Abstract
Purpose
The alpine meadow has received mounting attention due to its degradation resulting from overgrazing on the Tibetan Plateau. However, belowground biotic characteristics under varied grazing stresses in this ecosystem are poorly understood.
Materials and methods
Here, the responses of soil protozoan abundance, community composition, microbial biomass, and enzyme activity to five grazing patterns including (1) artificial grassland without grazing (AG), (2) winter grazing (WG), (3) grazing for 7 months within a fence (GF), (4) continuous grazing for a whole year (CG), and (5) natural heavy grazing (HG) were investigated for two continuous years. Soil protozoan community composition was investigated using the most possible number (MPN) method, and soil microbial biomass and enzyme activity were analyzed using chloroform fumigation extraction and substrate utilization methods, respectively. Multivariate statistical analysis, the analysis of variance (ANOVA), multiple comparisons, and correlation analysis were together performed.
Results and discussion
The WG treatment had the highest abundance of total protozoa (2342–2524 cell g−1). Compared with AG treatment, HG treatment significantly reduced the abundance of soil total, flagellate and ciliate protozoa, and protease activities in 2012 and 2013. Significantly, lower soil microbial biomass nitrogen (MBN) was also observed in the HG (6.60 and 14.6 mg N kg−1) than those in other four treatments (22.3–82.9 mg N kg−1) both in 2012 and 2013, whereas significantly higher microbial biomass carbon (MBC) was observed in HG than that in AG treatment in 2012. Moreover, significantly positive correlations were detected between the abundance of soil protozoa and soil moisture, pH, organic C, total N, and MBN. Our results indicated that soil protozoa showed a negative response to increasing grazing intensities and therefore, suggesting that aboveground grazing practices also exerted strong impact on belowground protozoa, not only on soil microbial characteristics.
Conclusions
Soil protozoan community composition was apparently different between the HG treatment and other four grazing patterns and was potentially impacted by altered soil properties and MBC and/or MBN. Our results suggested that moderate grazing may sustain better belowground biotic diversity and ecosystem functioning in this alpine meadow on the Tibetan Plateau.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Livestock overgrazing is one of the primary factors that elicit grassland degradation and desertification (Akiyama and Kawamura 2007; Wu et al. 2009). Aboveground and belowground ecosystems are directly or indirectly affected by livestock feeding, trampling, and feces extraction (Bardgett et al. 1998; Neilson et al. 2002; Wang et al. 2006; Qi et al. 2011; Kuijper and Bakker 2012). For example, increased herbivore trampling and feces extraction decreased soil moisture and pH but increased soil bulk density (Ekelund and Ronn 1994; Holt 1997; Li et al. 2005). Livestock herbivores have altered plant community structure and productivity by selecting for dominant plant species with low nutrient requirements and low-quality leaf litter (Steffens et al. 2008; Teague et al. 2011; Wu et al. 2012a). Long-term intensive grazing reduces plant productivity and soil organic matter, leading to grassland degradation (Qi et al. 2011; Ramos et al. 2011). In addition, high grazing intensity also alters the community of soil biota, which maintains fundamental soil functions in terrestrial ecosystem. In correspondence with the “grazing optimization hypothesis”, previous studies have demonstrated that soil microorganisms generally show significant variation among grazing intensities and have higher microbial biomass and diversity under light grazing because of improved plant litter quality and increased soil nutrient availabilities (Dyer et al. 1986; Neilson et al. 2002; Li et al. 2005; Qi et al. 2011; Stark et al. 2015).
Soil protozoa are an indispensable part of the “microbial loop” around plant roots and release nearly one-third of available N that ultimately stimulates plant growth (Bonkowski and Brandt 2002). Soil protozoa are regarded as a model bioindicator for monitoring belowground ecosystems because of their delicate cell-membranes, rapid reproduction, short generation time, and ubiquity in most ecosystems (Ekelund and Ronn 1994; Foissner 1999). Soil protozoa also have the potential to indicate the variation in different soil environments, such as tundra, moorland, and polluted soil (Nguyen-Viet et al. 2007; Cebron et al. 2011; Tsyganov et al. 2011; Turner and Swindles 2012). The abundance of soil protozoa could be improved by stimulating aboveground plant productivity and increasing C and N inputs under light or moderate grazing intensity (Hamilton and Frank 2001; Wu et al. 2012a). Soil protozoan community has been observed to be negatively affected by grazing, primarily due to soil compaction and lower soil water content (Ekelund and Ronn 1994; Holt 1997). Furthermore, soil protozoa have been shown to be more sensitive to grazing pressure than soil microbes or soil nematodes in the semiarid steppe of Inner Mongolia (Li et al. 2005; Qi et al. 2011). However, the responses of the soil protozoan community to different grazing intensities in alpine meadow ecosystem remain poorly understood.
Soil microbes play a crucial role in soil nutrient cycling and organic matter mineralization in terrestrial ecosystems, and it is common to assess microbial activity and community function under contrasting land management practices based on analyzing soil enzyme activity and soil microbial biomass (Bardgett and McAlister 1999; King and Hutchinson 2007; García-Ruiz et al. 2008; Figuerola et al. 2012; Esch et al. 2013). Given the roles in maintaining soil function and regulating soil biogeochemical processes, such as organic matter transformation, mineralization, and nutrient recycling (García-Ruiz et al. 2009; Wu et al. 2012b), soil enzymes have been used to monitor early environmental changes induced by pollution and soil disturbance (Holt 1997; Badiane et al. 2001; Su et al. 2005; Karaca et al. 2011). For grasslands, grazing is the main type of disturbance and may influence soil enzyme activity by reducing organic matter input as well as decelerating soil C, N, and P cycling (Acosta-Martínez et al. 2010; Fterich et al. 2012). Low enzyme activity is generally detected in grazed pastures rather than in non-grazed pastures because of reduced organic matter input, although there was no significant variation in some enzymes under grazing (Acosta-Martínez et al. 2010; Fterich et al. 2012). In addition, moderate grazing pressure increased enzyme activity by improving microbial biomass (Bardgett et al. 1998; Singh and Rai 2004; Xu et al. 2007; Olivera et al. 2014).
Alpine meadows are widespread at altitudes from 3500 to 5500 m and cover approximately 1.2 × 106 km2, accounting for 48% of the plateau’s land area on the Tibetan Plateau (Cao et al. 2004). This grassland ecosystem is essential in maintaining the climate and biodiversity of the alpine region for sustainable pastoralism (Chen et al. 2008; Long et al. 2008; Zhang et al. 2012; Dorji et al. 2014). However, alpine meadow degradation, mainly induced by grazing pressure (i.e., increases in livestock), is a serious issue on the Tibetan Plateau. One-third of grassland areas have been recently reported to be suffering from different levels of degradation and even from desertification (Zhou et al. 2004; Wang et al. 2007; Wu et al. 2009; Feng et al. 2010; Harris 2010; Wu et al. 2012a). Although grazing effects on alpine meadow of the Tibetan Plateau have been previously documented in terms of plant cover biomass (Sun et al. 2011) and soil organic C and N mineralization (Wu et al. 2010; Rui et al. 2011), few studies focus on the soil microfauna (i.e., soil protozoa) and microbial (i.e., biomass and enzyme activity) responses to grazing patterns. A better understanding of soil biotic functioning linked to higher aboveground productivity is essential to prevent degradation and to manage sensitive ecosystems such as the grasslands on the Tibetan Plateau in a sustainable way (Ros et al. 2006).
In this study, soil samples from grasslands undergoing five different grazing intensities [artificial grassland without herbivore grazing (AG), winter grazing (WG), grazing within a fence (GF), continuous grazing (CG), and natural heavy grazing (HG)] were collected in 2012 and 2013. Soil protozoan abundance, soil microbial biomass C (MBC) and N (MBN), and the activities of four enzymes (catalase, cellulase, protease, and urease), which typically reflect C and N transformation in soil, were measured. The aims of this study were (1) to assess the effects of grazing intensity on the soil protozoan abundance and community, soil microbial biomass, and enzyme activities and (2) to reveal the relationships among soil protozoa abundance, microbial biomass, and soil physicochemical properties. We hypothesized that heavy grazing pressure would have a negative effect on soil protozoan community and microbial activity, and moderate grazing pressure could improve the biological property in alpine meadow according to “grazing optimization hypothesis” proposed by Dyer et al. (1986).
2 Materials and methods
2.1 Sampling sites
Soil samples were obtained from experimental plots at the Damxung Grassland Observation Station (30°29′-30°30′N, 91°04′E) in Damxung County, the Autonomous Region of Tibet, China. The average altitude of at the station is 4300 m. The experimental area has a continental climate with semi-dry monsoons. The annual mean amount of sunshine at the experimental site is 2881 h, and the annual mean solar radiation is 7528 MJ m−2. The annual mean precipitation in this area is 480 mm, and precipitation mainly occurs from June to September. The annual mean air temperature is 1.3 °C, ranging from a minimum of −10.4 °C in January to a maximum of 10.7 °C in July. Soil type is sandy loam. The vegetation is dominated by the perennial sedges Kobresia pygmaea and Carex montis-everestii and the grass Stipa capillacea, and accompanied by herbs such as Anaphalis xylorhiza and Potentilla bifurca. The mean canopy height is less than 10 cm (Xu et al. 2005; Zhang et al. 2009).
2.2 Experimental design
We focused on five grazing patterns which are listed by increasing intensity according to both number of livestock and the grazing time: (1) artificial grassland without herbivore grazing (AG), (2) winter grazing by 60 Tibetan yaks and 36 sheep (WG), (3) grazing by 60 Tibetan yaks and 36 sheep for 7 months of the year within a fence (GF), (4) continuous grazing by 60 Tibetan yaks and 36 sheep over a whole year (CG), and (5) heavy grazing by 120 Tibetan yaks and 72 sheep (HG). The research area was highly uniform with vegetation cover and all grazing experiments were initiated in 2008. Three 10 × 10 m plots (>2 m from each other) were randomly established in each grazing experimental sites for soil sampling.
2.3 Sample collection and preprocessing
Soil samples were collected twice from the upper 15 cm in each experimental treatment in August of 2012 and 2013. Fifteen soil cores (3.5 cm diameter, 15 cm depth) were collected from each plot and then mixed thoroughly to obtain a composite soil sample, resulting in a total of 30 soil samples (5 grazing treatments × 3 replicates × 2 years). Samples were stored in a cooler and immediately transported to the laboratory. Samples were passed through a 2-mm sieve to remove roots and rock particles and then divided into two portions. One subsample was air dried at room temperature for soil physicochemical analyses. The other part was kept moist at 4 °C for microbial and protozoan community analyses.
2.4 Soil protozoan community analyses
The quantity of soil protozoa was counted by using the method of three series of 10-fold dilutions with three replicates. The soil suspensions of each dilution were examined after culture on the 4th, 7th, and 11th days, and the number of amoebae, flagellates, and ciliates in each dilution was counted based on the presence of protozoa in three dilution gradients by the most possible number (MPN) method and recorded according to their shapes, sizes, and movement patterns (Yin 1998).
2.5 Measurement of soil physicochemical properties and soil microbial biomass C and N
Soil moisture was measured gravimetrically using the oven drying method. Soil pH was determined by a soil-to-water ratio of 1:2.5 (Lu 1999). Soil microbial biomass C and N (MBC and MBN) were determined using the chloroform fumigation extraction method, which measured the differences between organic C and N extracted with 0.5 M K2SO4 from chloroform-fumigated and un-fumigated soil samples. Soil MBC and MBN were calculated using the conversion factors K ec (0.38) and K en (0.54), respectively (Vance et al. 1987). The soil samples were cultured at 25 °C for 1 week, and water holding capacity was regulated at approximately 50%. The assays of the soil organic carbon (OC) and total nitrogen (TN) were carried out using a liquid model TOC analyzer (Vario, Elementar, Germany) and continuous flow analyzer (AA3, SEAL, Germany).
2.6 Soil enzyme activity analysis
The activities of soil catalase, cellulase, protease, and urease were determined in this study. Catalase activity was determined by back-titrating residual H2O2 with 0.02 M KMnO4 (Johnson and Temple 1964). The results were expressed as milliliter (0.1 mol/L KMnO4) (h g)−1. Cellulase activity was assayed using the method of Guan (1996), where 1% carboxy methyl cellulose solution was used as a substrate for measuring cellulase activity, which was expressed in terms of milligrams glucose per gram of dry soil in 72 h (Guan 1996). Soil protease activity was determined using casein as the substrate according to the method described by Wu and Lin (2006), and the results were expressed as micrograms tyrosine per gram of dry soil per hour. Urease activity was measured by the colorimetric method using 10% urea (Guan 1986) and was expressed as the amount of enzyme that produces 1 mg NH4 +-N g−1 dry soil in 24 h at 37 °C.
2.7 Statistical analyses
Two-way analysis of variance (ANOVA) was performed to analyze the effect of grazing, collection time, and their interaction on the soil protozoan community and enzyme activity. Multiple comparisons of groups among treatments were performed using one-way ANOVA in SPSS (version 16.0; SPSS Inc., Chicago, IL), and significant differences in protozoan abundance, soil properties, enzyme activities, and soil microbial biomass were assessed using Fisher’s Least Significant Difference (LSD) at P < 0.05. The correlations among different parameters were tested using the Pearson correlation coefficients. The relationships between the soil protozoan community and environmental factors were assessed for redundancy analysis (RDA) with the package “Vegan” in R, and Monte Carlo 999 permutation tests were used to examine the significance of the environmental factors and ordination axes at P < 0.05. The soil protozoan abundance was Hellinger-transformed to down-weight the influence of rare protozoan group. The interrelationships among the soil protozoan community composition, microbial activities, and soil factors were further investigated by Mantel tests utilizing the “mantel” function in the “Vegan” package (Oksanen et al. 2015).
3 Results
3.1 Soil physicochemical properties
Compared with the AG treatment, soil moisture was observed to be significantly lower in the treatments of WG, GF, CG, and HG in 2012 and just be significantly lower in the HG treatment in 2013 (Table 1). Overall, soil moisture declined from 14.63 to 2.68% and from 8.67 to 4.67% with increasing grazing intensities in 2012 and 2013, respectively (Table 1). The HG resulted in higher soil pH compared with those of the other four grazing treatments in 2013, whereas this parameter was only significantly higher in HG than AG in 2012. Soil OC and TN contents in the HG were significantly lower than those in the AG, WG, GF (except TN of 2012), and CG treatments. The HG treatment also resulted in the lowest C/N ratio in 2012 and 2013 (Table 1).
3.2 Soil enzyme activity and microbial biomass C and N
Grazing intensity had significant effects on soil enzyme activities (P < 0.05), although there was no significant effect of collection year on cellulase, in contrast to other enzymes. Protease and urease activity showed higher activity in the CG treatment, while the lower activity of cellulose in 2012 and of catalase in 2012 and 2013 was found in CG treatment (Table 2). Protease showed a significantly decreasing activity in HG treatment. In contrast, cellulase, catalase, and urease had higher activities in the HG than those of treatments that received light grazing, WG, with the exception of urease activity in 2013, which was the lowest activity observed under heavy grazing (Table 2).
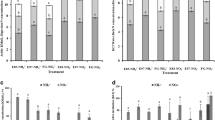
Soil MBC and MBN ranged from 180.65 to 268.59 mg C kg−1 and 14.66 to 80.4 mg N kg−1 in 2012 (Fig. 1a), and from 142.61 to 304.68 mg C kg−1 and 6.6 to 82.91 mg N kg−1 in 2013 (Fig. 1b), respectively. The MBC was significantly affected by grazing, and MBN was significantly affected by both grazing and sampling time (P < 0.05, Table 3). For instance, in 2012, significantly higher MBC was observed in WG and CG, followed by those in GF and HG, and the lowest MBC detected in the AG treatment (Fig. 1a). However, compared with AG, the MBC were significantly (P < 0.05) increased by GF and CG treatments, but no significant difference caused by treatments of WG and HG in 2013 (Fig. 1b). The MBN was significantly decreased with increasing grazing intensity with the highest and the lowest MBN observed in the AG and HG treatments, respectively, in 2012 (Fig. 1a). This parameter, however, was only significantly decreased by the HG treatment compared with AG treatment, whereas no difference was found among the treatments of AG, WG, GF, and CG (Fig. 1b).
Effects of grazing intensities on soil microbial biomass carbon and nitrogen in an alpine meadow in Damxung County, Tibet in 2012 (a) and 2013 (b) (means ± SD, n = 3). Multiple comparisons of groups among treatments were performed with LSD for soil microbial biomass under different grazing intensities within 2012 and 2013, respectively. Different letters within each grazing intensity indicate significant differences at the 0.05 level. AG artificial pasture without grazing, WG winter grazing (36 sheep and 60 yaks in winter), GF grazing with fence (36 sheep and 60 yaks in 7 months), CG continuous grazing (36 sheep and 60 yaks in a year), HG natural heavy grazing (72 sheep and 120 yaks in a year)
3.3 Soil protozoan abundance and community composition
The abundance of amoeba was significantly (P < 0.05) influenced by grazing and those of flagellate and ciliate were significantly (P < 0.05) affected by both grazing and collection year (Table 3). There was a significantly interactive effect of grazing and collection year on the abundance of amoeba (Table 3). The WG treatment had the highest abundance of total protozoan and the HG treatment significantly decreased this parameter compared with other four treatments (Fig. 2). The abundance of amoeba was higher in the CG treatment in 2012, but no significant difference was observed in comparison with those of other four treatments in 2013. The WG and HG treatments had the highest and lowest flagellate abundance, respectively, and no significant difference in flagellate abundance was observed among AG, WG, and CG treatments in both 2012 and 2013. Compared with the AG, significantly lower ciliate abundances were detected in treatments of WG, GF, and HG in 2012 (Fig. 2a), and lower in WG and HG in 2013 (Fig. 2b). No significant difference in ciliate abundances was observed among WG, GF, CG, and HG in 2012 (Fig. 2a) and among WG, GF, and CG in 2013 (Fig. 2b).
Effects of grazing intensities on soil protozoa in an alpine meadow in Damxung County, Tibet in 2012 (a) and 2013 (b) (means ± S.D., n = 3). Multiple comparisons of groups among treatments were performed with LSD for each soil protozoan group under different grazing intensity within 2012 and 2013, respectively. Different letters within each grazing intensity indicate significant differences at the 0.05 level. AG artificial pasture without grazing, WG winter grazing (36 sheep and 60 yaks in winter), GF grazing with fence (36 sheep and 60 yaks in 7 months), CG continuous grazing (36 sheep and 60 yaks in a year), HG natural heavy grazing (72 sheep and 120 yaks in a year)
Redundancy analysis indicated that the soil protozoan community composition was distinctly different among the five grazing treatments in 2012 (Fig. 3a). The AG, WG, CG, and HG treatments were clearly separated from each other along the RDA 1 axis, which accounted for 80.52% of the variation in the soil protozoan community in 2012, whereas only the HG treatment was relatively significantly separated from other treatments along RDA 1 (49.33%) reflecting the variation in the soil protozoan community in 2013 (Fig. 3b). Moreover, the soil protozoan community was found to be significantly affected by MBN (P = 0.001), SM (P = 0.003), pH (P = 0.012), OC (P = 0.012), and TN (P = 0.048) in 2012 (Fig. 3a) and by MBN (P = 0.014) and MBC (P = 0.022) in 2013 (Fig. 3b).
Ordination diagram of redundancy analysis (RDA) showing relationships between physiochemical parameters, microbial activities, and abundance of soil protozoa in an alpine meadow of the Tibetan Plateau in (a) 2012 and (b) 2013. AG artificial pasture without grazing, WG winter grazing (36 sheep and 60 yaks in winter), GF grazing with fence (36 sheep and 60 yaks in 7 months), CG continuous grazing (36 sheep and 60 yaks in a year), HG natural heavy grazing (72 sheep and 120 yaks in a year), MBC soil microbial biomass C, MBN soil microbial biomass N, SM soil moisture, OC organic carbon, TN total nitrogen. *P < 0.05 and **P < 0.01
3.4 Relationships among soil protozoa, microbes, and soil properties
Mantel tests indicated that significant relationships were observed between protozoan community and microbial activity (microbial biomass and enzyme activity) (r = 0.175, P = 0.025) and soil factors (r = 0.528, P = 0.001), as well as between microbial activity and soil factors (r = 0.252, P = 0.002), which were consistent with the results of RDA and correlation analyses (Table 4). Amoeba had a significant relationship with soil pH, OC, and TN (P < 0.05); flagellate was significantly associated with SM, OC, TN, and MBN (P < 0.05); and ciliate was significantly related to SM and pH (P < 0.05) (Table 4). Soil protease activity was positively correlated with SM, OC, TN, and MBN, but negatively correlated with soil pH (Table 4). Soil urease significantly associated with soil TN and MBN, whereas cellulase was significantly negatively correlated with only SM (Table 4). Furthermore, the MBC was found to significantly positively relate with OC and TN; and MBN significantly positively correlated with SM, OC, and TN, but negatively correlated with pH (Table 4).
4 Discussion
4.1 Effects of grazing intensity on the soil protozoan community
Soil protozoa generally have a special distribution and community composition based on the type of terrestrial ecosystem in which they occur (Foissner 1999; Esteban et al. 2006; Bates et al. 2013). Our results demonstrated that the soil protozoan community in alpine meadow on the Tibetan Plateau was primarily composed of three broad taxonomic groups: amoebas, flagellates, and ciliates. Amoeba and flagellate groups were dominant components and accounted for 89–95% of the total abundance of protozoa, while ciliates had the smallest population in the research area. Similar results have been reported on the semiarid steppe in Inner Mongolia and the alpine grassland in Qinghai Province (Ning and Shen 1998; Li et al. 2005; Qi et al. 2011). Consistent with previous findings, the abundance of soil protozoa was correlated with protozoan taxonomy, which determined protozoan distribution in the soil of the alpine meadow ecosystem on the Tibetan Plateau (Ning and Shen 1998; Foissner 1999; Esteban et al. 2006).
Soil protozoa representing important soil components have been proposed to be sensitive indicators for human perturbations (Ekelund and Ronn 1994; Foissner 1999; Nguyen-Viet et al. 2007; Maharning et al. 2009; Papadimitriou et al. 2013). Long-term grazing may influence soil ecosystem processes through altering the structure of the soil protozoan community. In this study, soil protozoan abundance had a tendency to decline with increasing grazing intensity in the alpine meadow ecosystem, which supported our hypothesis that soil protozoan community was negatively affected by heavy grazing. Similarly, high livestock stocking caused a significant decrease in protozoan abundance in a semiarid steppe of Inner Mongolia (Li et al. 2005; Qi et al. 2011). Particularly, the three taxonomic groups of amoebas, flagellates, and ciliates showed similar dynamics in response to grazing, indicating that soil protozoa declined under high grazing stress. Previous studies also showed that grazing could result in a decrease in soil protozoan abundance owing to soil compaction (Holt 1997). However, Li et al. (2005) reported that flagellate was more abundant under heavy grazing because their body size was suitable for small pores in the Inner Mongolia grassland, but this could also be caused by higher soil moisture in the heavy grazing intensity. In our study, protozoan abundance varied among different grazing intensities, and soil moisture significantly influenced the abundance of flagellate (P < 0.05) and ciliate (P < 0.001) protozoa.
Redundancy analysis demonstrated that the soil protozoan community was significantly correlated with grazing intensity and soil properties, such as SM, pH, OC, and TN. These soil properties could affect the response of the soil protozoan community to grazing, which supported the previous findings in grassland ecosystem (Li et al. 2005, Qi et al. 2011). Soil protozoa have been reported to be largely modulated by soil compaction and water content (Holt 1997), due to water content as the crucial constraint for protozoan life history in terrestrial ecosystems (Ekelund and Ronn 1994; Ning and Shen 1998; Foissner 1999; Esteban et al. 2006; Warner et al. 2007). Soil pH is another important environmental factor to affect protozoan cell osmotic pressure and was increased under heavy grazing intensity possibly due to increasing urea input in livestock waste. In this study, we found that soil amoeba and ciliate were negatively associated with soil pH, supporting the similar results observed between testate amoebas and soil pH by Lamentowicz et al. (2013). Moreover, soil amoeba and flagellate were observed to be positively correlated with OC and TN, which indicated that soil protozoa play a crucial ecological function in stimulating soil carbon and nitrogen cycling and could improve the turnover of soil organic matter in terrestrial ecosystem by preying on rhizosphere bacteria and increasing aboveground plant productivity (Foissner 1999; Esteban et al. 2006).
Soil microbes could have a bottom-up effect on the soil protozoan community in terrestrial ecosystems because bacteria and fungi are major nutrient sources for most of the soil protozoa in the “microbial loop”, and protozoan abundance could be enhanced with increasing microbial activity (Muller et al. 2013; Zhao and Xu 2013). In this study, we found a significantly positive relationship between the soil protozoan community and microbial characteristics. In particular, soil protozoa exhibited a certain dependence on MBN and MBC, which could regulate the soil protozoan community in combination with soil abiotic property. Moreover, correlation analysis between soil protozoa and environmental parameters also indicated that soil protozoa responded significantly to grazing intensity, possibly due to drastic variation in soil properties and microbial activity caused by livestock herding. Similar results have been reported by other researchers, suggesting that soil abiotic and biotic properties play a crucial role in the composition of the soil protozoan community in grassland ecosystems (Bardgett et al. 1998; Qi et al. 2011). Therefore, grazing could change soil properties and decrease soil microbial activity as a result of livestock trampling, feces extraction, and variation in plant cover in this low-productivity region on the Tibetan Plateau (Wang et al. 2006; Ramos et al. 2011; Kuijper and Bakker 2012). Altogether, the community structure of soil protozoa would be directly affected by soil attributes and indirectly by microbial variation resulting from grazing in the alpine meadow.
4.2 Effects of grazing on soil microbial biomass and soil enzyme activity
Both soil MBC and MBN were significantly reduced with increasing grazing pressure in the current study, and significantly positive correlations were observed between soil microbial biomass (MBC, MBN) and organic matter (OC and TN) (Table 4). Consistently, previous researches showed that heavy grazing could cause a dramatic decrease in soil microbial biomass due to lower plant litter input and reduction of OC and TN (Holt 1997; Li et al. 2005; Wang 2006; Qi et al. 2011; Fu et al. 2012). Therefore, lower soil MBC and MBN detected in the heavy grazing treatments, compared with the lighter grazing treatments (e.g., AG, WG, GF, and CG), probably resulting from decreased OC and TN negatively affected certain microbial groups and thus decreased soil microbial biomass.
In the current study, soil enzyme activities showed different responses to grazing patterns depending on enzyme type. The activities of protease declined with increasing grazing intensity, which may be in relation to the changes in soil C and N conditions. This concurs with results documented by other researchers (Holt et al. 1997; Esch et al. 2013; Olivera et al. 2014). Particularly, Olivera et al. (2014) suggested that grazing could lead to declining protease activity if MBN and TN declined as a source and substrate for soil enzymes. Soil moisture and OC also contributed to variation in soil protease activity, possibly due to low humidity and nutrient input (Karaca et al. 2011; Burns et al. 2013; Olivera et al. 2014). However, cellulase and catalase showed higher activity in the HG treatment compared with those of the other treatments, probably due to increasing C and N mineralization for microbes when given sufficient available substrate as reported previously (Xu et al. 2007; Prieto et al. 2011; Wu et al. 2012b; Esch et al. 2013). In our study, increased herbivore feces inputs under the HG treatment could have effectively offset the negative effect of MBC and OC decline and improved cellulase and catalase activity. Urease activity involved in soil N cycling had different variations under grazing between two collection years, possibly due to different soil TN trend. In addition, significant correlations between protease and soil factors indicated that soil protease responded more sensitively to environmental variation inducing by grazing than the other three enzymes.
5 Conclusions
In conclusion, our results indicated that soil protozoa showed a negative response to increasing grazing intensities in alpine meadow on the Tibetan Plateau. Heavy grazing pressure resulted in significant negative effect on soil physicochemical properties (SM, OC, and TN) and soil microbial characteristics (MBN, MBC, and protease activity), which potentially influenced the soil protozoan community composition. The winter grazing at moderate intensity was proposed to be an appropriate grazing strategy for sustaining soil biological properties in this alpine meadow due to little disturbance to the alpine meadow ecosystem. However, given that this study was conducted only in August (the rainy season), even though for two continuous years, further studies on seasonal dynamics of soil protozoa, microbial biomass, and activities are required to fully assess the consequences of grazing intensity on belowground biotic variables.
References
Acosta-Martínez V, Bell CW, Morris BEL, Zak J, Allen VG (2010) Long-term soil microbial community and enzyme activity responses to an integrated cropping-livestock system in a semi-arid region. Agric Ecosyst Environ 137:231–240
Akiyama T, Kawamura K (2007) Grassland degradation in China: methods of monitoring, management and restoration. Grassl Sci 53:1–17
Badiane NNY, Chotte JL, Pate E, Masse D, Rouland C (2001) Use of soil enzyme activities to monitor soil quality in natural and improved fallows in semi-arid tropical regions. Appl Soil Ecol 18:229–238
Bardgett RD, McAlister E (1999) The measurement of soil fungal: bacterial biomass ratios as an indicator of ecosystem self-regulation in temperate meadow grasslands. Biol Fert Soils 29(3):282–290
Bardgett RD, Wardle DA, Yeates GW (1998) Linking above-ground and below-ground interactions: how plant responses to foliar herbivory influence soil organisms. Soil Biol Biochem 30(14):1867–1878
Bates ST, Clemente JC, Flores GE, Walters WA, Parfrey LW, Knight R, Fierer N (2013) Global biogeography of highly diverse protistan communities in soil. ISME J 7(3):652–659
Bonkowski M, Brandt F (2002) Do soil protozoa enhance plant growth by hormonal effects? Soil Biol Biochem 34(11):1709–1715
Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013) Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem 58:216–234
Cao GM, Tang YH, Mo WH, Wang YA, Li YN, Zhao XQ (2004) Grazing intensity alters soil respiration in an alpine meadow on the Tibetan plateau. Soil Biol Biochem 36(2):237–243
Cebron A, Cortet J, Criquet S, Biaz A, Calvert V, Caupert C, Pernin C, Leyval C (2011) Biological functioning of PAH-polluted and thermal desorption-treated soils assessed by fauna and microbial bioindicators. Res Microbiol 162(9):896–907
Chen J, Yamamura Y, Hori Y, Shiyomi M, Yasuda T, Zhou HK, Li YN, Tang YH (2008) Small-scale species richness and its spatial variation in an alpine meadow on the Qinghai-Tibet Plateau. Ecol Res 23:657–663
Dorji T, Moe SR, Klein JA, Totland O (2014) Plant species richness, evenness, and composition along environmental gradients in an alpine meadow grazing ecosystem in central Tibet, China. Arct Antarct Alp Res 46(2):308–326
Dyer MI, DeAngelis DL, Post WM (1986) A model of herbivore feedback on plant productivity. Math Biosci 79:171–184
Ekelund F, Ronn R (1994) Notes on protozoa in agricultural soil with emphasis on heterotrophic flagellates and naked amoebae and their ecology. FEMS Microbiol Rev 15(4):321–353
Esch EH, Hernández DL, Pasari JR, Kantor RSG, Selmants PC (2013) Response of soil microbial activity to grazing, nitrogen deposition, and exotic cover in a serpentine grassland. Plant Soil 366:671–682
Esteban GF, Clarke KJ, Olmo JL, Finlay BJ (2006) Soil protozoa—an intensive study of population dynamics and community structure in an upland grassland. Appl Soil Ecol 33:137–151
Feng RZ, Long RJ, Shang ZH, Ma YS, Dong SK, Wang YL (2010) Establishment of drooping wildrye grass (Elymus natans) improves soil quality of a heavily degraded alpine meadow in Qinghai-Tibetan Plateau, China. Plant Soil 327:403–411
Figuerola ELM, Guerrero LD, Rosa SM, Simonetti L, Duval MAE, Galantini JA, Bedano JC, Wall LG, Erijman L (2012) Bacterial indicator of agricultural management for soil under no-till crop production. PLoS One 7:e510755
Foissner W (1999) Soil protozoa as bioindicators: pros and cons, methods, diversity, representative examples. Agric Ecosyst Environ 74(1–3):95–112
Fterich A, Mahdhi M, Mars M (2012) Impact of grazing on soil microbial communities along a chronosequence of Acacia tortilis subsp raddiana in arid soils in Tunisia. Eur J Soil Biol 50:56–63
Fu G, Shen ZX, Zhang XZ, Zhou YT (2012) Response of soil microbial biomass to short-term experimental warming in alpine meadow on the Tibetan Plateau. Appl Soil Ecol 61:158–160
García-Ruiz R, Ochoa V, Hinojosa MB, Carreira JA (2008) Suitability of enzyme activities for the monitoring of soil quality improvement in organic agricultural systems. Soil Biol Biochem 40(9):2137–2145
García-Ruiz R, Ochoa V, Viñegla B, Hinojosa MB, Peña-Santiago R, Liebanas G, Linares JC, Carreira JA (2009) Soil enzymes, nematode community and selected physico-chemical properties as soil quality indicators in organic and conventional olive oil farming: influence of seasonality and site features. Appl Soil Ecol 41(3):305–314
Guan SM (1986) Soil enzyme and research methods. China Agriculture Press, Beijing (In Chinese)
Hamilton EW, Frank DA (2001) Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology 82(9):2397–2402
Harris RB (2010) Rangeland degradation on the Qinghai-Tibetan plateau: a review of the evidence of its magnitude and causes. J Arid Environ 74:1–12
Holt JA (1997) Grazing pressure and soil carbon, microbial biomass and enzyme activities in semi-arid northeastern Australia. Appl Soil Ecol 5(2):143–149
Johnson JI, Temple KL (1964) Some variables affecting the measurement of catalase activity in soil. Soil Sci Soc Am Proc 28:207–216
Karaca A, Cetin SC, Turgay OC, Kizilkaya R (2011) Soil enzymology. In: Shukla G (ed) Soil enzymes as indication of soil quality, 1st edn. Springer, Berlin Heidelberg, pp 119–148
King KL, Hutchinson KJ (2007) Pasture and grazing land: assessment of sustainability using invertebrate bioindicators. Aust J Exp Agr 47(4):392–403
Kuijper DPJ, Bakker JP (2012) Below- and above-ground vertebrate herbivory and abiotic factors alternate in shaping salt-marsh plant communities. J Exp Mar Biol Ecol 432:17–28
Lamentowicz M, Bragazza L, Buttler A, Jassey VEJ, Mitchell EAD (2013) Seasonal patterns of testate amoeba diversity, community structure and species–environment relationships in four Sphagnum-dominated peatlands along a 1300 m altitudinal gradient in Switzerland. Soil Biol Biochem 67:1–11
Li Q, Mayzlish E, Shamir I, Pen-Mouratov S, Sternberg M, Steinberger Y (2005) Impact of grazing on soil biota in a Mediterranean grassland. Land Degrad Dev 16:581–592
Long RJ, Ding LM, Shang ZH, Guo XH (2008) The yak grazing system on the Qinghai-Tibetan plateau and its status. Rangel J 30(2):241–246
Lu R (1999) Analytical methods for soil and agricultural chemistry. China Agricultural Scientech Press, Beijing (in Chinese)
Maharning AR, Mills AAS, Adl SM (2009) Soil community changes during secondary succession to naturalized grasslands. Appl Soil Ecol 41:137–147
Muller MS, Scheu S, Jousset A (2013) Protozoa drive the dynamics of culturable biocontrol bacterial communities. PLoS One 8(6):e66200
Neilson R, Robinson D, Marriott CA, Scrimgeour CM, Hamilton D, Wishart J, Boag B, Handley LL (2002) Above-ground grazing affects floristic composition and modifies soil trophic interactions. Soil Biol Biochem 34(10):1507–1512
Nguyen-Viet H, Bernard N, Mitchell E, Cortet J, Badot PM, Gilbert D (2007) Relationship between testate amoeba (Protist) communities and atmospheric heavy metals accumulated in Barbula indica (Bryophyta) in vietnam. Microbial Ecol 53(1):53–65
Ning YZ, Shen YF (1998) Soil protozoa in typical zones of China. Ecological Study 44(3):271–276 (in Chinese)
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2015) Vegan: community ecology package. R package version 2:3–2 http://CRAN.R-project.org/package=vegan
Olivera NL, Prieto L, Carrera AL, Cisneros HS, Bertiller MB (2014) Do soil enzymes respond to long-term grazing in an arid ecosystem? Plant Soil 378(1–2):35–48
Papadimitriou CA, Petridis D, Zouboulis AI, Samaras P, Yiangou M, Sakellaropoulos GP (2013) Protozoans as indicators of sequential batch processes for phenol treatment; an autoecological approach. Ecotox Environ Safe 98:210–218
Prieto LH, Bertiller MB, Carrera AL, Olivera NL (2011) Soil enzyme and microbial activities in a grazing ecosystem of Patagonian Monte, Argentina. Geoderma 162(3–4):281–287
Qi S, Zheng H, Lin Q, Li G, Xi Z, Zhao X (2011) Effects of livestock grazing intensity on soil biota in a semiarid steppe of Inner Mongolia. Plant Soil 340(1–2):117–126
Ramos ME, Robles AB, Sanchez-Navarro A, Gonzalez-Rebollar JL (2011) Soil responses to different management practices in rainfed orchards in semiarid environments. Soil Till Res 112(1):85–91
Ros M, Pascual JA, García C, Hernandez MT, Insam H (2006) Hydrolase activities, microbial biomass and bacterial community in a soil after long-term amendment with different composts. Soil Biol Biochem 38(12):3443–3452
Rui YC, Wang SP, Xu ZH, Wang YF, Chen CR, Zhou XQ, Kang XM, Lu SB, Hu YG, Lin QY (2011) Warming and grazing affect soil labile carbon and nitrogen pools differently in an alpine meadow of the Qinghai-Tibet Plateau in China. J Soils Sediments 11(6):903–914
Singh SK, Rai JPN (2004) Soil microbial population and enzyme activity related to grazing pressure in alpine meadows of Nanda Devi Biosphere Reserve. J Environ Biol 25(1):103–107
Stark S, Mannisto MK, Eskelinen A (2015) When do grazers accelerate or decelerate soil carbon and nitrogen cycling in tundra? A test of theory on grazing effects at contrasting levels of habitat fertility. Oikos 124(5):593–602
Steffens M, Kolbl A, Totsche KU, Kogel-Knabner I (2008) Grazing effects on soil chemical and physical properties in a semiarid steppe of Inner Mongolia (PR China). Geoderma 143(1–2):63–72
Su YZ, Li YL, Cui JY, Zhao WZ (2005) Influences of continuous grazing and livestock exclusion on soil properties in a degraded sandy grassland, Inner Mongolia, northern China. Catena 59(3):267–278
Sun DS, Wesche K, Chen DD, Zhang SH, Wu GL, Du GZ, Comerford NB (2011) Grazing depresses soil carbon storage through changing plant biomass and composition in a Tibetan alpine meadow. Plant Soil Environ 57(6):271–278
Teague WR, Dowhower SL, Baker SA, Haile N, DeLaune PB, Conover DM (2011) Grazing management impacts on vegetation, soil biota and soil chemical, physical and hydrological properties in tall grass prairie. Agric Ecosyst Environ 141(3–4):310–322
Tsyganov AN, Nijs I, Beyens L (2011) Does climate warming stimulate or inhibit soil protist communities? A test on testate amoebae in high-arctic tundra with free-air temperature increase. Protist 162(2):237–248
Turner TE, Swindles GT (2012) Ecology of testate amoebae in moorland with a complex fire history: implications for ecosystem monitoring and sustainable land management. Protist 163(6):844–855
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wang KH, McSorley R, Bohlen P, Gathumbi SM (2006) Cattle grazing increases microbial biomass and alters soil nematode communities in subtropical pastures. Soil Biol Biochem 38(7):1956–1965
Wang G, Wang Y, Li Y, Cheng H (2007) Influences of alpine ecosystem responses to climatic change on soil properties on the Qinghai-Tibet Plateau, China. Catena 70(3):506–514
Warner BG, Asada T, Quinn NP (2007) Seasonal influences on the ecology of testate amoebae (Protozoa) in a small Sphagnum peatland in Southern Ontario, Canada. Microbial Ecol 54(1):91–100
Wu JS, Lin QM (2006) Soil microbial biomass—method and application. China Meteorological Press, Beijing (in Chinese)
Wu GL, Du GZ, Liu ZH, Thirgood S (2009) Effect of fencing and grazing on a Kobresia-dominated meadow in the Qinghai-Tibetan Plateau. Plant Soil 319(1–2):115–126
Wu GL, Liu ZH, Zhang L, Chen JM, Hu TM (2010) Long-term fencing improved soil properties and soil organic carbon storage in an alpine swamp meadow of western China. Plant Soil 332(1–2):331–337
Wu H, Wiesmeier M, Yu Q, Steffens M, Han X, Kögel-Knabner I (2012a) Labile organic C and N mineralization of soil aggregate size classes in semiarid grasslands as affected by grazing management. Biol Fert Soils 48(3):305–313
Wu XD, Zhao L, Fang HB, Chen J, Pang QQ, Wang ZW, Chen MJ, Ding YJ (2012b) Soil enzyme activities in permafrost regions of the western Qinghai-Tibetan Plateau. Soil Sci Soc Am J 76(4):1280–1289
Xu LL, Zhang XZ, Shi PL, Yu GR, Sun XM (2005) Net ecosystem carbon dioxide exchange of alpine meadow in the Tibetan Plateau. Acta Ecol Sin 25(8):1948–1952 (in Chinese)
Xu YQ, Li LH, Wang QB, Chen QS, Cheng WX (2007) The pattern between nitrogen mineralization and grazing intensities in an inner Mongolian typical steppe. Plant Soil 300(1–2):289–300
Yin W (1998) Pictorial keys to soil animals of China. Science Press, Beijing (in Chinese)
Zhang BS, Shi PL, He YT, Zhang XZ, Li Q (2009) The climate feature of Damxung alpine meadow carbon flux research station on the Tibetan Plateau. J M Sci 27(1):88–95 (in Chinese)
Zhang H, John R, Peng Z, Yuan J, Chu C, Du G, Zhou S (2012) The relationship between species richness and evenness in plant communities along a successional gradient: a study of sub-alpine meadows of the eastern Qinghai-Tibetan Plateau, China. PLoS One 7(11):e49024
Zhao F, Xu KD (2013) Microbial genetic diversity and ciliate community structure along an environmental gradient in coastal soil. Eur J Protistol 49(4):516–525
Zhou HK, Zhao XQ, Gu TS, Zhou L (2004) Alpine grassland degradation and its control in the source region of the Yangtze and Yellow Rivers, China. Grassl Sci 51:191–203
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (No. 41101237). The authors greatly appreciate the staff of the Damxung Grassland Observation Station for their assistance with the fieldwork and soil sampling. We appreciate CAS member, Prof. Weibo Song of Ocean University of China for his support on protozoan taxonomy. We also greatly thank Prof. Qimei Lin of China Agricultural University for his valuable suggestions and comments.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Chengrong Chen
Rights and permissions
About this article
Cite this article
Lin, B., Zhao, X., Zheng, Y. et al. Effect of grazing intensity on protozoan community, microbial biomass, and enzyme activity in an alpine meadow on the Tibetan Plateau. J Soils Sediments 17, 2752–2762 (2017). https://doi.org/10.1007/s11368-017-1695-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1695-3