Abstract
The study area is among Changsha, Zhuzhou, and Xiangtan cities, which was under agricultural use and natural conditions about 10 years ago and now is becoming part of the metropolis because of the urban expansion. This study aims to investigate the mechanisms and capabilities of the local alluvial soil layer for protecting the local shallow groundwater from arsenic pollution by field surveys and batch experiments. The field surveys showed that there was an acidic tendency of the groundwater, and phosphate, nitrate, and arsenic in the groundwater significantly increased comparing to their reference values. It indicates that the disturbance of the former agricultural land due to the change of land use may be responsible for these changes. From the experimental results, the maximum adsorption capacity of the soil for As(V) was as low as 0.334 mg/g, and lower As(V) adsorption capacities were obtained at higher As(V) concentration, higher pH, and lower temperature. The presence of H2PO4 − and SiO3 2− posed negative, while HCO3 − slight positive, and SO4 2−, NO3 − and Cl− negligible influences on the As(V) adsorption. The surface-derived organic matter played a negative role in the adsorption process, and low specific surface area influenced adsorption capacity of the soil. The study reveals that the local soil layer shows poor potential for protection of the local shallow groundwater from As(V) pollution, and the change trends of the groundwater environments due to more intensive anthropogenic activities will further weaken this potential and increase the risk of the groundwater contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) pollution in groundwater and the related threats to people health and grain growth are increasingly causing serious global concerns (Tong et al. 2014; Chakraborti et al. 2016). Apart from natural sources, human activities including industrial waste discharges and utilization of arsenical herbicides and pesticides are the primary sources for presence of As in shallow groundwater. Soils are the natural barriers to prevent pollutants from entering the shallow groundwater, and their adsorption capacities play a significant role in As transporting into shallow groundwater.
It has been proved that As(V) adsorption from aqueous phase by a soil primarily depends on the content of clay minerals, metal (hydr)oxides, organic matter (OM), cation exchange capacity (CEC), specific surface area (SSA), and point of zero charge (pHPZC) of a soil, as well as the competitive ions (Suda et al. 2016; Moghal et al. 2017). Clay minerals, because of the ion substitution in the crystal lattice and the following electrovalent balance by cations in the interlayer, usually have a permanent negative charge and CEC (Bhattacharyya and Gupta 2008). The negative As(V) species such as H2AsO4 −, HAsO4 2−, and AsO4 3−, which are common oxyanions in the shallow groundwater, can be adsorbed onto the clay minerals through the cation bridges (Cornu et al. 2003), and show a linear, reversible and non-specific adsorption process. Besides, the edges of clay minerals can also host charges due to the broken bonds in the silica-aluminum units and can adsorb As(V) oxyanions by electrostatic, non-specific, or specific adsorption (Wainipee et al. 2013; Ghorbanzadeh et al. 2015).
Metal bearing minerals, especially iron and aluminum (hydr)oxides in a soil are another important minerals responsible for the As(V) adsorption (Adegoke et al. 2014). Lots of studies have confirmed that As(V) ions have affinity toward iron and aluminum compounds (Rahman et al. 2013; Kovačević et al. 2013; Dai et al. 2016), and primarily bind as inner sphere complexes onto the surface of these matters (Mikutta et al. 2014; Zhang et al. 2014). Moreover, at the lower acidic medium, the dissolved Fe3+ may precipitate with As(V) and decrease mobility of As(V) (Liu et al. 2016).
OM, in spite of the lower content in most soils, can play a crucial role in As(V) adsorption on the soil minerals. Because of presence of iron or aluminum, OM such as humic acids can perform Al or Fe-OM complexes by metal bridging and occupy the surface sites of iron or aluminum (hydr)oxides when OM coexists with As(V) in the solution (Luo et al. 2015; de Oliveira et al. 2015). The OM-metal complexes can also bind strongly with As(V) to form soluble ternary complexes like As-metal-OM (Liu et al. 2011; Mikutta et al. 2011). These processes may be influenced by As/Fe molar ratio in a given pH condition (Mikutta et al. 2014). Moreover, some studies stated that the young surface-derived and old source of OM in a soil may show relatively rapid and slow rate of As release, respectively (Fendorf et al. 2010; Al Lawati et al. 2012).
CEC, SSA, and pHPZC are considered to be the important properties of a soil to adsorb As(V) in solution, which depend on many factors such as soil texture, soil minerals, particle size, crystallinity, OM, soil pH, and so on (Chutia et al. 2009). Therefore, due to the differences of the weathering, erosion, transport, and material sources, soils in different topographies show apparent different physiochemical properties.
The common anions in groundwater, including phosphate, bicarbonate, silicate, nitrate, sulfate, and chloride, may pose to some extent impacts on the As(V) adsorption on a soil. Some studies reported that co-existing of phosphate, silicate, and (bi)carbonate with As(V) ions in solution had a detectable negative effect on As(V) adsorption on a soil, the intensity of which depends on pH of the medium, concentration of co-existing anions and OM (Gao et al. 2013a; Biswas et al. 2014), whereas nitrate and chloride had negligible effects on As(V) adsorption processes, and sulfate exhibited an intermediate behavior (Frau et al. 2010).
Human activities may influence the adsorptive properties of a soil by changing the soil texture, OM, pH, concentration of target pollutant, and competitive ions. According to the construction planning of Changsha-Zhuzhou-Xiangtan city group in 2007, these three cities will be combined together in the future. The area among these cities was under agricultural use and natural conditions about 10 years ago and has been under more intensive anthropogenic activities because of the urban expansion. The change of land use and groundwater environments will influence the adsorptive properties of the local soil layer and increase the risk of the local shallow groundwater contamination. In order to understand the possible mechanisms and potential of the local alluvial soil layer for protecting the local shallow groundwater from As(V) pollution, field surveys and laboratory batch experiments were conducted. (1) Field surveys can help to understand the local shallow groundwater environment and its change trends by comparing the environmental conditions before the city expansion. (2) Batch experiments of effects of initial As(V) concentration, contact time, pH, temperature, and competitive anions on the As(V) adsorption by the alluvial soil were employed to investigate the influences of the environment change on the soil adsorptive properties. The field survey and the batch experiments will help to evaluate the risk of As(V) contamination in the local shallow groundwater with the construction of Changsha-Zhuzhou-Xiangtan city group.
Field survey
Groundwater sample collection
In order to understand the change of the local shallow groundwater in the study area in the past 10 years, field surveys were conducted in 2014, and the monitoring data of the groundwater within 1998 to 2000 were collected as the reference values. Groundwater samples were collected from 11 open wells ranging in depth between 1.0 and 5.0 m among Changsha, Zhuzhou, and Xiangtan cities in 2014. The sampling sites are illustrated in Fig. 1. Each sample was collected by acid-washed 1000-ml polyethylene bottle. The bottle was washed three times using groundwater before sampling and was completely filled with water in case of air bubble trapped in the sample. The samples were put into a case and carefully sent to the laboratory within 8 h and stored at a temperature below 4 °C prior to analysis in the laboratory. The monitoring data within 1998 to 2000 are from 4 of the 11 open wells.
Groundwater sample measurement
Eight parameters including pH, water temperature, phosphate, sulfate, bicarbonate, nitrate, chloride, and arsenic, which are considered to be the main impact factors on the As(V) adsorption on a soil, were presented in the study. The pH and water temperature were measured in the field using pH meter and thermometer. The other parameters were analyzed in the laboratory of Hunan Environmental Monitoring Center. The analysis methods given by Ministry of Environmental Protection of the People’s Republic of China (HJ/T164-2004) were applied.
Adsorption experiments
Materials
The alluvial soil employed in this study was collected from Muyun, southern Changsha, Hunan province. It is located in the east bank of Xiangjiang River, and the straight distance to the river is about 1.82 km and the geographic coordinate is 28° 03′ 05.79″ N, 112° 57′ 12.40″ E (Fig. 1). The sampling depth range was 10–20 cm. After removing roots, coarse sands, and gravels, the soil sample was air-dried, crushed, and screened through 0.180-mm nylon sieve. The screened soil was divided into two parts. One part was for measurement of the physiochemical properties and another for the batch experiments. The soil sample for batch experiments was previously washed with KH2PO4 (0.5 M, liquid-solid ratio 4:1) for 8 h to remove As and then washed with ultrapure water for 8 h. The prepared soil sample was stored in a capped bottle and labeled for the subsequent measurements and experiments.
As(V) stock solution (1000 mg/l) was prepared by dissolving Na3AsO4 (analytical reagent) in ultrapure water, and a working solution for all the experiments was freshly prepared from the stock solution. Standard acid (0.1 M HNO3, guaranteed reagent) and base solution (0.1 M NaOH, analytical reagent) were used for pH adjustment. The matrix modifier Ni(NO3)2 (10 mg/L) for arsenic analysis was prepared by dissolving Ni(NO3)2·6H2O (analytical reagent) in ultrapure water. NaCl, NaNO3, KH2PO4, Na2SiO3, Na2SO4, and NaHCO3 for the experiments of effect of co-existing anions are analytical grade.
Analytical methods
The soil sample was characterized by XRD (Bruker D8 Advance, German) with a high power CuKα radioactive source (λ = 0.154 nm) at 35 kV/40 mA and was measured at 3°–80° of 2θ. The chemical compositions were determined by X-ray fluorescence spectrometer (XRF, Bruker S4 Pioneer, German). CEC was determined by the method of ammonium acetate centrifugal exchange and SSA by a BET Analyzer (NOVA-3000, Quantachrome, USA) after degassing overnight at 100 °C and using nitrogen and multipoint analysis. OM, pH, and pHPZC of the soil sample were measured using improved potassium dichromate volumetric weight method, titration, and potentiometric titration method, respectively. As(V) remaining in the solution of adsorption experiments was measured by graphite furnace atomic absorption spectrometry (GFAAS) with 10 mg/L Ni(NO3)2 as a matrix modifier. The measurements of XRD and XRF were finished in Changsha Research Institute of Mining and Metallurgy Co., Ltd. and the others in Hunan Provincial Key Laboratory of Water and Sediment Science and Water Hazard Prevention, Changsha University of Science and Technology.
Batch experiments
Batch experiments were conducted by adding 1 g (accurate to 0.0001 g) soil sample into 50 ml As(V) solutions and agitating at 180 rpm on a shaker with temperature controller at a designed temperature for a predetermined equilibrium time of 24 h. The isotherms were investigated by varying the initial As(V) concentrations in the range of 0.05–20.0 mg/l with initial pH of the solutions 7.0 and the temperature 293 ± 1 K. The adsorption kinetics was studied at different time intervals with As(V) concentration of 1.50 mg/l, pH 7.0, and temperature 293 ± 1 K. The effects of pH on As(V) adsorption were performed by changing pH of the solutions from 2 to 11 adjusted using 0.1 M HNO3 and 0.1 M NaOH as mentioned above, with the As(V) concentration 1.50 mg/L and solution temperature 293 ± 1 K. The adsorption thermodynamics was investigated at different temperatures (278 ± 1, 283 ± 1, 293 ± 1, 303 ± 1, 313 ± 1 K) with the initial As(V) concentration 1.50 mg/l and pH 7.0. The effects of co-existing anions on As(V) adsorption were also investigated in such a way that each ion was separately added to a container of 50 ml solution of 1.5 mg/l As(V) concentration and was agitated at 180 rpm for 24 h. The suspension was centrifuged for 20 min and was filtered through a 0.45-μm membrane filter. The arsenic in aqueous solutions was determined by using GFAAS. All glasswares were pre-soaked in 2% HNO3 for at least 24 h and rinsed with ultrapure water before use.
The amount of As(V) adsorbed on the soil sample (mg/g) was calculated by the following equation:
where C 0 and C e are initial and final concentration of As(V) in solution (mg/l), respectively. V is the volume of solution (ml), and m the mass of adsorbent (g).
Judgment of precision of modeling
The accuracy of the modeling applied in the study was judged from the correlation coefficient (R 2) between the measured data and the modeled data, as well as the mean absolute percentage error (MAPE) depicting the deviation between the experimental data and the modeled values, which is defined in Eq. (2). The SPSS 20.0 was employed for the non-linear regression.
Results and discussion
Description of study area and the shallow groundwater
The study area is among Changsha, Zhuzhou, and Xiangtan cities and is between Xiangjiang River and Liuyanghe River (Fig. 1). Alluvial soil is the main soil types in this area. This area was under agricultural use and natural conditions before 2004, and now are gradually becoming parts of the cities because of the urban expansion. The abundant shallow groundwater is still the main water resources of the local people. Precipitation and surface water recharge are the primary source of the shallow groundwater. Therefore, the alluvial soil layer is the natural barrier for the protection of the shallow groundwater from pollution. The change of land use due to the city expansion is varying the soil and groundwater environments and may influence the protection potential of the soil for the shallow groundwater.
Parameters of the local shallow groundwater in the study area were tabulated in Table 1. It can be observed that the pH, phosphate, nitrate, and arsenic in the groundwater showed significant changes, bicarbonate and chloride slightly increased, and water temperature and sulfate slightly decreased, comparing to their reference values. The decrease of the pH of the groundwater indicates an acidic trend of the groundwater. The significant increases of the concentration of phosphate, nitrate, and arsenic in the groundwater suggest that more intensive anthropogenic activities because of the urban expansion would be responsible for these changes. The slight increase of bicarbonate in the groundwater may be derived from the breaking of the carbonate equilibrium of the groundwater due to the change of the concentration of CO2. The increase of chloride may be due to the domestic pollution.
The change of pH value of the groundwater, concentration of the anions, and arsenic in the groundwater may pose influences on As(V) adsorption on the soil and cause more pollutants release into the groundwater (Szolnoki et al. 2013; Arco-Lázaro et al. 2016).
Characterization of soil
The XRD pattern of the soil sample is illustrated in Fig. 2, and the minerals, physiochemical properties, and chemical composition are shown in Tables 2 and 3. The major constituents of the soil sample are quartz, montmorillonite, illite, kaolinite, and iron (hydr)oxides, and silicon, iron, and aluminum are the predominant compositions of the soil sample, which may facilitate As(V) adsorption. The OM in the soil sample is primarily surface derived due to the property of the alluvial soil and agricultural discharges and may have a relatively fast rate of As release for the soil (Lawson et al. 2016). The CEC is moderate compared to some soils reported (Alshaebi et al. 2010; Feng et al. 2013). The ASS is lower than some soils such as Hainan soil (27.20 m2/g), West Bengal soil, India (15.365 m2/g), and most soil samples from Mediterranean coast of Morocco (22.4–119 m2/g) and may influence the As(V) capacity efficiency of the soil sample (Maji et al. 2007; Feng et al. 2013; Bentahar et al. 2016). The pHPZC plays a significant role in adsorption process because the adsorption of multivalent ions effectively takes place at pH values below pHPZC. The pHPZC of the soil sample is 8.10 and is favorable for the As(V) adsorption in most natural conditions.
Effect of initial As(V) concentration
To determine the adsorption efficiency and to investigate the variation of the equilibrium adsorption with the initial As(V) concentration, the adsorption of As(V), which existed predominantly as HAsO4 2− in the solution at the given pH, on the soil sample was studied as a function of 16 initial As(V) concentrations, and the results are illustrated in Fig. 3. It can be seen that the equilibrium As(V) adsorption percentage is lower than many soils reported (Maji et al. 2008; Smith and Naidu 2009; Feng et al. 2013), and it drops rapidly from 74.78 to 54.23% with the initial As(V) concentration increasing from 0.05 to 2.0 mg/L and then decreases relatively slowly to 26.63% as the initial As(V) concentration further increases to 20.0 mg/L. It is reasonable that the As(V) adsorption percentage decreases with increase of the initial As(V) concentration, because there are certain surface active sites for a given amount of an adsorbent. However, it is unusual that the curve shows apparent two segments as shown in Fig. 3. In order to study this phenomenon, Freundlich and Langmuir models were used to fit three datasets, which are 0.05–20.0, 0.05–2.0, and 2.0–20.0 mg/l, respectively, and the modeling results are shown in Table 4 and Fig. 4.
It can be observed in Table 4 and Fig.4a that Langmuir model fits better to all the isotherm adsorption data with R 2 0.974 and MAPE 14.62 than Freundlich model with R 2 0.950 and MAPE 61.90. This indicates that the overall reaction may be primary a monolayer adsorption. However, Freundlich and Langmuir models both fit well to the lower initial As(V) concentration dataset of 0.05–2.0 mg/l (Table 4 and Fig.4b), which means that the adsorption is more complex in the lower initial As(V) concentration range. Electrostatic adsorption through cation bridge of the clay minerals and inner sphere complexes derived from the iron and aluminum components in the soil sample both play a significant role in the adsorption processes (Michael et al. 2016). As for the higher initial As(V) concentration datasets (2.0–20.0 mg/l), Langmuir model fits much better (Table 4 and Fig.4c). It reveals that the reaction is characterized by monolayer adsorption in the higher initial As(V) concentration range, and the inner sphere complex resulted from the iron and aluminum (hydr)oxides may control the adsorption (Kumar et al. 2016). The very small R L suggests that an irreversible and specific adsorption occurs (Simsek and Beker 2014). So, the moderate CEC probably makes the soil preferentially adsorb As(V) as the As(V) concentration is lower because of its rapid reaction process, while the inner sphere complex gradually enhances with the initial As(V) concentration increasing. Moreover, the relatively small SSA makes the soil sample have lower maximum adsorption capacity. The OM in the soil sample is surface derived and may occupy the surface active sites and block As(V) adsorption onto the soil, especially when the amount of As(V) in the solution is higher (Al Lawati et al. 2012; Lawson et al. 2016). Some studies stated that the release of As(V) ions resulted from OM increased by formation of the soluble OM-Fe and As-Fe-OM complexes with As/Fe molar ratio of the solution increasing (Tareq et al. 2013; Kim et al. 2015). This reaction is possibly another cause that the As(V) removal efficiency decrease with the initial As(V) concentration increasing.
From the experimental results, the lower SSA and the surface-derived OM may be responsible for the low As(V) adsorption efficiency of the local soil. Higher initial As(V) concentration is unfavorable for As(V) adsorption on the soil. Therefore, change of the soil texture, OM, and increase of the arsenic concentration in the groundwater resulted from the more intensive human activities, may weaken the potential of the soil layer for the protection of the local shallow groundwater, and increase the risk of the groundwater suffering from As pollution.
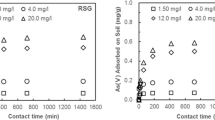
Effect of contact time
The kinetics of As(V) adsorption onto the soil sample is illustrated in Fig. 5. It can be observed that the adsorption capacity increases rapidly to 0.040 mg/g with adsorption efficiency 53.76% within the initial 180 min and then increases slowly to 0.0465 mg/g and achieve equilibrium with the maximum adsorption efficiency 61.94%. To study the adsorption kinetics, pseudo-first-order, pseudo-second-order, intra-particle diffusion, and diffusion chemisorption model were applied to fit the experimental data. Table 5 summarizes the calculated parameters of kinetic modeling, R 2 and MAPE. As can be seen in Table 5, pseudo-second-order model fits the kinetics better with R 2 0.995 and MAPE 11.08 than the other three models, which suggests that the overall rate of As(V) adsorption is controlled by a chemisorption process (Ivan et al. 2015). Under the given pH condition, the chemisorption of the As(V) ion adsorption onto the soil sample is primarily the As(V) adsorption on the clay minerals owing to the broken bonds in the edge of clay mineral crystals, and the inner sphere adsorption of As(V) onto the iron and aluminum (hydr)oxides (Kim et al. 2014; Ghorbanzadeh et al. 2015).
Under natural conditions, kinetics of As(V) adsorption onto soil particles is influenced by many factors. Fluctuation of water table, concentration variation of arsenic, or the other ions like phosphate in the groundwater (Neupane et al. 2014; Mukhopadhyay et al. 2015), which usually resulted from anthropogenic activities, will break this equilibrium and cause the release of arsenic from the soil into groundwater.
Effect of pH
Effect of pH on the adsorption of As(V) onto the soil sample is shown in Fig. 6. It can be observed that the As(V) equilibrium adsorption capacity maintains relatively high level in the pH range of 2.0–7.0 and then decreases rapidly with the pH further increasing to 11.0. The pH value influences the metal ions adsorption onto an adsorbent by changing the surface charge of the adsorbent and the species of adsorbates (Ivan et al. 2015). For minerals of the main components of the soil sample such as clay minerals and iron (hydr)oxides (Table 2), the surface charge is determined by proton transfer of the amphoteric hydroxyl group present on their surface, and the surface ionization reaction depends directly on the pH value of the solution. The surface protonation is promoted in acidic medium, while the deprotonation under basic condition (Mamindy-Pajany et al. 2009). These two reactions are depicted as follows,
With pH value increasing, the As(V) species transform gradually from H2AsO4 − to HAsO4 2− and then AsO4 3−, and H2AsO4 − is the predominant species in the pH range of 2.3–6.8, while HAsO4 2−, 6.8–11.5 (Sharma and Sohn 2009). Considering the pHPZC (8.10) of the soil sample (Table 2), the surface of the adsorbent is more positively charged at lower pH, where H2AsO4 − is the predominant As(V) species and then the HAsO4 2− (Panagiotaras et al. 2015). So, more As(V) oxyanions are adsorbed on the surface of the soil solid through the electrostatic attraction (Michael et al. 2016). With the pH approaching to 7.0, H2AsO4 − decreases and HAsO4 2− increases, and the adsorption efficiency decreases because of the higher adsorption free energy of HAsO4 2− (Chowdhury and Yanful 2010). On the contrary, as pH values are higher than pHPZC, HAsO4 2− becomes the main species and AsO4 3− increases gradually (Smedley and Kinniburgh 2002). Therefore, more As(V) anions remain in the solution due to electrostatic repulsive forces between the negatively charged surface of the soil and the As(V) anions. In addition, precipitation is possibly another kind of As(V) removal mechanism by the soil sample. In much lower acidic medium, As(V) may precipitate with Fe3+ dissolved from the iron oxides (Langmuir et al. 2006; Liu et al. 2016) and is favorable for the As(V) removal. With the solution changing gradually to basic condition, the pre-formed precipitants start to dissolve and cause the release of As(V) ions.
The effects of pH on the As(V) adsorption onto the soil revealed that lower pH (<7.0) was favorable for the adsorption. The present pH values of the local shallow groundwater and the soil (Tables 1 and 2), as well as the acidic trend of the groundwater, are favorable for the arsenic adsorption on the soil. But other issues resulted from the acidification of the groundwater must be concerned.
Effect of temperature
To explore the influence of temperatures on As(V) adsorption by the soil sample, the experiments were conducted at 5 different temperatures. The experimental results are illustrated in Fig. 7, and the thermodynamic parameters are listed in Table 6, which are calculated by the following equations:
where ΔG is standard Gibbs free energy (kJ/mol). ΔH is enthalpy change (kJ/mol), and ΔS is entropy change (J/mol K). R is the gas constant (0.008314 kJ/mol K). T is the solution temperature (K). K c is the equilibrium constant, and the C Ae and C Se are the equilibrium concentrations of As(V) on the adsorbent and in the solution, respectively.
As can be seen in Fig. 7, the As(V) removal efficiency increases with the temperature increasing, revealing that increase of the temperature in the studied temperature range is favorable for the As(V) adsorption. The increasing K c value with increase of the temperature (Table 6) agrees with an endothermic adsorption reaction, which testifies the trend observed in the experiments. ΔG represents the spontaneity of an adsorption reaction, and the negative values of ΔG and their decreasing tendency with temperature increasing indicates that higher temperature is thermodynamically favorable for the As(V) adsorption. ΔH is usually employed to distinguish physisorption and chemisorption, and the absolute magnitude of the heat of physisorption usually changes from 2 to 30 kJ/mol, while ΔH value for chemisorption always falls in the range of 40–200 kJ/mol (Yazdani et al. 2016). The ΔH values of 5.13 kJ/mol indicates that physisorption may be an important mechanism of As(V) adsorption on the soil in the lower initial As(V) concentration. Further, the positive ΔS reveals an increased randomness at the solid-liquid adsorption system (Israelachvili 1991).
Although the increase of temperature is favorable for the As(V) adsorption by the soil, the relatively stable groundwater temperature (around 292 K) in the study area cannot pose a significant impact on the adsorptive properties of the local soil for As(V).
Effect of co-existing anions
Six anions including H2PO4 −, SiO3 2−, SO4 2−, HCO3 −, NO3 −, and Cl− were employed to investigate the influences of the presence of individual anion on the As(V) adsorption onto the soil sample. The selection and concentrations of the anions (0, 20, 80, 200 mg/L) were determined according to the field surveys and the present research results (Rouwane et al. 2016; Arco-Lázaro et al. 2016). The experimental results are shown in Fig. 8. As can be seen in Fig. 8, H2PO4 − and SiO3 2− in the solution can cause an apparent decrease in the As(V) adsorption on the soil sample, and the presence of HCO3 − slightly promotes the As(V) adsorption, whereas the other co-existing anions do not interfere perceptibly with the As(V) adsorption. The interference of phosphate on As(V) adsorption on the soil sample is expected because phosphate and arsenate have similar chemical structure and behavior. Hence, the competition of phosphate with arsenate for the active sites on the surface of the adsorbent decreases the As(V) adsorption. Many studies have the same conclusions (Neupane et al. 2014; Arco-Lázaro et al. 2016). In soil and groundwater environments, silicate tends to present as colloid-like [SiO3 2−] n and is in favor of adsorbing onto the soil minerals such as Fe-(hydr)oxides and Al-(hydr)oxides through surface complex between the –SiOH group and the surface groups of the soil particles, and also suppresses the As(V) adsorption onto the soil sample. This experimental result is in agreement with the previous studies (Feng et al. 2013; Zhang et al. 2015). Different from H2PO4 − and SiO3 2−, the presence of HCO3 − in the solution made the As(V) adsorption rate slightly increase with the added HCO3 − amount increasing, which is different from some studies (Meng et al. 2002; Gao et al. 2013b). The possible reason is that the increase of the concentration of H+ ions due to the bicarbonate dissociation changes the adsorption equilibrium between the As(V) ions and the soil surface, which is favorable for the As(V) adsorption. The reactions can be depicted as the following equations
The presence of phosphate in the local groundwater may pose small influences on the release of As(V) from the soil because of its very low concentration (Table 1). However, the increasing trend of the phosphate in groundwater obtained from the field survey indicates that the land development may change the situation because the study area was under agricultural use before. Silicate in the groundwater was not investigated this time. Different from some studies (Gao et al. 2011; Gao et al. 2013b), this study showed that the presence of bicarbonate played a slight positive effects on the As(V) adsorption onto the soil. So, further investigation should be done in the coming study.
Conclusions
In order to understand the potential of the local alluvial soil for protecting the shallow groundwater from arsenic pollution in the study area, the field survey and laboratory batch experiments were conducted. The adsorptive properties of the alluvial soil were studied by measurement of its minerals, chemical compositions, and physicochemical properties and by investigating the effects of initial As(V) concentration, contact time, pH, temperature, and co-existing anions on the As(V) adsorption by the soil. The field survey showed that there was an acidic trend of the groundwater, and the concentration of phosphate, nitrate, and arsenic in the groundwater increased in contrast to their reference values. It indicates that the disturbance of the former agricultural land due to the change of land use may be responsible for these changes. The experimental results indicate that As(V) adsorption onto the soil depends primarily on the clay minerals, iron (hydr)oxides, OM, and some key physicochemical properties, where OM plays a negative role because of its mainly surface-derived origin. Relatively low SSA may make the soil have low adsorption capacity. Lower adsorption capacities were obtained at higher initial As(V) concentration, at higher pH and at lower temperatures. The pseudo-second-order model can be used to describe the adsorption kinetics well. The presence of H2PO4 − and SiO3 2− in the solution poses negative effects on the As(V) adsorption, while HCO3 − slight positive, and SO4 2−, NO3 − and Cl− negligible influences. The study reveals, to some extent, that the soil layer in the study area shows relatively poor potential for protecting the local shallow groundwater. The change trends of the local groundwater environments due to more intensive human activities will probably weaken the arsenic adsorption capacities of the soil and increase the risk of the groundwater contamination.
References
Adegoke HI, Adekola FA, Fatoki OS, Ximba BJ (2014) A comparative study on sorption of As (V) ions on hematite, goethite and magnetite nanoparticles. Nanotech 1:184–187
Al Lawati WM, Rizoulis A, Eiche E, Boothman C, Polya DA, Lloyd JR, Berg M, Vasquez-Aguilar P, van Dongen BE (2012) Characterisation of organic matter and microbial communities in contrasting arsenic-rich Holocene and arsenic poor Pleistocene aquifers, Red River Delta. Vietnam Appl Geochem 27:315–325
Alshaebi FY, Yaacob WZW, Samsudin AR (2010) Removal of arsenic from contaminated water by selected geological natural materials. Aust J Basic Appl Sci 4(9):4413–4422
Arco-Lázaro E, Agudo I, Clemente R, Bernal MP (2016) Arsenic(V) adsorption-desorption in agricultural and mine soils: effects of organic matter addition and phosphate competition. Environ Pollut 216:71–79
Bentahar Y, Hurel C, Draoui K, Khairoun S, Marmier N (2016) Adsorptive properties of Moroccan clays for the removal of arsenic(V) from aqueous solution. Appl Clay Sci 119:385–392
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interf Sci 140:114–131
Biswas A, Gustafsson JP, Neidhardt H, Halder D, Kundu AK, Chatterjee D, Berner Z, Bhattacharya P (2014) Role of competing ions in the mobilization of arsenic in groundwater of Bengal Basin: insight from surface complexation modeling. Water Res 55:30–39
Chakraborti D, Rahman MM, Ahamed S, Dutta RN, Pati S, Mukherjee SC (2016) Arsenic groundwater contamination and its health effects in Patna district (capital of Bihar) in the middle Ganga plain, India. Chemosphere 152:520–529
Chowdhury SR, Yanful EK (2010) Arsenic and chromium removal by mixed magnetite-maghemite nanoparticles and the effect of phosphate on removal. J Environ Manag 91:2238–2247
Chutia P, Kato S, Kojima T, Satokawa S (2009) Arsenic adsorption from aqueous solution on synthetic zeolites. J Hazard Mater 162:440–447
Cornu S, Breeze D, Saada A, Baranger P (2003) The influence of pH, electrolyte type, and surface coating on arsenic(V) adsorption onto kaolinites. Soil Sci Soc Am J 67:1127–1132
Dai M, Xia L, Song SX, Peng CS, Lopez-Valdivieso A (2016) Adsorption of As(V) inside the pores of porous hematite in water. J Hazard Mater 307:312–317
De Oliveira LK, Melo CA, Goveia D, Lobo FA, Hernández MAA, Fraceto LF, Rosa AH (2015) Adsorption/desorption of arsenic by tropical peat: influence of organic matter, iron and aluminium. Environ Technol 36(2):149–159
Fendorf S, Michael HA, van Geen A (2010) Spatial and temporal variations of groundwater arsenic in south and Southeast Asia. Science 328:1123–1127
Feng QZ, Zhang ZY, Chen Y, Liu LY, Zhang ZJ, Chen CZ (2013) Adsorption and desorption characteristics of arsenic on soils: kinetics, equilibrium, and effect of Fe(OH)3 colloid, H2SiO3 colloid and phosphate. Procedia Environ Sci 18:26–36
Frau F, Addari D, Atzei D, Biddau R, Cidu R, Rossi A (2010) Influence of major anions on as(V) adsorption by synthetic 2-line ferrihydrite. Kinetic investigation and XPS study of the competitive effect of bicarbonate. Water Air Soil Pollut 205:25–41
Gao XD, Root RA, Farrell J, Ela W, Chorover J (2013a) Effect of silicic acid on arsenate and arsenite retention mechanisms on 6-L ferrihydrite: a spectroscopic and batch adsorption approach. Appl Geochem 38:110–120
Gao XB, Su CL, Wang YX, Hu QH (2013b) Mobility of arsenic in aquifer sediments at Datong Basin, northern China: effect of bicarbonate and phosphate. J Geochem Explor 135:93–103
Gao XB, Wang YX, Hu QH, Su CL (2011) Effects of anion competitive adsorption on arsenic enrichment in groundwater. J Environ Sci Health A 46:1–9
Ghorbanzadeh N, Jung W, Halajnia A, Lakzian A, Kabra AN, Jeon B-H (2015) Removal of arsenate and arsenite from aqueous solution by adsorption on clay minerals. Geosystem Eng 18(6):302–311
Israelachvili JN (1991) Intermolecular and surface forces, 2 edn. Academic, New York
Ivan A, Diana NHT, Shervin K, Sara A, Marijana M, Dusan L (2015) Graphene aerogels decorated with α-FeOOH nanoparticles for efficient adsorption of arsenic from contaminated waters. Appl Mater Interfaces 7:9758–9766
Kim EJ, Hwang BR, Baek K (2015) Effects of natural organic matter on the coprecipitation of arsenic with iron. Environ Geochem Health 37(6):1029–1039
Kim EJ, Yoo J-C, Baek K (2014) Arsenic speciation and bioaccessibility in arsenic-contaminated soils: sequential extraction and mineralogical investigation. Environ Pollut 186:29–35
Kovačević D, Džakula BN, Hasenay D, Nemet I, van Rončević S, Dékány I, Petridis D (2013) Adsorption of arsenic on MgAl layered double hydroxide. Croat Chem Acta 86(3):273–279
Kumar R, Kumar R, Mittal S, Arora M, Babu JN (2016) Role of soil physicochemical characteristics on the present state of arsenic and its adsorption in alluvial soils of two agri-intensive region of Bathinda, Punjab, India. J Soils Sediments 16(2):605–620
Langmuir D, Mahoney J, Rowson J (2006) Solubility products of amorphous ferric arsenate and crystalline scorodite (FeAsO4·2H2O) and their application to arsenic behavior in buried mine tailings. Geochimca et Cosmochimica Acta 70:2942–2956
Lawson M, Polya DA, Boyce AJ, Bryant C, Ballentine CJ (2016) Tracing organic matter composition and distribution and its role on arsenic release in shallow Cambodian groundwaters. Geochim Cosmochim Acta 178:160–177
Liu G, Fernandez A, Cai Y (2011) Complexation of arsenite with humic acid in the presence of ferric iron. Environ Sci Technol 45:3210–3216
Liu YK, Hu P, Zheng JT, Wu MB, Jiang B (2016) Utilization of spent aluminum for p-arsanilic acid degradation and arsenic immobilization mediated by Fe(II) under aerobic condition. Chem Eng J 297:45–54
Luo C, Xie YW, Li F, Jiang T, Wang Q, Jiang ZM, Wei SQ (2015) Adsorption of arsenate on iron oxides as influenced by humic acids. J Environ Qual 44(6):1729–1737
Maji SK, Pal A, Pal T (2008) Arsenic removal from real-life groundwater by adsorption on laterite soil. J Hazard Mater 151:811–820
Maji SK, Pal A, Pal T, Adak A (2007) Adsorption thermodynamics of arsenic on laterite soil. J Surf Sci Technol 22(3–4):161–176
Mamindy-Pajany Y, Hurel C, Marmier N, Romeo M (2009) Arsenic adsorption onto hematite and goethite. Comptes Rendus Chimie 12:876–881
Meng XG, Korfiatis GP, Bang S, Bang KW (2002) Combined effects of anions on arsenic removal by iron hydroxides. Toxicol Lett 133:103–111
Michael A, Donn B, David D (2016) Arsenic in the soil environment: a soil chemistry review. Int J Appl Agric Res 11:1–28
Mikutta C, Kretzschmar R (2011) Spectroscopic evidence for ternary complex formation between arsenate and ferric iron complexes of humic substances. Environ Sci Technol 45(22):9550–9557
Mikutta R, Lorenz D, Guggenberger G, Haumaier L, Freund A (2014) Properties and reactivity of Fe-organic matter associations formed by coprecipitation versus adsorption: clues from arsenate batch adsorption. Geochim Cosmochim Acta 144:258–276
Moghal AAB, Reddy KR, Mohammed SAS, Al-Shamrani MA, Zahid WM (2017) Retention studies on arsenic from aqueous solutions by lime treated semi-arid soils. Int J GEOMATE 12(29):17–24
Mukhopadhyay S, Hashim MA, Allen M, Sen Gupta B (2015) Arsenic removal from soil with high iron content using a natural surfactant and phosphate. Int J Environ Sci Technol 12:617–632
Neupane G, Donahoe RJ, Arai Y (2014) Kinetics of competitive adsorption/desorption of arsenate and phosphate at the ferrihydrite–water interface. Chem Geol 368:31–38
Panagiotaras D, Papoulis D, Stathatos E, (2015) Origin of arsenic toxicity–geochemistry. Chapter 4, in Arsenic toxicity: prevention and treatment. Editor: Narayan Chakrabarty, CRC Press, Taylor & Francis publishers.
Rahman IMM, Begum ZA, Sawai H, Maki T, Hasegawa H (2013) Decontamination of spent iron-oxide coated sand from filters used in arsenic removal. Chemosphere 92(2):196–200
Rouwane A, Rabiet M, Grybos M, Bernard G, Guibaud G (2016) Effects of NO3− and PO4 3− on the release of geogenic arsenic and antimony in agricultural wetland soil: a field and laboratory approach. Environ Sci Pollut Res 23:4714–4728
Sharma VK, Sohn M (2009) Aquatic arsenic: toxicity, speciation, transformations, and remediation. Environ Int 35:743–759
Simsek EB, Beker U (2014) Equilibrium arsenic adsorption onto metallic oxides: isotherm models, error analysis and removal mechanism. Korean J Chem Eng 31(11):2057–2069
Smedley PL, Kinniburgh DG (2002) A review of the source, behavior and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smith E, Naidu R (2009) Chemistry of inorganic arsenic in soils: kinetics of arsenic adsorption–desorption. Environ Geochem Health 31:49–59
Suda A, Makino T (2016) Functional effects of manganese and iron oxides on the dynamics of trace elements in soils with a special focus on arsenic and cadmium: a review. Geoderma 270:68–75
Szolnoki Z, Farsang A, Puskás I (2013) Cumulative impacts of human activities on urban garden soils: origin and accumulation of metals. Environ Pollut 177:106–115
Tareq SM, Maruo M, Ohta K (2013) Characteristics and role of groundwater dissolved organic matter on arsenic mobilization and poisoning in Bangladesh. Phys Chem Earth 58–60:77–84
Tong JT, Guo HM, Wei C (2014) Arsenic contamination of the soil–wheat system irrigated with high arsenic groundwater in the Hetao Basin, Inner Mongolia, China. Sci Total Environ 496:479–487
Wainipee W, Cuadros J, Sephton MA, Unsworth C, Gill MG, Strekopytov S, Weiss DJ (2013) The effects of oil on As(V) adsorption on illite, kaolinite, montmorillonite and chlorite. Geochim Cosmochim Acta 121:487–502
Yazdani M, Tuutijärvi T, Bhatnagar A, Vahala R (2016) Adsorptive removal of arsenic from aqueous phase by feldspars: kinetics, mechanism, and thermodynamic aspects of adsorption. J Mol Liq 214:149–156
Zhang MY, He GZ, Pan G (2014) Structure and stability of arsenate adsorbed on α-Al2O3 single-crystal surfaces investigated using grazing-incidence EXAFS measurement and DFT calculation. Geochim Cosmochim Acta 144:258–276
Zhang WS, Singh P, Issa TB (2015) The effect of Cl−, PO4 3−, and SiO3 2− on the adsorption of As(V) and As(III) on bauxite in water. Int J Eng Sci Technol 7(4):30–36
Acknowledgements
The authors would like to send sincere appreciation to China Hunan Provincial Science and Technology Department for the funds, Prof. Quanxi Shao of the Commonwealth Scientific and Industrial Research Organization (CSIRO), and the reviewers for their good proposals for the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Rights and permissions
About this article
Cite this article
Chen, H., Mei, J., Luo, Y. et al. Adsorptive properties of alluvial soil for arsenic(V) and its potential for protection of the shallow groundwater among Changsha, Zhuzhou, and Xiangtan cities, China. Environ Sci Pollut Res 24, 4018–4028 (2017). https://doi.org/10.1007/s11356-016-8150-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8150-7