Abstract
Inorganic arsenic (As) pesticides have been widely used for decades in many countries. However, insufficient data are available on the chemical speciation of inorganic arsenicals in tropical paddy soils. Inorganic As-containing pesticides were used in tropical countries, a few decades ago, however, their fate have not been studied. Hence, the objective of this study was to determine fractionation of inorganic arsenicals and to assess As lability with/without fertilizer application using a static incubation experiment. Eight soils from wet and dry regions of Sri Lanka were amended with 1,000 mg/kg arsenate for this purpose. The FT-IR and XRF results suggested that soils in the wet region were rich in Fe/Al-oxides. Paddy soils in the dry zone showed high As lability. These low-humic gley soils have low Fe/Al oxyhydroxide and alkaline pH. In contrast, the wet zone had soils with higher As retention capacity, high amounts of Fe/Al oxyhydroxide, and acidic pH. Arsenic lability increased considerably 30 days after fertilizer application. Overall, As lability was mainly influenced by soil mineralogical and chemical properties, i.e., Fe/Al oxyhydroxide, pH, organic matter, and fertilizer application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic activities are major contributors to arsenic (As) found in soils (Cullen and Reimer 1989; Davis et al. 2001; Almaroai et al. 2012; Usman et al. 2012). Repeated and widespread use of inorganic As pesticides has contributed to extensive contamination of soils in the USA (Murphy and Aucott 1998; Folkes et al. 2001; Belluck et al. 2003). During the 1970s, nearly 80 % of the world’s As consumption was attributed to agriculture (Mandal and Suzuki 2002). It was only two decades ago that inorganic As pesticides were banned in the USA and elsewhere. This has contributed to a reduction in the use of inorganic As-based agrochemicals from 80 to 40 % (Adriano 2001). However, the past use of inorganic As agrochemicals must have contaminated soils in many countries, although it has mostly been recorded in the USA. No data are found in the tropical countries for the use and fate of inorganic arsenical compounds which is similar in the case of Sri Lanka.
The potential risk from As-contaminated soils depends on factors that influence its mobility and availability (Almaroai et al. 2013). Soil components, such as organic matter, iron oxides, and clay minerals strongly influence the processes involved in As mobility (Adriano 2001; Balasoiu et al. 2001; Lee et al. 2013a). Consequently, As retention within soils reveals the fundamental necessity for contaminant risk assessment (Fendorf et al. 2004; Usman et al. 2012). Bioaccessibility related to metal toxicity decreases with soil aging (Tang et al. 2007; Ahmad et al. 2012c). However, changes in soil redox potential, pH conditions, and the presence of other ions, such as phosphate and humic matter (HM) may change As mobility in soil (Cao et al. 2003; Masscheleyn et al. 1991; Wang and Mulligan 2006; Lim et al. 2013).
Baseline concentrations of As in soils are generally 5–10 mg/kg (Smedley and Kinniburgh 2002). Boyle and Jonasson (1973) quoted an average baseline As concentration in world soils of 7.2 mg/kg. Rice is sometimes cultivated in agricultural lands that have accumulated elevated levels of As, much higher than the baseline values (Zhu et al. 2008). In the case of inorganic As compounds, most studies have reported As phytotoxicity to rice grown in soils with As concentrations of 0.5–150 mg/kg (Sheppard 1992). The effects of soil type, arsenic speciation, and soil aging on bioaccessibility of inorganic and organic As in treated soils have been studied extensively in various crops (Datta et al. 2006; Fendorf et al. 2004; Sarkar and Datta 2003; Tang et al. 2007; Quazi et al. 2011). Many studies have been performed to understand the effect of phosphate fertilizers on As uptake by plants (Cozzolino et al. 2010; Geng et al. 2005; Lu et al. 2010); however, it is evident that many additional factors, such as tillage and water management may affect As mobilization (Talukder et al. 2011), but the studies are limited on these.
Only a limited amount of work has focused on the effect of other fertilizers and soil physicochemical characteristics on As bioavailability (Chen et al. 2008; Signes-Pastor et al. 2007; Wang and Mulligan 2006; Quazi et al. 2011). These studies have shown that the formation of soluble and strong HM-As complexes facilitates the mobility of As-contaminated soils. Some studies have suggested that nitrate inhibits Fe(III) reduction, leading to As co-precipitation with, or adsorption to Fe(III) minerals in soil. Adding nutrients to soil may cause competition between elements for fixation sites in the soil, and adding N, P, and K can significantly affect the concentrations of available As in soil (Signes-Pastor et al. 2007; Abd El-Azeem et al. 2013). Plant–soil interactions make a system more complex; thereby, making it more difficult to investigate soil–As interactions especially under different fertilizer amendments. All these studies are focused on temperate world where the soil system is distinct from the tropical region. It is, therefore, appropriate to investigate the fractionation and lability of As in tropical paddy soils under controlled conditions in order to eliminate the effect of other factors. In Sri Lanka, rice is grown under flooded culture and reducing conditions. Soil properties under these conditions may sufficiently differ from other agricultural soils. The current study elucidated the fractional speciation and lability of As in inorganic As pesticide-amended paddy soils treated with different fertilizers. The As concentrations selected for this study represented the worst case scenarios resulting from repeated application of As pesticides (Quazi et al. 2011).
Materials and methods
Soil sampling procedures
Eight typical paddy soils were collected from wet [Pelmadulla (soil 4), Warakapola (soil 5), and Matara (soil 6)] and dry zone (Galgamuwa (soil 1), Thambuththegama (soil 2), Habarana (soil 3), Eppawala (soil 7), and Medirigiriya (soil 8) paddy fields in Sri Lanka (Fig. 1). Soil samples were taken from the arable layer (0–15 cm deep), combined into one sample, and transported to the laboratory for further treatment and chemical analysis. In the laboratory, soil samples were air-dried at room temperature and crushed with a wooden roller to pass through a 2-mm sieve. A small portion of the crushed (≤2 mm particle size) soils was further ground with an agate mortar to pass through a <63-μm sieve for X-ray fluorescence (XRF) analysis (Ahmad et al. 2012a). This method of analysis was selected to obtain the major element concentrations in the soils.
Determination of soil physicochemical properties
Total digestion of the crushed soil was carried out using a temperature-controlled microwave heating system (Milestone ETHOS PLUS lab station with HRP-1000/10S high pressure segmented rotor). The following proportions of acids were used on 1 g of soil sample: 16 mL HNO3, 10 mL HCl, and 3 mL HF. Minor elements determination was done using the microwave method as the same method was used for the digestion of the residual fraction of sequential extraction method. Fourier-transform-infrared spectroscopy (FT-IR) was used to investigate the differences between soil samples by determination of OH, Al and Fe oxides that are mainly responsible for As sorption in different fractions. Air-dried and sieved (2 mm) soils were used to determine pH, electrical conductivity (EC), and organic matter content using standard protocols (Sumner and Miller 1996; Jung et al. 2011). Soils were extracted in 1 M ammonium acetate solution and 1 M potassium chloride solution for cation exchange capacity (CEC) analysis (Anderson and Ingram 1993).
Phosphate concentrations in the extracts and digests were determined colorimetrically using the molybdate–ascorbic acid method (Watanabe and Olsen 1965) and 1,10-phenanthroline-reagent method (Olson and Ellis 1982), respectively, using an ultraviolet–visible light spectrometer (Shimadzu 2400 PC) which is the common standard practice. The concentrations of Ca, Mg, and Al in the extracts/digests were analyzed using a GBC 93AAA atomic-absorption spectrophotometer in the flame mode. The digests were analyzed for As using a hydride generation atomic-absorption spectrophotometer.
Samples (50 g) of each of the eight soils were spiked with 50 mL Na3AsO4 solution to artificially increase the total As concentration in the soils by approximately 1,000 mg/kg. After addition of As, the pesticide was mixed with the soils, which were then stored in a closed environment. The soils were aerated, and constant water content was maintained for 30 days. Three separate sets for each of the eight soils were prepared with the same As concentrations and treated with phosphorus at 75 mg P/kg from triple super phosphate (TSP), 100 mg/kg N from urea, or 40 mg/kg K from muriate of potash (MoP). All soils were stored in a 25 °C incubator to maintain 70 % moisture content. A small amount of sample (1 g) was taken for sequential extraction at different times (1 h, and 1, 4, 10, 20, and 30 days).
Sequential extraction methodology
The sequential extraction method selected for this study was based on a comparison of methods. The modified Hall method (Hall et al. 1996) was the most suitable and frequently used among the studied sequential extraction methods for As fractionation in mineral mixtures (Mihaljevič et al. 2003). Five different fractions were considered: exchangeable (1 M NaOAc, pH 5, 12 h), bound to amorphous Fe oxyhydroxides (0.25 M NH2OH·HCl in 0.25 M HCl, 2 h, 60 °C, 0.5 h), bound to crystalline Fe oxyhydroxides (1 M NH2OH·HCl in 25 % HOAc, 3 h, 90 °C, 1.5 h), bound to organic matter (15 mL 30 % H2O2 and 3 mL 0.02 M HNO3, 85 °C, 2 h and 3.2 M NaOAc, 5 mL, 85 °C, 3 h) and bound to the mineral matrix (also called the “residual fraction”, digestion with a mixture of HF, HNO3, and HClO4). Between successive extractions, separation was effected by centrifuging at 3,500 rpm for 15 min. The supernatant was filtered through a 0.45-μm filter paper and analyzed for As using atomic-absorption spectrophotometry (Model GBC 933 AA, Australia), while the residue was rinsed with deionized water. The total concentration of arsenate in the “residual fraction” and in individual synthetic mixtures was determined by dissolving the solid samples in a mixture of HCl and HNO3 at a ratio of 5:1. All solutions were stabilized with concentrated nitric acid (final HNO3 concentration, 2 % v/v). Before the As concentrations were determined using hydride generation atomic-absorption spectrophotometry, arsenate was reduced to arsenite using 20 % HCl and 200 ppm KI, and maintained for 2 h to complete the reaction.
Results and discussion
Soil properties
The soils were classified as Low-Humic Gley (soils 1, 2, 7, and 8), Reddish Brown Earth (soil 3), Reddish Brown Latosol (soil 4), Immature Brown Loam (soil 5), and Red Yellow Podosol (soil 6) (De Alwis and Panabokke 1972). The characterization data for the eight soil samples are given in Table 1.
The pH of the soil samples depicted the characteristic differences between wet and dry zone paddy soils. Soils 1, 2, and 7 were moderately alkaline to alkaline, indicating accumulation of base-forming cations, such as Ca and Mg in the soil cation exchange complex due to fluctuations in ground water level during rainy and dry seasons. This indicates that acid-forming cations, such as Fe and Al exist in inorganically bound forms (Ok et al. 2007; Yang et al. 2009). Soils from dry zone regions (soils 1, 2, and 7) and the wet zone (soil 6) showed high EC levels, which indicate the potential for development of soil salinity. High salinity was expected for soil 6, because it was from a coastal zone (Fig. 1).
The CEC of the soils was 15–30 meq/100 g, which is ideal for an agricultural soil (10–30 meq/100 g). Although soil organic matter content is positively related to CEC, no significant relationship was observed in these samples. Thus, the variation in CEC may be due to a difference in the quality and quantity of the clay fraction in the colloidal complex. Therefore, the amount of clay in soils could have contributed to the randomness of the CEC data. However, the CEC of all soils was greater than the characteristic CEC of the kaolinite clay mineral (3–15 meq/100 g), which is the most abundant type of clay mineral in Sri Lanka (Ok et al. 2011a; Lee et al. 2013b). Therefore, it is evident that the CEC of these soils is maintained at a higher level by the accumulation of organic matter.
Total soil carbon was higher (>3 %) in the wet zone (soils 4, 5, and 6) than that in the dry zone. This was attributed to rapid decomposition of organic matter and nutrient mineralization in the dry zone by microbial activity favored by high temperatures, and by better aeration when soil moisture was available. Soil organic carbon in typical paddy soils is <5 %, which is characteristic for tropical soils. More than 70 % of total soil carbon in these samples was derived from soil organic carbon. Soil organic matter content in these soils was 2–7 %. Lack of organic matter in soil is reflected by a high soil C:N ratio (10–15:1), which is favorable for microbial activity and depicts the continuous decomposition of organic matter and rapid C mineralization (Awad et al. 2012; Ok et al. 2011b).
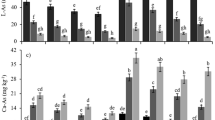
Soil available phosphate was highest in soils 2 and 7 and this was attributed to the inherently high P in parent material. The high amount of soil Ca indicates that these areas were located in close proximity to a rock phosphate ore. In addition, the other dry zone soils (1, 3, and 8) had higher available P than soils in the wet zone areas. This can be explained by the inverse relationship between available phosphate and soil moisture. Under low soil moisture conditions, phosphorus is fixed due to shrinkage of amorphous Fe- and Al-oxides. Even though P availability is limited in tropical soils, these soils were not deficient in available P.
Spectroscopic evidence
Some selected infrared absorbance spectra of the soils are shown in Fig. 2. Soils 1, 3, 4, and 6 were compared, as the other samples appeared similar to these four (data not shown). Several components of the wet and dry zone soils were easily recognizable from their spectral features. Some characteristic spectral features were recognized in the mixture spectra, such as the doublet at 912–937 cm−1 corresponding to the Al–OH bending vibrations of kaolinite (Marel and Krohmer 1969), and the peak at 1,030 cm−1 attributed to Fe–O bonding vibrations in iron-bearing minerals present in the soils (Fig. 2). Soils 1 and 3 did not show Al–OH bending vibrations of kaolinite, whereas this was apparent in soils 4 and 6 (Fig. 2). This could be due to the low concentrations of Al in soils 1 and 3. The bands at 3,698, 3,650, and 3,620 cm−1 were attributed to structural OHs of kaolinite. The bands at 3,526 cm−1 indicate the presence of gibbsite (Bertaux et al. 1998). The band at 3,650 cm−1 in soil 1 for structural OH was noticeable as a shoulder; however, in soil 3, it was slightly observable, suggesting less structural OH in soils 3 (Fig. 2). All samples showed amorphous silica, which was recognized by bands present at 800 and 1,100 cm−1 (Fröhlich 1989). Amorphous silica showed three fundamental vibration modes for silica, and all three were apparent in all soil samples, namely Si–O stretching at 1,100, Si–O–Si stretching at 800 cm−1, and Si–O bending at 470 cm−1 (Bertaux et al. 1998). The FT-IR analysis revealed that the soils were mixed composites of Fe/Al/Si oxides.
Other than the silicates and Fe/Al-oxides bands, some bands were attributed to soil organic carbon. However, not many bands were associated with soil organic carbon. The transmission FT-IR band at 1,630 cm−1 is significantly correlated with soil organic C content (Haberhauer and Gerzabek 1999). The broad intense band at about 3,400 cm−1 was due to a stretching vibration of the bonded and non-bonded hydroxyl groups (Ahmad et al. 2012b). According to the chemical analysis, it was observed that the three most abundant elements, after C, were Si, Al, and Fe. The FT-IR spectral information showed the compositional differences between the wet and dry zone soils.
Labile As with regard to soil characteristics
Labile As was affected by both fertilizer and soil type (Table 3). The labile As concentrations in soils 1, 2, and 7 were generally high, which was expected due to the higher soil pH and lower contents of iron and aluminum in the soil structure than those in the other soils (Table 2), reducing the ability of soil particles to bind As (Woolson et al. 1971; Johnston and Barnard 1979; Liversy and Huang 1981). Soil 3, which had neutral pH and moderate Fe/Al, showed moderate labile As concentrations (Table 3). Fe and Al are the primary solid phases that affect soil-As solubility (Woolson et al. 1971; Johnston and Barnard 1979; Liversy and Huang 1981).
After 30 days, the highest labile As concentration was recorded from soils 2 and 7. Arsenic lability also depends on the phosphorus concentration of soils. Because As and P have similar chemical properties, they often compete for similar sorption sites, resulting in increased As mobility and availability (Manning and Goldberg 1996). The soil analysis showed that As lability was directly related to phosphate concentrations in soil samples. Samples with high levels of available P demonstrated high As lability (soils 2 and 7) and low retention capacity. The soils were categorized into three groups based on the labile As concentrations: highly labile (soils 2 and 7), moderately labile (soils 1, 3, and 8), and low labile soils (soils 4, 5, and 6). Soils from the wet zone had high As retention capacity; i.e., low lability, whereas dry zone soils, which were characterized as low-humic gley, had low retention. The cause for the differences in As lability may be partly due to the pH and organic matter content in the soils. This was supported by the results that clearly showed high organic matter (OM), total carbon, and total nitrogen concentrations in low labile soils (Table 2). Soils rich in OM and phosphate suppress arsenate adsorption (Grafe et al. 2001; Liu et al. 2001). Pearson’s correlation coefficient (r) between labile arsenic and each of soil OM, TC, TN and phosphate content supported the above observation. Labile arsenic was negatively correlated with OM (r = −0.79), TC (r = −0.75), and TN (r = −0.73) indicating inverse relationships between labile As and each of the three soil parameters. A positive correlation (r = 0.76) was observed between phosphate and labile As.
As fractionation in soil
The availability and mobility of As in soil–water systems are determined not only by the soil characteristics but also by the speciation and partitioning of As in the soil solution and soil matrix (Han et al. 2004). Table 3 shows the distribution of different As concentrations found in different fractions at 1 and 30 days. Arsenic was mainly retained by the amorphous Fe-oxyhydroxide and crystalline Fe-oxyhydroxide fractions of all soils. The lowest retention was by the sulfide and organic matter fractions. The As concentration in the Fe-oxyhydroxide fraction varied greatly (10–110 mg/kg) depending on the soil composition. Similar observations were also apparent in other fractions. Retention of As in the sulfide and organic matter fractions was 0.25–6.0 mg/kg. The amount of silicate-bound As was slightly higher than that bound to sulfide. The amount of As bound to organic matter was 0.25–40 mg/kg. Hence, the maximum total retention capacity of As in the paddy soils was about 10 % of the initial application of 1,000 mg/kg. However, this was less for the low-humic gley soils of the dry zone.
Figure 3 shows the average As concentrations extracted using the sequential extraction procedure for soils based on As lability. Exchangeable As concentrations showed a slight decrease with time in the low labile soils from the wet zone. However, exchangeable As concentrations increased slightly with time in high labile soils and also in one of the moderately labile soils. A slow release of As that is loosely bound to the silicate fraction may have resulted in this observation. Because the point of zero charge for silicates is pH 3–4, silicates are not supportive of anionic metal adsorption since their surface is negatively charged at environmental pH (Jacobs et al. 1970). As a result, the outer spherically bound neutral species and surface-precipitated As are released slowly. A slow reduction in As in the silicate fraction was also apparent (data not shown).
The As concentrations in the amorphous Fe-oxyhydroxide fraction increased with time in all soils (Fig. 3). Arsenic strongly adsorb onto Fe and Al-oxides (Jacobs et al. 1970), thereby rendering As mobile. The soils with high amounts of native Fe/Al oxyhydroxides (soils 4–6) contained less labile As due to high retention capacity even though the rate of As retention differed between these soils. The highest rates of As retentions were observed in soils rich in Fe/Al oxyhydroxides, whereas the lowest rates were observed for soils low in Fe/Al. The rate of increase of As concentration was high in the beginning and then reached equilibrium after 15 days for most soils (Fig. 3). The main observation from the fractionation results was that the wet zone soils had higher As concentrations in all fractions, except the exchangeable fraction at all times as compared to dry zone soils.
Effect of fertilizer on As fractionation
Muriate of potash is the most common potassium source used in agriculture, while urea and TSP are frequently used as nitrogen and phosphorous species, respectively. Hence, we selected these three fertilizers to determine their effect on As mobilization in the soil systems. All eight soils treated with MoP, TSP, and urea showed low As adsorption at 30 days as compared to the native soils (Fig. 4a). This effect was more pronounced for the wet zone soils than for the dry zone soils. Studies conducted on As adsorption in the presence of potassium have reported no effect on As adsorption (Shipley et al. 2009). However, the increase in the salinity due to the presence of MoP may have reduced adsorption compared to that in native soils. A few studies have reported a slight decrease in As retention due to an increase in salinity (Gupta and Chen 1978; Chan 1999). Thus, the reduction in As retention observed in this study could be due to an increase in soil salinity caused by the application of MoP (Costello et al. 2003).
It is a well-known fact that the presence of phosphorous reduces As retention in soils (Manning and Goldberg 1996; Violante and Pigna 2002; Jain and Loeppert 2000). This can be attributed to competition between the two species for soil adsorption sites. The reduction in As retention in soils treated with TSP was observed in the present study. Interestingly, MoP showed a slightly lower arsenic retention than that of TSP and, this finding may need further investigation.
Urea applied soils showed different results compared to the TSP and MoP applied soils (Fig. 4a). Arsenic retention was less in TSP and MoP applied soils compared to soils applied with urea. This was noticeable in the amorphous fraction of the soil (Fig. 4b). When urea is added to moist soils, it dissolves and transforms to ammonium carbonate, which rapidly decomposes into ammonia, carbon dioxide, and water. In aqueous solution, ammonia acts as a base acquiring hydrogen ions from H2O to yield ammonium and hydroxide ions (Kettering et al. 2013). The formation of OH− ions increases the pH of the soil, which then reduces As adsorption onto soil particulate surfaces (Jacobs et al. 1970). This temporary change in pH may reduce As retention and the soil pH may ultimately decrease with the formation of NO3 −. However, urea treatment decreased As concentration within each fraction over time. The concentration of As in amorphous Fe oxyhydroxides was high soon after urea application (Table 3), which may have been due to complexation activity of urea acting as a chelating agent on As (Urík et al. 2009). It is evident that the presence of urea fertilizer reduced As retention capacity of the Fe/Al oxyhydroxides. Interestingly, the wet zone model soil (soil 4) showed a threefold reduction in 30 day As retention capacity with all three fertilizers compared to its As retention capacity without fertilizers. The plausible mechanisms of As interaction with soils based on the observations from the incubation experiment with different fertilizers are summarized and given in Fig. 5.
Conclusion
Comparative studies on different soils contaminated with inorganic As pesticides are limited. This study evaluated the relationship between soil properties and labile As after fertilizer application. The amount of labile As was affected by differences in soil physicochemical properties, particularly the pH and Fe-bearing minerals. Soils containing high levels of available P demonstrated high As lability (soils 2 and 7) and low retention capacity. High levels of As were found in the labile soil fraction from the dry zone, which resulted in much lower levels of As in surface soil. Wet zone soils rich in Fe/Al oxyhydroxides were an As pool. The minimum and maximum As retention in soils were varied from 10 to 110 mg/kg depending on the soil composition followed by organic matter fraction (0.25–40 mg/kg). Maximum As retention was observed from wet zone soils of about 10 % of the initial application of 1,000 mg/kg. Labile As varied with soil type and fertilizer application. Urea and TSP had a positive effect on the As concentrations of the different fractions particularly the exchangeable, amorphous, and crystalline Fe fractions. MoP reduced the soil-As retention ability considerably. The As concentrations were high soon after fertilizer application, but decreased with time. Hence, the fertilizer application was confirmed to play a role in promoting the release of As from the soil to water. Careful studies must be undertaken to reveal the mechanisms behind the As interactions in tropical paddy soils using macro and molecular level techniques towards reducing lability.
References
Abd El-Azeem SAM, Ahmad M, Usman ARA, Kim K-R, Oh S-E, Yang JE, Lee SS, Ok YS (2013) Changes of biochemical properties and heavy metal bioavailability in soil treated with natural liming materials. Environ Earth Sci. doi:10.1007/s12665-013-2410-3
Adriano DC (2001) Trace elements in the terrestrial environments: biogeochemistry bioavailability, and risks of metals. Springer, New York
Ahmad M, Hashimoto Y, Moon DH, Lee SS, Ok YS (2012a) Immobilization of lead in a Korean military shooting range soil using eggshell waste: an integrated approach. J Hazard Mater 209–210:392–401
Ahmad M, Lee SS, Dou X, Mohan D, Sung JK, Yang JE, Ok YS (2012b) Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour Technol 118:536–544
Ahmad M, Lee SS, Yang JE, Ro HM, Lee YH, Ok YS (2012c) Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil. Ecotoxicol Environ Saf 79:225–231
Almaroai YA, Usman ARA, Ahmad M, Kim KR, Moon DH, Lee SS, Ok YS (2012) Effects of synthetic chelators and low molecular weight organic acids on chromium, copper, and arsenic uptake and translocation in maize (Zea mays L.). Soil Sci 177(11):655–663
Almaroai YA, Usman ARA, Ahmad M, Kim K-R, Vithanage M, Sik Ok Y (2013) Role of chelating agents on release kinetics of metals and their uptake by maize from chromated copper arsenate-contaminated soil. Environ Technol 34(6):747–755
Anderson JM, Ingram JSI (1993) Tropical soil biology and fertility. A handbook of methods. CAB International: Wallingford
Awad YM, Blagodatskaya E, Ok YS, Kuzyakov Y (2012) Effects of polyacrylamide, biopolymer and biochar on decomposition of soil organic matter and plant residues as determined by 14C and enzyme activities. Eur J Soil Biol 48:1–10
Balasoiu CF, Zagury GJ, Deschênes L (2001) Partitioning and speciation of chromium, copper, and arsenic in CCA-contaminated soils: influence of soil composition. Sci Total Environ 280(1–3):239–255
Belluck DA, Benjamin SL, Baveye P, Sampson J, Johnson B (2003) Widespread arsenic contamination of soils in residential areas and public spaces: an emerging regulatory or medical crisis? Int J Toxicol 22(2):109–128
Bertaux J, Froehlich F, Ildefonse P (1998) Multicomponent analysis of FTIR spectra; quantification of amorphous and crystallized mineral phases in synthetic and natural sediments. J Sediment Res 68(3):440–447
Boyle RW, Jonasson IR (1973) The geochemistry of Arsenic and its use as an indicator element in geochemical prospecting. J Geochem Explor 2(3):251–296
Cao X, Ma LQ, Shiralipour A (2003) Effects of compost and phosphate amendments on arsenic mobility in soils and arsenic uptake by the hyperaccumulator, Pteris vittata L. Environ Pollut 126(2):157–167
Chan FY (1999) The sorption of arsenic species to Moira lake sediments. University of Toronto, Toronto
Chen X-P, Zhu Y-G, Hong M-N, Kappler A, Xu Y-X (2008) Effects of different forms of nitrogen fertilizers on arsenic uptake by rice plants. Environ Toxicol Chem 27(4):881–887
Costello LR, Perry EJ, Matheny NP, Henry JM, Geisel PM (2003) Abiotic disorders of landscape plants and a diagnostic guide. University of California Agriculture and Natural Resources
Cozzolino V, Pigna M, Di Meo V, Caporale AG, Violante A (2010) Effects of arbuscular mycorrhizal inoculation and phosphorus supply on the growth of Lactuca sativa L. and arsenic and phosphorus availability in an arsenic polluted soil under non-sterile conditions. Appl Soil Ecol 45(3):262–268
Cullen WR, Reimer KJ (1989) Arsenic speciation in the environment. Chem Rev 89(4):713–764
Datta R, Sarkar D, Sharma S, Sand K (2006) Arsenic biogeochemistry and human health risk assessment in organo-arsenical pesticide-applied acidic and alkaline soils: an incubation study. Sci Total Environ 372(1):39–48
Davis A, Sherwin D, Ditmars R, Hoenke KA (2001) An analysis of Arsenic records of decision. Environ Sci Technol 35(12):2401–2406
De Alwis K, A, Panabokke CR (1972) Handbook of the soils of Sri Lanka. J Soil Sci Soc Ceylon 2:43
Fendorf S, La Force MJ, Li G (2004) Temporal changes in soil partitioning and bioaccessibility of arsenic, chromium, and lead. J Environ Qual 33(6):2049–2055
Folkes DJ, Kuehster TE, Litle RA (2001) Contributions of pesticide use to urban background concentrations of arsenic in Denver, Colorado, U.S.A. Environ Forensics 2(2):127–139
Fröhlich F (1989) Deep-sea biogenic silica: new structural and analytical data from infrared analysis—geological implications. Terra Nova 1(3):267–273
Geng C-N, Zhu Y-G, Liu W-J, Smith SE (2005) Arsenate uptake and translocation in seedlings of two genotypes of rice is affected by external phosphate concentrations. Aquat Bot 83(4):321–331
Grafe M, Eick MJ, Grossl PR (2001) Adsorption of Arsenate (V) and Arsenite (III) on goethite in the presence and absence of dissolved organic carbon. Soil Sci Soc Am J 65(6):1680–1687
Gupta SK, Chen KY (1978) Arsenic removal by adsorption. J Water Pollut Control Fed 50(3):493–506
Haberhauer G, Gerzabek MH (1999) Drift and transmission FT-IR spectroscopy of forest soils: an approach to determine decomposition processes of forest litter. Vib Spectrosc 19(2):413–417
Hall GEM, Vaive JE, Beer R, Hoashi M (1996) Selective leaches revisited, with emphasis on the amorphous Fe oxyhydroxide phase extraction. J Geochem Explor 56(1):59–78
Han FX, Kingery WL, Selim HM, Gerard PD, Cox MS, Oldham JL (2004) Arsenic solubility and distribution in poultry waste and long-term amended soil. Sci Total Environ 320(1):51–61
Jacobs LW, Syers JK, Keeney DR (1970) Arsenic sorption by soils. Soil Sci Soc Am J 34(5):750–754
Jain A, Loeppert RH (2000) Effect of competing anions on the adsorption of Arsenate and Arsenite by ferrihydrite. J Environ Qual 29(5):1422–1430
Johnston SE, Barnard WM (1979) Comparative effectiveness of fourteen solutions for extracting Arsenic from four Western New York soils. Soil Sci Soc Am J 43(2):304–308
Jung K, Ok YS, Chang SX (2011) Sulfate adsorption properties of acid-sensitive soils in Athabasca oil sands region in Alberta, Canada. Chemosphere 84:457–463
Kettering J, Ruidisch M, Gaviria C, Ok YS, Kuzyakov Y (2013) Fate of fertilizer 15N in intensive ridge cultivation with plastic mulching under a monsoon climate. Nut Cycling Agroecosystem 95(1):57–72
Lee K-Y, Moon DH, Lee S-H, Kim K-W, Cheong K-H, Park J-H, Ok YS, Chang YY (2013a) Simultaneous stabilization of arsenic, lead, and copper in contaminated soil using mixed waste resources. Environ Earth Sci. doi:10.1007/s12665-012-2013-4
Lee SS, Lim JE, Abd El-Azeem SAM, Choi B, Oh SE, Moon DH, Ok YS (2013b) Heavy metal Immobilization in soil near abandoned mines using eggshell waste and rapeseed residue. Environ Sci Pollut Res 20:1719–1726
Lim JE, Ahmad M, Usman ARA, Lee SS, Jeon W-T, Oh S-E, Yang JE, Ok YS (2013) Effects of natural and calcined poultry waste on Cd, Pb and As mobility in contaminated soil. Environ Earth Sci 69:11–20. doi:10.1007/s12665-012-1929-z
Liu F, De Cristofaro A, Violante A (2001) Effect of pH, phosphate and oxalate on the adsorption/desorption of Arsenate on/from Geothite. Soil Sci 166(3):197–208
Liversy NT, Huang PM (1981) Adsorption of Arsenate by soils and its relation to selected chemical properties and anions. Soil Sci 131(2):88–94
Lu K-L, Liu C-W, Wang S-W, Jang C-S, Lin K-H, Liao VH-C, Liao C-M, Chang F-J (2010) Primary sink and source of geogenic arsenic in sedimentary aquifers in the southern Choushui River alluvial fan. Taiwan. Appl Geochem 25(5):684–695
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58(1):201–235
Manning BA, Goldberg S (1996) Modeling competitive adsorption of Arsenate with phosphate and molybdate on oxide minerals. Soil Sci Soc Am J 60(1):121–131
Marel HW, Krohmer P (1969) O–H stretching vibrations in kaolinite, and related minerals. Contrib Mineral Petrol 22(1):73–82
Masscheleyn PH, Delaune RD, Patrick WH (1991) Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ Sci Technol 25(8):1414–1419
Mihaljevič M, Poňavič M, Ettler V, Šebek O (2003) A comparison of sequential extraction techniques for determining arsenic fractionation in synthetic mineral mixtures. Anal Bioanal Chem 377(4):723–729
Murphy EA, Aucott M (1998) An assessment of the amounts of arsenical pesticides used historically in a geographical area. Sci Total Environ 218(2–3):89–101
Ok YS, Chang SX, Feng YS (2007) Sensitivity to acidification of forest soils in two watersheds with contrasting hydrological regimes in the oil sands region of Alberta. Pedosphere 17(6):747–757
Ok YS, Lim JE, Moon DH (2011a) Stabilization of Pb and Cu contaminated soils and soil quality improvements using waste oyster shells. Environ Geochem Health 33:83–91
Ok YS, Usman ARA, Lee SS, Abd El-Azeem SAM, Choi BS, Hashimoto Y, Yang JE (2011b) Effects of rapeseed residue on lead and cadmium availability and uptake by rice plants in heavy metal contaminated paddy soil. Chemosphere 85(4):677–682
Olson RV, Ellis JR (1982) Iron. In: Miller RH, Keeney DR (eds) Methods of soil analysis, vol 2., 2American Society of Agronomy and Soil Science Society of America, Madison, pp 301–312
Quazi S, Datta R, Sarkar D (2011) Effects of soil types and forms of arsenical pesticides on rice growth and development. Inter J Environ 8(16):445–460
Sarkar D, Datta R (2003) A modified in vitro method to assess bioavailable arsenic in pesticide-applied soils. Environ Pollut 126(3):363–366
Sheppard SC (1992) Summary of phytotoxic levels of soil arsenic. Water Air Soil Pollut 64(3):539–550
Shipley HJ, Yean S, Kan AT, Tomson MB (2009) Adsorption of arsenic to magnetite nanoparticles: effect of particle concentration, pH, ionic strength, and temperature. Environ Toxicol Chem 28(3):509–515
Signes-Pastor A, Burló F, Mitra K, Carbonell-Barrachina AA (2007) Arsenic biogeochemistry as affected by phosphorus fertilizer addition, redox potential and pH in a west Bengal (India) soil. Geoderma 137(3–4):504–510
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17(5):517–568
Sumner ME, Miller WP (1996) Cation exchange capacity and exchange coefficients. In: Sparks DL (ed) Methods of soil analysis: Part 3. Chemical methods., vol 5. ASA and SSSA, Madison, pp 1201–1229
Talukder ASMHM, Meisner CA, Sarkar MAR, Islam MS (2011) Effect of water management, tillage options and phosphorus status on arsenic uptake in rice. Ecotoxicol Environ Saf 74(4):834–839
Tang X-Y, Zhu Y-G, Shan X-Q, McLaren R, Duan J (2007) The ageing effect on the bioaccessibility and fractionation of arsenic in soils from China. Chemosphere 66(7):1183–1190
Urík M, Littera P, Ševc J, Kolenčík M, Čerňanský S (2009) Removal of arsenic (V) from aqueous solutions using chemically modified sawdust of spruce (Picea abies): Kinetics and isotherm studies. Int J Environ Sci Tech 6(3):451–456
Usman ARA, Lee SS, Awad YM, Lim KJ, Yang JE, Ok YS (2012) Soil pollution assessment and identification of hyperaccumulating plants in chromated copper arsenate (CCA) contaminated sites, Korea. Chemosphere 87(8):872–878
Violante A, Pigna M (2002) Competitive sorption of Arsenate and Phosphate on different clay minerals and soils. Soil Sci Soc Am J 66(6):1788–1796
Wang S, Mulligan CN (2006) Occurrence of arsenic contamination in Canada: sources, behavior and distribution. Sci Total Environ 366(2–3):701–721
Watanabe FS, Olsen SR (1965) Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci Soc Am Proc 29:677–678
Woolson EA, Axley JH, Kearney PC (1971) Chemistry and phytotoxicity of arsenic in soils. I. Contaminated field soils. Soil Sci Soc Am Proc 35(6):938–943
Yang JE, Lee WY, Ok YS, Skousen J (2009) Soil nutrient bioavailability and nutrient content of pine trees (Pinus thunbergii) in areas impacted by acid deposition in Korea. Environ Monit Assess 157:43–50
Zhu Y-G, Williams PN, Meharg AA (2008) Exposure to inorganic arsenic from rice: a global health issue? Environ Pollut 154(2):169–171
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1B3001409). Instrumental analysis was partly supported by the Korea Basic Science Institute, the Central Laboratory and Environment Research Institute of Kangwon National University in Korea. Mr. Pathirana and Ms. Wanigathunge at IFS, Sri Lanka helped soil sampling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vithanage, M., Rajapaksha, A.U., Wijesekara, H. et al. Effects of soil type and fertilizer on As speciation in rice paddy contaminated with As-containing pesticide. Environ Earth Sci 71, 837–847 (2014). https://doi.org/10.1007/s12665-013-2486-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2486-9