Abstract
Purpose
Arsenic (As) contamination of groundwater has received significant attention recently in district Bathinda, due to consequent health risk in this region. Soil is the one of the primary medium for arsenic transport to groundwater. Thus, there is an essential requirement for understanding the retention capacity and mobility of arsenic in the soils to ensure sustainability of the groundwater in the locality. Arsenic interaction with various physicochemical properties of soil would provide a better understanding of its leaching from the soil.
Materials and methods
Fifty-one soil samples were collected from two regions of Bathinda district with extensive agricultural practices, namely, Talwandi Sabo and Goniana. The soils were analyzed for arsenic content and related physicochemical characteristic of the soil which influence arsenic mobility in soil. Adsorption studies were carried out to identify the arsenic mobilization characteristic of the soil. SEM-EDX and sequential extraction of arsenic adsorbed soil samples affirmed the arsenic adsorption and its mobility in soil, respectively. Multiple regression models have been formulated for meaningful soil models for the prediction of arsenic transport behavior and understand the adsorption and mobilization of arsenic in the soil matrices.
Results and discussion
Region-wise analysis showed elevated levels of arsenic in the soil samples from Goniana region (mean 9.58 mg kg−1) as compared to Talwandi Sabo block (mean 3.38 mg kg−1). Selected soil samples were studied for As(V) and As(III) adsorption behavior. The characteristic arsenic adsorption by these soil samples fitted well with Langmuir, Freundlich, Temkin, and D-R isotherm with a q max in the range of 45 to 254 mg kg−1 and 116 to 250 mg kg−1 for As(III) and As(V), respectively. Adsorption isotherms indicate weak arsenic retention capacity of the soil, which is attributed to the sandy loam textured soil and excessive fertilizer usage in this region. PCM and MLR analysis of the soil arsenic content and its adsorption strongly correlated with soil physicochemical parameters, namely, Mn, Fe, total/available phosphorus, and organic matter.
Conclusions
Manganese and iron content were firmly established for retention of arsenic in soil, whereas its mobility was influenced by organic matter and total/available phosphorus. The poor adsorptive characteristic of these soils is the primary cause of higher arsenic concentration in groundwater of this region. A strong correlation between monitored arsenic and adsorbed As(III) with manganese suggests As(III) as the predominant species present in soil environment in this region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Arsenic (As) contamination of soil is one of the great environmental concern and serious problem worldwide (Smedley and Kinniburgh 2002), due to its high toxicity and carcinogenicity potential. Even, a low arsenic content in natural soil is toxic because of its ease of solubility (Fernández et al. 2005). The current permissible limit of arsenic in agricultural soils is 20 mg kg−1. Various natural and anthropogenic sources are responsible for the presence of arsenic in soil. Natural sources include weathering of rocks and minerals, geothermal activities, and volcanic eruption, further complicated by the characteristic geology, geomorphology, and hydrology of the locality (Smedley and Kinniburgh 2002; Wang and Mulligan 2006). However, major anthropogenic sources of arsenic include industrial waste, sewage sludge, incineration, wood treatment, mining/smelting operations, and use of agricultural chemicals, which are a common practices throughout the world (Smedley and Kinniburgh 2002; Wang and Mulligan 2006). Soil behaves as a central medium for the environmental cycle of arsenic. Soil behaves as a medium, whether arsenic is transported to groundwater or food chain by plants. Soil medium plays a significant role in the retention and mobilization of arsenic, influenced by arsenic species and soil physicochemical properties.
The toxicity and mobility of arsenic depend upon its speciation and oxidation state (Smedley and Kinniburgh 2002). Arsenic exists mainly as two species, namely, arsenate [As(V)] and arsenite [As(III)], which are transported to surface and groundwater by soil and sediment mineral surfaces. Adsorption is the one of the main mechanism for controlling the mobility and transport of arsenic in the soil-water system. The adsorption of both the species in soil is governed by physicochemical characteristics, like iron (Fe), manganese (Mn), aluminum (Mn), organic matter (OM), clay content, pH, and phosphate (Dias et al. 2009; Mello et al. 2006; Feng et al. 2013; Sharma et al. 2010). The dynamic changes in these physicochemical characteristics under the influence of various anthropogenic/natural activities leads to the leaching of arsenic from the soil. Thus, soil’s physicochemical characteristics and its binding affinity for arsenic are essential for understanding the dynamic behavior in a particular local environment.
Iron as its oxide/hydroxide form is key component involved in the immobilization of arsenic on to the soil. Iron oxides, hydroxides, and oxyhydroxides have affinity for arsenic and increase its adsorption in soil (Adegoke et al. 2013). Naturally occurring iron oxy-hydroxy surfaces, as in cases of goethite (Sun and Doner 1996), hematite (Sherman and Randall 2003), and ferrihydrite (Sherman and Randall 2003), have affinity to bind and immobilize both arsenate and arsenite ions. Extended X-ray absorption fine structure (EXAFS) and Fourier transform infrared spectroscopy (FTIR) studies of minerals have revealed the formation of monodentate or bidentate inner-sphere surface complexes for both forms of arsenic [As(V) and As(III)] with iron-oxide via a ligand exchange mechanism (Cances et al. 2005; Sun and Doner 1996). EXAFS study also showed that adsorption on oxide surfaces play a critical role in the mobility of As in the soil environment (Cances et al. 2005). Apart from iron, manganese (Mn) in its oxide form has affinity for binding and immobilizing arsenic in the soil. However, Mn differs in the mode of immobilization and uptake of both As(III) and As(V) (Deschamps et al. 2003) takes place with oxidation of As(III) to As(V) (Sun and Doner 1996; Deschamps et al. 2003; Ouvrard et al. 2005). Phosphorus on the other hand competitively inhibits the binding of metal oxide sites for both As(V) and As(III) and enhance their mobilization in soil (Cui and Weng 2013). Organic matter (OM) has a dual role in regulating arsenic mobility in soil. OM may compete strongly with As(V) and As(III) for active adsorption sites on mineral surfaces with enhanced mobilization of arsenic (Sharma et al. 2010; Kar et al. 2011). However, OM can immobilize arsenic by either direct adsorption or by assisting adsorption on to the surface of iron oxides as a chelating ligand (Ritter et al. 2006). These studies have been extended to arsenic adsorption by soil using analytical and statistical approaches (Dias et al. 2009; Martin et al. 2014; Moreno-Jiménez et al. 2013; Sahoo et al. 2013; Yin et al. 2015).
Several studies on arsenic in groundwater and soil have already been reported in the alluvial Gangetic plains in Bihar, West Bengal, and Bangladesh (Hossain et al. 2008; Saha et al. 2010; Majumder et al. 2014). Groundwater in Punjab (India), particularly, the alluvial plains of southwest region of Sutlej, including Bathinda district, is affected by arsenic (Hundal et al. 2007, 2013; Sharma et al. 2013). Significant health risk prevails in Bathinda district, with an increase in the incidence of premature graying of hairs, ageing, infertility, abortion, development delay, and cancer (Halder 2007; Mittal et al. 2014). These health risks are associated with elevated levels of arsenic in the groundwater of this region ranging from 5 to 688 μg l−1 (Hundal et al. 2007, 2013; PGIMER 2007; Vicky-Singh et al. 2010). The presence of arsenic in groundwater is attributed to various anthropogenic interventions including excessive usage of agricultural fertilizers (Farooqi et al. 2009; Atafar et al. 2010), pesticides (Hundal et al. 2013), coal fly-ash (EPRI, USA 2009) from thermal power plant, water stagnated paddy field (Roberts et al. 2012; Hundal et al. 2013; Sahoo et al. 2013), and geological and geomorphological background of the district. However, in the majority of these sources, soil behaves as a medium for transport of arsenic in groundwater. Two independent studies by Hundal et al. (2007) and Vicky-Singh et al. (2010) showed a large variation in the soil arsenic content in this region. Recently, Hundal et al. (2013) studied the arsenic mobilization in the paddy fields of Punjab and attributed the mobility to the change in the oxidation-reduction potential (ORP) of the flooded lands. Hundal et al. (2009) carried out preliminary study of arsenic adsorption behavior of the alluvial soil in this region in presence of iron filling and found significantly increased adsorption of arsenic in the soil samples due to the presence of iron oxide. However, these results were preliminary and a more comprehensive study on monitoring and the leaching behavior of the arsenic in soil with relation to various physicochemical parameters of soil are required to be investigated. However, adsorption based physicochemical approach may provide a solution to the arsenic mobility in the soil of in this region. Thus, in the present work, two agriculturally intensive regions namely Talwandi Sabo and Goniana region of Bathinda district were selected for arsenic monitoring of arsenic and its adsorption behavior in soil. Further, a statistical correlation has been derived between various physicochemical properties of soil, which influence the arsenic adsorption and mobilization. The arsenic adsorption characteristic of soil is confirmed by the sequential extraction analysis and scanning electron microscopy energy-dispersive X-ray (SEM-EDX) analysis.
2 Materials and methods
2.1 Site description
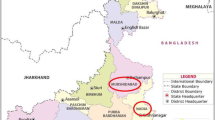
The sampling sites of Bathinda district lie between latitudes 29° 85′ N to 30° 32′ N and longitudes 74° 67′ E to 75° 28′ E. Talwandi Sabo block is situated southeast of Bathinda city, whereas Goniana is located northwest of Bathinda city. The area of Talwandi Sabo was selected based on the previous reports on the presence of arsenic in groundwater in this region. This area has scorching summers, cold winter, and moderate rainy seasons. The average annual rainfall and temperature of Bathinda district is between 20 to 40 mm and 1–49 °C, respectively. The height from sea level is in the range of 200–202 m. The locations of sampling sites were mapped using Arc GIS 10.1 software (Fig. 1).
2.2 Soil sampling
Total of 51 soil samples were collected from agricultural fields during October–December, 2013, to ensure both geographic and taxonomic representation. Twenty-nine villages were selected in the Talwandi Sabo block, whereas 22 villages in Goniana region of Bathinda block. Soil samples were collected in polythene bag from agricultural fields with the help of soil corer from a depth of 5–15 cm. Soil sample (2 kg) were taken from each sampling site. All soil samples were kept in plastic bags, double sealed, coded, and transported to the laboratory. Soil samples were air-dried at room temperature and sieved through 2.36 mm sieve, transferred to new polythene bags and physicochemical characterizations were carried out. However, bulk density was analyzed directly using soil corer, collected from the field without drying.
The texture of the soil found to be predominantly sandy loam to silt. Four types of soil profiles have been identified in the study area in both regions with the help of Indian standard method (IS 1985) and soil map provided by the Department of Soil and Water Conservation, Govt. of Punjab (DSWC 2014). These soil profiles are as given below with their respective codes (i), (ii), (iii), and (iv). The soil profiles of all 51 soil samples are provided individually in the Electronic Supplementary Material (S1.3).
-
1.
Coarse loamy soil, well drained (Ustic Hamlocambids)/sandy soils, excessively drained (Ustic Torripsamments)—14 samples
-
2.
Coarse loamy soil, well drained (Ustic Hamlocambids)/sandy over loamy soils, somewhat well drained (Ustic Hamlocambids)—23 samples
-
3.
Sandy soil, excessively drained (Ustic Torripsamments)/coarse loamy soil, well drained (Ustic Hamlocambids)—11 samples
-
4.
Coarse loamy soil, well drained (Typic Ustochrepts)/fine loamy soil, well drained (Typic Ustochrepts)—3 samples
2.3 Physicochemical analysis of soil samples
pH and electrical conductivity (EC) of soil samples were determined by IS method (IS 1987, 2000). Soil organic carbon and organic matter were determined by Walkley-Black rapid titration method (Walkley 1947). Total phosphorus and available phosphorus of soil samples were determined using Allen’s method (Jackson 1973) and Olsen method (Olsen 1954), respectively. Soil sample of each sample (0.5 g) was added to 10 ml of 1:2 (v/v) mixtures of nitric acid and perchloric acid and digested till the appearance of white fumes (Amin et al. 2014). The solution was cooled by adding 25 ml double-distilled water and filtered using Whatman filter paper no. 1. The residue was repeatedly washed with distilled water and the recovered filterate was diluted to 100 ml. Arsenic in micrograms per liter was analyzed by atomic absorption spectrophotometer (AAS), using graphite furnace analyzer (GFA), whereas Mn and Fe in milligrams per liter were analyzed by flame atomic absorption method (Nan et al. 2002).
2.4 Batch adsorption of soil for arsenate [As(V)] and arsenite [As(III)]
Ionic strength adjusted As(V) and As(III) solutions of concentration 10, 25, 50, 75, 100, 150, and 200 mg l−1 in 5 mM CaCl2 medium were prepared. To a 25 ml of As(V) or As(III) solution in 100-ml conical flask was added 5 g of soil. The samples were equilibrated for 48 h on an orbital shaker maintained at 150 rpm at 25 ± 2 °C.
Upon equilibration, the samples were transferred to a centrifuge tube and centrifuged at 6000 rpm for 10 min. The solution was filtered and filtrate was analyzed using flame atomic absorption spectrometry. The data was fitted to various isotherms, namely, Langmuir, Freundlich, Tempkin, and Dubinin–Radushkevich isotherms to derive kinetic and thermodynamic parameters for the adsorption of As(III) and As(V) to the soil samples. The details of Langmuir, Freundlich, Tempkin, and Dubinin–Radushkevich isotherms are given in the Electronic Supplementary Material.
2.5 Determination of arsenate [As(V)] in saturated soil by sequential extraction procedure
The sequential extraction procedure was carried out to determine the distribution and mobility of arsenic in different soil fractions. Batch experiment was performed for various soil samples (10 g) by equilibration with 100 ml arsenate (1000 mg l−1) solution. One gram of arsenate adsorbed soil sample was taken in a 50-ml centrifuge tube, and 25 ml of different extraction reagents was added sequentially and stirred for the respective time intervals (Table 1). The supernatant solution was separated by centrifugation (6000 rpm, 10 min) between each successive extraction step and filtered through Whatman paper. The extractants were kept at 4 °C in refrigerator until analyzed using atomic absorption spectrometry. The residue was further used for subsequent sequential extraction procedure.
2.6 Microscopic characterization of soil
The soil samples were examined for morphological and elemental analysis by SEM-EDX analysis using ZEISS Merlin Compact Field Emission SEM equipped with Oxford X Maxn EDX system. Prior to imaging and elemental analysis, the samples were transferred to carbon tape and gold-coated using Q150R ES (QUORUM Inc.) vacuum plasma coating.
2.7 Statistical analysis
Box plot was used for the comparison of arsenic content in Talwandi Sabo block and Goniana region and plotted using Origin software v8.1. Multiple linear regression (MLR) and Pearson correlation matrix (PCM) analysis were performed for the statistical analysis of arsenic adsorption efficiency of As and its association with soil parameters. MLR and PCM analyses were carried out by SPSS software v18 and Excel Microsoft office 2010, respectively. The stepwise backward and forward procedures were followed in MLR analysis. In this study, the forward stepwise linear regression was employed as the regression procedure, which means that the best regressor was the first one to be included in the model, followed by the second variable and so on. The cutoff value 0.5 is used in this procedure. This model is counter checked by backward regression procedure at a significance level of 0.05. In backward linear regression model, all the independent variables were included and the nonsignificant variable subsequently omitted from the model.
3 Results and discussion
3.1 Monitoring of arsenic content and its correlation with physicochemical characteristics of soil
The physicochemical characterization of soil samples, namely pH, electrical conductivity (EC), bulk density, organic matter (OM), organic carbon (OC), total phosphorus (TP), available phosphorus (Ava P), arsenic (As), manganese (Mn), and iron (Fe) were carried out. The region-wise summary of the physicochemical characteristics of the soil samples is given in Table 2. However, the details of the soil type and physicochemical characteristics of soil samples are provided in the Electronic Supplementary Material (Table A1).
The pH analyses of these soils indicate that all the soil samples were predominantly alkaline in nature. The EC of the soil samples were in the range 0.08–0.99 dS m−1, which is <1 dS m−1. Thus, the soil samples from both the regions were non-saline and had no impact on crops as well as soil microbial processes (USDA-NRCS 2014b). The bulk density of these soil samples lie between 1.10 and 1.20 g cm−3, indicating soil suitability for agriculture (USDA-NRCS 2014a), due to its ideal compactness in both the regions. The soil samples from Talwandi Sabo and Goniana region upon comparison show similar physicochemical properties in case of pH, bulk density, and EC. However, the difference exists in the parameters like OC/OM, total P, available P, Fe, Mn, and As. Total and available phosphorus are high in soil samples collected from Talwandi Sabo as compared to Goniana region. The mean Mn was found to be higher in soil samples from Goniana region than Talwandi Sabo region, whereas Fe content is found to be higher in soil samples of Talwandi Sabo region. The arsenic content is found to be significantly higher in soil samples of agricultural fields in Goniana region than Talwandi Sabo block (Table 2 and Fig. 2). In spite of high Fe content, arsenic is found to be low in Talwandi Sabo, which is attributed to the higher total/Ava P and low Mn found in this region. Total/Ava P leads to the leaching of immobilized arsenic and subsequent mobilization from the soil samples (Smith and Naidu 2009; Vithanage et al. 2014).
Agricultural practices like groundwater irrigation, paddy cultivation, and fertilizer usage account for the difference in the arsenic content of both regions. Most parts of agricultural fields in Talwandi Sabo block are irrigated by canal water mainly from the Sirhind canal system (Kotla branch) and Bhakra canal (main branch) system (Thomas et al. 1995; CGWB 2007), whereas, agricultural fields in Goniana region depend more on groundwater as compared to canal water for irrigation. This is further supported by the higher gross groundwater draft for irrigation in Bathinda block (12,729 ha m) than Talwandi Sabo region (6395 ha m) (CGWB 2011), which in case of 2004 was under safe limits for groundwater exploitation (CGWB 2007). The presence of significant amount of arsenic in the groundwater has already been reported in Bathinda district (Vicky-Singh et al. 2010; Hundal et al. 2013; Sharma et al. 2013). Thus, groundwater irrigation is one of the probable sources of arsenic in agricultural soils of this region (Vicky-Singh et al. 2010). Further, land cover under paddy cultivation is more in Goniana region as compared to Talwandi Sabo (DCP Bathinda 2012-13). Paddy cultivation requires large quantities of water for irrigation, which may cause arsenic contamination of the soil (Roberts et al. 2012; Sahoo et al. 2013). Apart from this, paddy fields are characterized by high clay content, which leads to increased surface area and subsequently higher arsenic adsorption (Manning and Goldberg 1997; Weiss and Khondoker 2013).
3.2 Spatial arsenic distribution and its statistical correlation with physicochemical properties of soil
The interaction of physicochemical properties of soil with arsenic was statistically correlated, using PCM and MLR analysis. All the soil parameters, namely, pH, OM, TP, Ava P, Fe, Mn, and As were included in PCM and MLR analysis of all the soil samples as given in Table 3 and Eq. (1), respectively. Further, for the assessment of variation in arsenic retention, the soils were segregated region-wise and analyzed by PCM and MLR analysis [Tables 4 and 5, Eqs. (2) and (3)], respectively.
PCM analysis of all 51 soil samples showed a positive correlation between total As and Mn content (Table 3). MLR analysis also confirmed the findings of PCM (Eq. (1)). The statistical analysis revealed that Mn is associated with arsenic retention in the soil, while phosphorus is responsible for the transport of arsenic in these soils.
The PCM analysis (Tables 4 and 5) of both the regions (Talwandi Sabo and Goniana) carried out individually, showed a positive correlation of Mn and Fe with total arsenic content of soil samples. However, a strong correlation was found in the case of Goniana region. MLR also reaffirmed the findings of the PCM analysis, whereby Mn plays a significant role in retaining arsenic. Soil monitoring studies based on MLR and PCM analysis revealed similar correlation between soil immobilized arsenic and the physicochemical characteristics of soil (Moreno-Jiménez et al. 2013; Sahoo et al. 2013; Martin et al. 2014; Yin et al. 2015). In particular, Martin et al. (2014) showed that arsenic content of alluvial soils in Bangladesh significantly correlated with Fe and Mn in these soil samples. However, the soil monitoring of arsenic do not provide any information on arsenic retention and transport behavior of the soil. Thus, for the ratification of results of the above findings, adsorption and sequential extraction were carried out for selected soil samples in this region.
3.3 Studies in the arsenic adsorption behavior of soil from Bathinda district
The adsorption of arsenic by the soil was accessed by batch adsorption. Ten soil samples, namely, PBT-2, PBT-8, PBT-11, PBT-14, PBT-23, PBT-24, PBT-34, PBT-45, PBT-49, and PBT-50 were selected for the adsorption study, on the basis of their soil physicochemical properties. Adsorption studies were performed for both arsenate and arsenite species. All the soil samples showed significant adsorption of As(V) and As(III) from ionic strength maintained aqueous As(V) and As(III) solution (0–200 mg l−1). The equilibrium adsorption (q e ) of all the soil samples were found to increase with increase in the equilibrium concentration of As(V) and As(III) solution (Electronic Supplementary Material, A2 section). However, the adsorption behavior of soil samples PBT-2 (Talwandi Sabo region) and PBT-49 (Goniana region) are depicted in Fig. 3a, b, for As(V) and As(III), respectively.
The equilibrium data of As(V) and As(III) adsorption in soil samples were analyzed using linear isotherm fitting. The linearized form of Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich (D-R) isotherms equations were used for comprehensive analysis of As(III) and As(V) adsorption behavior in soil (Electronic Supplementary Material, S2). The thermodynamic and kinetic parameters for adsorption were obtained from Langmuir, Freundlich, Temkin, and D-R isotherms, as given in Tables 6 and 7, for arsenate and arsenite, respectively.
The Langmuir isotherm model was selected for the estimation of maximum adsorption capacity corresponding to complete monolayer coverage on the soil surface (adsorbent). The specific sorption (C e /q e ) was plotted against the equilibrium concentration C e for the soil samples of arsenite adsorbed samples, whereas 1/q e versus 1/C e was plotted in case of arsenate adsorption equilibrium for the soil samples (Ho et al. 2005), as shown in the Electronic Supplementary Material (Fig. S3). However, the Langmuir isotherm plots of sample PBT-2 and PBT-49 upon adsorption of arsenate and arsenite are depicted in Fig. 4a, b. The linear isotherm constants q m and K L , and the regression coefficient R 2, are as given in Tables 6 and 7 for arsenate and arsenite, respectively. The maximum adsorption of the soil samples was found to lie in the range 120 to 250 mg kg−1for As(V) (Table 6), while in case of As(III), it was found to vary between 45 to 254 mg kg−1 (Table 7). High R 2 value found in Langmuir isotherms plots suggested that the monolayer behavior is linear over the entire concentration range. Separation factor (S F ) provides information about favorability of adsorption. The separation parameters (S F ) for all soil samples for both arsenic species lie below 1, which indicates that soil is an appropriate adsorbent for arsenic. Region-wise analysis of arsenic adsorption, soil samples from Talwandi Sabo region, namely, PBT-2, PBT-8, PBT-11, PBT-14, PBT-23, and PBT-24 showed higher adsorption (q m ) for As(III) and As(V) than the soil samples from Goniana region (PBT-34, PBT-45, PBT-49, PBT-50). Further, soil samples from Goniana region showed high adsorption for As(V) in comparison to As(III) and the reverse trend was observed in soil samples from Talwandi Sabo.
The Freundlich model was used to estimate the adsorption intensity for the soil adsorption of arsenate and arsenite ions. The adsorption intensity 1/n was found to lie below 1 in all the soil samples for both the arsenic species, which confirmed normal adsorption for both the arsenic species. The low 1/n shows nonlinear behavior of adsorption for entire concentration range (Manning and Goldberg 1997; Zhang and Selim 2005; Yolcubal and Akyol 2008). Further, low 1/n values for all the soil samples indicate the extensive heterogeneity of adsorption sites in these soils (Zhang and Selim 2005). The intensity factor further indicates strong multilayer adsorption in case of As(III) as compared to As(V). This is due to the existence of nonionic species in comparison to negatively charged As(V) species under similar condition leading to electrostatic repulsion in the later (Dias et al. 2009).
Langmuir and Freundlich isotherms do not explain mechanism of adsorption; thus, Temkin and D-R isotherms were fitted for the thermodynamic analysis of arsenate and arsenite adsorption by soil samples. Temkin isotherm contains a factor that explicitly takes into account the thermodynamics of adsorbate–adsorbent interactions. By ignoring the extremely low and large value of concentrations, the model assumes that heat of adsorption (function of temperature) of all molecules in the layer would decrease linearly rather than in a logarithmic coverage. Arsenic implied in the equation, its derivation is characterized by a uniform distribution of binding energies (up to some maximum binding energy) was carried out by plotting the quantity sorbed q e against ln C e . The adsorption data of As(V) is good fit in Temkin isotherm model. Adsorption potentials (A T ) and constant for energy of adsorption (B) were calculated from Temkin adsorption isotherm model (Shahmohammadi-Kalalagh et al. 2011). The soil samples showed lower R 2 value for arsenic(III) adsorption by the soil samples PBT-24, PBT-34, PBT-45, PBT-49, and PBT-50, thus do not fit the Temkin isotherm in this particular case. The low values for adsorption potential and constant B indicate a weak interaction between adsorbate [As(V) and As(III)] and soil.
D-R isotherm is generally applied to express the adsorption mechanism with a Gaussian energy distribution onto a heterogeneous surface. D-R isotherm introduces the term Polanyi potential (ε), which is a function of C e and temperature (T). The equilibrium data for soil adsorption of As(III) and As(V) was fitted to the D-R isotherm model. The plot between ln q e (arsenic adsorbed per unit weight of adsorbent) and ε 2 (square of Polanyi potential) is shown in the Electronic Supplementary Material (Section S3.4). The D-R isotherm model showed the mean free energy of adsorption to lie in the range of 2.33–4.77 and 1.95–5.59 kJ mol−1 for adsorption of As(V) and As(III), respectively. However, PBT-2 showed an exceptionally higher value for E of 12.91 kJ mol−1, which indicates ion exchange type adsorption of arsenite in this soil sample. The values of E in all other soil samples were <8 kJ mol−1 for adsorption of both the arsenic species. So, adsorption in these soil samples was accounted to be physiosorption-type binding of arsenate and arsenite to these soil samples. Thus, the D-R isotherm fitting of the equilibrium adsorption data indicates weak van der Waals force of attraction between adsorbent (soil) and adsorbate (As(III) and As(V)).
The weak attraction between soil and arsenic is accounted to the excessive use of fertilizer for agricultural practices in this region leading to increase in the available phosphorus content in the soil (Dhawan et al. 2014). Dhawan et al. (2014) reported the fertilizer usage of more than twice the amount of urea and 50 % for DAP above the recommended levels for cotton cropping in Bathinda district. Phosphate displaces arsenic effectively (Peryea 1998) and increases leachability of arsenic in soil (Vithanage et al. 2014). However, urea reduces arsenic retention capacity of iron oxide by increasing the pH or by lowering electrostatic force of attraction (Dias et al. 2009; Kettering et al. 2013; Vithanage et al. 2014) of soil solution leading to subsequent formation of OH− ion, which competes for soil adsorption surface (Kettering et al. 2013). Thus, urea and phosphate mobilize arsenic in the soil and enhance its leaching from iron oxide surface. Further, alkaline condition with pH >8.5 increase desorption of arsenic from iron oxide surfaces spontaneously (Dzombak 1990).
3.4 Regression analysis of As(V) or As(III) adsorption with physicochemical characteristics of the soil in Bathinda district
3.4.1 Regression model of As(V) and As(III) adsorption
The MLR analysis of As(V) and As(III) adsorption characteristics of the soil were studied. The analysis established relation between maximum adsorption capacity (q m ) obtained from Langmuir adsorption isotherm and various physicochemical parameters of the soil. The predicted regression models were determined by forward regression procedure, as given in Tables 8 and 9. The expression for the regression analysis of As(V) and As(III) are given in Eqs. (4) and (5), respectively.
The results suggest As(V) adsorption by soil samples were strongly correlated to available phosphorus, Fe, and OM. A positive correlation was observed with Fe, whereby an increase in the Fe content increased the As(V) adsorption. However, a negative correlation was found between adsorbed As(V) with OM and available phosphorus in the soil. Thus, available phosphorus and OM enhance the mobility of As(V) in these soil samples (Vithanage et al. 2014). Similarly, MLR analysis of As(III) adsorption by soil samples showed Mn as an important soil physicochemical parameter, followed by total Fe, OM, and Ava P. Mn and Fe are most important factors for As(III) adsorption, whereas available P and OM are responsible for the mobility of arsenite species (Eq. (5)).
3.5 Sequential extraction studies on bound arsenic in soil samples from Bathinda district
Sequential extraction is a widely used technique for the identification of binding sites and potential mobility of arsenic in soil. In the present study, sequential extraction studies for As(V) were carried out using arsenic saturated soil samples to elucidate the oxanion binding to various phases in the soil. Further, these results would substantiate the findings of the statistical analysis. The results of the sequential extraction of As(V) from these soil samples are as given in Table 10.
The study showed extraction in step F1 to be in the range of 34.27–87.77 % for the analyzed soil samples. F1 step represents easily exchangeable arsenic, which binds to soil and form outer sphere complexes. The study shows that maximum amount of arsenic is released in this step, suggesting mobile nature of arsenic bound to the soil, which can be released easily into soil water through ion exchange (Zhang et al. 2013). Further, significant extraction of arsenic was found in step F2 and F5. In case of step F2, the range of extraction varied from 5.59–25 %, which indicates the formation of strongly bound inner-sphere surface complex between arsenic and metal oxide, which is extracted by phosphate with competitive ligand exchange (Wenzel et al. 2001; Yolcubal and Akyol 2008). The selective extraction in step F5 indicates strongly adsorbed arsenic bound to Fe oxyhydroxide surface (Yolcubal and Akyol 2008), with an extraction of up to 27.83 % arsenic in this stage. Thus, the extraction in step F5 indicates the significant role of Fe in the retention or immobilization of As(V) oxoanion. These results corroborate with our statistical analysis of the adsorption data, particularly in case of Fe participation in the binding of arsenate [As(V)] (Eq. (4)). Further, the sequential extraction indicates that carbonate and Mn adsorbed As(V) showed lower extractable As(V) in comparison with Fe and Al, and other complexation phenomenon. This could partially be explained by the significant participation of Fe in comparison to manganese for complexation of As(V). The soil samples studied show the percentage adsorbed arsenic present in the sample decreased in the order resin extractable > Fe, Al oxide > inner-sphere complex. Thus, the soil samples show a very low retention of As(V), which leads to its enhanced transport to groundwater.
The soil pH, presence of minerals like Fe and Mn, competitive anions like phosphate and organic matter, affect arsenic retention and its mobility. Iron (hydr)oxides act as a preferential substrate for arsenic immobilization in soils by the adsorption phenomenon (Sun and Doner 1996; Sherman and Randall 2003). Statistical analysis of adsorption study also indicates the affinity to Fe for both arsenic species (arsenate and arsenite). Further, sequential extraction studies confirmed that significant amount of As(V) is bound to surface by complexation (Table 10) (Zhang and Selim 2005). The role of Mn is quite evident for arsenic immobilization in both monitoring and As(III) adsorption. It is further supported by sequential extraction experiment (Table 10), whereby it was inferred that Mn participates to a lesser extent in As(V) adsorption. The presence of manganese oxides and hydroxides increase the adsorption of arsenic (Cai et al. 2002; Sun and Doner 1996). Recently, Martin et al. (2014) reported immobilization of arsenic in soil due to its co-precipitation with Fe and Mn (hydr)oxides in alluvial soils. Organic matter containing organic acid (humic and fulvic acid) soluble in alkaline condition, causes stronger competition with As(V) and As(III) for the available adsorption sites (Sharma et al. 2010). Organic matter is a primary source of electron donor or chelating ligand, which leads to leaching of Fe and Mn oxide immobilized arsenic (Fendorf et al. 2010). Available phosphorus emerged as one of the significant factors that induce mobility (Vithanage et al. 2014). This available phosphorus is an indicator of the phosphate fertilizers used in agricultural practices of this region. Several studies have shown inhibition of arsenic uptake by PO4 3− application in soil (Cui and Weng 2013; Feng et al. 2013).
Generally, it was found that the adsorption of As(V) is more in acidic condition, whereas As(III) adsorption predominates in alkaline conditions (Dias et al. 2009; Goh and Lim 2004). Soil samples PBT-8, PBT-34, PBT-45, PBT-49, and PBT-50 showed higher adsorption for As(V) than As(III) from aqueous solution. The alkalinity of soil shows that the soil carbonate plays a vital role in As(V) adsorption; however, the surface adsorption by iron oxide remains as a key factor in the arsenic retention in these soil samples (Yolcubal and Akyol 2008). The sequential extraction results reveal a significant As binding with Fe and confirmed the presence carbonate and its binding with As(V) in these soil samples (Table 10).
Arsenite adsorption is found to be higher than arsenate in three samples namely PBT-2, PBT-11, and PBT-23, whereas PBT-8 and PBT-14 showed similar adsorption of both As(III) and As(V). As(III) is adsorbed by negatively charged surface groups on soil particles and the adsorption increases with an increase of surface charge upon increasing pH of the soil (Dias et al. 2009). The role of Mn is quite evident in arsenic retention (Mello et al. 2006), found statistically in monitored and adsorbed soil samples (Eq. (5)). The solubility of Mn decreases in alkaline condition (Nádaská et al. 2010). Mn oxidizes As(III) effectively into As(V), commonly in soil and sediments (Deschamps et al. 2003; Yang et al. 2005) under alkaline condition (Feng et al. 2006; Babaeivelni et al. 2014), and the reductive dissolution of Mn leads to the increased adsorption of As(V), as indicated by several studies with synthetic and natural Mn oxides (Feng et al. 2006).
3.6 SEM-EDX study
SEM-EDX study performed for confirmation of our environmental monitoring, adsorption, and sequential extraction based correlation between soil physicochemical parameters with As(III) and As(V). SEM analysis was carried out at ×200 magnification to know the morphological and elemental information of soil and their arsenic adsorbed residues (Fig. 5). The samples selected for analysis were PBT-2 (collected from Talwandi Sabo), PBT-49 (collected from Goniana) and their corresponding As(V), and As(III) adsorbed residues. The contents of soil and arsenic adsorbed soil were revealed by EDX spectral analysis. The constituents of the sites in soil samples are as given in Table 11. The SEM and EDX analysis revealed that arsenic in soil samples was not sufficiently traceable. However, upon adsorption of As(III) and As(V) in the soil, the observed level of arsenic was quite related to the presence of Mn and Fe in the soil constituents and confirmed our experimental findings of both the adsorption and sequential extraction studies. Thus, the SEM analysis of the samples corroborate with the findings of the adsorption and sequential extraction studies for the correlation between As(III) and As(V) adsorption on to the surface of the soil.
3.7 Relation between arsenic concentration in soil and groundwater of Bathinda district
The arsenic concentration in groundwater is found to be higher than permissible limit in this region with up to 11–688 μg l−1 (Table 12). Vicky-Singh et al. (2010) studied the correlation between the arsenic in the groundwater in Bathinda district with that of the soil samples in the region. They found a moderate correlation between the surface soil arsenic content and tube well water. Hundal et al. (2007) had accounted the evaporative concentration of the salts in groundwater to the arid conditions prevailing in this region, as one of the major causes of elevated level of arsenic in groundwater. However, the present study reveals the unsaturation of arsenic in soil samples as indicated by the adsorption studies. This unsaturation could be accounted partly to the sandy loam to silt texture (Fuller 1978) and excessive use of phosphate fertilizer in this region. The evaporative concentration of the soil arsenic is not effective on soil as indicated by the salinity of the soil samples being <1 dS m−1. On the other hand, the mobilizing characteristics of the soil in this region aggravate the arsenic concentration in groundwater due to enhanced evaporative water losses in irrigational practices of this region . Further quantitative assessment of the relation between the evaporative water loss and soil mobilization, with suitable modeling, would provide a basis for the dynamic assessment of arsenic cycling in environment of this region.
4 Conclusions
Soil samples from Talwandi Sabo and Goniana region were studied for arsenic and various physicochemical parameters. Both monitoring and adsorption study revealed a strong correlation between soil manganese and iron on the immobilization of arsenic in soil. However, the mobility of arsenic in the soil samples is influenced by organic matter and total/available phosphorus. Adsorption studies revealed physisorption-type binding of arsenic to soil with poor adsorption efficiency observed in these soils. The poor adsorptive efficiency of arsenic in these soils is the primary reason for arsenic transported through the soil and leading to a higher concentration in groundwater of Bathinda district. Soils from Talwandi Sabo showed better As(III) and As(V) adsorption capacity in comparison to Goniana, which is a consequence of arsenic binding iron being higher in this region. Sequential extraction studies with As(V) established Fe oxide as a principle complexing site in the soil. A strong correlation between monitored arsenic and adsorbed As(III) with manganese suggests As(III) as the predominant species in the present soil environment of both the regions. This is further supported by the absence of any correlation between manganese with As(V) adsorption and sequential extraction. However, we intend to investigate the As(III) presence in the soil in the district with its depth profiling for determination of flux of arsenic from soil to groundwater in this region.
References
Adegoke H, Adekola FA, Fatoki OS, Ximba BJ (2013) Sorptive interaction of oxyanions with iron oxides: a review. Pol J Environ Stud 22:7–24
Amin N, Ibrar D, Alam S (2014) Heavy metals accumulation in soil irrigated with industrial effluents of Gadoon Industrial Estate, Pakistan and its comparison with fresh water irrigated soil. J Agr Chem Environ 3:80–87
Atafar Z, Mesdaghinia A, Nouri J, Homaee M, Yunesian M, Ahmadimoghaddam M, Mahvi AH (2010) Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess 160:83–89
Babaeivelni K, Khodadoust AP, Bogdan D (2014) Adsorption and removal of arsenic (V) using crystalline manganese (II, III) oxide: kinetics, equilibrium, effect of pH and ionic strength. J Environ Sci Hlth, Part A 49:1462–1473
Cai Y, Cabrera JC, Georgiadis M, Jayachandran K (2002) Assessment of arsenic mobility in the soils of some golf courses in South Florida. Sci Total Environ 291:123–134
Cances B et al (2005) XAS evidence of As (V) association with iron oxyhydroxides in a contaminated soil at a former arsenical pesticide processing plant. Environ Sci Technol 39:9398–9405
CGWB, Central Ground Water Board report (2007) Ministry of Water Resources New Delhi. http://cgwb.gov.in/District_Profile/Punjab/Bathinda.pdf. Accessed 12 Aug. 2014
CGWB, Central Ground Water Board report (2011) Ministry of Water Resources New Delhi. http://www.cgwb.gov.in/documents/Dynamic%20GW%20Resources%20-2009.pdf. Accessed 14 May 2014
Cui Y, Weng L (2013) Arsenate and phosphate adsorption in relation to oxides composition in soils: LCD modeling. Environ Sci Technol 47:7269–7276
DCP, District Credit Plan Bathinda District (2012-13) http://www.bathinda.gov.in/html/District_Plan/District%20Credit%20Plan-2012-13%20Bathinda/6.%20BLOCK%20PROFILE.pdf. Accessed 7 June 2014
DSWC (2014) Department of Soil & Water Conservation, Govt. of Punjab, Punjab. http://dswcpunjab.gov.in/contents/punjab_soils.htm. Accessed 24 Mar 2014
Deschamps E, Ciminelli VS, Weidler PG, Ramos AY (2003) Arsenic sorption onto soils enriched in Mn and Fe minerals. Clay Clay Miner 51:197–204
Dhawan AK, Mukherjee J, Kang GS, Singh H (2014) Fertilization unsuitability index for assessing fertilizer induced contamination risk in soils of cotton-wheat system of South Punjab districts. Indian J Ecol 41(2):262–268
Dias FF, Allen HE, Guimarães JR, Taddei MHT, Nascimento MR, Guilherme LRG (2009) Environmental behavior of arsenic (III) and (V) in soils. J Environ Monit 11:1412–1420
Dzombak DA (1990) Surface complexation modeling: hydrous ferric oxide. John Wiley & Sons
EPRI, Electric Power Research Institute USA (2009) Coal ash: characteristics, management and environmental issues. http://www.whitehouse.gov/sites/default/files/omb/assets/oira_2050/2050_meeting_101609-2.pdf. Accessed 5 July 2014
Farooqi A, Masuda H, Siddiqui R, Naseem M (2009) Sources of arsenic and fluoride in highly contaminated soils causing groundwater contamination in Punjab, Pakistan. Arch Environ Contam Toxicol 56:693–706
Fendorf S, Michael HA, van Geen A (2010) Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 328:1123–1127
Feng XH, Zu YQ, Tan WF, Liu F (2006) Arsenite oxidation by three types of manganese oxides. J Environ Sci 18:292–298
Feng Q, Zhang Z, Chen Y, Liu L, Zhang Z, Chen C (2013) Adsorption and desorption characteristics of arsenic on soils: kinetics, equilibrium, and effect of Fe (OH)3 colloid, H2SiO3 colloid and phosphate. Procedia Environ Sci 18:26–36
Fernández P, Sommer I, Cram S, Rosas I, Gutiérrez M (2005) The influence of water-soluble As(III) and As (V) on dehydrogenase activity in soils affected by mine tailings. Sci Total Environ 348:231–243
Fuller WH (1978) Investigation of landfill leachate pollutant attenuation by soils. EPA-600/2-28-158. U.S. Environmental Protection Agency. Office of Research and Development, Cincinnati
Goh KH, Lim TT (2004) Geochemistry of inorganic arsenic and selenium in a tropical soil: effect of reaction time, pH, and competitive anions on arsenic and selenium adsorption. Chemosphere 55:849–859
Halder A (2007) Premature greying of hairs, premature ageing and predisposition to cancer in Jajjal, Punjab: a preliminary observation. J Clin Diagn Res 1:577–580
Ho Y-S, Chiu W-T, Wang C-C (2005) Regression analysis for the sorption isotherms of basic dyes on sugarcane dust. Bioresource Technol 96:1285–1291
Hossain M, Jahiruddin M, Panaullah G, Loeppert R, Islam M, Duxbury J (2008) Spatial variability of arsenic concentration in soils and plants, and its relationship with iron, manganese and phosphorus. Environ Pollut 156:739–744
Hundal H, Kumar R, Singh K, Singh D (2007) Occurrence and geochemistry of arsenic in groundwater of Punjab, Northwest India. Commun Soil Sci Plant Anal 38:2257–2277
Hundal H, Singh K, Singh D (2009) Adsorption of arsenate on coarse loamy mixed hyperthermic Fluventic Haplustept soil of Punjab, Northwest India. Commun Soil Sci Plant Anal 40:3015–3022
Hundal H, Singh K, Singh D, Kumar R (2013) Arsenic mobilization in alluvial soils of Punjab, North–West India under flood irrigation practices. Environ Earth Sci 69:1637–1648
IS (1985) Methods of test for soils (Second revision). Grain Size Analysis. IS: 2720 (Part 4) -1985, Bureau of Indian Standards, New Delhi
IS (1987) Methods of test for soils (Second revision). Determination of pH value. IS: 2720 (Part 26) -1987, Bureau of Indian Standards, New Delhi
IS (2000) Determination of the specific electrical conductivity of soils-method of test. IS: 14767-2000, Bureau of Indian Standards, New Delhi
Jackson ML (ed) (1973) Soil chemical analysis. Prentice Hall of India Pvt. Ltd, New Delhi
Kar S et al (2011) Role of organic matter and humic substances in the binding and mobility of arsenic in a Gangetic aquifer. J Environ Sci Health, Part A Tox Hazard Subst Environ Eng 46(11):1231–1238. doi:10.1080/10934529.2011.598796
Kettering J, Ruidisch M, Gaviria C, Ok YS, Kuzyakov Y (2013) Fate of fertilizer 15N in intensive ridge cultivation with plastic mulching under a monsoon climate. Nutr Cycl Agroecosys 95:57–72
Majumder S, Nath B, Sarkar S, Chatterjee D, Roman-Ross G, Hidalgo M (2014) Size-fractionation of groundwater arsenic in alluvial aquifers of West Bengal, India: the role of organic and inorganic colloids. Sci Total Environ 468:804–812
Manning BA, Goldberg S (1997) Arsenic(III) and arsenic(V) adsorption on three California soils. Soil Sci 162:886–895
Martin M, Bonifacio E, Hossain K, Huq S, Barberis E (2014) Arsenic fixation and mobilization in the soils of the Ganges and Meghna floodplains. Impact of pedoenvironmental properties. Geoderma 228:132–141
Mello J, Roy W, Talbott J, Stucki J (2006) Mineralogy and arsenic mobility in arsenic-rich Brazilian soils and sediments. J Soils Sediments 6:9–19
Mittal S, Kaur G, Vishwakarma GS (2014) Effects of environmental pesticides on the health of rural communities in the Malwa Region of Punjab, India: a review. Hum Ecol Risk Assess 20:366–387
Moreno-Jiménez E, Clemente R, Mestrot A, Meharg AA (2013) Arsenic and selenium mobilisation from organic matter treated mine spoil with and without inorganic fertilisation. Environ Pollut 173:238–244
Nádaská G, Lesný J, Michalík I (2010) Environmental aspect of manganese chemistry. Hungarian Journal of Sciences ENV-100702-A, 1-16
Nan Z, Zhao C, Li J, Chen F, Sun W (2002) Relations between soil properties and selected heavy metal concentrations in spring wheat (Triticum aestivum L.) grown in contaminated soils. Water Air Soil Pollut 133:205–213
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate USDA Circular 939:1-19. Gov. Printing Office, Washington DC
Ouvrard S et al (2005) Natural manganese oxide: combined analytical approach for solid characterization and arsenic retention. Geochim Cosmochim Acta 69:2715–2724
Peryea F (1998) Phosphate starter fertilizer temporarily enhances soil arsenic uptake by apple trees grown under field conditions. Hortscience 33:826–829
PGIMER Epidemiological study of cancer cases in Talwandi Sabo block. PGIMER Chandigarh. Sept. 2007
Ritter K, Aiken GR, Ranville JF, Bauer M, Macalady DL (2006) Evidence for the aquatic binding of arsenate by natural organic matter-suspended Fe (III). Environ Sci Technol 40:5380–5387
Roberts LC et al (2012) Arsenic Contamination of Paddy Fields through Groundwater Irrigation in Bangladesh: Risks for Rice Production and Mitigation Perspectives
Saha D, Sarangam SS, Dwivedi SN, Bhartariya KG (2010) Evaluation of hydrogeochemical processes in arsenic-contaminated alluvial aquifers in parts of Mid-Ganga Basin, Bihar, Eastern India. Environ Earth Sci 61:799–811
Sahoo PK, Zhu W, Kim S-H, Jung MC, Kim K (2013) Relations of arsenic concentrations among groundwater, soil and paddy from an alluvial plain of Korea. Geosci J 17:363–370
Shahmohammadi-Kalalagh S, Babazadeh H, Nazemi A, Manshouri M (2011) Isotherm and kinetic studies on adsorption of Pb, Zn and Cu by kaolinite. Caspian J Environ Sci 9:243–255
Sharma P, Rolle M, Kocar B, Fendorf S, Kappler A (2010) Influence of natural organic matter on As transport and retention. Environ Sci Technol 45:546–553
Sharma C, Mahajan A, Garg UK (2013) Assessment of arsenic in drinking water samples in south-western districts of Punjab-India. Desalin Water Treat 51:5701–5709
Sherman DM, Randall SR (2003) Surface complexation of arsenic (V) to iron (III)(hydr) oxides: structural mechanism from ab initio molecular geometries and EXAFS spectroscopy. Geochim Cosmochim Acta 67:4223–4230
Singh K, Singh D, Hundal HS, Khurana MP (2013) An appraisal of groundwater quality for drinking and irrigation purposes in southern part of Bathinda district of Punjab, northwest India. Environ Earth Sci 70:1841–1851
Smedley P, Kinniburgh D (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smith E, Naidu R (2009) Chemistry of inorganic arsenic in soils: kinetics of arsenic adsorption–desorption. Environ Geochem Health 31:49–59
Sun X, Doner HE (1996) An investigation of arsenate and arsenite bonding structures on goethite by FTIR. Soil Sci 161:865–872
Thomas A, Verma V, Sood A, Litoria P, Sharma P, Ravindran K (1995) Hydrogeology of Talwandi Sabo Tehsil, Bathinda District (Punjab): A Remote Sensing Approach Journal of the. Indian Society of Remote Sensing 23:47-56 Accessed 12 Sept. 2014
USDA-NRCS (2014a) United State Department of Agriculture - Natural Resources Conservation Services. Soil bulk density – Soil Quality Kit (Guides for Educators). http://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_053260.pdf. Accessed 12 Sept. 2014
USDA-NRCS (2014b) United State Department of Agriculture-Natural Resources Conservation Services. Soil Electrical Conductivity – Soil Quality Kit (Guides for Educators). http://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_053280.pdf. Accessed 12 Sept. 2014
Vicky-Singh BM, Preeti-Sharma MS (2010) Arsenic in water, soil, and rice plants in the Indo-Gangetic plains of northwestern India. Commun Soil Sci Plant Anal 41:1350–1360
Vithanage M, Rajapaksha AU, Wijesekara H, Weerarathne N, Ok YS (2014) Effects of soil type and fertilizer on As speciation in rice paddy contaminated with As-containing pesticide. Environ Earth Sci 71:837–847
Walkley A (1947) A critical examination of a rapid method for determining organic carbon in soils-Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63:251–264
Wang S, Mulligan CN (2006) Effect of natural organic matter on arsenic release from soils and sediments into groundwater. Environ Geochem Health 28:197–214
Weiss D, Khondoker R (2013) Evaluation of As (III) and Sb (III) adsorption to paddy soils from irrigated rice fields in Bangladesh. J Braz Chem Soc 24:690–694
Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, Lombi E, Adriano DC (2001) Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta 436:309–323
Yang J, Barnett MO, Zhuang J, Fendorf SE, Jardine PM (2005) Adsorption, oxidation, and bioaccessibility of As(III) in soils. Environ Sci Technol 39:7102–7110
Yin N, Cui Y, Zhang Z, Wang Z, Cai X, Wang J (2015) Bioaccessibility and dynamic dissolution of arsenic in contaminated soils from Hunan, China. J Soils Sediments 15:584–593
Yolcubal I, Akyol NH (2008) Adsorption and transport of arsenate in carbonate-rich soils: coupled effects of nonlinear and rate-limited sorption. Chemosphere 73:1300–1307
Zhang H, Selim H (2005) Kinetics of arsenate adsorption-desorption in soils. Environ Sci Technol 39:6101–6108
Zhang C, Wu P, Tang C, Han Z, Sun J (2013) Assessment of arsenic distribution in paddy soil and rice plants of a typical karst basin affected by acid mine drainage in Southwest China. Environ Pollut 2:27
Acknowledgments
We thank Central University of Punjab, Bathinda, India, for providing facility and infrastructure. We acknowledge the Central Instrumentation Facility and Central university of Punjab, Bathinda for the instrumentation requirements for the monitoring and analysis. We are also thankful to Dr. Puneeta Pandey at Centre for Environmental Science and Technology, Central University of Punjab for her help in mapping this region. Sunil Mittal is thankful to UGC for financial assistance under the Faculty Startup Grant. Ravishankar Kumar and Rabindra Kumar are thankful to CUPB and UGC for scholarship under Ph.D. program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Dong-Mei Zhou
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 728 kb)
Rights and permissions
About this article
Cite this article
Kumar, R., Kumar, R., Mittal, S. et al. Role of soil physicochemical characteristics on the present state of arsenic and its adsorption in alluvial soils of two agri-intensive region of Bathinda, Punjab, India. J Soils Sediments 16, 605–620 (2016). https://doi.org/10.1007/s11368-015-1262-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1262-8