Abstract
Purpose
The aim of the study was to investigate the patterns of soil nitrogen (N)-cycling functional gene abundance along a precipitation gradient on the Mongolian Plateau, and the effects of grazing on the population size of microbial functional group under different precipitation regimes.
Materials and methods
Soil samples were taken from grazing and non-grazing plots of meadow steppe, typical steppe, and desert steppe plots on the Mongolian Plateau for soil gravimetric moisture content, pH, and soil organic carbon (SOC), total N, and inorganic N (NH4 +-N and NO3 −-N) concentrations, and the abundance of functional genes associated with N2 fixation (nifH gene), nitrification (AOA and AOB genes), and denitrification (narG, nirS, nirK, and nosZ genes) was studied. The relationships between environmental variables, soil physicochemical properties, and functional microbial abundance were examined.
Results and discussion
Soil properties (soil moisture, pH, soil organic carbon, total nitrogen, NH4 +-N, and NO3 −-N content) and abundance of N-cycling groups all varied with precipitation. Compared with desert steppe, precipitation significantly decreased the abundance of nifH gene by 1 order of magnitude, but markedly increased the abundance of AOA and AOB genes by 1.32 to 4.72 times and denitrifying genes narG, nirS, nirK, and nosZ by 0.66 to 9.02 times in meadow steppe. Grazing significantly decreased the abundance of functional groups in desert steppe and typical steppe (p < 0.001), while there was no difference between grazing and non-grazing treatments in meadow steppe which had the highest precipitation level. Soil pH was the main factor affecting the abundance of nifH gene according to simple linear regression (R 2 = 0.934, p < 0.001), while moisture was positively related with population sizes of nitrifier and denitrifier groups, explaining 53.8–92.34 % of the variation in the abundance of AOA, narG, nirS, and nosZ genes in all three steppes.
Conclusions
Soil pH was the major factor that significantly affected the gene abundance of nitrogen fixation process, and soil moisture was the dominant factor controlling the gene abundance of nitrification and denitrification process along the precipitation gradient. Grazing had no effect on the gene abundance of N-cycling process in meadow steppe but decreased it in desert and typical steppe. Our results suggest that grazing may not necessarily be associated with a reduction in microbial functional potentials when soil moisture was relatively good but will decrease the soil microbial functional potentials in a more arid environment in northern China grasslands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrogen (N) cycling is one of the most important biogeochemical processes in terrestrial ecosystems. The transfer of nitrogen into, within, and out of soil requires the interplay of microorganisms performing N2 fixation, nitrification, and denitrification (De Boer and Kowalchuk 2001; Vitousek et al. 2002). For example, N fixer converts atmospheric N2 into NH4 +, nitrifier oxidizes it to NO2 − and then NO3 −, and denitrifier reduces NO3 − to NO2 −, NO, N2O, and N2. Therefore, changes in microbial composition or the abundance of special functional groups can alter N availability to plants or N loss from the ecosystem (Lindsay et al. 2010). A number of studies have demonstrated that the abundance of soil microorganisms is regulated by a wide range of biotic and abiotic factors such as soil moisture, temperature, and litter inputs (Fierer and Jackson 2006; Walker et al. 2008; Sheik et al. 2011; Yergeau et al. 2012); however, most studies focused on one process of the N cycle, i.e., either on nitrification or on denitrification (Chroňáková et al. 2009; Di et al. 2010; Philippot et al. 2009), Therefore, they were unable to completely describe the whole N-cycling process.
Precipitation, directly affecting the moisture availability in the soil, is a key factor regulating the spatial and temporal patterns of microbial communities in arid and semiarid terrestrial ecosystems (Reynolds and Stafford Smith 2002; Sorensen et al. 2013). Water availability can directly affect the abundance of soil microorganisms and indirectly affect it through regulating the substrate availability, diffusion of oxygen, and soil pH (Griffiths et al. 2003). N2 fixation typically occurs at water contents between 20 and 60 % (Belnap et al. 2004); nitrification prefers relatively dry soil condition (Maag and Vinther 1996; Luo et al. 2008), but denitrification usually increases in wetter soils as the soils become more anaerobic (Dobbie and Smith 2001; Bateman and Baggs 2005; Diba et al. 2011). All these researches focused on the effect of soil water on N cycling through observed soil conditions and their effects on carbon (C) and N cycling. Recently, there were some studies that have been considerate on the role that microorganisms play in N cycling especially along a precipitation gradient (Adair and Schwartz 2008; Forbes et al. 2009; Mao et al. 2013). Many researches showed that population size of some functional groups are (e.g., narG and AOB) significantly related to variations in rainfall amount (Adair and Schwartz 2008; Forbes et al. 2009), while others showed that the N-cycling groups were independent of precipitation gradient (Mao et al. 2013). We still lack a clear understanding on soil N-cycling microbial functional potentials along a precipitation gradient in Inner Mongolia grassland.
Grazing, as a common land-use type in grassland ecosystems, can profoundly impact N cycling through reducing vegetation cover and altering soil water and energy balance (Leriche et al. 2001), increasing soil compaction or reducing soil aeration by tramping (Oenema et al. 2007; Houlbrooke et al. 2008), or changing the quantity and quality of soil organic matter and mineral N content by the deposition of dung and urine (Saggar et al. 2004). Recently, there were large of studies that have been conducted in grasslands on N cycling and its microbial process under different managements (Patra et al. 2006; Le Roux et al. 2008; Xu et al. 2008). These studies showed an interesting result that for most of managed temperate grassland under humid climate, animal grazing would increase N-cycling rates because of the deposition of dung and urine (Le Roux et al. 2008; Chroňáková et al. 2009; Di et al. 2010; Keil et al. 2011), while for most of arid and semiarid grasslands, animal grazing generally decreased N-cycling rates by the grazing-induced reduction in soil organic matter and soil moisture (Xu et al. 2007, 2008; Wolf et al. 2010). This difference of responses to grazing may be partially driven by an interaction between climate and grazing impacting nutrient cycling through changes in microbe population, which has not been explored so far.
In this study, we focus on the effects of animal grazing on the abundance of functional genes associated with N cycling along a low-precipitation gradient set composed of three natural arid and semiarid ecosystem sites across the Inner Mongolia region of China (Fig. 1). The Inner Mongolia grassland covers an area of about 8.67 × 107 hm2 and is one of the most well-known rangelands in the eastern part of the Eurasian steppe (Coupland 1993), from east to west, presenting a natural precipitation gradient, which is extremely water-limited (Bai et al. 2008). Chen et al. (2014) indicated that the important impact of precipitation on microbial community can be based on phospholipid fatty acid (PLFA), but this technology was limited to detect the biomass of living microorganism; the population size of microbial functional groups has not been explored so far. Grazing-induced grassland degradation has profoundly affected the grassland ecosystems and led to significant depletion in soil organic matter and biomass production (Li et al. 1997, 2008). Previous study only focused on the impact of animal grazing on ecosystem functioning and stoichiometry (Bai et al. 2012). For microbial functional groups, researchers reported the effect of grazing on the abundance of functional microbial genes in one precipitation regime (Zhong et al. 2014), but no information is available along precipitation gradient. Therefore, we address the following research questions: First, how does the abundance of functional genes associated with N-cycling change along a precipitation gradient across the arid and semiarid steppe? Second, how does grazing affect the population of microbial functional group? And third, does the effect of grazing on the abundance of functional genes vary with different precipitation?
2 Materials and methods
2.1 Site description
This study was conducted at sites for long-term ecological research in Inner Mongolia grassland that represent three different types of grassland: desert steppe, typical steppe, and meadow steppe. We took advantage of a region-scale transect across a low-precipitation gradient on Mongolian Plateau. This transect is approximately 1,200 km long and at 30° longitude.
The desert steppe was located in Siziwang Banner near the Ulanqab Grassland Ecosystem Research Station (41°47′N, 111°53′E, 1,456 m above sea level (a.s.l.)). The climate of this experimental area was semiarid, with annual mean (1982–2008) precipitation of 280 mm and annual mean temperature of 3.5 °C (Lin et al. 2010). The soil type is Kastanozem (FAO soil classification system) with a loamy-sand texture. The dominant species in this desert-steppe grassland are Stipa breviflora, and other prevalent species include Agropyron desertorum, Cleistogenes songorica, Artemisia frigid, and Salsola collina. Previously, this experimental region had been grazed at a relatively light stocking density before the increasing number of sheep leads to grassland degradation about two decades ago. The enclosure was established in 2004 to investigate the effect of grazing on organisms, including aboveground plants and belowground microorganisms and where grazing density was moderately grazed (1.82 sheep ha−1).
The typical steppe was located in Xilin River Catchment near the Inner Mongolia Grassland Ecosystem Research Station (43°38′N, 116°42′E, 1,200 m a.s.l.). This area is continental and has semiarid climate, with annual mean (1982–2008) precipitation of 335 mm and annual mean temperature of 0.7 °C (Schönbach et al. 2010). The major soil types of this area are calcic chestnuts and calcic chernozems, with fine-sand texture. The dominant species in this typical-steppe grassland are Stipa grandis, and other prevalent species include Leymus chinensis, Stipa krylovii, Cleistogenes squarrosa, Agropyron cristatum, A. frigid, and Caragana microphylla. The enclosure was established in 2005 in order to investigate the effect of grazing on organisms, including aboveground plants and belowground microorganisms and where grazing density was moderate (4.5 sheep ha−1).
The meadow steppe was located in Xieertala Ranch near the Hulunber Grassland Ecosystem Research Station of the Chinese Academy of Agriculture Sciences (49°19′N, 119°55′E, 628 m a.s.l.). This area is characterized by semiarid climate, with annual mean (1982–2010) precipitation of 400 mm and annual mean temperature of 0 °C (Chen et al. 2012). The soil type is dark chestnut (Chinese classification) or Calcicorthic Aridisol (US soil taxonomy classification system). The native meadow-steppe grassland is dominated by Stipa baicalensis, and other prevalent species include L. chinensis and Filifolium sibiricum. Previously, this region had been grazed at a very low stocking density before the density increased to “moderate to heavy” level four and five decades ago. The enclosure was established in 2006 in order to investigate the effect of grazing on organisms, including aboveground plants and belowground microorganisms and where grazing density was moderate (about 0.34 cattle ha−1).
2.2 Climate condition, soil sampling, and plant community analysis
Total rainfall of 2011 was 317.4, 226.7, and 241.9 mm in meadow steppe, typical steppe, and desert steppe, respectively, with rainfall amount being lower than long-term mean annual precipitation of the three grassland types (400, 335, and 280 mm). Interestingly, the precipitation in July in meadow steppe accounted for 58.2 % of the total rainfall, while typical steppe and desert steppe represented 34.1 and 36.4 %, showing an extremely humid soil condition during the soil sampling time in meadow steppe. The monthly mean air temperature was similar in the three grassland types, which was highest from June to August and lowest in January.
We sampled soil from grazing enclosure experiment in meadow steppe, typical steppe, and desert steppe to investigate the effect of grazing on aboveground plants, soil properties, and belowground microorganisms in similar enclosing time to reduce the possibility of other factors biasing the results. Soil samples were taken in late-July 2011 when all experimental regions were in peak plant biomass in the growing season. There were several precipitation events (for a total rainfall of 21, 30.4, and 87.5 mm in a week in desert steppe, typical steppe, and meadow steppe, respectively) before soil sampling. At each site, this sampling was performed on triplicate grazed and non-grazed plots of 1 × 1 m in size. We sampled soil at a depth of 0–10 cm, using a 5-cm-diameter soil core sampler. At each plot, five randomly selected subsamples were taken after the removal of the vegetation litter with a rake. Five subsamples were pooled together and passed through a 2-mm sieve and then subsequently maintained at 4 °C during transport from the field to the laboratory for molecular and chemical analyses.
We analyzed aboveground plant community at the same time as soil sampling. Coverage was measured by visual estimation method, and plant aboveground standing biomass was determined in three 1 × 1-m quadrates at each site. All the plant materials were oven-dried at 65 °C for 48 h and weighed for each plant species. The Shannon-Wiener index estimating diversity of plant community was used to calculated H′ with the following equation:
where S is species number and p i is the proportion of individuals belonging to the ith species in the dataset of interest.
2.3 Soil analyses
Gravimetric moisture content was determined by oven-drying at 105 °C to a constant mass. Soil pH was measured using a pH meter (Oakton, California, USA). The concentrations of NH4 +-N and NO3 −-N were determined by extraction with 2 M KCl (Abu-Qaoud et al. 1991) on an Alpkem Flow Solution III (OI Analytical, Oregon, USA). Total soil organic carbon content was analyzed using the potassium dichromate heating method, and total nitrogen content was analyzed using the semi-micro Kjeldahl method with a Vario EL III elemental analyzer (Elementar, Germany).
2.4 DNA extraction and quantification of functional genes by real-time PCR
DNA was extracted from the soil using the PowerSoil® DNA Isolation Kit (MO BIO, California, USA) according to the manufacturer’s instructions. Functional genes encoding an enzyme involved in nitrogen fixation (nifH), archaeal and bacterial ammonia monooxygenase (AOA, AOB), nitrate reductase (narG), nitrite reductase (nirK, nirS), and nitrous oxide reductase (nosZ) were quantified in triplicate by quantitative real-time PCR using an ABI 7500 FAST system (Applied Biosystems, California, USA). For qPCR, 2 μl of template DNA, 12.5 μl of 2× SYBR Green qPCR Master Mix (Takara, Japan), 9.5 μl of ddH2O, and 1 μl of primer (Table 1) were mixed into a total reaction volume of 25 μl. The reaction efficiencies of qPCRs were 86 % for nifH, 88 % for AOA, 89 % for AOB, 66 % for narG, 84 % for nirK, 74 % for nirS, and 79 % for nosZ, and the R 2 values were 0.99 for all runs. The abundances of all functional genes were finally calculated as copy number per gram of dry soil.
2.5 Statistical analyses
First, we used one-way ANOVA to analyze the effect of grazing on soil properties and abundance of functional genes. Means were contrasted post hoc by Tukey’s studentized range (HSD) for comparing the samples from grazing and non-grazing treatments. Then, two-way ANOVA was performed testing the main and interactive effects of treatment (grazing or non-grazing) and precipitation (meadow steppe, typical steppe, and desert steppe) on soil properties and abundance of functional genes. Statistix, version 8.0 (Analytical Software, Tallahassee, USA) was used for all ANOVA analyses. Second, a model based on stepwise regression was made predicting the relationship between environmental variables (soil, plant, and precipitation data) and functional groups of nitrogen cycling. All these analyses were performed with SAS software, version 8.0 (SAS Institute, North Carolina, USA).
3 Results
3.1 Plant community analysis
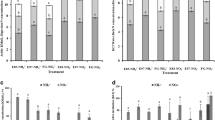
Significant treatment, precipitation, and interactions of treatment and precipitation were observed for aboveground standing biomass and the Shannon-Wiener index. A significant effect of treatment was found for plant coverage in three grasslands (Fig. 2). The plant coverage was significantly decreased by 44.2 % under grazing treatment in meadow steppe (F = 16.41, p = 0.016), but there was no significant difference between grazing treatments in desert steppe and typical steppe (Fig. 2a). Grazing significantly decreased aboveground standing biomass by 43.6, 46.6, and 87.5 % (Fig. 2b), but it markedly increased the Shannon-Wiener index by 0.77, 1.55, and 0.87 times in desert steppe, typical steppe, and meadow steppe, respectively (Fig. 2c).
Plant coverage (a), aboveground biomass (b), and Shannon index (c) in desert steppe, typical steppe, and meadow steppe along low-precipitation gradient. NG denotes non-grazing treatment and G denotes grazing treatment. Values (mean ± SE) followed by different letters are significantly different within each set of sample data. T treatments, P precipitation, T*P treatment×precipitation
3.2 Soil analysis
The moisture content ranged from 3.6 to 34.4 % (Table 2) in the three types of grassland and was significantly lower under grazing treatment in desert steppe (F = 55.78, p = 0.002) and typical steppe (F = 325.87, p = 0.001). Soil pH was significantly higher under grazing treatment in desert steppe (F = 9.49, p = 0.037), but was lower under grazing treatment in typical steppe (F = 8.29, p = 0.045). Total C content was significantly decreased by 16.7, 15.3, and 18.4 % under grazing treatment in desert steppe (F = 14.99, p = 0.018), typical steppe (F = 16.91, p = 0.015), and meadow steppe (F = 67.27, p = 0.001), while the same phenomenon was only observed in typical steppe (F = 736.11, p < 0.001) for total N, decreasing from 0.17 to 0.13 %. NO3 −-N concentration was higher under grazing than non-grazing treatment, from 16.93 to 36.0 mg/kg in desert steppe (F = 23.0, p = 0.009) and 6.87 to 10.33 mg/kg in meadow steppe (F = 13.94, p = 0.02). Compared with NO3 −-N content, grazing increased NH4 +-N content by 24.1 % in typical steppe (F = 9.97, p = 0.034) while it decreased by 42.9 % in desert steppe (F = 738.17, p < 0.001). It was clearly showed that all measured soil properties demonstrated a significant relationship with precipitation (p < 0.001) and precipitation×treatment (p < 0.001), while grazing treatment did not change the characteristics of soil in the three grassland types (Table 3).
3.3 Abundance of nitrogen-cycling microorganisms
Similar to the soil properties in the three grasslands, the abundance of N-cycling microorganisms measured in both grazing treatments varied significantly with precipitation (p < 0.001) and precipitation×treatment effect (p < 0.001) (Figs. 3, 4, and 5). nifH gene abundance varied from 2.4 × 105 to 2.9 × 106 copies g−1 of dry soil and was significantly lower under grazing than non-grazing treatment in desert steppe (F = 22.5, p = 0.009) and typical steppe (F = 11.61, p = 0.027), but there was no significant difference in meadow steppe (Fig. 3).
Abundance of nifH gene (copies per gram of dry soil) in desert steppe, typical steppe, and meadow steppe along low-precipitation gradient. NG denotes non-grazing treatment and G denotes grazing treatment. Values (mean ± SE) followed by different letters are significantly different within each set of sample data. T treatments, P precipitation, T*P treatment×precipitation
Abundance of AOA (a) and AOB (b) genes (copies per gram of dry soil) in desert steppe, typical steppe, and meadow steppe along low-precipitation gradient. NG denotes non-grazing treatment and G denotes grazing treatment. Values (mean ± SE) followed by different letters are significantly different within each set of sample data. T treatments, P precipitation, T*P treatment×precipitation
Abundance of narG (a), nirK (b), nirS (c), and nosZ (d) genes (copies per gram of dry soil) in desert steppe, typical steppe, and meadow steppe along low-precipitation gradient. NG denotes non-grazing treatment and G denotes grazing treatment. Values (mean ± SE) followed by different letters are significantly different within each set of sample data. T treatments, P precipitation, T*P treatment×precipitation
The abundance of nitrifier groups was quantified by analysis of the ammonia monooxygenase subunit A (amoA) gene (Fig. 4). AOB gene abundance was outnumbered by AOA gene by 1 to 2 orders of magnitude in all samples. The abundance of AOB gene was significantly lower under grazing treatment, from 4.2 × 105 to 2.3 × 105 copies g−1 in desert steppe (F = 11.97, p = 0.026) and 5.6 × 105 to 2.8 × 105 copies g−1 in typical steppe (F = 40.54, p = 0.003), but there was no significant effect between grazing treatment in meadow steppe (Fig. 4b). The abundance of AOA gene measured in grazed soil was significantly lower in typical steppe (F = 12.36, p = 0.025), but was not significantly different in both desert steppe and meadow steppe (Fig. 4a).
Four functional genes associated with denitrification were quantified in this study (Fig. 5). The abundance of narG gene under grazing treatment (1.5 × 107 copies g−1) increased to double compared to that under non-grazing treatment (8.3 × 106 copies g−1), but there was no significant difference in meadow steppe (Fig. 5a). The abundance of nirK gene in meadow steppe was two times of magnitude greater than in the other two sites, but there was no significant difference between the grazing and non-grazing treatments (Fig. 5b). As another gene encoding nitrite reductase, nirS gene has higher abundance under grazing than in non-grazing treatment, from 3.6 × 106 to 5.8 × 106 copies g−1 in meadow steppe (F = 9.28, p = 0.038), but markedly decreased from 4.0 × 106 to 2.3 × 106 copies g−1 in typical steppe (F = 102.81, p < 0.001) (Fig. 5c). For nosZ gene, grazing significantly decreased the abundance of nosZ gene by 27.8 % in desert steppe (F = 18.31, p = 0.013), but did not significantly influence the abundance of nosZ gene in both meadow steppe and typical steppe (Fig. 5d).
3.4 Environmental variables controlling functional groups associated with nitrogen cycling
In 2011, soil moisture was positively related with the abundance of AOA, narG, nirS, and nosZ genes at meadow steppe, typical steppe, and desert steppe (Table 4), suggesting that soil moisture was a primary factor controlling population sizes of N-cycling groups. Soil pH, concentration of NO3 −-N, and precipitation together explained 98.16 % of the variation in the abundance of nifH gene. Soil moisture, pH, and mean annual precipitation explained 94.06 % of the variation of AOA gene abundance, while soil organic C explained 75.1 % of that of AOB gene abundance. Soil moisture and organic C together accounted for 75.29 % of the total variance of narG gene abundance. Total N explained 95.68 % of the variation of nirK group, while soil moisture and organic C explained 74.5 % of the variation of the nirS group. Soil moisture and Shannon diversity index represented 96.46 % of the variations of nosZ gene abundance.
4 Discussion
4.1 Changes in the abundance of functional genes associated with N-cycling change along a low-precipitation gradient
A series of researches focus on the change of ecosystem process along this precipitation gradient such as primary production (Bai et al. 2008), stable C and N (Ma et al. 2012), and soil microbial communities (Chen et al. 2014) in Inner Mongolia, and all these studies showed the precipitation or soil moisture was the primary environmental factor controlling the ecosystem process. In our study, we found that the plant variables (aboveground standing biomass and Shannon-Wiener index) kept a significant relationship with precipitation in meadow steppe, typical steppe, and desert steppe (Fig. 2b, c). The result was consistent with most studies in arid and semiarid grasslands (Bai 1999; Bai et al. 2000; Liu et al. 2007), indicating the importance of precipitation in water-limited grassland ecosystem.
In N-fixation process, we found that nifH gene abundance significantly increased with the increase of soil pH (Table. 4), indicating a close linkage between N-fixation group and soil pH. This result was different from that obtained in Australia (Lindsay et al. 2010) and Europe (Meyer et al. 2013) where nifH gene abundance was mainly controlled by total N in nitrogen-rich grassland (Meyer et al. 2013). In our result, soil pH can explain 93.15 % of the variance of nifH gene abundance (Table 4), suggesting that soil pH was the main ecological factor for nifH gene in Inner Mongolia, which is most likely associated with that in other ecosystem. Acidic soil would inhibit dinitrogenase reductase and decreased the gene abundance of N-fixer groups (Pereira e Silva et al. 2013). Our research supports the results of Zhang et al. (2013) that the abundance of N-fixer groups was directly controlled by soil pH in Inner Mongolia grassland.
In nitrification and denitrification process, the abundance of nitrifier and denitrifier groups all increased with the increase of rainfall amount (Figs. 4 and 5) and soil moisture was the main ecological factor changing AOA, narG, nirS, and nosZ gene abundance except AOB and nirK genes (Table 4), indicating that precipitation regulates the soil moisture which changes the abundances of nitrifier and denitrifier groups in arid and semiarid grasslands. This finding was also consistent with other ecosystem such as forest ecosystem (Szukics et al. 2010). Szukics et al. (2010) investigated the response of nitrifier and denitrifier to a change in moisture and found that AOA abundance rapidly responded to water content while nirK gene abundance increased remarkably after short-term incubation under wet soil condition, suggesting the sensitivity of nitrifier and denitrifier groups to soil moisture. However, our result was inconsistent with recent research works in Europe, revealing plant diversity (Lange et al. 2014). pH and substrate (Yao et al. 2013) were the most important factors controlling the microbial functional groups. This difference with those researches in Europe might be due to the difference in precipitation in the two research regions, with the mean annual precipitation in Europe being abundant, ranging from 501 mm to approximately 2,000 mm per year, implying that microbial functional groups might be more responsive to other abiotic and biotic factors than moisture, while in Inner Mongolia, the precipitation (280∼400 mm per year) which limits the growth of plants, microbial community, and ecosystem functioning (Bai et al. 2004, 2008), suggesting moisture as the primary factor in arid and semiarid grasslands. Compared with other size of functional groups significantly relating with moisture, our result indicated that the abundance of AOB gene was positively related with soil organic C (Table 4). Mineralization of nitrogen collaborating with pool of soil organic C supplied NH4 +-N, a kind of substrate used for nitrification by ammonia-oxidizing bacteria (Forbes et al. 2009). Soil organic C in our research explained the largest statistically proportion of variation in AOB gene abundance among abiotic and biotic factors, suggesting the important role of soil organic carbon in the process of nitrification. The copper nitrite reductase and cytochrome cd 1 nitrite reductase respectively encoded by nirK and nirS genes are involved in the reduction of NO2 − to NO (Zumft 1997). Our result showed that the abundance of the nirS groups significantly increased with increasing soil moisture, while the nirS groups appeared to be more related with total nitrogen (Table 4), indicating the difference in phylogenetic diversity between nirK and nirS denitrifiers (Philippot et al. 2009).
4.2 Effect of grazing on nitrogen fixer, nitrifier, and denitrifier groups along a low-precipitation gradient
For all functional groups, our finding showed that grazing significantly decreased the abundance of nifH, AOA, AOB, narG, nirK/S, and nosZ genes in typical steppe and desert steppe, but did not affect them in meadow steppe (Figs. 3, 4, and 5). This result was different from that obtained in the studies made by Mirza et al. (2014) and Chroňáková et al. (2009) in Brazil and Europe, respectively, where the precipitation or soil moisture condition was higher than that in our site. In these recent reports, the abundance of functional genes involved in N cycling was significantly higher in grazed soil than in non-grazed soil, indicating the effect of variation in C and N supply induced by grazing on sizes of microbial population. In meadow steppe, our result demonstrated that there was no significant difference in the abundance of functional genes between grazing and livestock exclusion. The mechanism behind this pattern remained unclear, and we assumed that the different response of functional groups to grazing might be explained by the following two reasons: (1) some researchers suggested that the abundance of functional genes was more responsive to soil characteristics than to present grazing pasture (Wakelin et al. 2008, 2009; Lindsay et al. 2010). Wakelin et al. (2009) reported that the abundance of functional genes was not significantly different under pasture treatment but varied with variation in soil properties such as pH or nutrient content. As mentioned above, the abundance of nifH genes was mainly affected by soil pH while other genes associated with nitrification and denitrification processes were closely related to moisture. In this case, soil pH and moisture were not significantly different in grazing and non-grazing treatments, which might attribute to the observed similarity in size of N-cycling groups. (2) In our result, the experimental region in meadow-steppe grassland received much higher amount of precipitation (87.5 mm) in a week before soil sampling compared to desert steppe (21 mm) and typical steppe (30.4 mm), so that soil moisture (31.3 and 34.4 % in grazed and non-grazed soil) was similar to that in Europe with moisture ranging from about 30 to 60 % (Chroňáková et al. 2009). We speculated that grazing imposing no significant impact on the abundance of functional genes might due to a buffering effect induced by moisture on grazing. Furthermore, our finding was consistent with a study in meadow steppe that grazing did not significantly change gene abundance of nitrifier and denitrifier groups (Zhong et al. 2014). In this research, the abundance of AOA and narG genes was not obviously different between grazing and livestock exclusion in July while a significant difference was observed in May and September, confirming that moisture condition controls the effect of grazing on soil N-cycling microbial functional potentials. In typical steppe and desert steppe, our finding shows that the abundance of functional genes was lower in grazing treatment than in non-grazing treatment. In Inner Mongolia, although studies which focus on the effect of grazing on the abundance of functional genes are rare, several researches reported the effect of grazing on C, N, and P content (Wen et al. 2013) and plant communities (Wang et al. 2014), and all these studies showed the negative effect of grazing on ecosystem process in typical steppe and desert steppe. Our result had a similar trend that grazing decreased the abundance of functional genes, indicating the vulnerability of functional groups to grazing under low-moisture condition.
5 Conclusions
In summary, by investigating the effect of grazing on N-cycling gene abundances in three different grassland types in Inner Mongolia, our result showed that soil pH controlled the nitrogen fixation process and soil moisture was the dominant factor controlling the gene abundance of nitrification and denitrification process along the precipitation gradient. Grazing had no effect on the gene abundance of N-cycling process in meadow steppe but decreased it in desert and typical grasslands. This suggests that grazing may not necessarily be associated with a reduction in microbial functional potentials in meadow grassland because of the relatively good soil moisture condition but it will decrease the soil microbial functional potentials in a more arid environment in northern China grasslands.
References
Abu-Qaoud H, Skirvin RM, Below FE (1991) Influence of nitrogen form and NH4 +-N/:NO3 −-N ratios on adventitious shoot formation from pear (Pyrus communis) leaf explants in vitro. Plant Cell Tiss Org 27:315–319
Adair KL, Schwartz E (2008) Evidence that ammonia-oxidizing archaea are more abundant than ammonia-oxidizing bacteria in semiarid soils of northern Arizona, USA. Microb Ecol 56:420–426
Bai YF (1999) Influence of seasonal distribution of precipitation on primary productivity of Stipa krylovii community. Acta Phytoecologica Sinica 23(2):155–160 (in Chinese)
Bai YF, Li LH, Wang QB, Zhang LX, Zhang Y, Chen ZZ (2000) Changes in plant species diversity and productivity along gradients of precipitation and elevation in the Xilin River Basin, Inner Mongolia. Acta Phytoecologica Sinica 24:667–673 (in Chinese)
Bai YF, Wu JG, Clark CM, Pan QM, Zhang LX, Chen SP, Wang QB, Han XG, Wisley B (2012) Grazing alters ecosystem functioning and C:N:P stoichiometry of grasslands along a regional precipitation gradient. J Appl Ecol 49:1204–1215
Bai YF, Wu JG, Xing Q, Pan QM, Huang JH, Yang DL, Han XG (2008) Primary production and rain use efficiency across a precipitation gradient on the Mongolia plateau. Ecology 89:2140–2153
Bai YF, Han XG, Wu JG, Chen ZZ, Li LH (2004) Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 431:181–184
Bateman EJ, Baggs EM (2005) Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol Fertil Soils 41:379–388
Belnap J, Phillips SL, Miller ME (2004) Response of desert biological soil crusts to alterations in precipitation frequency. Oecologia 141:306–316
Braker G, Fesefeldt A, Witzel KP (1998) Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl Environ Microbiol 64:3769–3775
Bru D, Sarr A, Philippot L (2007) Relative abundances of proteobacterial membrane-bound and periplasmic nitrate reductases in selected environments. Appl Environ Microbiol 73:5971–5974
Chen DM, Mi J, Chu PF, Cheng JH, Zhang LX, Pan QM, Xie YC, Bai YF (2014) Patterns and drivers of soil microbial communities along a precipitation gradient on the Mongolian Plateau. Landscape Ecol. doi:10.1007/s10980-014-9996-z
Chen YM, Gao JX, Feng CY, Jia XY (2012) Temporal and spatial distribution of vegetation net primary productivity (NPP) in the years from 1982 to 2010 in Hulunbeier. J Ecol Rural Environ 28:647–653 (in Chinese)
Chroňáková A, Radl V, Čuhel J, Šimek M, Elhottová D, Engel M, Schloter M (2009) Overwintering management on upland pasture causes shifts in an abundance of denitrifying microbial communities, their activity and N2O-reducing ability. Soil Biol Biochem 41:1132–1138
Coupland RT (1993) Ecosystems of the World. In: Coupland RT (ed) Natural grasslands: Eastern Hemisphere and resume. Elsevier, Amsterdam, pp 3–59
De Boer W, Kowalchuk GA (2001) Nitrification in acid soils: micro-organisms and mechanisms. Soil Biol Biochem 33:853–866
Di HJ, Cameron KC, Sherlock RR, Shen JP, He JZ, Winefield CS (2010) Nitrous oxide emissions from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia-oxidizing bacteria and archaea. J Soils Sediments 10:943–954
Diba F, Shimizu M, Hatano R (2011) Effects of soil aggregate size, moisture content and fertilizer management on nitrous oxide production in a volcanic ash soil. Soil Sci Plant Nutr 57:733–747
Dobbie KE, Smith KA (2001) The effects of temperature, water-filled pore space and land use on N2O emissions from an imperfectly drained gleysol. Eur J Soil Sci 52:667–673
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:626–631
Forbes MS, Broos K, Baldock JA, Gregg AL, Wakelin SA (2009) Environmental and edaphic drivers of bacterial communities involved in soil N-cycling. Aust J Soil Res 47:380–388
Griffiths RI, Whiteley AS, O’Donnell AG, Bailey MJ (2003) Physiological and community responses of established grassland bacterial populations to water stress. Appl Environ Microbiol 69:6961–6968
Henry S, Bru D, Stres B, Hallet S, Philippot L (2006) Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl Environ Microbiol 72:5181–5189
Houlbrooke DJ, Littlejohn RP, Morton JD, Paton RJ (2008) Effect of irrigation and grazing animals on soil quality measurements in the North Otago Rolling Downlands of New Zealand. Soil Use Manage 24:416–423
Keil D, Meyer A, Berner D, Poll C, Schutzenmeister A, Piepho HP, Vlasenko A, Philippot L, Schloter M, Kandeler E, Marhan S (2011) Influence of land-use intensity on the spatial distribution of N-cycling microorganisms in grassland soils. FEMS Microbiol Ecol 77:95–106
Lange M, Habekost M, Eisenhauer N, Roscher C, Bessler H, Engels C, Oelmann Y, Scheu S, Wilcke W, Schulze ED, Gleixner G (2014) Biotic and abiotic properties mediating plant diversity effects on soil microbial communities in an experimental grassland. PLoS ONE 9(5):e96182
Le Roux X, Poly F, Currey P, Commeaux C, Hai B, Nicol GW, Prosser JI, Schloter M, Attard E, Klumpp K (2008) Effects of aboveground grazing on coupling among nitrifier activity, abundance and community structure. ISME J 2:221–232
Leriche H, LeRoux X, Gignous J, Tuzet A, Fritz H, Abbadie L, Loreau M (2001) Which functional processes control the short-term effect of grazing on net primary production in grasslands? Oecologia 129:114–124
Li LH, Chen ZZ, Wang QB, Liu XH, Li YH (1997) Changes in soil carbon storage due to over-grazing in Leymus chinensis steppe in the Xilin River Basin of Inner Mongolia. J Environ Sci 9:486–490 (in Chinese)
Li YH, Wang W, Liu ZL, Jiang S (2008) Grazing gradient versus restoration succession of Leymus chinensis (Trin.) Tzvel. grassland in Inner Mongolia. Restor Ecol 16:572–583
Lin Y, Hong M, Han GD, Zhao ML, Bai YF, Chang SX (2010) Grazing intensity affected spatial patterns of vegetation and soil fertility in a desert steppe. Agr Ecosyst Environ 138:282–292
Lindsay EA, Colloff MJ, Gibb NL, Wakelin SA (2010) The abundance of microbial functional genes in grassy woodlands is influenced more by soil nutrient enrichment than by recent weed invasion or livestock exclusion. Appl Environ Microbiol 76:5547–5555
Liu ZF, Liu GH, Fu BJ, Zheng XX (2007) Relationship between plant species diversity and soil microbial functional diversity along a longitudinal gradient in temperate grasslands of Hulunbeir, Inner Mongolia, China. Ecol Res 23:511–518
Luo J, Ledgard SF, Lindsey SB (2008) A test of a winter farm management option for mitigating nitrous oxide emissions from a dairy farm. Soil Use Manage 24:121–130
Ma JY, Sun W, Liu XN, Chen FH (2012) Variation in the stable carbon and nitrogen isotope composition of plants and soil along a precipitation gradient in northern China. PLoS ONE 7(12):e51894
Maag M, Vinther FP (1996) Nitrous oxide emission by nitrification and denitrification in different soil types and at different soil moisture contents and temperatures. Appl Soil Ecol 4:5–14
Mao YJ, Yannarell AC, Davis SC, Mackie RI (2013) Impact of different bioenergy crops on N-cycling bacterial and archaeal communities in soil. Environ Microbiol 15:928–942
Meyer A, Focks A, Radl V, Keil D, Welzl G, Schoning I, Boch S, Marhan S, Kandeler E, Schloter M (2013) Different land use intensities in grassland ecosystems drive ecology of microbial communities involved in nitrogen turnover in soil. PLoS ONE 8(9):e73536
Michotey V, Mejean V, Bonin P (2000) Comparison of methods for quantification of cytochrome cd1-denitrifying bacteria in environmental marine samples. Appl Environ Microbiol 66:1564–1571
Mirza BS, Potisap C, Nüsslein K, Bohannan BJM, Rodrigues JLM (2014) Response of free-living nitrogen-fixing microorganisms to land use change in the Amazon rainforest. Appl Environ Microbiol 80:281–288
Oenema O, Oudendag D, Velthof GL (2007) Nutrient losses from manure management in the European Union. Livest Sci 112:261–272
Okano Y, Hristova KR, Leutenegger CM, Jackson LE, Denison RF, Gebreyesus B, Lebauer D, Scow KM (2004) Application of real-time PCR to study effects of ammonium on population size of ammonia-oxidizing bacteria in soil. Appl Environ Microbiol 70:1008–1016
Patra AK, Abbadie L, Clays-Josserand A, Degrange V, Grayston SJ, Guillaumaud N, Loiseau P, Louault F, Mahmood S, Nazaret S, Philippot L, Poly F, Prosser JI, Le Roux X (2006) Effects of management regime and plant species on the enzyme activity and genetic structure of N-fixing, denitrifying and nitrifying bacterial communities in grassland soils. Environ Microbiol 8:1005–1016
Pereira e Silva MC, Schloter-Hai B, Schloter M, van Elsas JD, Salles JF (2013) Temporal dynamics of abundance and composition of nitrogen-fixing communities across agricultural soils. PLoS ONE 8(9):e74500
Philippot L, Cuhel J, Saby NP, Cheneby D, Chronakova A, Bru D, Arrouays D, Martin-Laurent F, Simek M (2009) Mapping field-scale spatial patterns of size and activity of the denitrifier community. Environ Microbiol 11:1518–1526
Reynolds JF, Stafford Smith D (2002) Do humans cause deserts? Global desertification: do humans cause deserts? Dahlem workshop report 88. Dahlem University Press, Berlin, pp 1–21
Rosch C, Bothe H (2005) Improved assessment of denitrifying, N2-fixing, and total-community bacteria by terminal restriction fragment length polymorphism analysis using multiple restriction enzymes. Appl Environ Microbiol 71:2026–2035
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Saggar S, Bolan NS, Bhandral R, Hedley CB, Luo J (2004) A review of emissions of methane, ammonia, and nitrous oxide from animal excreta deposition and farm effluent application in grazed pastures. New Zeal J Agr Res 47:513–544
Schönbach P, Wan HW, Gierus M, Bai Y, Müller K, Lin LJ, Susenbeth A, Taube F (2010) Grassland responses to grazing: effects of grazing intensity and management system in an Inner Mongolian steppe ecosystem. Plant Soil 340:103–115
Sheik CS, Beasley WH, Elshahed MS, Zhou X, Luo Y, Krumholz LR (2011) Effect of warming and drought on grassland microbial communities. ISME J 5:1692–1700
Sorensen PO, Germino MJ, Feris KP (2013) Microbial community responses to 17 years of altered precipitation are seasonally dependent and coupled to co-varying effects of water content on vegetation and soil C. Soil Biol Biochem 64:155–163
Szukics U, Abell GC, Hodl V, Mitter B, Sessitsch A, Hackl E, Zechmeister-Boltenstern S (2010) Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil. FEMS Microbiol Ecol 72:395–406
Vitousek PM, Hattenschwiler S, Olander L, Allison S (2002) Nitrogen and nature. Ambio 31:97–101
Wakelin SA, Gregg AL, Simpson RJ, Li GD, Riley IT, McKay AC (2009) Pasture management clearly affects soil microbial community structure and N-cycling bacteria. Pedobiologia 52:237–251
Wakelin SA, Macdonald LM, Rogers SL, Gregg AL, Bolger TP, Baldock JA (2008) Habitat selective factors influencing the structural composition and functional capacity of microbial communities in agricultural soils. Soil Biol Biochem 40:803–813
Walker JK, Egger KN, Henry GH (2008) Long-term experimental warming alters nitrogen-cycling communities but site factors remain the primary drivers of community structure in high arctic tundra soils. ISME J 2:982–995
Wang ZW, Jiao SY, Han GD, Zhao ML, Ding HJ, Zhang XJ, Wang XL, Ayers EL, Willms WD, Havsatad K, Liu YZ (2014) Effects of stocking rate on the variability of peak standing crop in a desert steppe of Eurasia grassland. Environ manage 53:266–273
Wen HY, Niu DC, Fu H, Kang J (2013) Experimental investigation on soil carbon, nitrogen, and their components under grazing and livestock exclusion in steppe and desert steppe grasslands, Northwestern China. Environ Earth Sci 70:3131–3141
Wolf B, Zheng XH, Bruggemann N, Chen WW, Dannenmann M, Han XG, Sutton MA, Wu HH, Yao ZS, Butterbach-Bahl K (2010) Grazing-induced reduction of natural nitrous oxide release from continental steppe. Nature 464:881–884
Xu YQ, Li LH, Wang QB, Chen QS, Cheng WX (2007) The pattern between nitrogen mineralization and grazing intensities in an Inner Mongolian typical steppe. Plant Soil 300:289–300
Xu YQ, Wan SQ, Cheng WX, Li LH (2008) Impacts of grazing intensity on denitrification and N2O production in a semi-arid grassland ecosystem. Biogeochemistry 88:103–115
Yao HY, Campbell CD, Chapman SJ, Freitag TE, Nicol GW, Singh BK (2013) Multi-factorial drivers of ammonia oxidizer communities: evidence from a national soil survey. Environ Microbiol 15:2545–2556
Yergeau E, Bokhorst S, Kang S, Zhou JZ, Greer CW, Aerts R, Kowalchuk GA (2012) Shifts in soil microorganisms in response to warming are consistent across a range of Antarctic environments. ISME J 6:692–702
Zhang XM, Liu W, Schloter M, Zhang GM, Chen QS, Huang JH, Li LH, Elser JJ, Han XG (2013) Response of the abundance of key soil microbial nitrogen-cycling genes to multi-factorial global changes. PLoS ONE 8(10):e76500
Zhong L, Du R, Ding K, Kang XM, Li FY, Bowatte S, Hoogendoorn CJ, Wang YF, Rui YC, Jiang LL, Wang SP (2014) Effects of grazing on N2O production potential and abundance of nitrifying and denitrifying microbial communities in meadow-steppe grassland in northern China. Soil Biol Biochem 69:1–10
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol R 61:533–616
Acknowledgments
This study was supported by the National Sciences Foundation of China (30830026), the Knowledge Innovation Program of the China Academy of Science (Y12501BES2), and the National Youth Science Foundation of China (grant no. 31300417).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jizheng He
Rights and permissions
About this article
Cite this article
Ding, K., Zhong, L., Xin, X.P. et al. Effect of grazing on the abundance of functional genes associated with N cycling in three types of grassland in Inner Mongolia. J Soils Sediments 15, 683–693 (2015). https://doi.org/10.1007/s11368-014-1016-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-014-1016-z