significantly affect the surface pressure. We also added enzymes to the flour to evaluate in what way the surface-active properties of the liquor components can be affected. These results were compared to the effect of adding a surfactant [diacetyl tartaric esters of mono- and diglycerides (DATEM)]. Biobake 10804, a xylanase that increased the arabinoxylan content of the dough liquor, decreased the surface pressure and increased the dilational modulus in lipid-poor liquor. This effect was not observed with the liquor including the lipids. Lipopan 50 BG, a 1,3-specific lipase, increased the surface pressure of the liquor that included the lipids. Lipopan F BG, which converts polar lipids to their lyso form, strongly increased the surface pressure not only in the lipid-containing liquor but also in the lipid-poor liquor. DATEM, as expected, increased the surface pressure while strongly decreasing the dilational modulus. Results of these studies were used to help explain changes in loaf volume observed in a series of baking tests, using the same enzymes and additives. This led to the conclusion that the effect of surface-active components alone cannot account for the larger loaf volumes observed. Clearly, both the effect of bulk and interfacial rheological properties should be considered together when explaining gas cell stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The structure of bread dough can be described as a foam in which individual gas cells are separated by a continuous gluten film. Bread-making quality is largely determined by the gas cell stability of the proofing dough. During the bread-making process, a continuous protein network is formed in which gas cells are entrapped. Gas cell nuclei are introduced in the aqueous phase during mixing and the mixing conditions will influence the bubble size distribution in the dough. Previous studies have mainly focused on the role of gluten proteins in determining the gas holding ability of dough.1 , 2 In this respect, the strain hardening properties of dough/gluten are considered relevant.1 , 2 However, some studies indicated that the contribution of a liquid film surrounding the gas cells is also relevant.3 – 7 In the latter case, formation and stabilization of gas/liquid interfaces during bread making could be a fundamental mechanism to control the gas holding capacity of the dough.
The stability of gas cells is influenced by the composition and mechanical properties of the layer formed at the air/liquid interface. Surface-active materials may be able to counteract instability mechanisms such as coalescence and disproportionation (Ostwald ripening) by stabilizing the interface. Due to compression of the surface on bubble shrinkage, the interface concentration of surface stabilizer may increase. This causes an increase in surface pressure. This change in surface pressure as a function of surface deformation may be expressed by the surface dilational modulus.8 The gas cell surface can be stabilized either by proteins or lipids/surfactants, but the stabilization mechanism is different. Proteins can form elastic networks by unfolding and reacting with their neighbors at the surface of the bubbles; thus providing mechanical strength to the layer around the gas bubble.9 Lipids and surfactants present a concentration effect—the Gibbs/Marangoni mechanism. Wheat flour proteins are generally classified into four fractions depending on their solubility according to the so-called Osborne fractionation scheme. Albumins are water-soluble, globulins are salt water-soluble, but the majority of wheat proteins, consisting of monomeric gliadins and disulfide-linked glutenin polymer aggregates have no or only a poor solubility in water. Previous studies10 showed that gliadins were the most surface-active wheat protein fraction. Although polar lipids are more efficient than proteins in reducing the interfacial tension, it is not clear to what extent lipids could compete for a position at the interface, when interface is occupied with protein. Compared to lipids, proteins have a larger contact area per molecule at the interface, and are able to develop strong intermolecular interactions. Also, bulk interactions (protein–lipid, starch–lipid) as well as bulk viscosity could prevent or delay surface-active materials such as lipids from entering the interface and thus taking part in the foaming properties of the dough.6
Other components considered relevant for dough foam stability are arabinoxylans and surfactants. Arabinoxylans are considered to stabilize protein foams by increasing the viscosity of the interlamellar liquid and by the mediation of interactions and cross-links between proteins in the adsorbed layer.9 Elofsson et al.12 showed that water-extractable nonstarch polysaccharides (NSP) (arabinoxylans and arabinogalactans) are surface-active. Also, an electrostatic interaction between NSP and gliadins was demonstrated by sequential adsorption experiments. Surfactants such as diacetyl tartaric esters of mono- and diglycerides (DATEM) are often added to a dough formulation to improve bread quality. DATEM produces bread of a larger volume and a finer crumb structure. DATEM is a surfactant that produces a high surface pressure. This would cause the air bubbles entrapped during the mixing process to break up into smaller ones.9 So far, no clear description of the role of DATEM in bread making exists, although several theories6 , 13 have described their role via protein and lipid interactions.
This study was aimed to investigate the interfacial properties of components that make up the water-soluble phase of dough, the dough liquor. How do these proteins interact and compete with other flour components (e.g., lipids and arabinoxylans) and surfactants to form (dough) foams, and how will this affect bread volume.
The effect of arabinoxylans was studied by using a xylanase. Xylanase, which cleaves the backbone of arabinoxylans, was used to hydrolyze water-insoluble arabinoxylans into water-soluble arabinoxylans, thereby increasing the arabinoxylan content in the dough liquor. The surface properties of endogenous wheat flour lipids were subsequently modified by lipase treatment. Lipases with activity toward triglycerides (1,3-specific lipases) and lipases with activity toward polar lipids (such as phospholipids and galactolipids) were used to modify the endogenous wheat flour lipids.
Addition of enzymes or DATEM during dough processing will lead to changes in the composition of the dough liquor. With drop tensiometry, we can study how this affects the surface layer properties of a gas cell in the dough liquor. Infrared (IR) spectroscopy allows us to study the composition of the air/liquor interface formed. Through this process, we intended to identify the key components determining gas cell interfacial properties and understand how these would contribute to overall gas cell stability as expressed by a higher loaf volume.
Materials and methods
Materials
Flour of two varieties, Soissons and Spring (Meneba Meel BV, Rotterdam, the Netherlands) were used. These flours have different protein strength and their composition14 is shown in Table 1.
Lipopan 50 BG and Lipopan F BG were kindly provided by Novozymes (Denmark). Lipopan 50 BG is a 1,3-specific lipase from Aspergillus oryzae, with an activity of 50,000 lipase units/g (LU/g). Lipopan F BG is a lipase that acts on polar lipids (phospholipids and galactosyl glycerides) and on triglycerides, with an activity of 25,000 LU/g. According to the manufacturer, 1 lipase unit is the amount of enzyme that releases 1 mmol titratable butyric acid/min at pH = 7.
Biobake 10804, kindly provided by Quest International Ltd., is an endoxylanase with an activity of 2500 units/g (Megazyme endoxylanase assay procedure, XYL 9/95). One unit of activity of enzyme activity is the amount of enzyme required to release 1 μmol or reducing sugar equivalents from arabinoxylan per minute under standard assay conditions (40°C and pH 4.7).
Additives
DATEM was provided by Kerry Bio-Science (the Netherlands).
Methods
Dough mixing
Dough was mixed (30°C) to optimum mixing time in a 50-g Brabender Farinograph (Brabender, Duisburg, Germany). Dough formulation included 100 g flour, 52.5/56.5 mL H2O (for Soissons and Spring flour, respectively) and 2 g NaCl. Control dough (without enzyme addition) and dough containing enzymes (with Soissons flour) were prepared. The enzymes and dosages used were: Biobake 10804 (5 mg/100 g flour), Lipopan F BG (3 mg/100 g flour), and Lipopan 50 BG (1.5 mg/100 g flour). DATEM was used at 0.3% on flour weight.
Isolation of dough liquor
Directly after mixing, dough was transferred into an ultracentrifuge tube (∼12.6 g dough/tube) and rested for 60 min at 30°C. The water-soluble phase of dough (dough liquor) was obtained by ultracentrifugation from dough at 150,000×g for 90 min at 20°C5 , 6 , 15 in a Beckman L60 Ultracentrifuge. After centrifugation, six phases can be distinguished (Figure 1): a top layer of solid lipids, dough liquor (liquid phase), gel, gluten, starch, and unseparated dough.15 Dough liquor with the top layer of solid lipids were collected together; this material is called dough liquor with lipids (DLL).
To obtain dough liquor without the top layer of solid lipids, hexane (0.5 ml) was placed on top of the sample just before ultracentrifugation. This helped us to better separate the lipid phase from the dough liquor phase after centrifugation. The hexane phase was removed by using a small suction device connected to a water pump. The liquid fraction (lipid-poor liquor) is called dough liquor (DL).
It is noteworthy that, in the presence of DATEM, the separation of where DL from the hexane containing lipids phase was impaired; therefore with DATEM only DLL was used.
Protein analysis
Protein content of DL and DLL was determined by using the DUMAS method with an NA2100 Nitrogen and Protein Analyser (CE Instruments). Methionine was used as a standard.
Electrophoresis SDS-PAGE
SDS-PAGE was performed using a Bio-Rad Mini Protean II system (Bio-Rad, Hercules, CA, USA) with 15% acrylamide gels (15×10 cm). Sample buffer consisted of 0.5 M Tris (pH 6.8), 10% SDS (w/v), glycerol, 0.05% bromophenol blue, and distilled water. Reduced and nonreduced proteins (5 μL) were run on 4% stacking gel and 15% separating gel. Electrophoresis was performed at 80 V during 15 min, and next at 200 V until the Bromophenol blue marker reached the end of the gel.
Staining of gels was performed with 5 mL PhastGel Blue R, dispersed in ethanol, in 100 mL 10% trichloroacetic acid (TCA) (w/v). Destaining was performed with 10% TCA (w/v).
Sugar content (Dubois)
Total neutral sugar content of DL and DLL was determined by using the phenol–sulfuric acid method.16
Monosaccharide composition
Monosaccharide composition was determined via high pH anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) using a Dionex Carbo-Pak PA-100 column. Glucose, fructose, arabinose, xylose, and galactose were used as standards.
Lipid content
To determine the lipid content, the isolated lipids (hexane phase) were purified. Hexane was removed by evaporation. Then lipids were freeze-dried to remove any remaining water, and weighed. Lipid content was expressed as percentage in dough (w/w).
Lipid analysis by qualitative thin-layer chromatography
Separation of lipids contained in DL and DLL was performed on high-performance thin-layer chromatography (HPTLC) silica plates (10×10 cm, Silica gel 60 F254 with concentrating zone 10×2.5 cm; Merck, Germany). DL and DLL (35–40 mg) were dissolved in chloroform/methanol/water (1 mL; 65:25:4), and an aliquot of the sample (25 μL) was loaded onto the silica plate.
Separation of polar lipids
To resolve polar lipids, the plates were developed with chloroform/methanol/water (65:25:4). l-α-Lysophosphatidylcholine (R f = 0.17–0.23), l-α-phosphatidylcholine (R f = 0.37–0.52), and digalactosyl diglyceride (DGDG) (R f = 0.71–0.76) (Larodan, Sweden) were used as a reference.
Separation of nonpolar lipids
To resolve nonpolar lipids, the plates were developed with diethylether/toluene/ethanol/acetic acid (40:50: 2:0.2). Monoolein (R f = 0.27), 1,3-diolein (R f = 0.75–0.78), and triolein (R f = 0.92–0.98) (Larodan) were used as a reference.
The separated bands were detected by using iodine vapor staining.
Circular dichroism
Far-UV circular dichroism (CD) spectra of Soissons dough liquor (protein concentration: 0.1 mg/mL in 10 mM MES, pH 5.7) was recorded at 220 nm from 20 to 90°C on a Jasco J-715 spectropolarimeter (Jasco Corp., Japan). The scanning speed was 1°C/min. Quartz cuvettes with an optical path of 1 mm were used.
Physical properties of the air/liquor interface
The air/liquor interface was studied by using an automated drop tensiometer (ADT; I.T. Concept). ADT, which is described elsewere,17 calculates the interfacial tension of a bubble by analyzing its shape. ADT in rising drop configuration was used to measure the surface tension (γ) of DL and DLL at the air/water interface. The surface tension is calculated by drop shape analysis of a bubble of air created in a cuvette containing dough liquor. The bubble is illuminated with a uniform light source, and the image is digitized by a CCD camera connected to a computer. The drop profile is processed according to the fundamental Laplace equation applied to the drop profile.17
Surface pressure–time curves
DL and DLL were diluted in 10 mM MES, pH 5.7 buffer (protein concentration 0.9 mg/mL), and surface tension was studied by using the ADT. Surface pressure (Π) was obtained as the surface tension of the buffer minus the surface tension of the dough liquor. The increase in surface pressure was followed during 3600 s after bubble formation (volume: 5 μl, area: 14 mm2).
Surface rheology
Dilational modulus (E) was obtained by dynamic oscillation of the bubble area. During the time of the experiment (6000 s), compression–expansion cycles (5) with a 3% change in area and a frequency of 0.01 s−1 were followed by a cycle where the area was kept constant. Rates of expansion relevant during dough proving have been calculated2 between 0.01 and 0.0001 s−1.
Dilational modulus is determined by measuring the change in interfacial tension due to a specific change in interfacial area (\( {\delta \gamma } \mathord{\left/ {\vphantom {{\delta \gamma } {\delta A}}} \right. \kern-\nulldelimiterspace} {\delta A}\)), whereas the shape of the surface area remains the same. Dilational modulus is a useful tool to quantify the mechanical strength of a viscoelastic protein system, because it measures the resistance to compression and expansion of the adsorbed layer. IT also provides information about the composition of the layer. The rheology of an interfacial film can provide information about the nature of the molecules from which the film was formed.6
Infrared reflection absorption spectroscopy
Infrared reflection absorption spectroscopy (IRRAS) provides a novel way to obtain information on molecular composition at the air/water interface.18 The spectral resolution makes it possible to distinguish between proteins, lipids, and carbohydrates in a surface layer. IRRAS spectra can be acquired using an Equinox 55 Fourier transform infrared (FTIR) spectrometer (Bruker, Karlsruhe, Germany) attached to the IRRAS accessory (XA500, Bruker, Ettlingen, Germany)). The latter is equipped with a broadband mercury cadmium telluride (MCT) detector (cryostatted with liquid nitrogen) to collect the specular reflected IR beam from the sample. The accessory has two movable arms with which to adjust the angle of incidence and reflection. Polarization of the incidence light can be adjusted by rotation of the polarizer.
The IRRAS accessory contains a Langmuir trough, which consists of two compartments—one for the reference or buffer solution (260 × 50 × 15 mm, volume: 200 ml) and one for the sample (260 × 100 × 15 mm, volume: 400 ml). It can be moved in horizontal (x–y) and vertical (z) directions to change between measurement of the reference or sample, to focus the light beam at the air/water interface, or to move the trough out of the light beam in order to measure a reference signal of the optics.
Spectra were acquired from 1000 to 4000 cm−1 by using s-polarized light. The angle of incidence was 30°. Data were collected at 2 cm−1 resolution, and typically 100 scans (scanning velocity 20 kHz) were averaged. Zero filling was applied prior to Fourier transformation. Reflection signals were measured relative to that of a buffer (−log I/I R). The spectrum was smoothed over 8 cm−1.
Bread making
Crispy rolls were baked at the TNO Baking Laboratory (Zeist, The Netherlands). Wheat flour (3000 g), water (1695 mL for Soissons based on farinograph water absorption, ICC 115/1, 1991), yeast (50 g), salt (70 g), and ascorbic acid (20 ppm) were blended in a mixer (Kemper SP 15, Kemper, The Netherlands). Ingredients (temperature: 5°C) except water were blended for 1 min at low speed (140 rpm). Next, water (10°C) was added and mixed at low speed for 2 min, then the dough was kneaded at high speed (280 rpm) until a final dough temperature of 26°C. After mixing, the dough was set aside for 15 min. Then it was divided and rounded. Proofing was performed at 30°C and 80% relative humidity until a fixed volume of gas produced (500 mL SJA). The breads were baked at 215°C for 25 min in a Rototherm RE oven (Haton, The Netherlands).
Control breads (from Soissons and Spring flour, no enzyme addition) were prepared as well as bread (prepared with Soissons) including single addition of enzymes on the formulation: Biobake 10804 (5 mg/100 g flour), Lipopan F BG (3 mg/100 g flour), Lipopan 50 BG (1.5 mg/100 g flour), and DATEM (0.3% in flour weight).
Bread specific volume was determined in triplicate by using rape seed displacement (standard deviation was lower than 0.15 mL/g).
Results
Effect of flour quality
Two types of flour of known different bread-making performance were used in order to observer if this different performance was reflected in the gas cell surface properties of the DL and DLL. The aqueous phase obtained by ultracentrifugation contained water-soluble proteins, nonstarch lipids, and sugars. Sugars identified were glucose, fructose, arabinose, xylose, and galactose (results not shown). The different flour characteristics were reflected in the composition of DL and DLL (Table 2). Dough liquor obtained from Spring, the flour of better bread-making quality, contained greater amounts of proteins and sugar.
Proteins in dough liquor were further characterized by SDS-PAGE (Figure 2). Both water-soluble (albumins) and salt-soluble (globulins) proteins were found in the liquor, and likely some remaining gliadins and glutenins. Qualitatively, Spring DL contained more protein than Soissons DL. This is in agreement with the larger protein content of Spring DL.
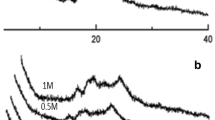
Surface activity of the DL as well as the DLL extracted from a control dough sample prepared with Soissons flour is shown in Figure 3. Soissons control showed an equilibrium surface pressure of 27.6±0.3 and 27.1±0.5 mN/m for DL and DLL, respectively. Fast adsorption kinetics was observed for both DL and DLL. The equilibrium surface pressure from Spring was comparable to that of Soissons (27.4±0.2 and 27.6±0.2 mN/m for DL and DLL, respectively). Also, the dilational modulus of DL in Spring and in Soissons were comparable, with a maximum value of 50 and 47 mN/m, respectively. However, the inclusion of lipids in DLL showed a different behavior between the two flour samples. The dilational modulus of DLL was slightly lower than that of DL, likely due to the higher proportion of lipids. A maximum value of 44 mN/m for the dilational modulus was found for both Soissons and Spring liquor with lipids, but Spring reached a larger surface pressure (29 mN/m). The lipid composition of DLL, studied via TLC (Figure 4b), showed some differences among the nonpolar lipids of DLL from Spring with respect to Soissons. Two new spots were separated, R f = 0.48 and 0.18, in lipids extracted from Spring.
TLC plates of DLL lipids. (A) Detail of the separation of polar lipids using eluent 1 (chloroform/methanol/water, 65:25:4). Line 1: DLL Soissons control; line 2: DLL Soissons Lipopan F BG; line 3: DLL Soissons Lipopan 50 BG. (B) Detail of the separation of nonpolar lipids using eluent 2 (diethylether/toluene/ethanol/acetic acid, 40:50:2:0.2). Line 1: DLL Soissons control; line 2: DLL Soissons Lipopan F BG; line 3: DLL Soissons Lipopan 50 BG; line 4: DLL Spring.
Effect of temperature
The surface activity properties of the dough liquor were studied at 30°C to simulate the proving stage. Dobraszczyk et al.2 reported a high correlation between dough bubble stability parameters and baking quality at elevated temperatures. This leads to the question whether the surface activity of liquor components changes when the temperature starts to increase at the beginning of baking.
We therefore performed a temperature sweep between 20 and 90°C, and studied protein structural changes using CD. Figure 5 demonstrates that denaturation of dough liquor proteins starts from 55°C onwards, as illustrated by the sigmoidal part of the curve (Figure 5). Based on this finding, we also studied the surface activity of the dough liquor at 50°C, just below the denaturation midpoint. At this temperature, the equilibrium surface pressure and the kinetics of surface pressure development appeared to be comparable to values observed at 30°C (27±1 mN/m) (Figure 6). This indicated that results of studies performed at 30°C are representative for proving and the initial baking stage (oven rise).
Effect of enzymes and surfactants
We modulated the surface properties of the dough liquor by including enzymes and DATEM in the dough formulation. The observed differences in surface properties were related to the modified components. The enzymes/surfactant used, lipases and DATEM, did not significantly modify the protein and sugar content, except for xylanase. Addition of xylanase decreased the protein content and increased the total neutral sugar content of DL and DLL, thereby increasing the sugar/protein ratio (Table 2).
The amount of free and bound lipids (to proteins and starch) changes during the bread-making process. Addition of lipid modifying enzymes and emulsifiers can alter this proportion. This explains why the lipid content was affected, mainly, by DATEM (Table 2). However, DATEM increased the lipid content due to the presence of DATEM itself in this phase. The lipid composition of DLL was modified by lipases (Figure 4). The different activity of Lipopan F BG and Lipopan 50 BG was clearly reflected in the observed changes in composition of polar lipids (Figure 4A) (at R f = 0.85, 0.57 and R f = 0.85, 0.79, for Lipopan F BG and Lipopan 50 BG, respectively) as well as changes in nonpolar lipids (Figure 4B). Furthermore, Lipopan F BG (Figure 4A) showed an increased intensity of the spot corresponding to lysophosphatidylcholine (R f = 0.17) and a decreased intensity of the spot corresponding to DGDG (R f = 0.74). In the case of nonpolar lipids, Lipopan 50 BG was more active showing clear increases at R f = 0.24 and 0.18. This is the result of the hydrolysis of triglycerides. The lipid composition of DL was also studied (results not shown). DL contained a low amount of polar lipids. Only DL Lipopan F BG showed a change in polar lipid composition (R f = 0.9.4, 0.87, 0.78, and 0.58). This is likely the result of hydrolysis of DGDG (R f = 0.74)/MGDG into either DGMG or MGMG. Nonpolar lipids were not detected in DL.
The effect of enzymes and DATEM on the surface activity of DL is shown in Figure 7. While xylanase (Biobake 10804) significantly decreased the surface pressure of DL to 26.6 mN/m, Lipopan 50 BG did not significantly change the surface pressure (27.6 mN/m). Lipopan F BG, on the other hand, increased the surface pressure of dough liquor to 33 mN/m. This difference in surface pressure indicated changes in composition of the surface layer. The surface pressure of DLL is also shown in Figure 7. In this case, the presence of lipids cancelled the decrease in surface pressure observed with xylanase, but lower kinetics was observed with respect to the control DLL. Lipids modified by Lipopan 50 BG increased the surface pressure (28.4 mN/m). DATEM led to a larger surface pressure (28.5 mN/m) than that of control, indicating the adsorption of DATEM (or protein–DATEM complexes) at the interface. Again, a large effect of Lipopan F BG (37 mN/m) was observed.
Network-forming properties of proteins at the interface were assessed in terms of dilational modulus. Dilational modulus is associated with adsorption/desorption of the surface-active component together with the interactions between molecules. The dilational modulus of DL with Biobake 10804 was larger than that of control (Figure 8A). Lipopan 50 BG had a dilational modulus similar to that of control, but Lipopan F BG showed a decrease in dilational modulus accompanied by a larger surface pressure. Values obtained by Lipopan F BG suggest the presence of more polar lipids or protein–lipid complexes at the interface. The dilational modulus of DLL (Figure 8B) showed the same trend with some exceptions. The decrease in surface pressure obtained with Biobake 10804 disappeared when lipids were included, and the surface pressure increased when the lipids treated with Lipopan 50 BG were included with the dough liquor (Figure 8B). DATEM increased the surface pressure and decreased the dilational modulus, indicating a more lipid-like interface.
Infrared reflection absorption spectroscopy
Surface rheological measurements indicated changes in composition of the surface layer as a result of enzyme/surfactant treatments. To test this, IRRAS spectra of the dough liquor were recorded. This allows us to measure the composition of the surface layer at the air/water interface (Figure 9). Spectra from the dough liquor show protein bands, centered around 1650 and 1550 cm−1 for the amide I (primarily protein C=O stretching) and amide II (majority protein NH bending), as well as small bands for the CH stretching region corresponding to lipids as well. These latter bands were found at 2959, 2920, and 2856 cm−1 for the CH3 stretching asymmetric, CH2 stretching asymmetric, and CH2 stretching symmetric, respectively. The presence of these small bands clearly demonstrates that besides the proteins, a small amount of lipids is also found at the interface. The CH bands should also be accompanied by a vibration band for the ester carbonyl at 1750 cm−1, but—probably because of the small contribution of lipids in the surface layer—the carbonyl band cannot be observed.
Spectra for DLL showed bands both for the CH stretching and for the proteins, but also a band for the ester carbonyl stretching at 1750 cm−1 (Figure 10). This indicates that mixed systems of protein–lipids/DATEM are present at the air/water interface. IRRAS does not allow differentiation of the nature (polarity) of the lipids present at the interface. From a qualitative analysis of the spectra,18 it can be deduced that the formed layers can be regarded as monomolecular in thickness. These components will be present both at the interface and in the bulk, but with a higher concentration in the interfacial layer.
Bread making
A bread-making test with Soissons flour showed an effect on the bread volume for all the enzymes/additive included in this study. Figure 11 shows the percentage increase in volume of breads prepared with Soissons flour compared to the control bread (Soissons). The largest increases were observed with the xylanase enzyme (Biobake 10804) and DATEM (+28% and 26%, respectively). Both lipases, Lipopan F BG and Lipopan 50 BG, increased the bread volume by ca. 16%. In general, control bread prepared from Spring flour showed a 22% larger volume compared to that of Soissons.
Discussion
The stability of the gas bubbles in dough has been related to the properties of the liquid film surrounding the gas cells.3 – 7 , 19 Scanning electron microscopy showed3 that during the baking process some bubbles grow to such an extent that they are separated from neighboring cells only by thin films. In this study, the surface activity of the soluble phase of the dough, dough liquor (lipid-free and with lipids), has been investigated and its composition modified by adding enzymes/additive in the dough formulation. Dough liquor contains surface-active compounds as proteins, polar lipids, and arabinoxylans. We attempted to study the effect of these compounds on the surface activity of the dough liquor by using enzymes.
At the surface layer surrounding the gas cells, a competition between these surface-active compounds—proteins, polar lipids and surfactants—must exist. This is the first time a spectroscopic technique, IRRAS, has been used to analyze the molecular composition of dough liquor components at an air/water interface. IRRAS showed unique results; a protein layer containing a small amount of lipids is present at the interface in the dough liquor. The control dough liquor with the lipids provided surface pressure equilibrium values comparable to those of the control dough liquor itself (Figure 3). IRRAS spectra showed a mixed system (protein–lipid) at the interface. Some displacement of proteins by lipids occurred at the interface.
The ezymes used in this study can have two effects: more material is becoming available at the liquor phase and/or the material is changed. Xylanase can hydrolyze water-insoluble arabinoxylans present in the dough, leading to the release of soluble arabinoxylans in the dough liquor. With the methods used, we could not observe a significant effect of lipases on the quantity of lipids in the dough liquor. However, clear differences in the composition were observed, leading to a higher polarity of the lipids present.
Dilational experiments were performed to test the stabilizing effects of dough liquor components. Either proteins or surfactants can stabilize the interface, but the stabilization mechanism is different. The dilational modulus of a foam with a viscoelastic protein is a measure of the mechanical strength of the interfacial layer. Typically, when a surfactant is added to the system, a decrease in dilational modulus is obtained, as shown here with DATEM (Figure 8).
Arabinoxylans are thought to stabilize protein foams,7 , 11 , 12 not because of their surface activity (Π=12 mN/m) but by the mediation of interactions and cross-links between proteins in the adsorbed layer at the interface. The xylanase used increased the sugar content of the dough liquor. This is likely attributable to the hydrolysis of insoluble wheat flour arabinoxylans (Table 2). The increased arabinoxylan content was paralleled by an increased elasticity of the dough liquor (Figure 8) under conditions resembling proving. A previous work20 reported that arabinoxylans stabilized protein foams after heating (90°C), although that condition does not seem to be relevant for this study. Xylanase (Biobake 10804) led to a high increase in the bread volume (Figure 11). This could be explained by the (small) change in surface properties and/or by a large reported effect of arabinoxylans on the bulk rheological properties of the dough.21 – 24 Which of these effects is the most important remains to be unraveled.
The involvement of lipids and lipid–protein complexes in the stability of dough foam has been previously studied.25 In wheat flour, nonpolar lipids, triglycerides, free fatty acids, and polar lipids (phospholipids and glycolipids) are found. The first three are said to be detrimental for foam stability.25 Polar lipids are considered positive.25 Lipases are useful tools to modify the polarity of lipids. In this study, two types of lipases have been studied. Lipopan 50 BG is a 1,3-specific lipase that hydrolyzes nonpolar triglycerides to yield more polar compounds. Lipopan F BG hydrolyzes the endogenous wheat polar lipids (phospholipids and galactosyl glycerides) into the corresponding more polar monoacyl lipids. This work shows clearly that they form different products (Figure 4) with very distinct surface properties (Figures 7, 8). The lipids resulting from Lipopan F BG treatment would be more polar than the lipids produced by Lipopan 50 BG. Therefore, they (or their complex with proteins) would produce a higher increase in surface pressure of the dough liquor (Figure 7). However, the effect on bread volume was the same for both enzymes. This suggests that the effect on surface properties of the dough liquor is not the only mechanism by which lipase addition leads to an increased bread volume. Interactions of lipids and gluten proteins, as modified by these enzymes, as well as effects of these enzymes on gas inclusion during mixing need to be taken into account as well. DATEM is included in bread formulations because of its beneficial effect on bread quality. So far, however, there is no consensus on the mechanism of DATEM in bread making. Interactions with gluten proteins affecting bulk properties have been described, along with effects that would affect surface properties such as the sequestering of native wheat lipids.6 , 13 Contradictory effects have been described for the effect of DATEM on surface properties. Sahi6 reported that no effect of DATEM on surface tension was observed. On the other hand, Kokelaar9 showed good surface properties of DATEM. We observed an increase in surface pressure of DLL with a decreased elasticity when DATEM was included in the formulation.
Lipid and proteins can compete for the interface, as shown in a previous work.26 It was concluded, by using lipid depleted flour and by performing lipid readdition experiments, that a nondefatted leavened dough can be described as a lipid-stabilized foam. The IRRAS data unambiguously showed that mixed protein–lipid surface layers were formed. In protein and lipid/surfactant mixed layers, either their interaction (in solution or at the interface) or the displacement of proteins by the lipids/surfactants is responsible for properties of the system.27 Direct adsorption of protein–lipid/small surfactant complexes at the air/water interface can occur for lipid-binding proteins, and the enhancement of the foaming properties is observed when both lipid-binding proteins and lipids are present in the interfacial layer.28
However, differences in bread-making performance could not be completely explained by differences in surface properties. We therefore propose that, next to surface properties, bulk rheological properties of dough are important for stabilization of the foam structure of the dough. Again, results in the literature are contradictory in this area. Kokelaar9 suggested that differences in baking performance between flour of different bread-making quality could not be attributed to surface properties. Sahi5 showed that a low bread-making quality flour produced a larger increase in surface pressure than a flour of good bread-making quality. However, the difference in gluten content/properties (changing bulk properties) between these two wheat cultivars would better explain their different performance. Depending on the rheological properties of the bulk phase, the gas cells may be stabilized against dissolution by interfacial rheological properties, bulk rheological properties, or a combination of both.8
In this study, we showed that lipases, especially Lipopan F BG, effectively change the surface activity of the dough liquor. We also demonstrated clear differences in mode of action that are related to different substrate specificities of the enzymes. With IRRAS, we demonstrated that both proteins and lipids/surfactant are present at the interface. The presence of proteins leads to a more elastic interface, whereas the presence of surfactant hardly led to the development of an elastic interface. However, these differences do not allow us to explain the differences in bread volume observed. Clearly, the effects of enzymes and surfactant on bulk rheological properties also need to be studied to obtain a full explanation of the observed effects on baking quality.
References
T. Vliet, A.M. Jansssen, A.H. Bloksma, P.Walstra, J Texture Stud 23, 439 (1992)
B.J. Dobraszczyk, J. Smewing, M. Albertini, G. Maesmans, J.D. Schofield, Cereal Chem 80, 218 (2003)
Z. Gan, R. Angold, M.R. Williams, P.R. Ellis, J.G. Vaughan, T. Galliard, J Cereal Sci 12, 15 (1990)
Z. Gan, P.R. Ellis, J.D. Shofield, J Cereal Sci 2, 215 (1995)
S.S. Sahi, J Cereal Sci 20, 119 (1994)
S.S. Sahi, J Cereal Sci 37, 205 (2003)
E.N.C. Mills, P.J. Wilde, L.J. Salt, P. Skeggs, Trans Ichem Em 81C, 189 (2003)
W. Kloek, T. van Vliet, M. Meinders, J Colloid Interf Sci 237, 158 (2001)
J.J. Kokelaar, Physics of breadmaking, Ph.D. thesis, Wageningen Agricultural University, Wageningen, The Netherlands, 1994
R.C.A. Keller, R. Orsel, R.J. Hamer, J Cereal Sci 25, 175 (1997)
D.K. Sarker, P.J. Wilde, D.C. Clark, Cereal Chem 75, 493 (1998)
U. Elofsson, A.C. Eliasson, M. Wahlgren, A.M.A. Loosveld, C.M. Courtin, J.A. Delcour, Cereal Chem 77, 679 (2000)
N.O. Carr, and P.J. Frazier, In: Wheat Structure, Biochemistry and Functionality, edited by J.D. Shofield. (Royal Society of Chemistry, London, UK 1995), p. 280
ICC International Association for Cereal Chemistry. Ash nr101/1. Protein nr. 167. Water absorption, farinograph (Brabender) nr 115/1. Energy input, alveograph (Chopin) nr 121. Starch nr 128/1. Fat nr 136.
H. Larsson, A.C. Eliasson, Cereal Chem 73, 18 (1996)
M. Dubois, K.A. Gilles, J.K. Hamilton, D.A. Rebers, F. Smith, Anal Chem 28, 350 (1956)
J. Benjamins, A. Cagna, E.H. Lucassen-Reynders, Colloids Surf A 114, 245 (1996)
M.B.J. Meinders, G.G.M. van den Bosch, H.H.J. de Jongh, Trends in Food Sci Technol 11, 218 (2000)
L. Dubreil, S. Melinole, H. Chiron, J.P. Compoint, L. Quillen, G. Branlard, D. Marion, Cereal Chem 75, 222 (1998)
M. Izydorczyk, C.G. Biliaderis, W. Bushuk, Cereal Chem 68, 145 (1991)
C. Primo-Martín, R. Valera, M.A. Martínez-Anaya, J Agric Food Chem 51, 4673 (2003)
C. Primo-Martín, M. Wang, W. Lichtendonk, J.J. Plijter, R.J. Hamer, J Sci Food Agric 85, 1186 (2005)
M.W. Wang, R.J. Hamer, T. van Vliet, G.J. Oudgenoeg, Cereal Sci 36, 25 (2002)
M.W. Wang, T. van Vliet, R.J. Hamer, J Cereal Sci 39, 395 (2004)
D. Marion, D.C. Clark, In: Wheat Structure, Biochemistry and Functionality, edited by J.D. Shofield. (Royal Society of Chemistry, London, UK 1995) p. 245
F. MacRitchie, Cereal Chem 50, 292 (1973)
M.A. Bos, T. van Vliet, Adv Colloid Interface Sci 91, 437 (2001)
P.J. Wilde, D.C. Clark, D. Marion, J Agric Food Chem 41, 1570 (1993)
Acknowledgements
The authors would like to thank Jolan de Groot for her experimental contribution. Also, Peter Wierenga is kindly acknowledged for his help with IRRAS measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Primo-Martín, C., Hamer, R.J. & de Jongh, H.H.J. Surface Layer Properties of Dough Liquor Components: Are They Key Parameters in Gas Retention in Bread Dough?. Food Biophysics 1, 83–93 (2006). https://doi.org/10.1007/s11483-006-9008-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-006-9008-1