Abstract
Alzheimer’s disease (AD) is an age-associated neurodegenerative disease. As the population ages, the increasing prevalence of AD threatens massive healthcare costs in the coming decades. Unfortunately, traditional drug development efforts for AD have proven largely unsuccessful. A geroscience approach to AD suggests that since aging is the main driver of AD, targeting aging itself may be an effective way to prevent or treat AD. Here, we discuss the effectiveness of geroprotective interventions on AD pathology and cognition in the widely utilized triple-transgenic mouse model of AD (3xTg-AD) which develops both β-amyloid and tau pathologies characteristic of human AD, as well as cognitive deficits. We discuss the beneficial impacts of calorie restriction (CR), the gold standard for geroprotective interventions, and the effects of other dietary interventions including protein restriction. We also discuss the promising preclinical results of geroprotective pharmaceuticals, including rapamycin and medications for type 2 diabetes. Though these interventions and treatments have beneficial effects in the 3xTg-AD model, there is no guarantee that they will be as effective in humans, and we discuss the need to examine these interventions in additional animal models as well as the urgent need to test if some of these approaches can be translated from the lab to the bedside for the treatment of humans with AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With improvements in medicine and public health, the number of aged individuals has increased dramatically [1]. The number of individuals 65 years or older in the USA is expected to reach 88 million by 2050; this population already numbered 55 million in 2019 [2]. As age increases, so does susceptibility to age-related diseases such as Alzheimer’s disease (AD). AD is a neurodegenerative disease in which patients exhibit impaired memory, motor function, and language due to neuronal damage [2]. In 2019, approximately 5.8 million people were living with AD in the USA, resulting in a total healthcare cost of $290 billion [2]. By 2050, those healthcare costs are expected to be over $1 trillion [3]. Due to the high prevalence and cost of this disease, it is imperative to determine effective ways to prevent, delay, or treat AD.
There are two different types of AD. The most common is “late-onset”, also known as “sporadic”, in which AD develops late in life. Of the 5.8 million Americans living with Alzheimer’s disease in 2019, approximately 95% were late-onset [4]. The cause of late-onset AD is not known, but aging and possessing an apolipoprotein E4 allele (apoE4) are both risk factors [4]. In contrast, the other 5% of cases of AD were in younger adults and are characterized as “early-onset” or “familial” AD (FAD). FAD is primarily characterized by inherited amyloid precursor protein (APP), presenilin-1 (PSEN1), or presenilin-2 (PSEN2) mutations [4, 5]. Though FAD occurs due to one or more specific genetic mutations, and specific allelic variants increase the risk of late-onset AD, age is the greatest risk factor for all forms of AD [6].
Pathology of Alzheimer’s disease
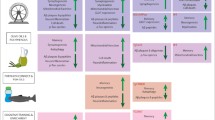
The progression of both types of AD are similar and are characterized by several different physiological changes (summarized in Figure 1). In healthy brains, tau is associated with microtubules and acts to stabilize them [7]. Binding of tau to microtubules is regulated by post-translational modifications, primarily phosphorylation [7, 8]. Phosphorylation of tau decreases its affinity toward microtubules, so varying amounts of phosphorylation allow for intra-axonal sorting, with phosphorylated tau aggregating closer to the soma and dephosphorylated tau located near the growth cone [7]. When hyperphosphorylated, tau completely dissociates from the microtubules and aggregates together to form neurofibrillary tangles (NFTs) [8]. NFT aggregation is strongly associated with neuronal death [9, 10]. Another contributing factor to AD development is microRNA (miRNA) dysregulation [11]. Dicer is an miRNA-processing enzyme which is upregulated during exercise, and ablation of this enzyme lowers miRNA abundance, leading to neuronal loss, tau hyperphosphorylation, and changes in behavior—similar phenotypes as seen in AD pathology, leading researchers to conclude that normal miRNA abundance and Dicer processing are important in overall brain health [12,13,14].
Characteristics of AD pathology in the central nervous system. During the progression of AD, many changes occur in the brain. Microtubules disintegration occurs due to the hyperphosphorylation of tau and formation of NFTs. Meanwhile, Aβ clearance is disrupted leading to aggregation into plaques. Astrocytes and microglia are activated, and pro-inflammatory cytokines are released. Each of these factors then contribute to synaptic dysfunction and neuronal death, which ultimately result in cognitive deficits. Created with BioRender.com
Another aspect of AD pathology is the accumulation of β-amyloid (Aβ) plaques. In the healthy brain, APP is cleaved into Aβ, which acts to decrease synaptic activity and regulate potassium and calcium channel production to control excitability and cell survival [15]. However, problems arise when too much Aβ is produced or clearance mechanisms are no longer sufficient to keep Aβ at a healthy level (reviewed in [16]). One potential mechanism for excessive Aβ production is beta-site APP cleaving enzyme 1 (BACE1), which cleaves APP to produce Aβ [17, 18]. Patients with AD have higher BACE1 levels, and deletion of BACE1 in mice prevents Aβ accumulation and ameliorates AD pathology [17,18,19]. Decreased autophagy [20, 21] and a disruption of insulin signaling [22,23,24] are both thought to contribute to the accumulation of Aβ to form extracellular plaques. During aging, two specific enzymes that are responsible for the majority of Aβ degradation are downregulated: neprilysin and insulin-degrading enzyme [25]. The downregulation of these two enzymes may contribute greatly to the accumulation of Aβ.
Aβ pathogenicity differs somewhat between FAD and late-onset AD, with FAD exhibiting altered Aβ ratios while late-onset AD does not [26]. There are a variety of different Aβ isoforms, with Aβ40 levels being significantly more abundant than Aβ42. Aβ42 is the more hydrophobic and fibrillogenic of the Aβ isoforms, and is greatly increased in the brain during FAD, leading to the formation of amyloid plaques (reviewed in [25]). Transgenic mouse models have shown that amyloid deposition is primarily due to Aβ42, and that Aβ increases NFT production. Aggregated Aβ also contributes to immune (microglia) activation which leads to high levels of neuroinflammation and may exacerbate neuronal stress.
There is considerable interplay between the different pathological features of AD, some of which are still not well known [27]. Current findings suggest that Aβ contributes to tau pathology and tau pathology in return may promote Aβ pathology. In addition, NFTs may increase Aβ toxicity, suggesting that tau pathology may indeed may be a major driver of AD pathology [28].
During the preclinical stage, there are no clinical systems evident, but there are changes in biomarkers [2, 27]. Evident biomarkers in the preclinical stage may include reduced Aβ1-42 in the cerebrospinal fluid and increased amyloid tracers during PET scans, increased phosphorylated tau (p-tau) levels in the cerebrospinal fluid, and changes in MRI scans [27]. As preclinical AD progresses, clinical symptoms eventually appear, though this occurs in humans at least 15 years after the beginning of Aβ accumulation [27]. Clinical symptoms start as mild cognitive impairment, such as memory lapses and difficulty thinking, and then progress to AD dementia [2, 27]. AD dementia patients may have trouble communicating and performing routine tasks as well as deficits in motor control and swallowing. They may also exhibit personality and behavior changes, suspiciousness, and agitation [2]. AD has dramatic negative impacts not only on the quality of life of the patient, but also greatly affects those who must care for the individual.
Another compounding factor in the use and cost of caregiving for AD patients is the prevalence of comorbidity with a number of other age-related diseases, including cardiovascular disease, diabetes, kidney disease, and cancer [2]. Coexisting conditions such as these increase caregiving time and cost and may exacerbate the symptoms and progression of AD.
Diabetes and Alzheimer’s disease
In humans, the development of AD is often closely tied to diabetes and obesity. In 2014, 37% of Medicare beneficiaries who were 65 or older with dementia also had diabetes [2]. The high prevalence of comorbidity between these two diseases has led to the suggestion that AD development may be linked to dysfunctional insulin signaling and glucose homeostasis [2, 29]. While the exact relationship between glucose metabolism and AD is unclear, the risk of AD is increased by type 2 diabetes, and glucose metabolism is impaired in individuals with AD (reviewed in [30]). Glucose metabolism is disrupted in the brains and neurons of AD patients, with defects in glucose uptake (reviewed in [30]) and mitochondrial dysfunction, which may stem from disruption of the TCA cycle, electron transport chain, or fission-fusion [31,32,33]. It has been well established that there is impaired insulin signaling in the brain during AD, so much so that AD is often referred to as type III diabetes [34,35,36,37,38,39,40]. In many tissues, insulin acts to stimulate glucose uptake via translocation of glucose transporters [39]. This allows tissues such as muscle to take up blood glucose, thereby lowering post-prandial blood glucose and allowing the tissue to utilize the glucose for energy.
In the CNS, insulin has a number of different functions such as signaling food intake and energy regulation in the hypothalamus and effecting synaptic plasticity in the hippocampus [34, 39]. Insulin also greatly influences learning and memory, neurotransmitter signaling, and neuronal development [38, 39]. Insulin promotes neuronal glucose uptake as well [39]. In insulin-resistant patients, the inability of tissues to respond to insulin and initiate glucose uptake leads to hyperinsulinemia [38]. Peripheral hyperinsulinemia in turn leads to downregulation of insulin receptors in the blood brain barrier, leading to decreased uptake of insulin into the brain [34, 38]. Decreased insulin function in the brain is detrimental to learning and memory but also leads to decreased glucose uptake into both the brain and neurons themselves [39, 40]. Decreased glucose metabolism has been found in AD patients and correlates with a reduction in cognitive performance [40].
In 3xTg mice, a number of studies have examined how the progression of AD pathology correlates with disruptions in insulin signaling and glucose homeostasis [29, 41,42,43]. Older 3xTg mice exhibit impaired glucose tolerance as well as elevated fasting blood glucose levels [42, 43], and diet-induced obesity accelerates the cognitive decline of 3xTg mice [44]. According to Vandal et al., impaired glucose tolerance is evident by 10 months of age, but not at 6 months of age [42]. In contrast, Velazquez et al. did not find differences in glucose tolerance at 10 months of age but did by 16 months of age [43]. Inconsistencies in timing between these two studies may be due in part to differences in the glucose tolerance test protocol [42, 43]; but in both studies, 3xTg mice do exhibit an age-dependent increase in glucose intolerance. However, 3xTg mice do not show impaired performance on an insulin tolerance test [42]. In the central nervous system, 3xTg-AD mice exhibit an age-dependent increase in insulin resistance, occurring prior to peripheral insulin resistance [43]. These results suggest that as in humans, insulin signaling and glucose homeostasis are likely linked to AD, although how these are linked remains to be determined. To accelerate the process of determining these links between AD and metabolic health, as well as developing treatments for AD, scientists have developed animal models of AD to aid research into the development, progression, and treatment of the disease.
The 3xTg-AD mouse model
There are over 180 mouse models for AD, many of which have only a single gene mutation; as a result, these models exhibit only either Aβ or tau pathology, and do not encompass the full scope of neurodegenerative issues that occur in the brain of an AD patient [4, 45]. The 3xTg-AD mouse model, developed by Oddo and colleagues in 2003, was made by comicroinjection of two independent transgenes containing APPSwe and tauP301L into the embryos of homozygous mutant PS1M146V knock-in mice [46]. The 3xTg-AD model is useful for studying AD treatments as they exhibit a full range of AD pathologies.
These mice exhibit robust Aβ pathology, with an age-dependent increase in Aβ40 as well as the longer, more amyloidogenic Aβ42 form [42, 46, 47]. By 3–4 months of age, 3xTg-AD mice exhibit intracellular Aβ immunoreactivity in parts of the hippocampus and cortex, with extracellular Aβ plaque accumulation seen starting at 12–15 months of age [46,47,48,49]. Tau pathology develops later, with the first signs of human tau immunoreactivity appearing at 12 months of age; more than 6 months after Aβ pathology begins [46, 49]. Once tau pathology begins to develop, it progresses in a fashion very similar to what is observed in humans. First, tau hyperphosphorylation is evident in the hippocampus, particularly in the pyramidal neurons of the CA1 subfield, and developing in the cortex at later stages of the pathology [46]. In humans, tau pathology in the cerebrospinal fluid correlates much more strongly with neuronal death and cognitive defects than Aβ, but Aβ is thought to be a primary driver for the overall pathology of AD [4, 49]. Thus, the appearance of Aβ pathology prior to tau pathology follows a similar trend to human AD.
In humans, synaptic loss in the frontal cortex is primarily responsible for cognitive defects and occurs early in the onset of AD [50,51,52]. 3xTg mice also display synaptic dysfunction and impaired long-term potentiation by 6 months of age, likely due to tau pathology [46]. Inflammation, another hallmark of AD pathogenesis, is also present in 3xTg-AD mice, with microglia activation beginning by 6–12 months of age, and astrogliosis present by 12 months [47, 53]. There is some inconsistencies in the performance of 3xTg-AD mice in motor coordination tests, but most reports agree that the mice appear to exhibit impaired motor function starting at about 6 months of age, which is consistent with motor function impairments seen in some AD patients [2, 54].
Although in humans, increased Aβ levels in the brain may have been seen up to 22 years prior to exhibiting symptoms [2], the accumulation of Aβ and cognitive deficits are more concurrent in 3xTg mice, which exhibit mild cognitive defects in spatial learning and memory by 4–6 months of age [47, 48, 55]. Deficits have also been found in contextual fear memory [48]. Overall, the 3xTg mouse model displays a progression of Aβ and tau pathology, along with changes in neuronal function and inflammatory state, that closely mimics the progression of AD in humans, though the timing in some of these aspects are skewed, likely by the much shorter lifespan of mice.
One avenue of interest in potential AD treatments is repurposing dietary and pharmaceutical interventions that are effective in other age-related diseases in the treatment of AD. Aging is the primary risk factor for both late-onset and FAD, so geroprotective interventions may be highly beneficial in the regulation of AD. The 3xTg model has been widely used in examining the effects of these interventions in AD, which is the topic of this review and summarized in Table 1.
mTOR signaling and Rapamycin
One potential link between insulin signaling and the progression of AD is the protein kinase mechanistic Target Of Rapamycin Complex 1 (mTORC1) (Table 1, Figure 2). mTORC1 acts as a central hub for nutrient signaling, integrating nutritional information as well as hormonal cues to regulate the activation of anabolic processes essential for cell and organismal growth, including protein translation, ribosomal biogenesis, lipogenesis and nucleotide biogenesis; mTORC1 also negatively regulates autophagy [56, 57]. mTORC1 is a key downstream effector of insulin signaling and provides feedback inhibition of insulin signaling through its substrates S6K1 and Grb10, thus promoting the degradation of insulin receptor substrate [58,59,60]. As such, sustained activation of mTORC1 by nutrient overload, obesity, and aging has been suggested as an underlying molecular mechanism that drives the development of insulin resistance.
Potential pathways to target in the treatment or prevention of AD. FGF21, mTORC1, and SIRT1 are three of the most promising pathways for influencing AD pathology. Downregulation of mTORC1 may improve AD pathology through increased autophagy, leading to improved clearance of Aβ and p-tau. Upregulation of FGF21 may improve a variety of AD characteristics directly, as well as through inhibition of both BACE1 and NF-κB. SIRT1 upregulation may improve AD pathology via inhibition of both apoptosis and NF-κB as well as promotion of ADAM10 to reduce Aβ plaque formation and neuron death. Due to the interplay between the different aspects of AD pathology, even treatments that only target one aspect may have long-reaching effects on overall AD pathology. Created with BioRender.com
mTORC1 signaling has been linked to age-related diseases and lifespan in diverse species including worms, flies, and mammals [56, 61]. Genetically reducing mTORC1 signaling extends the lifespan of yeast, worms, flies, and mice [62,63,64,65,66,67,68]. Studies by multiple independent laboratories and groups have shown that the mTOR inhibitor rapamycin extends lifespan in worms, flies, and multiple strains of wild-type mice, even when treatment is intermittent or conducted for only a relatively short period of time [69,70,71,72,73,74,75,76,77,78]. Rapamycin improves multiple parameters of healthspan in mice [79]. mTORC1 signaling has been implicated in cognitive decline with normal, non-pathogenic aging, and treatment of wild-type mice with rapamycin ameliorates deficits in learning and memory, perhaps in part by preserving cerebral blood flow, and blunts age-related declines in synaptic density [80, 81].
mTORC1 signaling is elevated in the brains of human AD patients [82,83,84,85], and genetic depletion of S6K1, a substrate and effector of mTORC1, is sufficient to improve memory and reduce AD pathology in 3xTg mice [86]. Similarly, treatment with the mTOR inhibitor rapamycin prevents the progression of AD pathology and prevents cognitive deficits in 3xTg mice, perhaps in part due to increased autophagy [85] (Table 1). In 3xTg-AD mice, autophagy improvement was seen following both short and long-term rapamycin treatment, with increases in the autophagy proteins ATG5 and ATG7, which are both necessary for autophagy induction, as well as increased LC3-II, a marker of autophagy that is incorporated into the autophagosome membrane [85].
Rapamycin-treated 3xTg-AD mice performed better than untreated 3xTg-AD mice as well as wild-type control mice in Morris Water Maze (MWM), a hippocampal-dependent task [85, 87]. Novel Object Recognition (NOR) also showed improvements in 3xTg-AD mice treated with rapamycin [87]. Overall Aβ pathology was improved in treated 3xTg-AD mice, as shown by decreased soluble and insoluble Aβ40 and Aβ42 levels and decreased Aβ deposition [85, 87]. Tau pathology was likewise improved, and tau phosphorylation was decreased at multiple residues [85, 87]. Finally, rapamycin-treated 3xTg-AD mice showed lower levels of activated microglia, and as such, lower levels of brain inflammation [87]. Although autophagy is increased in both short and long-term rapamycin treatment, beneficial effects of rapamycin treatment on Aβ and tau pathology is only seen when treatment begins before the formation of plaques and tangles, suggesting a preventative effect rather than curative [87].
As a result of the potent effects of rapamycin in the 3xTg mouse model of AD, as well as similar beneficial effects of rapamycin in other early onset AD mouse models [88,89,90], rapamycin has been proposed as a potential prophylactic therapy for AD [91]. However, there are potential safety concerns associated with the long-term use of rapamycin, which is FDA-approved as an immunosuppressant agent, and which also causes a variety of metabolic side effects when rodents or people are treated for long periods of time, particularly if high doses are used (reviewed by [92]). Additionally, it has been suggested that administration of rapamycin in late-stage AD may actually be detrimental due to impairment of the lysosomal system [93]. Though there is not yet any data on how AD patients may respond to rapamycin treatments, there are currently two clinical trials in progress at the University of Texas Health Science Center at San Antonio to determine the safety, tolerability, and cognitive effects of rapamycin administration in older adults with mild cognitive impairment or AD [94, 95].
Dietary interventions in the 3xTg mouse model of AD
Caloric restriction (CR) has represented the gold standard for geroprotective dietary interventions for almost a century, robustly extending lifespan in numerous species ranging from yeast, worms, and flies to mice, rats, dogs, and even non-human primates (reviewed in [96]). CR is an attractive option when considering AD prevention due to the beneficial effects of CR on many age-related diseases and its ability to decrease age-related disease-associated mortality [96, 97]. However, while dietary interventions such as CR may be efficacious in the laboratory, the ability of people to adhere to such an abstemious diet in the real world is limited. As such, research has begun to focus more strongly on other dietary interventions that may be easier to maintain long-term. The three dietary interventions that have been studied in the 3xTg-AD mouse model that we will discuss here are CR, protein restriction (PR), and branched-chain amino acid restriction (BCAA-R) (summarized in Table 1).
Calorie restriction
In addition to extending lifespan, CR also extends healthspan, with benefits including decreased adiposity, increased insulin sensitivity, decreased inflammation, decreased reactive oxidative species (ROS), and decreased lipids in the bloodstream, as well as reduced rates of diabetes and beneficial effects on brain function [96, 98,99,100,101]. Due to both the metabolic and geroprotective benefits of CR, there has been substantial interest in the use of CR as a way to treat or prevent AD. It is important to note, however, that fasting is an important aspect of many CR models. Most laboratory CR models involve feeding a reduced amount of food once a day, which results in an unintentional prolonged fasting period after the food is quickly consumed. Pak et al. examined whether fasting is required for the beneficial effects of CR [102]. They found that daily prolonged fasting without the restriction of calories recapitulated many of the benefits of a typical laboratory CR diet. In contrast, when mice were fed a diet that modeled CR without prolonged fasting, there was a large reduction in the beneficial effects of the CR diet.
Notably, mTORC1 is thought to be a key effector of the response to CR, and in yeast, deletion of TOR1 is epistatic with CR [62]. However, studies of CR and mTOR in worms and flies have not clearly demonstrated an epistatic relationship; in flies, lifespan is extended by rapamycin at every level of calorie intake [103,104,105,106,107]. Extensive mammalian “omics” studies suggest that rapamycin and CR have distinct, largely non-overlapping effects [108,109,110,111,112,113].
In various models of AD, CR decreases both Aβ and tau pathology, decreases neuroinflammation, improves neurogenesis and neuroplasticity, and improves cognition [100, 114,115,116,117,118,119]. Some mechanisms by which CR may induce these beneficial effects include attenuated immune and hormone changes, increased damage repair, changes in gene expression, increased autophagy and apoptosis, altered insulin/insulin-like growth factor-1 (IGF-1) signaling, altered mTORC1 signaling, activation of sirtuins, decreased metabolic rate, and attenuated ROS generation [96, 100, 117]. In the 3xTg mouse model of AD, CR ameliorates age-related deficits in Morris Water Maze (MWM) performance and open-field activity [120]. CR also decreases Aβ and tau pathology in the hippocampus, as evidenced by decreases in both Aβ40 and Aβ42 levels and decreased tau phosphorylation at Ser202. Halagappa et al. also looked at intermittent fasting alone and found that there were similar improvements in MWM and open-field, but observed no change in Aβ or p-tau levels as a result of intermittent fasting [120]. This suggests that CR may be a useful tool in controlling the pathology of AD, but unlike with the metabolic benefits of CR, fasting alone may not recapitulate all neurological improvements.
Rangan et al. utilized a fasting-mimicking diet (FMD) composed of low calories, low protein, and high unsaturated fats which produces similar longevity-related markers as water-only fasting but provides various nutrients to lessen the burden of prolonged fasting [121]. In the first experiment, FMD was administered starting at 3.5 months of age in 4–5 day cycles, depending on sex. FMD mice had improvements in both Y-maze and NOR performance, particularly in males, at 10.5 months of age. At 18 months of age, FMD female 3xTg mice performed better in the Barnes Maze (BM). Aβ load, p-tau, and microglia levels were decreased and neurogenesis was increased in FMD mice. In the second experiment, FMD was administered to 6.5-month-old mice, which are old enough that AD pathology can be observed. After 2 months of FMD, there was no change in Y-maze performance, but NOR recognition index was improved in males. Aβ and p-tau load was decreased in males, but unaltered in females. However, FMD female 3xTg mice had diminished microglial activation. Overall, FMD reduced inflammation, improved short-term memory, and ameliorated Aβ and tau pathology.
Emerging data suggests that CR may have beneficial effects for non-human primates as well as for humans. CR attenuates the progression of AD-type amyloidosis in Squirrel monkeys [122]. In humans, both clinical trials and observational studies have shown that caloric restriction, particularly when paired with exercise, ameliorates mild cognitive impairment and reduces risk of AD (reviewed in [123, 124]). Given this human data and supporting data from animal models, CR seems to be a promising avenue for AD patients, despite the difficulties in adherence to a CR diet.
Protein restriction
Ever since the very first studies of CR, scientists have wondered if the effects of CR on aging were due to reduced intake of calories generally, or due to a reduction in specific nutrients. Recently, dietary protein has been identified as a key macronutrient for aging, with studies in flies and rodents finding that animals consuming diets with less protein have better metabolic health and live longer [125,126,127,128,129]. While protein restriction (PR) has many of the same beneficial effects as CR, diets based on alterations in dietary macronutrients are thought to be easier to maintain than an abstemious, low-calorie CR regimen [96].
While PR has not yet been fully explored in the context of AD, 3xTg mice cycled on and off of a protein-free (PF) diet had improved working memory in the Y-maze and spatial memory in NOR [130]. Interestingly, while a CR diet reduces both Aβ and tau pathology, a PF diet specifically reduced tau pathology and phosphorylation, without altering Aβ immunoreactivity or Aβ plaque size in the hippocampus [120, 130]. Finally, a PF diet did not affect microglial activation, suggesting that PF does not influence inflammation in the brain [130]. PF did not lead to chronic low body weight in 3xTg mice, and did not significantly decrease blood glucose levels, but does reduce circulating levels of IGF-1 and IGF binding protein-3 (IGFBP-3) levels [130]. Administration of a cyclic 7-day 4% PR diet from 3.5 months to 18 months of age resulted in improved Y-maze and NOR performance, decreased Aβ and p-tau loads, and increased neurogenesis [121].
One potential mechanism by which reductions in dietary protein may prevent or treat AD is through reduced activity of mTORC1 signaling. As amino acids are potent agonists of mTORC1, it is logical that a reduction in dietary protein might limit mTORC1 signaling and have similar effects; PR has been shown to reduce mTORC1 signaling in vivo in multiple other tissues [128, 131], and preliminary work suggests that PR may be able to reduce mTORC1 signaling in the brain [132]. Though there is some contradicting evidence from clinical and observational studies in humans, many studies suggest that high protein intake is correlated with reduced risk of AD and improved cognition [133,134,135]. It is unclear if the differences between animal models and human studies are due to between-species effects or age-related differences. Many AD animal models, particularly in 3xTg mice, are utilized when they are still relatively young. It is possible that PR helps to delay the onset of AD pathology but is not sufficient to decrease AD prevalence. Conversely, it is likely that differing levels of protein intake are beneficial at different life stages. In this instance, PR may be more beneficial for the young, while increased protein intake may be beneficial late in life.
Restriction of branched-chain amino acids
The branched-chain amino acids (BCAAs; leucine, isoleucine, and valine) were identified as metabolically interesting in the late 1960s, when the blood levels of the BCAAs were found to be elevated in humans with obesity [136]. Since that time, blood levels of the BCAAs have been associated with cancer, cardiovascular disease and diabetes [137, 138]. As essential amino acids, blood levels of BCAAs are highly correlated with dietary levels, and increased consumption of BCAAs is associated with increases in both liver and blood levels of the BCAAs as well as the incidence of diabetes [128, 139, 140]. The BCAAs are potent agonists of mTORC1, and activation of mTORC1 has been suggested to be linked to their effects on insulin sensitivity as well as cancer [141, 142]. Dietary BCAA levels correlate negatively with lifespan in mice, with high BCAA diets shortening lifespan and BCAA restricted diets extending lifespan [127, 143].
It has been hypothesized that defects in BCAA catabolism, which elevate brain levels of the BCAAs, may contribute to the pathogenesis of AD by activating mTORC1 [137]. In the 3xTg-AD mouse model, increased BCAA intake is closely associated with diabetes and AD, possibly due to down-regulation of BCAA transaminase (BCAT) in the brain, which in turn leads to leucine accumulation [137]. Leucine accumulation is positively associated with risk for developing AD [137, 138]. Supplementation of BCAAs leads to increased cognitive deficits, increased tau phosphorylation, and, when paired with HFD, premature death of 3xTg mice [137, 138]. Conversely, restriction of BCAA intake improves cognitive performance of 3xTg mice in NOR, although it did not decrease tau phosphorylation [138].
While the BCAAs have most often been considered as a group, an emerging realization is that the individual BCAAs have distinct effects on metabolism and signaling. The valine catabolite 3-hydroxyisobutyrate (3-HIB) has been shown to promote insulin resistance in mice by stimulating fatty acid and glucose uptake into skeletal muscle [144, 145]. Isoleucine restriction improves glucose tolerance and reduces adiposity in mice, while in humans higher dietary levels of isoleucine are associated with greater body mass index, and higher blood levels of isoleucine are associated with increased mortality [146, 147].
When considering BCAA levels in humans, Tynkkynen et al. found that decreased BCAA levels are associated with increased risk of dementia and AD [148]. There have not been any studies examining the effect of individual BCAAs on cognition and AD pathology, however one study has found that a genetic predisposition to increased plasma isoleucine is positively associated with AD, suggesting that pharmacological or dietary interventions that reduce isoleucine levels may improve AD pathology [149]. Future research in animal models of AD may help to determine if the individual BCAAs have specific roles in driving the progression of AD.
Fgf21
All three of the dietary interventions discussed above — CR, PR, and BCAA restriction — have been shown to regulate Fibroblast growth factor 21 (FGF21), an endocrine hormone produced primarily by the liver but also by other tissues including adipose and skeletal muscle that was discovered over 20 years ago [150, 151]. FGF21 is strongly induced by protein restriction in both humans and rodents [152, 153], and is required for some of the effects of PR on lifespan and metabolic health [154,155,156], at least in C57BL/6J male mice [157]. While the effects of CR on FGF21 are not as clear, there are at least some reports that CR upregulates FGF21 signaling [158]. In mice, BCAA restriction upregulates FGF21, although the effect may depend upon the exact diet and length of restriction [127, 147, 152, 159, 160]. Restriction of isoleucine, which is the most critical BCAA for metabolic responses, strongly induces FGF21 in C57BL/6J males [147].
After release from the liver, FGF21 crosses the blood brain barrier to stimulate sympathetic nerve activity via β-klotho which results in an overall increase in energy expenditure and insulin sensitivity and decreased food intake and body weight [151, 153, 155, 156, 161,162,163]. Due to the ability of FGF21 to cross the blood brain barrier, its effects on cognition have been well studied. In humans, AD patients have lower FGF21 levels [164] and increased FGF21 expression correlates with improved cognition, learning ability, and both short- and long-term memory [165]. In Wistar rats, administration of FGF21 led to decreased systemic and neural inflammation, improved hippocampal synaptic plasticity, increased dendritic spine density, improved brain mitochondrial function, decreased oxidative stress, improved neurogenesis, and decreased tau pathology [166,167,168,169]. Interestingly, intraperitoneal FGF21 administration did not increase brain insulin sensitivity in Wistar rats [167]. Other studies have also found neuroprotective effects of FGF21. FGF21 is able to decrease Aβ toxicity and protects against oxidative stress and apoptosis in cultured neuroblastomas [170] and protected cultured neurons against excitotoxicity [171]. Given these many cognitive and neuroprotective improvements associated with increased FGF21 signaling, any interventions or pharmaceutical treatments that upregulate FGF21 are good candidates for improving AD pathology (Figure 2).
To summarize, dietary interventions have great potential for treatment of AD, but determining which components are most beneficial to increase or decrease in the daily diet is important, as well as identifying the molecular mediators. If increased FGF21 contributes to the beneficial effects of these diets on AD, other interventions which stimulate FGF21 — including diets low in methionine or chronic cold exposure [172,173,174,175] — may also be able to help preserve cognition and slow the development of AD pathology. In fact, Tournissac et al. examined the ability of repeated short cold exposure to protect against cold-induced tau phosphorylation [176]. Exposing 15-month-old 3xTg mice to short-term cold over a period of 4 weeks increased brown adipose tissue thermogenesis and plasma FGF21 levels, improved glucose tolerance, and decreased tau hyperphosphorylation. They found that plasma FGF21 levels were negatively correlated with p-tau levels in the hippocampus. These results suggest that increased FGF21 production via any mechanism may be beneficial for AD pathology. It is also possible that FGF21 may interact with other mechanisms to ameliorate AD pathology, as it has been reported that FGF21 may repress hepatic mTORC1 activity [177], while diminished mTORC1 activity in muscle may promote expression of FGF21 [178].
Dietary supplements
Carnosine
Carnosine (β-alanyl-L-histidine) is synthesized in the body from β-alanine and L-histidine, and increased ingestion of β-alanine leads to increased levels of carnosine [179]. β-alanine is found in high concentrations in meat, but due to the benefits of low-protein diets, consuming sufficient levels of β-alanine to significantly raise serum carnosine levels is impractical. This suggests that supplementation of β-alanine or carnosine may be more advantageous for the various metabolic and neurological benefits of carnosine. Carnosine, which is found in high concentrations in glial and neuronal cells throughout the brain, has been found in other models to function as a chelator for zinc as well as an antioxidant and free-radical scavenger; carnosine exerts anti-aging activity by attenuating the toxicity of byproducts of lipid peroxidation, and inhibits Aβ toxicity [179,180,181,182,183,184,185]. Mitochondrial dysfunction, which is prevalent as an early marker in AD, tends toward enhanced ROS generation, which in turn increases free zinc levels in neurons [180, 186,187,188]. This increase in zinc levels then leads to increased zinc-dependent ROS formation and Aβ oligomerization, all of which contribute individually to the development of AD pathology and each other [180, 187, 189,190,191]. Thus, carnosine’s activity as a chelator and antioxidant may be highly beneficial in AD. Additionally, carnosine acts as a rapamycin mimetic, and as such may construe beneficial effects in AD via mTOR inhibition [192].
Treatment of 3xTg male mice with carnosine for 11–13 months starting at 1 month of age did not significantly improve MWM performance or affect tau phosphorylation at the Thr231/Ser235 site, but did reduce intraneuronal Aβ in the hippocampus [180]. Carnosine supplementation also reversed age-dependent mitochondria deficits commonly associated with the 3xTg-AD mouse model [180, 188].
Human AD patients have reduced plasma carnosine levels [193]. In addition, several studies have found that carnosine supplementation is associated with improved cognition [194]. Though these data are limited, the potential for carnosine to reduce toxicity and improve cognition may result in long-term benefits for AD.
Curcumin
Curcumin is a component of the spice turmeric. Curcumin has been found to have anti-inflammatory effects, and is often used for arthritis, asthma, atherosclerosis, cancer, and diabetes [195, 196]. In vitro results have found that curcumin prevents neuronal damage, attenuates neuroinflammation, reduces oxidative damage, and ameliorates Aβ accumulation in the brain [196,197,198,199,200,201]. In the 3xTg-AD mouse model, curcumin has been studied in conjunction with DHA-rich fish oil as well as by itself [199]. Both curcumin and DHA reduced phosphorylation of c-Jun N-terminal kinase (JNK), insulin receptor substrate 1 (IRS-1), and tau (at Ser422), but did not affect performance on the Y-maze [199]. The combination treatment (curcumin + fish oil) had more significant effects on each of these parameters and also showed improvements in Y-maze performance [199].
In humans, consumption in diets high in curcumin are associated with lower incidence of AD [202, 203]. Several clinical studies have shown that curcumin has a number of health benefits in multiple organ systems. Specific to AD pathology, curcumin supplementation may prevent cognitive decline, induce Aβ clearance, and prevent some aspects of AD pathology (reviewed in [202, 203]). Potential mechanisms by which curcumin may induce beneficial effects on AD include the previously mentioned anti-inflammatory effects as well as stimulating FGF21 production [204], downregulating of mTOR signaling [205], increasing SIRT1 levels [206], and inhibiting Nuclear factor kappa B (NF-κB) [200].
Vitamin A
Vitamin A is a nutrient obtained through the food which is critical for proper brain function in adults, particularly in learning and memory processes—two aspects of cognition that are heavily deficient in AD [207,208,209,210]. Retinoids, including retinol, are vitamin A derivatives and are important in modulating the inflammatory response, including neuroinflammation, and contribute to normal brain function [211, 212]. Interestingly, circulating levels of vitamin A are decreased in elderly individuals concurrently with the risk of developing AD [207, 208, 213, 214], and retinol levels are also lower in AD patients [215,216,217]. This suggests that supplementation of vitamin A may help ameliorate AD pathology through the retinoid signaling pathway.
Male and female 3xTg mice show some improvements in AD pathology when vitamin is supplemented in diet from 8 to 14 months of age [207]. Specifically, vitamin A-supplementation increased discrimination between the familiar and novel arms of the Y-maze during the short-term memory test, without a change in visual acuity. Vitamin A supplementation decreased both Aβ and p-tau in males but not females, suggesting a sex-dependent effect in some aspects of retinoid signaling. Vitamin signaling did not affect A Disintegrin and Metalloproteinase 10 (ADAM10) or BACE1 signaling, nor did it alter the inflammatory environment in the brain.
All-trans retinoic acid (ATRA) has been FDA-approved for the treatment of acute promyelocytic leukemia and acne vulgaris. Recently, it has gained interest as a potential treatment for AD. It is thought that ATRA can modulate APP cleavage regulation via promotion of ADAM10 transcription [212]. Alternative possible mechanisms for the benefits of ATRA and other retinoids is through stimulating phospholipase activity, which plays an important part in neuronal membranes [212]. Several studies have been performed in the 3xTg-AD mouse model to determine the beneficial effects of ATRA on AD pathology [212, 218, 219]. Treatment with ATRA for 8 weeks starting at 7 months of age results in improved performance in the Morris radial arm water maze test as well as decreased Aβ levels [212]. Total tau levels were higher in ATRA-treated 3xTg-AD mice, but levels of phosphorylated tau were not measured [212]. Treatment of 3xTg mice with ATRA for 4 weeks starting at either 12 or 22 months had beneficial effects, including increased cell proliferation, decreased p-tau levels, and reduced microgliosis [218, 219].
Though there do not appear to be any clinical trials on the effects of vitamin A in AD patients, the decreased levels of vitamin A during AD and improved AD pathology in 3xTg mice suggest that vitamin A supplementation may be beneficial for AD patients. There are a number of ongoing clinical trials utilizing various retinoids or retinoid X receptor agonists [220,221,222]. One study on isotretinoin that has already been published in cognitively healthy subjects found that isotretinoin may have dose-dependent improvements in hippocampal-based learning [223]. Furthermore, a 1 year treatment of beta carotene (a provitamin A carotenoid) in individuals older than 65 years resulted in cognitive benefits, but short-term administration of beta carotene had no impact [224]. One potential hurdle in utilizing vitamin A or retinoids for AD is the potential negative side effect. Retinoids are potentially teratogenic, and as such would not be able to be used in pregnant or breastfeeding mothers [212]. In addition, prolonged usage of retinoids may lead to neurotoxicity or gastrointestinal hemorrhage in addition to arrhythmia, nausea, abnormal liver function, skin irritation, and depression [212]. As such, treatment dosage and period will both be important considerations when utilizing retinoids to combat AD.
Exercise
Physical exercise is well known for its beneficial effects on obesity, insulin resistance, and type 2 diabetes [225, 226], but importantly exercise also has a number of beneficial effects on the brain, promoting synaptic plasticity, neurotransmission, and neurogenesis, and inhibiting AD progression in mice [226,227,228,229]. In humans, physical exercise is correlated with decreased risk of developing MCI and improved cognition in both AD and MCI [227, 230]. Several studies on exercise have been performed in the 3xTg-AD mouse model, utilizing both aerobic and resistance exercise (summarized in Table 2).
Resistance exercise via weighted ladder climbs resulted in improved grip strength, increased hippocampal IGF-1, and decreased Aβ in females from 3 to 5 months of age [231]. In 9–10-month-old males, resistance exercise increased recognition index in NOR, improved Y-maze latency and errors, increased synaptic protein levels, reduced Aβ deposits, decreased tau hyperphosphorylation, reduced microglia activation, and decreased pro-inflammatory and increased anti-inflammatory cytokine levels [232]. When 3xTg mice are given free access to running wheels — a form of aerobic exercise — from 6 until 12 months of age, they exhibit decreased neophobia, decreased anxiety, improved MWM performance, decreased Aβ levels, decreased brain oxidative stress markers, and decreased mitochondrial DNA deficits [233]. Treadmill exercise 5 days a week for 9 weeks (beginning at 30 minutes per day and slowly increasing to 90 minutes per day) in 3-month-old females led to improved rotarod performance and increased hippocampal IGF-1 levels [231]. Forced running exercise on a motorized wheel bed for 12 weeks in 2.5 to 5.5 month old 3xTg-AD mice leads to reduced foot slips in the beam test, reduced removal time in adhesive test, and improved spatial recall in MWM [234]. Kim et al. found that aerobic exercise alleviates high fat diet-induced defects in metabolic function and insulin signaling while also improving cognitive function, decreasing Aβ plaque accumulation and Aβ42 levels, and reducing tau phosphorylation [226].
Dungan et al. used a combination resistance/aerobic exercise program called PoWeR (progressive weighted wheel running) where a running wheel is weighted with 2 g of resistance for the first 2 weeks then slowly increased to 5 g of resistance [19]. They began exercising female 3xTg-AD mice at 2 months of age and exercised them for 20 weeks. Though the authors did not look at hallmarks of AD pathology, they did look into potential mechanisms for how exercise may improve AD pathology, specifically BACE1, Dicer, and miRNA abundance. Endurance exercise has been found to downregulate BACE1, making it a possible mechanism for exercise-derived improvements in AD [17, 18]. PoWeR-exercised mice have lowered BACE1, increased Dicer expression, and overall beneficial changes in miRNA abundance, suggesting that these may be some of the mechanisms by which exercise improves AD [19].
Unfortunately for AD patients, late-stage AD pathology may lead to decreased motility and overall inability to exercise, making any benefits that exercise may have on AD pathology null. However, plasma from young, exercised individuals may be used to construe the same beneficial effects. Treating 12-month-old male 3xTg mice either young plasma or plasma from young, exercised mice for 1 month increased levels of synaptic proteins in the hippocampus, and mice treated with plasma from young, exercised mice had increased brain-derived neurotropic factor (BDNF), learning ability and both short- and long-term memory in MWM, increased neurogenesis. Young, exercised plasma reduced ROS, inhibited apoptosis and cell death in the hippocampus, and maintained mitochondria homeostasis [235].
Though more studies are needed to parse out the exact mechanisms by which exercise may ameliorate AD pathology, there is evidence that exercise promotes FGF21 expression and may activate the SIRT1 pathway [232]. These studies show that exercise alone may have a significant effect on AD. If the patient is unable to exercise, exercised plasma transfusions from another individual may be able to substitute for exercise, or small molecules which mimic the beneficial effects of exercise on AD may be developed. It is also possible that exercise may be combined with dietary interventions or pharmaceutical treatment to have even greater beneficial effects on cognition.
In humans, exercise has diverse metabolic and anti-aging benefits. It also has limited side effects compared to other geroprotective interventions and as such is well studied in human AD patients [225, 227, 230, 236,237,238,239,240,241,242,243,244,245,246,247,248,249,250]. Though there is a lot of inter-individual heterogeneity, the sheer number of clinical trials that have been performed allow meta-analyses to be performed and to determine that there are overall benefits to cognition, activities of daily living (ADL), and even AD risk (reviewed in [230, 239, 240, 244]). Of special note, the planned ExPlas study is unique from other exercise clinical trials as it will utilize plasma from exercise-trained donors [245]. If effective, plasma from exercised donors will be especially beneficial in AD treatment as AD is associated with motor impairments and AD patients may not be able to exercise effectively.
In addition to physical limitations of AD patients, exercise as a geroprotective intervention also faces a strong barrier in motivation to exercise. In an effort to encourage exercise in elderly adults, particularly those with AD, a number of programs have been developed, including telerehabilitation and community-based exercise programs [237, 241, 246]. Though it is hard to enforce participation in programs such as these, these programs should be readily available to patients as the benefits of exercise in old age and AD are strongly supported by both animal models and human clinical trials.
Type 2 diabetes mellitus drugs
Due to the high prevalence of AD and diabetes co-occurrence, the use of diabetes drugs to treat AD has been of considerable interest (diabetes drugs tested in 3xTg mice summarized in Table 3). Linagliptin is a Dipeptidyl peptidase 4 (DPP-4) inhibitor, and often one of the first drugs used to treat diabetes in patients due to its strong inhibitory effects [251, 252]. DPP-4 inactivates glucagon-like peptide (GLP) and glucose-dependent insulinotropic peptide (GIP), which regulate postprandial plasma glucose concentrations and sensitize pancreatic β-cells to glucose stimulation, respectively [252]. The GLP-1 receptor is highly expressed in the central nervous system, particularly in the hypothalamus and hippocampus [253, 254]. In addition to its direct effects on DPP-4, linagliptin may also ameliorate AD pathology via mTOR, SIRT1 [251, 255]. In the 3xTg-AD mouse model, females treated with linagliptin exhibited improved cognition and pathology, including reduced Aβ42 levels and reduced tau phosphorylation [256]. Linagliptin-treated 3xTg-AD mice had improved performance on both the MWM and Y-Maze, which suggests improvements in spatial learning and working memory compared to non-treated mice. Finally, linagliptin-treated 3xTg-AD female mice had decreased levels of glial fibrillary acidic protein (GFAP), a marker for inflammation [256].
Instead of blocking the action of DPP-4, an alternative way to activate GLP-1 signaling is with synthetic GLP-1 receptor agonists such as liraglutide and exenatide. Additional potential mechanisms for improvements by GLP-1 receptor agonists in AD may be due to increased FGF21 and SIRT1 activity, decreased inflammation, and increased insulin sensitivity [257, 258]. The effects of both liraglutide and exenatide have been examined in the 3xTg-AD mouse model [253, 259]. Liraglutide improved performance in MWM and attenuated tau phosphorylation at Thr231, Ser396, Ser214, and Ser199/202, but not Thr217 [253]. Liraglutide also protected the 3xTg-AD mice from neurodegeneration and promoted JNK and extracellular signal-regulated kinase (ERK) signaling [253]. Exenatide did not improve cognition or pathology in 3xTg mice, though it did improve cognition in a different mouse model of AD [259].
Pioglitazone is an insulin sensitizer used in T2DM treatment [251]. In addition to improving insulin sensitivity and as a result glycemic control, pioglitazone also downregulates NFκB transcription and mTOR signaling [260, 261]. Masciopinto et al. studied the effect of pioglitazone administered to 3xTg mice for 9 months starting at 3 months of age [262]. Pioglitazone did not improve memory performance on either MWM nor NOR; the mice also did not show any improvements in glucose metabolism or brain mitochondrial function [262]. This is at odds with other studies, which found that treatment with pioglitazone for 4 months starting at 10 months of age had a variety of benefits for 3xTg mice [260, 263]. These 14-month-old mice showed reduced soluble intracellular Aβ levels in the CA1 region of the hippocampus [260] along with decreased tau phosphorylation [260, 263]. While they did not show improvements in light/dark preference, spontaneous exploratory activity, locomotion, or anxiety, they did have improved avoidance learning and MWM performance [260, 263]. These mice also had improved long-term plasticity and increased membrane hyperpolarization, suggesting a younger neuronal phenotype [260]. Finally, pioglitazone-treated mice exhibited decreased astrocyte levels [263].
Rosiglitazone is another insulin sensitizer commonly used in T2DM patients with similar mechanisms as pioglitazone. When 10-month-old 3xTg-AD mice were treated with rosiglitazone for 4 months, they exhibited similar improvements as pioglitazone [263]. Pioglitazone-treated mice had improved MWM performance, decreased p-tau levels, and decreased astrocyte activation, but showed no changes in spontaneous exploratory activity, locomotion, or anxiety [263].
A novel GLP-1/GIP/Glucagon receptor (Gcg) triagonist developed by Finan et al. in 2015 may also have potential as an AD treatment [264]. It has been found to ameliorate obesity and diabetes in rodents through improvements in glucose homeostasis and increased FGF21 levels [264]. When tested in 7-month-old 3xTg mice, this novel triagonist improved long-term spatial memory in the MWM as well as working memory in the Y-maze and radial arm maze [265, 266]. Long-term potentiation and synaptic plasticity in the CA1 region of the hippocampus was also improved, along with decreased Aβ and tau aggregation in the hippocampus [265]. Synaptic markers synaptophysin and postsynaptic density protein 95 (PSD-95) were increased in the hippocampus [266]. Finally, the triagonist normalized spontaneous excitatory synaptic activities and hyperexcitability of hippocampal neurons [266].
Finally, something as simple as insulin may be beneficial in the treatment of AD. Insulin injections are commonly used in T2DM, and due to the prevalence of AD and T2DM occurring comorbidly, insulin injections may not only help with treating T2DM but AD as well, by improving insulin signaling in the brain. After placing 3xTg-AD mice on a HFD, they develop impaired glucose tolerance as well as greatly worsened Aβ pathology and cognitive function [267]. Injecting these much with a single injection of insulin improves memory in the novel object recognition tests, reduced soluble Aβ42 levels, and even decreased Aβ production and increased Aβ clearance [267]. Intranasal administration of insulin is an effective way of bypassing the blood-brain barrier and delivering insulin directly to the brain [268,269,270,271]. An important question to consider when examining the potential benefits of intranasal insulin administration is whether chronic administration is necessary or if acute treatment is sufficient. Chen et al. examined short-term intranasal insulin administration in 3xTg-AD mice and found that a 7-day treatment period was sufficient to increase levels of synaptic proteins and decrease bot microglia and Aβ levels, but was not able to decrease tau phosphorylation [268]. In HFD-fed 3xTg-AD mice, weekly intranasal insulin therapy for either 6 or 12 months ameliorated some aspects of HFD-exacerbated pathology [29]. HFD resulted in impaired spatial memory, which ameliorated by intranasal insulin in both 8 and 14-month-old mice [29]. In addition, intranasal insulin altered cytokine levels and was able to normalize some aspects of brain physiology [29]. These results suggest that insulin injections, both peripherally and intranasal, may be beneficial in AD, likely via improvements in insulin signaling.
These studies suggest that there is strong potential for already available T2D drugs to have benefits on AD pathology. Clinical trials in AD patients are needed to determine how translatable these benefits are as well as whether the benefits are due solely to improvements in glucose homeostasis or occur independently in metabolically healthy individuals. So far, some clinical trials utilizing T2D drugs in patients with AD and MCI have already been performed. Of the drugs discussed here, liraglutide treatment had neuroprotective effects, and both pioglitazone and rosiglitazone induced cognitive improvements in a number of studies (reviewed in [272]). Intranasal insulin has been widely studied in patients with AD and MCI (reviewed in [272]). As a whole, the findings of clinical trials on intranasal insulin have been largely positive. Intranasal insulin improves memory and cognition, and functional ability, and has additional neuroprotective effects [269,270,271,272,273]. Further research is needed to determine if benefits are solely due to improvements in glycemic control (and thus may only benefit patients with T2D) or if there are additional off-target effects of these drugs such via mechanisms like mTOR, SIRT1, and FGF21.
Anti-cancer therapeutics
As another age-related disease, therapies that are protective against various cancers may also be beneficial in the treatment of AD. Many of the aspects of AD pathology are shared with cancer such as microtubule disassembly and signaling pathway abnormalities. There have been preliminary studies on the efficacy of anti-cancer therapeutics in the 3xTg model that show promising results (Table 4).
Paclitaxel is a commonly used microtubule-stabilizing drug in cancer patients [274,275,276,277]. In cancer, microtubule-stabilizing drugs work by preventing microtubule disassembly to inhibit cellular mitosis and thus tumor growth [277, 278]. In AD, microtubule-stabilizing drugs may prevent neurodegeneration and preserve already damaged neurons [277]. Many microtubule-stabilizing drugs including paclitaxel are limited in their ability to cross the BBB, making delivery method an important consideration [277]. Cross et al. treated 3xTg mice intranasally as a non-invasive method of bypassing the BBB [277]. Two cohorts of mice were treated; a young cohort treated from 2 to 6.5 months to determine efficacy of preventing AD pathology and an old cohort treated from 11 to 17 months to determine the ability of paclitaxel to improve cognitive performance and reduce anxiety. In the young cohort, paclitaxel reduced p-tau levels, reactive gliosis, and astrocyte activation while also increasing axonal transport. In older mice with already developed AD pathology, paclitaxel improved performance in the radial water tread maze and decreased anxiety in the elevated plus maze.
Polyphillin I (PPI) is a drug isolated from plants that may inhibit the proliferation of cancer cells via downregulation of the expression of cancerous inhibitor of protein phosphatase 2A (CIP2A) which is upregulated in the brains of AD patients [279, 280]. Treatment of 10-month-old male 3xTg mice for 1 month results in improved object recognition performance and object location memory, improved spatial learning and memory in MWM, and rescued fear conditioning memory impairment [280]. PPI increased neuron and spine numbers while reducing Aβ loads and tau phosphorylation in the hippocampus. Zhou et al. confirmed that the reduction in tau phosphorylation was due directly to restored protein phosphatase 2A (PP2A) activity via in vitro experiments.
Radiotherapy is a well-known anti-cancer therapy. In adult patients with small cell lung cancer and pediatric leukemia, the standard of care frequently includes low dose prophylactic cranial radiation to decrease central nervous system relapse [281, 282]. Radiotherapy is successful in treating extracranial amyloidosis [282,283,284]. In 16 month old female 3xTg mice low dose irradiation results in decreases in both Aβ plaques and neurofibrillary tangles [282].
Interest in low dose irradiation as an AD treatment began with a single case study in 2015 in which a patient with advanced AD showed improvements in cognition, memory, and speech after consecutive CT scans [285]. Based on the results of that case, Cuttler et al. treated four patients with three consecutive treatments of low-dose irradiation and witnessed improvements in cognition and behavior for three out of the four patients [286]. There are a large number of additional ongoing clinical studies on the efficacy of low-dose radiotherapy in AD listed on clinicaltrials.gov. Neither paclitaxel nor PPI have been studied in humans with AD or MCI, however a number of other anti-cancer drugs have had AD or MCI-related clinical trials, the results of which have been extremely varied but promising (reviewed in [287]).
Histone deacetylase inhibitors
Histone deacetylases (HDACs) are enzymes that remove acetyl groups from lysine residues on histones [288]. HDAC enzymes are categorized into four classes, the Class I Rpd3-like proteins, Class II Hda1-like proteins, Class III Sir2-like proteins, and the Class IV protein [288]. In addition to deacetylation of histones, HDACs also deacetylate a number of non-histone substrates, primarily those involved in apoptosis, autophagy, and cell cycle regulation [289]. HDAC inhibitors increase histone acetylation and enhance memory and plasticity, leading them to be a potential modulator of AD pathology [290,291,292,293].
RGFP-966 is a selective HDAC3 (a class I HDAC) inhibitor that has been found to cross the blood-brain barrier [294]. HDAC3 is highest in brain regions involved in memory and learning, such as the hippocampus and cortex, where AD pathology is the highest [294,295,296]. Treatment with RGFP-966 in 9-month-old 3xTg-AD mice for 3 months improves spatial and recognition memory in the Y-maze, NOR, and BM tests [294]. RGFP-966 also improved motor function, as indicated by rotarod, treadmill and balance beam [294]. Aβ pathology was also ameliorated; Aβ42 levels were decreased in the brain and plasma, and there was increased activity of neprilysin, an Aβ degrading enzyme, suggesting improved Aβ degradation and clearance [294]. Finally, treatment with RGFP-966 reduced tau phosphorylation at Thr181 in the entorhinal cortex and phosphorylation at Ser396 in the entorhinal cortex and hippocampus [294].
Nicotinamide is released from NAD+ during deacetylation by sirtuins, a class III NAD+-dependent HDAC. It acts as a non-competitive inhibitor to sirtuins, as well as a Poly [ADP-Ribose] Polymerase I (PARP-1) inhibitor [290, 297]. PARP-1 typically functions as a DNA repair enzyme, but under intense DNA damage can lead to non-apoptotic cell death, which has been associated with a variety of neurodegenerative diseases [297]. Nicotinamide also plays a role in regulation of mTOR [298]. Administration of nicotinamide for 4 months to 4-month-old 3xTg mice improved both short- and long-term spatial learning in the MWM and improved contextual learning in the fear-conditioning assay [290]. No changes in Aβ pathology were found but tau pathology was ameliorated. In addition to ameliorating tau pathology and cognitive deficits, Nicotinamide treatment had a number of other beneficial effects on overall neuronal function. Interestingly, Green et al. also treated 20-month-old mice with nicotinamide for 4 months and found that once tau pathology has progressed to such an advanced stage, nicotinamide treatment does not induce any changes in cognitive function or tau phosphorylation, but did significantly reduce soluble Aβ42 levels. Sirtuin activity was reduced in the nicotinamide-treated mice.
The response of 3xTg mice to HDAC inhibitors were extremely positive, which means that HDAC inhibitors are a strong possible candidate in the treatment of AD. To our knowledge, there have not yet been any clinical studies utilizing RGFP-966 in AD but there are two clinical trials for the administration of nicotinamide to patients with AD or MCI have been completed but the results of which are not yet available [299]. One possible problem with utilizing HDAC inhibitors to treat AD is that HDAC I reduction is seen in AD patients, and decreased HDAC I levels are associated with neurodegeneration and cognitive decline [300]. In addition, SIRT1 is a class III HDAC of special interest in AD as its levels are decreased in AD patients [288, 301, 302]. Therefore, inhibition of HDACs may actually be detrimental in AD, which suggests the benefits of nicotinamide administration may be due primarily to its inhibition of PARP-1 or regulation of mTOR.
Sirtuin activators
Nicotinamide riboside (NR) is a NAD+ precursor. NAD+ depletion is an important aspect of mitochondria dysfunction, which is a hallmark of many neurodegenerative diseases including AD [303, 304]. NAD+ is a metabolite that is necessary for mitochondrial function, stem cell self-renewal, and neuronal stress resistance, and as such, is especially important in the brain [303, 305]. Neurons in the brain are thought to be especially sensitive to a depletion of NAD+ due to their high energy demands, so supplementation of a NAD+ precursor such as NR may improve neuronal function [303, 304]. As sirtuins are NAD+-dependent HDACs, increased NAD+ levels also lead to increased sirtuin activity. In addition, NR alleviates FGF21 resistance to improve FGF21 signaling [306]. Administration of NR for 6 months to 16–18-month-old 3xTg-AD mice increased the NAD+/NADH ratio in the cortex of the brain, which is typically lower in this model compared to wild-type controls; this suggests that orally administered NR crosses the blood-brain barrier to boost NAD+ levels in the brain [303]. NR treatment at this age also improved spatial learning and working memory on both the MWM and Y-maze as well as motor function in both rotarod and grip strength analysis. No changes in Aβ pathology were seen with NR treatment, but tau hyperphosphorylation was ameliorated. In addition to ameliorating tau pathology and cognitive deficits, NR treatment had a number of other beneficial effects on overall neuronal function, suppressed inflammatory responses, upregulated insulin-signaling pathways, improved long-term potentiation, decreased mitochondrial ROS levels, decreased DNA damage and decreased apoptosis.
Resveratrol is a natural compound found in grapes, red wine, peanuts, soy, and tea [307,308,309]. It has been found in animal models to have antioxidant, anti-inflammatory, and neuroprotective with longevity-promoting properties, making it a potential candidate for AD treatment [307, 309,310,311]. Resveratrol has been proposed to mimic the health benefits of caloric restriction through activation of SIRT1 [312,313,314].
Resveratrol treatment of 3xTg mice for 10 months starting at 2 months of age improved motor function as well as spatial and recognition memory in MWM and NOR assays [309]. Resveratrol also protected against both Aβ and tau pathology, as indicated by increased levels of the amyloid-degrading enzyme neprilysin, decreased intracellular APP and Aβ levels, and decreased tau phosphorylation at Ser202 [309]. Resveratrol did not improve neuroplasticity, unlike many of the other potential AD treatments, but did enhance the ubiquitin-proteasome system activity, which is important for Aβ and p-tau clearance [309]. When resveratrol was administered for only 5 months, treated mice exhibited decreased Aβ levels, inflammation, apoptosis, autophagy-related protein expression, BACE1 levels and NF-κB levels [315]. Resveratrol treatment also decreased α-synuclein, which can form amyloid-like fibrils under oxidative stress [315]. Resveratrol also increased neurotrophin and synaptic marker levels such as BDNF, NGF, synaptophysin, and PSD-95 levels. SIRT1 activation was increased, supporting the mechanism by which resveratrol may enact its beneficial effects [315]. These results suggest that even short-term resveratrol treatment can have beneficial effects on AD pathology, though it is still unknown how long lasting these benefits may be.
Recently, a novel formulation of resveratrol that has increased pharmacokinetic properties has been developed [316]. Dennison et al. performed both a sub-chronic reversal as well as a prophylactic study utilizing 3xTg mice to examine the potential benefits this drug may have on AD pathology [316]. In the sub-chronic reversal study, 15-month-old male 3xTg mice were treated for 36 days. At the end of the treatment, they exhibited increased ADAM10 in the hippocampus and decreased inflammatory cytokines, tumor necrosis factor alpha (TNFα), and interleukin 6 (IL-6) in the liver and spleen. In the prophylactic study, 4-month-old male and female 3xTg mice were treated for 9 months. The treated mice showed improvements in object location memory and NOR but had no improvements in spatial memory or sensorimotor behavior. Tau levels in the hippocampus and p-tau in the prefrontal cortex were decreased but Aβ42 levels were decreased only in the entorhinal cortex. AD-related gene expression as a whole was improved; particularly increased SIRT1 activity, increased Adam10 and Bdnf expression, and decreased Bace1 and Psen1. Inflammatory markers Tnf and Il6 were also decreased.
In summary, sirtuin activators are a promising avenue to explore in the treatment of AD pathology (Figure 2). Of the sirtuin activators studied in the 3xTg mouse model, there have been many beneficial effects and they may mimic many of the benefits of caloric restriction without the difficulties of a strict dietary regimen (Table 5). In addition to activating sirtuin, many of the sirtuin activators studied also inhibit NF-κB production, such as resveratrol [310, 311, 315]. In a clinical trial, nicotinamide riboside administration did not improve cognition, though the experimenters did see positive changes in brain function via fMRI [317]. In contrast, resveratrol showed much more promising results. Resveratrol was found to improve Aβ clearance, as shown by stabilized levels of Aβ in CSF and plasma, and modulated the inflammatory response [318]. Finally, there was a small amelioration of functional decline as quantified by the Alzheimer’s Disease Cooperative Study-Activities of Daily Living measure [318]. As such, both preclinical and clinical studies have shown sirtuin activators to be effective at ameliorating AD pathology.
Fingolimod
Sphingosine-1-phosphate (S1P) is a phospholipid that has been implicated in metabolic dysfunction such as in diabetes and obesity [319, 320]. S1P regulates many cellular processes through its paracrine and autocrine signaling. S1P is positively associated with obesity, but is thought to actually ameliorate obesity through energy homeostasis regulation [319]. In diabetes, S1P levels are increased, but it appears to have differing effects depending on which receptor it is bound to [319]. S1P1R activation ameliorates diabetes, whereas S1P2R activation exacerbates it. Furthermore, ceramide, a precursor of S1P, has important effects on cell growth, proliferation, cell migration, apoptosis, and differentiation [319, 320]. Ceramides have been found to inhibit insulin signaling and stimulate pancreatic β cell death [320]. S1P, however, appears to prevent cell death, suggesting that increased conversion of ceramide (and potentially other S1P precursors) may be beneficial in diabetes [320]. Further study on S1P signaling in obesity and diabetes is needed as there is controversy over whether increased S1P exacerbates metabolic syndrome or S1P is increased as a compensatory mechanism to combat metabolic syndrome [319, 320]. In AD, the S1P system is altered, suggesting that pharmaceuticals that normalize S1P system function, like fingolimod, may be beneficial in the treatment of AD [321, 322]. In AD, SIP kinase-1 and -2 levels are decreased and sphingosine lyase is increase, leading to a decrease in S1P [323]. S1P in AD models inhibits neuronal death, protects against Aβ toxicity, and reduced neuroinflammation [321, 323]. Ceramide levels are increased in AD, and may contribute to axonal degeneration and neuronal death via increased ROS production [323].
Fingolimod is an oral neuroimmunomodulatory drug used to treat patients with multiple sclerosis [322, 324]. It readily passes through the BBB to bind to the S1P receptors in the brain, except for S1P2 [322]. Fingolimod has been found to target S1P receptors on neuronal and glial cells, leading to regulation of neurogenesis, myelination, inflammation, and astrogliosis. Male 3xTg mice treated with fingolimod from 6 to 12 months of age had improved AD pathology and improved memory as assessed by the NOR and MWM tests [322]. Fingolimod also reduced microglial activation and the presence of both circulating and infiltrating CD3+ T cells. The inflammatory profile in the brain was improved, as shown by decreased IL-6 and increased interleukin 10 (IL-10) levels. The cortex had decreased p-tau and APP levels, suggesting an overall diminished AD phenotype. Although fingolimod has extremely promising results in 3xTg mice and other animal models, the number and severity of possible side effects introduce an inherent roadblock in utilizing fingolimod in AD patients [325, 326].
α7 Nicotinic acetylcholine receptor agonist
Cholinergic dysfunction is prevalent during neurodegenerative diseases such as AD [327, 328]. Of the cholinergic receptors, the α7 nicotinic acetylcholine receptors (α7 nAChRs) have been found to be closely linked with neurodegenerative and inflammatory diseases, particularly in the brain, as they are primarily expressed on neurons, macrophages, microglia, and astrocytes [196, 327, 329,330,331]. α7 nAChRs are important in AD because their levels are decreased in the cortex of AD patients and Aβ42 has been found to bind to α7 nAChRs, allowing it to accumulate in neurons [327, 332,333,334].
Both blocking and activating α7 nAChRs have been proposed as potential treatments for AD [327]. In 3xTg-AD mice, administration of A-582941, an α7 nAChR, restores learning and memory in the MWM, NOR, and contextual fear conditioning [327]. A-582941 treatment did increase the levels of memory-associated proteins in the brain, such as c-Fos levels and CREB, TrkB, and BDNF phosphorylation, but did not affect Aβ or tau pathology [327]. Finally, A-582941 treatment did not ameliorate neuroinflammation in the brains of 3xTg-AD mice [327]. These results suggest that while A-582941 treatment does improve some of the downstream cognition effects of AD pathology, it does not target the main causes, namely Aβ and tau accumulation. α7 nAChRs may be useful in treating symptoms of AD in later stage AD patients but may not have a beneficial effect on the disease progression itself.
In humans, some clinical trials of α7 nAChR modulators have shown promising results for use in the treatment of AD pathology [335, 336]. Deardoff et al. used EVP-6124 (also known as encenicline), an α7 nAChR partial agonist, and found improvements in both cognitive and functional measures [335]. Galantamine modulates nicotinic receptors and inhibits acetylcholinesterase to regulate nicotinic neurotransmission [336]. In AD patients, galantamine improves cognitive function and ADL scores but does not alter behavioral symptoms [336]. There is also an ongoing clinical trial to determine if daily transdermal nicotine impacts cognitive function in patients with MCI [337]. It is important to note that the majority of currently FDA approved therapeutics for AD target the cholinergic system, including galantamine, donepezil, rivastigmine, and tacrine, with mostly favorable results, which suggests that the cholinergic system and nAChR modulation may be promising targets for AD patients [338].
Immunotherapy and immunizations
Immunotherapy treatments targeting both Aβ and p-tau have been examined in the 3xTg-AD mouse model [339,340,341,342,343,344,345,346]. Oddo et al. looked at Aβ immunotherapy in the 3xTg mouse model by injecting anti-Aβ antibodies directly into the hippocampus [339]. By 7 days post-injection, there was a dramatic decrease in extracellular Aβ plaques, as well as a decrease in intracellular Aβ [339, 340]. Interestingly, anti-Aβ antibody treatment also reversed tau pathology, as shown by a complete reduction in intracellular tau immunoreactivity [339]. In older mice with already well-established Aβ and tau pathology, Aβ immunotherapy still cleared Aβ accumulation, but did not diminish tau aggregation. On the other hand, Walls et al. examined the effect of intrahippocampal phospho(Ser202/Thr205)-tau immunotherapy in the 3xTg-AD mouse model [340]. Within 7 days post-injection, 3xTg-AD mice showed decreased total tau levels [340]. p-Tau immunotherapy reduces pathological tau levels in both young and old mice but did not affect Aβ levels at any age [340].
St-Amour et al. examined the effects of a similar immune-based therapy: intravenous immunoglobulin (IVIg) [346]. IVIg is relatively safe and well tolerated, is being used in an increasing number of diseases, and is relatively easy to administer long-term, especially when compared to intrahippocampal immunotherapy injections [346, 347]. It has been found to improve cognition and reduce Aβ in cerebrospinal fluid of AD patients due to the naturally occurring anti-Aβ antibodies present in the blood of healthy individuals and thus IVIg preparations [346, 348,349,350]. 3xTg mice injected with IVIg for 1–3 months exhibited improved recognition memory in the NOR test, but spatial memory on the BM was not changed [346]. Soluble Aβ42 levels were decreased in 16-month-old mice, but not in 12-month-old mice, and no change was seen in insoluble Aβ levels or extracellular Aβ plaques [346]. Similarly, no changes were seen in tau phosphorylation at either the Thr181 or Ser396/Ser404 sites [346]. IVIg injections also modified the immune state of the 3xTg-AD mice, resulting in an overall decrease in inflammatory state as indicated by decreases in pro-inflammatory cytokines in the cortex [346].Vaccines for AD have also been heavily studied in the 3xTg mouse model [341,342,343,344,345]. Most studies focus on utilizing the Aβ-specific scFv-h3D6 vaccine, with promising results such as reduced Aβ plaques, decreased tau levels, ameliorated pro-inflammatory state as shown by decreased GFAP, ionized calcium binding adaptor molecule 1 (Iba1), and IL-6 levels, and improvements in spatial memory in the MWM [341, 344, 345]. Another study used a DNA epitope vaccine to produce anti-Aβ reactivity without the negative side effects that have been seen in some studies on Aβ vaccines or immunotherapy such as a T-cell mediated pro-inflammatory immune response or microhemorrhages in the brain [342]. The DNA epitope-vaccinated mice had decreased Aβ loads, improved spatial memory, decreased inflammation, and overall strong anti-Aβ-specific antibody production with an anti-inflammatory immune response [342]. In contrast, Rajamohamedsait et al. utilized a tau vaccine and found decreased tau pathology and Aβ plaque burden as well as decreased microgliosis [343]. The tau-vaccinated mice exhibited no change in astrocyte activation or neuronal density.
Though there is a lot more research that needs to be done on utilizing the immune response to combat the buildup of Aβ plaques and hyperphosphorylated tau during AD, the preliminary findings in the 3xTg model are promising (Table 6). Additionally, a number of clinical trials have already been performed using various immunotherapy strategies, with mixed results (reviewed in [351, 352]). Of the strategies discussed here, the IVIg studied by St-Amour et al. did not exhibit cognitive improvements as a whole, but subgroup analyses revealed that cognition was improved specifically in individuals with APOE4 [346]. Due to the increasing interest in immunotherapy approaches to AD, there are now two FDA-approved immunotherapy treatments [338]. The first immunotherapy to receive FDA approval was aducanumab (aduhelm), which was approved under the FDA’s accelerated approval process in June of 2021 [338]. Since its approval, aducanumab has received much controversy, with many patients developing amyloid-related imaging abnormalities, particularly individuals with APOE4. In January of 2023, a second anti-AD immunotherapy, lecanemab (leqembi), was approved by the FDA [338]. Although many clinical trials of lecanemab are still ongoing, preliminary results show a slowing of cognitive decline after lecanemab administration as well as ameliorated tau and amyloid pathology. Lecanemab has exhibited fewer negative side effects than aducanumab thus far. Further research is needed to determine the most effective immunotherapy approach that will produce a robust anti-Aβ or tau response in all individuals without increasing neuroinflammation.
Conclusion
There are many different mouse models of AD available, all with different strengths and weaknesses. Here, we have discussed studies performed using the 3xTg mouse model of AD. A major advantage of this strain is that they develop both Aβ and tau pathology. The main drawback to the 3xTg-AD model is that it is a model for early-onset AD, which is not nearly as common as late onset AD. While most AD rodent models have been developed via autosomal-dominant AD transgenes that have non-physiological expression patterns [353], there has recently been a push for developing mouse models with knocked in humanized Aβ or tau, though these knock-ins aren’t always sufficient to cause full AD pathology with severe cognition impairment as seen in human patients [353,354,355]. Another option for a more translatable model of late-onset AD is non-human primates, which closely resemble humans in the progression of cognitive decline and neuropathy during aging [356]. Non-human primates also display age-related Aβ plaque accumulation and tau pathology, making them a good option for modeling late-onset AD [356].
A number of different potential treatments — many of which have been demonstrated to function as geroprotectors, extending the lifespan of wild-type mice — have been discussed above. Many of these interventions, including rapamycin, are now being tested in humans to examine the potential efficacy against this deadly disease. Dietary and lifestyle interventions also have a lot of potential and are low-cost as compared to specialized pharmaceuticals; exercise in particular is extremely beneficial in the 3xTg model. However, a limitation of both dietary changes and exercise is that these may be particularly difficult for many aged individuals to follow.
The NIA Interventions Testing Program as well as other researchers have identified and continue to identify many different interventions that robustly extend the lifespan of mice. We have discussed some of these above, including rapamycin. Other interventions that have shown promise on their own or in combination with rapamycin include drugs for type 2 diabetes because there is such a close connection between AD and other metabolic disorders. These include acarbose, canagliflozin and metformin [357,358,359,360,361]. It will be important to test these and additional geroprotectors in mouse models of AD to determine if they might also have potential for the treatment or prevention of AD.
Regardless of the model species used, an important consideration in any animal model is genetic diversity. The human population is extremely genetically diverse, and in order for a model to be more representative of the human condition, sex and strain differences must be considered when drawing conclusions and attempting to translate results into humans. One great example of a genetically diverse AD model is the AD-BXD model proposed by Neuner et al. [362]. In this model, 5XFAD mice are bred to the well-studied BXD strains, allowing researchers to determine which sequence variants may contribute to exacerbated AD phenotype or protect against the development of AD. Though the 5XFAD is a model of early-onset AD, the AD-BXD model highlights the importance of genetic diversity in AD studies. Future genetically diverse mouse models of late-onset AD will be extremely useful in bringing a personalized medicine approach to the treatment of AD.
It is important to determine what the mechanisms are for the effectiveness of various dietary interventions, to make them as easy to stick to as possible. Diets based on altered levels of macronutrients are likely to be more sustainable than diets based on reduced energy intake; likewise, time restricted feeding is believed to be more sustainable for many than diets that involve calorie counting. While PR has performed well in short-term studies, the long-term adherence to such diets is unknown; there are also potential concerns as the elderly may have increased need for dietary protein to fight sarcopenia. Thus, diets reducing just once amino acid – for instance, isoleucine [147] — or drugs which mimic the effect of these restrictions, for example by altering BCAA uptake or catabolism — could allow many more individuals to benefit from these type of interventions. Likewise, exercise mimetics may be of particular interest for individuals who are not physically able to exercise.
Examining mechanisms by which dietary interventions may protect against AD pathology is of equal importance. Many of the dietary interventions discussed here, including CR, PR, and restriction of BCAAs, reduces activity of mTORC1; and as rapamycin delays or prevents AD in mouse models including 3xTg, it is logical to assume that these diets work at least in part by restricting mTORC1 signaling. Ketogenic diets also have been reported to reduce mTORC1 signaling but have not been tested in the 3xTg mouse model. However, several of the dietary interventions discussed promote SIRT1 and FGF21 activity, which may also be mechanisms by which they improve AD pathology. Further study of how these diets work, as well as the precise downstream effectors of their beneficial effects, may be critical to developing new pharmaceuticals to target AD.
In conclusion, due to advances in medical science and public health, the average person is now living much longer than in the past. This increase in lifespan has not been associated with an equal increase in healthspan, and as a result, we have witnessed an enormous increase in the prevalence of age-related diseases, including AD. Thus, there is now more than ever an urgent need to identify ways to effectively treat or prevent AD. Targeting the aging process itself may provide new and effective ways to target AD, and as such, there has been substantial research on the ability of geroprotective interventions to slow or prevent AD. Here, we have summarized research into the effect of many interventions on the development and progression of AD in the 3xTg mouse model. This model has provided important insights and will likely continue to be an important tool in the future due to its ability to model multiple aspects of AD; yet continued progress requires the development and use of mouse models that recapitulate late-onset AD in a genetically heterogenous population.
References
Burch JB, Augustine AD, Frieden LA, Hadley E, Howcroft TK, Johnson R, et al. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S1–3.
Alzheimer's Association. 2019 Alzheimer's disease facts and figures. Alzheimers Dement. 2019;15(3):321–87.
Sibener L, Zaganjor I, Snyder HM, Bain LJ, Egge R, Carrillo MC. Alzheimer's Disease prevalence, costs, and prevention for military personnel and veterans. Alzheimers Dement. 2014;10(3 Suppl):S105–10.
Kitazawa M, Medeiros R, LaFerla FM. Transgenic mouse models of Alzheimer disease: developing a better model as a tool for therapeutic interventions. Curr Pharm Des. 2012;18:1131–47.
Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, et al. Autosomal-dominant Alzheimer's disease: a review and proposal for the prevention of Alzheimer's disease. Alzheimers Res Ther. 2011;3(1):1.
Guerreiro R, Bras J. The age factor in Alzheimer's disease. Genome Med. 2015;7:106.
Sotiropoulos I, Galas MC, Silva JM, Skoulakis E, Wegmann S, Maina MB, et al. Atypical, non-standard functions of the microtubule associated Tau protein. Acta Neuropathol Commun. 2017;5(1):91.
Wegmann S, Biernat J, Mandelkow E. A current view on Tau protein phosphorylation in Alzheimer's disease. Curr Opin Neurobiol. 2021;69:131–8.
Le Corre S, Klafki HW, Plesnila N, Hubinger G, Obermeier A, Sahagun H, et al. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc Natl Acad Sci U S A. 2006;103(25):9673–8.
Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–81.
Wang WX, Huang Q, Hu Y, Stromberg AJ, Nelson PT. Patterns of microRNA expression in normal and early Alzheimer's disease human temporal cortex: white matter versus gray matter. Acta Neuropathol. 2011;121(2):193–205.
Cuellar TL, Davis TH, Nelson PT, Loeb GB, Harfe BD, Ullian E, et al. Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. Proc Natl Acad Sci U S A. 2008;105(14):5614–9.
De Pietri TD, Pulvers JN, Haffner C, Murchison EP, Hannon GJ, Huttner WB. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135(23):3911–21.
Hebert SS, Papadopoulou AS, Smith P, Galas MC, Planel E, Silahtaroglu AN, et al. Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum Mol Genet. 2010;19(20):3959–69.
Pearson HA, Peers C. Physiological roles for amyloid beta peptides. J Physiol. 2006;575(Pt 1):5–10.
Hampel H, Hardy J, Blennow K, Chen C, Perry G, Kim SH, et al. The amyloid-beta pathway in Alzheimer's disease. Mol Psychiatry. 2021;26(10):5481–503.
Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, et al. Mice deficient in BACE1, the Alzheimer's beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4(3):231–2.
Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A. 2008;105(17):6415–20.
Dungan CM, Valentino T, Vechetti IJ Jr, Zdunek CJ, Murphy MP, Lin AL, et al. Exercise-mediated alteration of hippocampal Dicer mRNA and miRNAs is associated with lower BACE1 gene expression and Abeta(1-42) in female 3xTg-AD mice. J Neurophysiol. 2020;124(6):1571–7.
Martinez-Vicente M, Cuervo AM. Autophagy and neurodegeneration: when the cleaning crew goes on strike. The Lancet Neurology. 2007;6(4):352–61.
Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13(7):805–11.
Correia SC, Santos RX, Perry G, Zhu X, Moreira PI, Smith MA. Insulin-resistant brain state: the culprit in sporadic Alzheimer's disease? Ageing Res Rev. 2011;10(2):264–73.
Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. Journal of Alzheimer's disease : JAD. 2005;8(3):247–68.
Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease - is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80.
Murphy MP, LeVine H 3rd. Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19(1):311–23.
Bali J, Gheinani AH, Zurbriggen S, Rajendran L. Role of genes linked to sporadic Alzheimer's disease risk in the production of beta-amyloid peptides. Proc Natl Acad Sci U S A. 2012;109(38):15307–11.
Aisen PS, Cummings J, Jack CR Jr, Morris JC, Sperling R, Frolich L, et al. On the path to 2025: understanding the Alzheimer's disease continuum. Alzheimers Res Ther. 2017;9(1):60.
Castillo-Carranza DL, Guerrero-Munoz MJ, Sengupta U, Hernandez C, Barrett AD, Dineley K, et al. Tau immunotherapy modulates both pathological tau and upstream amyloid pathology in an Alzheimer's disease mouse model. J Neurosci. 2015;35(12):1857–68.
Sanguinetti E, Guzzardi MA, Panetta D, Tripodi M, De Sena V, Quaglierini M, et al. Combined effect of fatty diet and cognitive decline on brain metabolism, food intake, body weight, and counteraction by intranasal insulin therapy in 3xTg mice. Front Cell Neurosci. 2019;13:188.
Duran-Aniotz C, Hetz C. Glucose metabolism: a sweet relief of Alzheimer's disease. Curr Biol. 2016;26(17):R806–9.
Newington JT, Harris RA, Cumming RC. Reevaluating metabolism in Alzheimer's disease from the perspective of the astrocyte-neuron lactate shuttle model. J Neurodegener Dis. 2013;2013:234572.
Krako N, Magnifico MC, Arese M, Meli G, Forte E, Lecci A, et al. Characterization of mitochondrial dysfunction in the 7PA2 cell model of Alzheimer's disease. J Alzheimers Dis. 2013;37(4):747–58.
Baek SH, Park SJ, Jeong JI, Kim SH, Han J, Kyung JW, et al. Inhibition of Drp1 ameliorates synaptic depression, abeta deposition, and cognitive impairment in an Alzheimer's disease model. J Neurosci. 2017;37(20):5099–110.
De Felice FG, Goncalves RA, Ferreira ST. Impaired insulin signalling and allostatic load in Alzheimer disease. Nat Rev Neurosci. 2022;23(4):215–30.
Ferreira LSS, Fernandes CS, Vieira MNN, De Felice FG. Insulin resistance in Alzheimer's disease. Front Neurosci. 2018;12:830.
Kellar D, Craft S. Brain insulin resistance in Alzheimer's disease and related disorders: mechanisms and therapeutic approaches. The Lancet Neurol. 2020;19(9):758–66.
Watson GS, Craft S. The role of insulin resistance in the pathogenesis of Alzheimer's disease: implications for treatment. CNS Drugs. 2003;17(1):27–45.
Wei Z, Koya J, Reznik SE. Insulin resistance exacerbates Alzheimer disease via multiple mechanisms. Front Neurosci. 2021;15:687157.
Gonzalez A, Calfio C, Churruca M, Maccioni RB. Glucose metabolism and AD: evidence for a potential diabetes type 3. Alzheimers Res Ther. 2022;14(1):56.
Kyrtata N, Emsley HCA, Sparasci O, Parkes LM, Dickie BR. A systematic review of glucose transport alterations in Alzheimer's disease. Front Neurosci. 2021;15:626–36.
Gali CC, Fanaee-Danesh E, Zandl-Lang M, Albrecher NM, Tam-Amersdorfer C, Stracke A, et al. Amyloid-beta impairs insulin signaling by accelerating autophagy-lysosomal degradation of LRP-1 and IR-beta in blood-brain barrier endothelial cells in vitro and in 3XTg-AD mice. Mol Cell Neurosci. 2019;99:103390.
Vandal M, White PJ, Chevrier G, Tremblay C, St-Amour I, Planel E, et al. Age-dependent impairment of glucose tolerance in the 3xTg-AD mouse model of Alzheimer's disease. FASEB J. 2015;29(10):4273–84.
Velazquez R, Tran A, Ishimwe E, Denner L, Dave N, Oddo S, et al. Central insulin dysregulation and energy dyshomeostasis in two mouse models of Alzheimer's disease. Neurobiol Aging. 2017;58:1–13.
Knight EM, Martins IV, Gumusgoz S, Allan SM, Lawrence CB. High-fat diet-induced memory impairment in triple-transgenic Alzheimer's disease (3xTgAD) mice is independent of changes in amyloid and tau pathology. Neurobiol Aging. 2014;35(8):1821–32.
Alzheimer's disease research models [Internet]. [cited June 4, 2020]. Available from: https://www.alzforum.org/research-models/alzheimers-disease.
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles. Neuron. 2003;39(3):409–21.
Belfiore R, Rodin A, Ferreira E, Velazquez R, Branca C, Caccamo A, et al. Temporal and regional progression of Alzheimer's disease-like pathology in 3xTg-AD mice. Aging Cell. 2019;18(1):e12873.
Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45(5):675–88.
Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003;24(8):1063–70.
DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27(5):457–64.
Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW Jr, et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurol. 2001;56(1):127–9.
Scheff SW, Price DA, Sparks DL. Quantitative assessment of possible age-related change in synaptic numbers in the human frontal cortex. Neurobiol Aging. 2001;22(3):355–65.
Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25(39):8843–53.
Stover KR, Campbell MA, Van Winssen CM, Brown RE. Analysis of motor function in 6-month-old male and female 3xTg-AD mice. Behav Brain Res. 2015;281:16–23.
Stover KR, Campbell MA, Van Winssen CM, Brown RE. Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer's disease. Behav Brain Res. 2015;289:29–38.
Simcox J, Lamming DW. The central moTOR of metabolism. Dev Cell. 2022;57(6):691–706.
Kennedy BK, Lamming DW. The mechanistic target of Rapamycin: the grand conducTOR of metabolism and aging. Cell Metab. 2016;23(6):990–1003.
Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332(6035):1317–22.
Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007;104(35):14056–61.
Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332(6035):1322–6.
Mota-Martorell N, Jove M, Pradas I, Berdun R, Sanchez I, Naudi A, et al. Gene expression and regulatory factors of the mechanistic target of rapamycin (mTOR) complex 1 predict mammalian longevity. Geroscience. 2020;42(4):1157–73.
Kaeberlein M, Powers RW 3rd, Steffen KK, Westman EA, Hu D, Dang N, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310(5751):1193–6.
Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14(10):885–90.
Powers RW 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20(2):174–84.
Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426(6967):620.
Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326(5949):140–4.
Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–43.
Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013;4(5):913–20.
Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66(2):191–201.
Arriola Apelo SI, Pumper CP, Baar EL, Cummings NE, Lamming DW. Intermittent administration of Rapamycin extends the life span of female C57BL/6J mice. J Gerontol A Biol Sci Med Sci. 2016;71(7):876–81.
Bitto A, Ito TK, Pineda VV, LeTexier NJ, Huang HZ, Sutlief E, et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife. 2016:5.
Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2(98):ra75.
Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176(5):2092–7.
Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10(24):4230–6.
Juricic P, Lu Y-X, Leech T, Drews LF, Paulitz J, Lu J, et al. Full geroprotection from brief rapamycin treatment by persistently increased intestinal autophagy. bioRxiv. 2022:2022.04.20.488884.
Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, et al. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15(5):713–24.
Shindyapina AV, Cho Y, Kaya A, Tyshkovskiy A, Castro JP, Deik A, et al. Rapamycin treatment during development extends life span and health span of male mice and Daphnia magna. Sci Adv. 2022;8(37):eabo5482.
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–5.
Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11(4):675–82.
Van Skike CE, Lin AL, Roberts Burbank R, Halloran JJ, Hernandez SF, Cuvillier J, et al. mTOR drives cerebrovascular, synaptic, and cognitive dysfunction in normative aging. Aging Cell. 2020;19(1):e13057.
Halloran J, Hussong SA, Burbank R, Podlutskaya N, Fischer KE, Sloane LB, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–13.
An WL, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K, et al. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer's disease. Am J Pathol. 2003;163(2):591–607.
Perluigi M, Pupo G, Tramutola A, Cini C, Coccia R, Barone E, et al. Neuropathological role of PI3K/Akt/mTOR axis in Down syndrome brain. Biochim Biophys Acta. 2014;1842(7):1144–53.
Tramutola A, Triplett JC, Di Domenico F, Niedowicz DM, Murphy MP, Coccia R, et al. Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem. 2015;133(5):739–49.
Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285(17):13107–20.
Caccamo A, Branca C, Talboom JS, Shaw DM, Turner D, Ma L, et al. Reducing ribosomal protein S6 Kinase 1 expression improves spatial memory and synaptic plasticity in a mouse model of Alzheimer's disease. J Neurosci. 2015;35(41):14042–56.
Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One. 2011;6(9):e25416.
Van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari Z, et al. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer's disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol. 2018;314(4):H693–703.
Van Skike CE, Hussong SA, Hernandez SF, Banh AQ, DeRosa N, Galvan V. mTOR Attenuation with Rapamycin reverses neurovascular uncoupling and memory deficits in mice modeling Alzheimer's disease. J Neurosci. 2021;41(19):4305–20.
Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5(4):e9979.
Kaeberlein M, Galvan V. Rapamycin and Alzheimer's disease: time for a clinical trial? Sci Transl Med. 2019;11(476)
Dumas SN, Lamming DW. Next generation strategies for geroprotection via mTORC1 inhibition. J Gerontol A Biol Sci Med Sci. 2020;75(1):14–23.
Carosi JM, Sargeant TJ. Rapamycin and Alzheimer disease: a double-edged sword? Autophagy. 2019;15(8):1460–2.
Gonzales M. Cognition, age, and RaPamycin effectiveness - downregulatIon of thE mTor pathway ((CARPE DIEM)) ClinicalTrials.gov: The University of Texas Health Science Center at San Antonio 2019 [updated 2/4/2022. Available from: https://clinicaltrials.gov/ct2/show/NCT04200911.
Gonzales M, Seshadri S. Rapamycin - effects on Alzheimer's and cognitive health (REACH) ClinicalTrials.gov: The University of Texas Health Science Center at San Antonio; 2020 [updated 10/12/2022. Available from: https://clinicaltrials.gov/ct2/show/NCT04629495.
Green CL, Lamming DW, Fontana L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol. 2021;23(1):56–73.
Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557.
Murphy T, Dias GP, Thuret S. Effects of diet on brain plasticity in animal and human studies: mind the gap. Neural Plast. 2014;2014:563160.
Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal. 2011;14(2):275–87.
Van Cauwenberghe C, Vandendriessche C, Libert C, Vandenbroucke RE. Caloric restriction: beneficial effects on brain aging and Alzheimer's disease. Mamm Genome. 2016;27(7-8):300–19.
Weiss EP, Fontana L. Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol. 2011;301(4):H1205–19.
Pak HH, Haws SA, Green CL, Koller M, Lavarias MT, Richardson NE, et al. Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice. Nat Metab. 2021;3(10):1327–41.
Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11(1):35–46.
Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8(2):113–27.
Syntichaki P, Troulinaki K, Tavernarakis N. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature. 2007;445(7130):922–6.
Henderson ST, Bonafe M, Johnson TE. daf-16 protects the nematode Caenorhabditis elegans during food deprivation. J Gerontol A Biol Sci Med Sci. 2006;61(5):444–60.
Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6(1):95–110.
Fok WC, Bokov A, Gelfond J, Yu Z, Zhang Y, Doderer M, et al. Combined treatment of rapamycin and dietary restriction has a larger effect on the transcriptome and metabolome of liver. Aging Cell. 2014;13(2):311–9.
Fok WC, Livi C, Bokov A, Yu Z, Chen Y, Richardson A, et al. Short-term rapamycin treatment in mice has few effects on the transcriptome of white adipose tissue compared to dietary restriction. Mech Ageing Dev. 2014;140:23–9.
Fok WC, Zhang Y, Salmon AB, Bhattacharya A, Gunda R, Jones D, et al. Short-term treatment with rapamycin and dietary restriction have overlapping and distinctive effects in young mice. J Gerontol A Biol Sci Med Sci. 2013;68(2):108–16.
Yu Z, Wang R, Fok WC, Coles A, Salmon AB, Perez VI. Rapamycin and dietary restriction induce metabolically distinctive changes in mouse liver. J Gerontol A Biol Sci Med Sci 2015;70(4):410-420.
Orenduff MC, Coleman MF, Glenny EM, Huffman KM, Rezeli ET, Bareja A, et al. Differential effects of calorie restriction and rapamycin on age-related molecular and functional changes in skeletal muscle. Exp Gerontol. 2022;165:111841.
Ham DJ, Borsch A, Chojnowska K, Lin S, Leuchtmann AB, Ham AS, et al. Distinct and additive effects of calorie restriction and rapamycin in aging skeletal muscle. Nat Commun. 2022;13(1):2025.
Mouton PR, Chachich ME, Quigley C, Spangler E, Ingram DK. Caloric restriction attenuates amyloid deposition in middle-aged dtg APP/PS1 mice. Neurosci Lett. 2009;464(3):184–7.
Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, et al. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol Aging. 2005;26(7):995–1000.
Schafer MJ, Alldred MJ, Lee SH, Calhoun ME, Petkova E, Mathews PM, et al. Reduction of beta-amyloid and gamma-secretase by calorie restriction in female Tg2576 mice. Neurobiol Aging. 2015;36(3):1293–302.
Sohal RS, Forster MJ. Caloric restriction and the aging process: a critique. Free Radic Biol Med. 2014;73:366–82.
Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, et al. Caloric restriction attenuates Beta-amyloid neruopathology in a mouse model of Alzheimer's disease. FASEB J. 2005;19(6):1–18.
Wu P, Shen Q, Dong S, Xu Z, Tsien JZ, Hu Y. Calorie restriction ameliorates neurodegenerative phenotypes in forebrain-specific presenilin-1 and presenilin-2 double knockout mice. Neurobiol Aging. 2008;29(10):1502–11.
Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2007;26(1):212–20.
Rangan P, Lobo F, Parrella E, Rochette N, Morselli M, Stephen TL, et al. Fasting-mimicking diet cycles reduce neuroinflammation to attenuate cognitive decline in Alzheimer's models. Cell Rep. 2022;40(13):111417.
Qin W, Chachich M, Lane M, Roth G, Bryant M, de Cabo R, et al. Calorie restriction attenuates Alzheimer's disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus). J Alzheimers Dis. 2006;10(4):417–22.
Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106(4):1255–60.
Mattson MP. Will caloric restriction and folate protect against AD and PD? Neurology. 2003;60(4):690–5.
Babygirija R, Lamming DW. The regulation of healthspan and lifespan by dietary amino acids. Transl Med Aging. 2021;5:17–30.
Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3(7):e223.
Richardson NE, Konon EN, Schuster HS, Mitchell AT, Boyle C, Rodgers AC, et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nat Aging. 2021;1(1):73–86.
Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19(3):418–30.
Solon-Biet SM, Mitchell SJ, Coogan SC, Cogger VC, Gokarn R, McMahon AC, et al. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Rep. 2015;11(10):1529–34.
Parrella E, Maxim T, Maialetti F, Zhang L, Wan J, Wei M, et al. Protein restriction cycles reduce IGF-1 and phosphorylated Tau, and improve behavioral performance in an Alzheimer's disease mouse model. Aging Cell. 2013;12(2):257–68.
Lamming DW, Cummings NE, Rastelli AL, Gao F, Cava E, Bertozzi B, et al. Restriction of dietary protein decreases mTORC1 in tumors and somatic tissues of a tumor-bearing mouse xenograft model. Oncotarget. 2015;6(31):31233–40.
Murphy ME, Narasimhan A, Adrian A, Kumar A, Green CL, Soto-Palma C, et al. Metabolism in the midwest: research from the midwest aging consortium at the 49(th) annual meeting of the american aging association. Geroscience. 2021;44(1):39–52.
Fernando W, Rainey-Smith SR, Gardener SL, Villemagne VL, Burnham SC, Macaulay SL, et al. Associations of dietary protein and fiber intake with brain and blood amyloid-beta. J Alzheimers Dis. 2018;61(4):1589–98.
Roberts RO, Roberts LA, Geda YE, Cha RH, Pankratz VS, O'Connor HM, et al. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J Alzheimers Dis. 2012;32(2):329–39.
Yeh TS, Yuan C, Ascherio A, Rosner BA, Blacker D, Willett WC. Long-term dietary protein intake and subjective cognitive decline in US men and women. Am J Clin Nutr. 2022;115(1):199–210.
Felig P, Marliss E, Cahill GF Jr. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281(15):811–6.
Li H, Ye D, Xie W, Hua F, Yang Y, Wu J, et al. Defect of branched-chain amino acid metabolism promotes the development of Alzheimer's disease by targeting the mTOR signaling. Biosci Rep. 2018;38(4)
Tournissac M, Vandal M, Tremblay C, Bourassa P, Vancassel S, Emond V, et al. Dietary intake of branched-chain amino acids in a mouse model of Alzheimer's disease: effects on survival, behavior, and neuropathology. Alzheimers Dement (N Y). 2018;4:677–87.
Ribeiro RV, Solon-Biet SM, Pulpitel T, Senior AM, Cogger VC, Clark X, et al. Of older mice and men: branched-chain amino acids and body composition. Nutrients. 2019;11(8)
Zheng Y, Li Y, Qi Q, Hruby A, Manson JE, Willett WC, et al. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol. 2016;45(5):1482–92.
Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–26.
Ericksen RE, Lim SL, McDonnell E, Shuen WH, Vadiveloo M, White PJ, et al. Loss of BCAA catabolism during carcinogenesis enhances mTORC1 activity and promotes tumor development and progression. Cell Metab. 2019;29(5):1151–65.
Solon-Biet SM, Cogger VC, Pulpitel T, Wahl D, Clark X, Bagley E, et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat Metab. 2019;1(5):532–45.
Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. 2016;22(4):421–6.
Bishop CA, Machate T, Henning T, Henkel J, Puschel G, Weber D, et al. Detrimental effects of branched-chain amino acids in glucose tolerance can be attributed to valine induced glucotoxicity in skeletal muscle. Nutr Diabetes. 2022;12(1):20.
Deelen J, Kettunen J, Fischer K, van der Spek A, Trompet S, Kastenmuller G, et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat Commun. 2019;10(1):3346.
Yu D, Richardson NE, Green CL, Spicer AB, Murphy ME, Flores V, et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab. 2021;33(5):905–22.
Tynkkynen J, Chouraki V, van der Lee SJ, Hernesniemi J, Yang Q, Li S, et al. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer's disease: a prospective study in eight cohorts. Alzheimers Dement. 2018;14(6):723–33.
Larsson SC, Markus HS. Branched-chain amino acids and Alzheimer's disease: a Mendelian randomization analysis. Sci Rep. 2017;7(1):13604.
Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492(1):203–6.
Hill CM, Berthoud HR, Munzberg H, Morrison CD. Homeostatic sensing of dietary protein restriction: a case for FGF21. Front Neuroendocrinol. 2018;51:125–31.
Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16(2):520–30.
Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124(9):3913–22.
Hill CM, Albarado DC, Coco LG, Spann RA, Khan MS, Qualls-Creekmore E, et al. FGF21 is required for protein restriction to extend lifespan and improve metabolic health in male mice. Nat Commun. 2022;13(1):1897.
Hill CM, Laeger T, Albarado DC, McDougal DH, Berthoud HR, Munzberg H, et al. Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Sci Rep. 2017;7(1):8209.
Hill CM, Laeger T, Dehner M, Albarado DC, Clarke B, Wanders D, et al. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Rep. 2019;27(10):2934–47.
Green CL, Pak HH, Richardson NE, Flores V, Yu D, Tomasiewicz JL, et al. Sex and genetic background define the metabolic, physiologic, and molecular response to protein restriction. Cell Metab. 2022;34(2):209–26.
Ruhlmann C. Long-term caloric restriction in ApoE-deficient mice results in neuroprotection via Fgf21-induced AMPK/mTOR pathway. Aging. 2016;8(11):2777–89.
Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol. 2018;596(4):623–45.
Maida A, Chan JSK, Sjoberg KA, Zota A, Schmoll D, Kiens B, et al. Repletion of branched chain amino acids reverses mTORC1 signaling but not improved metabolism during dietary protein dilution. Molecular metabolism. 2017;6(8):873–81.
Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide FGF21 can enter brain from blood. Peptides. 2007;28(12):2382–6.
BonDurant LD, Ameka M, Naber MC, Markan KR, Idiga SO, Acevedo MR, et al. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab. 2017;25(4):935–44.
Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014;20(4):670–7.
Conte M, Sabbatinelli J, Chiariello A, Martucci M, Santoro A, Monti D, et al. Disease-specific plasma levels of mitokines FGF21, GDF15, and Humanin in type II diabetes and Alzheimer's disease in comparison with healthy aging. Geroscience. 2021;43(2):985–1001.
Zanini D, Seper V, Javorac D, Stajer V, Cvetkovic M, Batez M, et al. Correlation between serum FGF21 and cognition in men and women over 60 years of age. Int J Gerontol. 2021;15(1):30–3.
Chen S, Chen ST, Sun Y, Xu Z, Wang Y, Yao SY, et al. Fibroblast growth factor 21 ameliorates neurodegeneration in rat and cellular models of Alzheimer's disease. Redox Biol. 2019;22:101133.
Sa-Nguanmoo P, Tanajak P, Kerdphoo S, Jaiwongkam T, Wang X, Liang G, et al. FGF21 and DPP-4 inhibitor equally prevents cognitive decline in obese rats. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;97:1663–72.
Sa-Nguanmoo P, Tanajak P, Kerdphoo S, Satjaritanun P, Wang X, Liang G, et al. FGF21 improves cognition by restored synaptic plasticity, dendritic spine density, brain mitochondrial function and cell apoptosis in obese-insulin resistant male rats. Horm Behav. 2016;85:86–95.
Wang Q, Yuan J, Yu Z, Lin L, Jiang Y, Cao Z, et al. FGF21 Attenuates high-fat diet-induced cognitive impairment via metabolic regulation and anti-inflammation of obese mice. Mol Neurobiol. 2018;55(6):4702–17.
Amiri M, Braidy N, Aminzadeh M. Protective effects of fibroblast growth factor 21 against Amyloid-Beta(1-42)-induced toxicity in SH-SY5Y cells. Neurotox Res. 2018;34(3):574–83.
Leng Y, Wang Z, Tsai LK, Leeds P, Fessler EB, Wang J, et al. FGF-21, a novel metabolic regulator, has a robust neuroprotective role and is markedly elevated in neurons by mood stabilizers. Mol Psychiatry. 2015;20(2):215–23.
Lees EK, Krol E, Grant L, Shearer K, Wyse C, Moncur E, et al. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell. 2014;13(5):817–27.
Wanders D, Forney LA, Stone KP, Burk DH, Pierse A, Gettys TW. FGF21 mediates the thermogenic and insulin-sensitizing effects of dietary methionine restriction but not its effects on hepatic lipid metabolism. Diabetes. 2017;66(4):858–67.
Forney LA, Fang H, Sims LC, Stone KP, Vincik LY, Vick AM, et al. Dietary methionine restriction signals to the brain through fibroblast growth factor 21 to regulate energy balance and remodeling of adipose tissue. Obesity (Silver Spring). 2020;28(10):1912–21.
Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19(2):302–9.
Tournissac M, Bourassa P, Martinez-Cano RD, Vu TM, Hebert SS, Planel E, et al. Repeated cold exposures protect a mouse model of Alzheimer's disease against cold-induced tau phosphorylation. Mol Metab. 2019;22:110–20.
Gong Q, Hu Z, Zhang F, Cui A, Chen X, Jiang H, et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology. 2016;64(2):425–38.
Tsai S, Sitzmann JM, Dastidar SG, Rodriguez AA, Vu SL, McDonald CE, et al. Muscle-specific 4E-BP1 signaling activation improves metabolic parameters during aging and obesity. J Clin Invest. 2015;125(8):2952–64.
Jukic I, Kolobaric N, Stupin A, Matic A, Kozina N, Mihaljevic Z, et al. Carnosine, small but mighty-prospect of use as functional ingredient for functional food formulation. Antioxidants (Basel). 2021;10(7)
Corona C, Frazzini V, Silvestri E, Lattanzio R, La Sorda R, Piantelli M, et al. Effects of dietary supplementation of carnosine on mitochondrial dysfunction, amyloid pathology, and cognitive deficits in 3xTg-AD mice. PLoS One. 2011;6(3):e17971.
Horning MS, Blakemore LJ, Trombley PQ. Endogenous mechanisms of neuroprotection: role of zinc, copper, and carnosine. Brain Res. 2000;852(1):56–61.
Kohen R, Yamamoto Y, Cundy KC, Ames BN. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci U S A. 1988;85(9):3175–9.
Preston JE, Hipkiss AR, Himsworth DT, Romero IA, Abbott JN. Toxic effects of beta-amyloid(25-35) on immortalised rat brain endothelial cell: protection by carnosine, homocarnosine and beta-alanine. Neurosci Lett. 1998;242(2):105–8.
Trombley PQ, Horning MS, Blakemore LJ. Carnosine modulates zinc and copper effects on amino acid receptors and synaptic transmission. Neuroreport. 1998;9(15):3503–7.
Trombley PQ, Horning MS, Blakemore LJ. Interactions between carnosine and zinc and copper: implications for neuromodulation and neuroprotection. Biochem Mosc. 2000;65(7):807–16.
Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc Natl Acad Sci U S A. 2009;106(47):20057–62.
Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci. 2009;10(11):780–91.
Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106(34):14670–5.
Bush AI, Pettingell WH, Multhaup G, d Paradis M, Vonsattel JP, Gusella JF, et al. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994;265(5177):1464–7.
Gazaryan IG, Krasinskaya IP, Kristal BS, Brown AM. Zinc irreversibly damages major enzymes of energy production and antioxidant defense prior to mitochondrial permeability transition. J Biol Chem. 2007;282(33):24373–80.
Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci U S A. 1999;96(5):2414–9.
Hipkiss AR, Baye E, de Courten B. Carnosine and the processes of ageing. Maturitas. 2016;93:28–33.
Schon M, Mousa A, Berk M, Chia WL, Ukropec J, Majid A, et al. The potential of carnosine in brain-related disorders: a comprehensive review of current evidence. Nutrients. 2019;11(6)
Caruso G, Godos J, Castellano S, Micek A, Murabito P, Galvano F, et al. The therapeutic potential of carnosine/anserine supplementation against cognitive decline: a systematic review with meta-analysis. Biomedicines. 2021;9(3)
Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197–212.
Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89(2):337–43.
Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J Pharmacol Exp Ther. 2008;326(1):196–208.
Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21(21):8370–7.
Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci. 2009;29(28):9078–89.
Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28(8):1765–73.
Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280(7):5892–901.
Chen M, Du ZY, Zheng X, Li DL, Zhou RP, Zhang K. Use of curcumin in diagnosis, prevention, and treatment of Alzheimer's disease. Neural Regen Res. 2018;13(4):742–52.
Voulgaropoulou SD, van Amelsvoort T, Prickaerts J, Vingerhoets C. The effect of curcumin on cognition in Alzheimer's disease and healthy aging: a systematic review of pre-clinical and clinical studies. Brain Res. 2019;1725:146476.
Zeng K, Tian L, Patel R, Shao W, Song Z, Liu L, et al. Diet polyphenol curcumin stimulates hepatic Fgf21 production and restores its sensitivity in high-fat-diet-fed male mice. Endocrinology. 2017;158(2):277–92.
Wang C, Zhang X, Teng Z, Zhang T, Li Y. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur J Pharmacol. 2014;740:312–20.
Ungurianu A, Zanfirescu A, Margina D. Regulation of gene expression through food-curcumin as a sirtuin activity modulator. Plants (Basel). 2022;11(13)
Biyong EF, Tremblay C, Leclerc M, Caron V, Alfos S, Helbling JC, et al. Role of Retinoid X Receptors (RXRs) and dietary vitamin A in Alzheimer's disease: evidence from clinicopathological and preclinical studies. Neurobiol Dis. 2021;161:105542.
Lane MA, Bailey SJ. Role of retinoid signalling in the adult brain. Prog Neurobiol. 2005;75(4):275–93.
Olson CR, Mello CV. Significance of vitamin A to brain function, behavior and learning. Mol Nutr Food Res. 2010;54(4):489–95.
Shearer KD, Stoney PN, Morgan PJ, McCaffery PJ. A vitamin for the brain. Trends Neurosci. 2012;35(12):733–41.
Behl T, Kaur D, Sehgal A, Singla RK, Makeen HA, Albratty M, et al. Therapeutic insights elaborating the potential of retinoids in Alzheimer's disease. Front Pharmacol. 2022;13:976799.
Lerner AJ, Gustaw-Rothenberg K, Smyth S, Casadesus G. Retinoids for treatment of Alzheimer's disease. Biofactors. 2012;38(2):84–9.
Mey J, McCaffery P. Retinoic acid signaling in the nervous system of adult vertebrates. Neuroscientist. 2004;10(5):409–21.
Ransom J, Morgan PJ, McCaffery PJ, Stoney PN. The rhythm of retinoids in the brain. J Neurochem. 2014;129(3):366–76.
Lopes da Silva S, Vellas B, Elemans S, Luchsinger J, Kamphuis P, Yaffe K, et al. Plasma nutrient status of patients with Alzheimer's disease: systematic review and meta-analysis. Alzheimers Dement. 2014;10(4):485–502.
Bourdel-Marchasson I, Delmas-Beauvieux MC, Peuchant E, Richard-Harston S, Decamps A, Reignier B, et al. Antioxidant defences and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing. 2001;30(3):235–41.
Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, et al. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003;24(7):915–9.
Takamura R, Watamura N, Nikkuni M, Ohshima T. All-trans retinoic acid improved impaired proliferation of neural stem cells and suppressed microglial activation in the hippocampus in an Alzheimer's mouse model. J Neurosci Res. 2017;95(3):897–906.
Watamura N, Toba J, Yoshii A, Nikkuni M, Ohshima T. Colocalization of phosphorylated forms of WAVE1, CRMP2, and tau in Alzheimer's disease model mice: involvement of Cdk5 phosphorylation and the effect of ATRA treatment. J Neurosci Res. 2016;94(1):15–26.
Cummings J. Bexarotene amyloid treatment for Alzheimer's disease (BEAT-AD) ClinicalTrials.gov: The Cleveland Clinic; 2013 [updated 02/12/2016.
Heuser I. Retinoic acid homeostasis in neuropsychiatric diseases (RAHND) ClinicalTrials.gov: Charite University; 2015 [updated 08/09/2021.
Miki T. Efficacy and safety of tamibarotene (OAM80) for Alzheimer's disease ClinicalTrials.gov: Osaka City University; 2010 [updated 07/22/2011.
Ormerod AD, Thind CK, Rice SA, Reid IC, Williams JH, McCaffery PJ. Influence of isotretinoin on hippocampal-based learning in human subjects. Psychopharmacology. 2012;221(4):667–74.
Grodstein F, Kang JH, Glynn RJ, Cook NR, Gaziano JM. A randomized trial of beta carotene supplementation and cognitive function in men: the Physicians' Health Study II. Arch Intern Med. 2007;167(20):2184–90.
Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–27.
Kim D, Cho J, Lee I, Jin Y, Kang H. Exercise attenuates high-fat diet-induced disease progression in 3xTg-AD mice. Med Sci Sports Exerc. 2017;49(4):676–86.
Geda YE, Silber TC, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, et al. Computer activities, physical exercise, aging, and mild cognitive impairment: a population-based study. Mayo Clin Proc. 2012;87(5):437–42.
Maesako M, Uemura K, Iwata A, Kubota M, Watanabe K, Uemura M, et al. Continuation of exercise is necessary to inhibit high fat diet-induced beta-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. PLoS One. 2013;8(9):e72796.
Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401(6748):63–9.
Strohle A, Schmidt DK, Schultz F, Fricke N, Staden T, Hellweg R, et al. Drug and exercise treatment of Alzheimer disease and mild cognitive impairment: a systematic review and meta-analysis of effects on cognition in randomized controlled trials. Am J Geriatr Psychiatry. 2015;23(12):1234–49.
Pena GS, Paez HG, Johnson TK, Halle JL, Carzoli JP, Visavadiya NP, et al. Hippocampal growth factor and myokine cathepsin B expression following aerobic and resistance training in 3xTg-AD mice. Int J Chronic Dis. 2020;2020:5919501.
Liu Y, Chu JMT, Yan T, Zhang Y, Chen Y, Chang RCC, et al. Short-term resistance exercise inhibits neuroinflammation and attenuates neuropathological changes in 3xTg Alzheimer's disease mice. J Neuroinflammation. 2020;17(1):4.
Garcia-Mesa Y, Gimenez-Llort L, Lopez LC, Venegas C, Cristofol R, Escames G, et al. Melatonin plus physical exercise are highly neuroprotective in the 3xTg-AD mouse. Neurobiol Aging. 2012;33(6):1124–e13.
Bareiss SK, Johnston T, Lu Q, Tran TD. The effect of exercise on early sensorimotor performance alterations in the 3xTg-AD model of Alzheimer's disease. Neurosci Res. 2022;178:60–8.
Kim TW, Park SS, Park JY, Park HS. Infusion of plasma from exercised mice ameliorates cognitive dysfunction by increasing hippocampal neuroplasticity and mitochondrial functions in 3xTg-AD mice. Int J Mol Sci. 2020;21(9)
Coelho FG, Vital TM, Stein AM, Arantes FJ, Rueda AV, Camarini R, et al. Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer's disease. J Alzheimers Dis. 2014;39(2):401–8.
Heisz JJ, Kovacevic A, Clark IB, Vandermorris S. Evaluation of a community-based exercise program for managing Alzheimer's disease. J Am Geriatr Soc. 2016;64(4):884–6.
Hoffmann K, Sobol NA, Frederiksen KS, Beyer N, Vogel A, Vestergaard K, et al. Moderate-to-high intensity physical exercise in patients with Alzheimer's disease: a randomized controlled trial. J Alzheimers Dis. 2016;50(2):443–53.
Lopez-Ortiz S, Lista S, Valenzuela PL, Pinto-Fraga J, Carmona R, Caraci F, et al. Effects of physical activity and exercise interventions on Alzheimer's disease: an umbrella review of existing meta-analyses. J Neurol. 2023;270(2):711–25.
Lopez-Ortiz S, Valenzuela PL, Seisdedos MM, Morales JS, Vega T, Castillo-Garcia A, et al. Exercise interventions in Alzheimer's disease: a systematic review and meta-analysis of randomized controlled trials. Ageing Res Rev. 2021;72:101479.
Menengiç KN, Yeldan I, Cinar N, Sahiner T. Effectiveness of motor-cognitive dual-task exercise via telerehabilitation in Alzheimer's disease: an online pilot randomized controlled study. Clin Neurol Neurosurg. 2022;223:107501.
Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, et al. Aerobic exercise for Alzheimer's disease: a randomized controlled pilot trial. PLoS One. 2017;12(2):e0170547.
Pitkala KH, Poysti MM, Laakkonen ML, Tilvis RS, Savikko N, Kautiainen H, et al. Effects of the Finnish Alzheimer disease exercise trial (FINALEX): a randomized controlled trial. JAMA Intern Med. 2013;173(10):894–901.
Rao AK, Chou A, Bursley B, Smulofsky J, Jezequel J. Systematic review of the effects of exercise on activities of daily living in people with Alzheimer's disease. Am J Occup Ther. 2014;68(1):50–6.
Tari AR, Berg HH, Videm V, Brathen G, White LR, Rosbjorgen RN, et al. Safety and efficacy of plasma transfusion from exercise-trained donors in patients with early Alzheimer's disease: protocol for the ExPlas study. BMJ Open. 2022;12(9):e056964.
Watson J, O'Keeffe N, West SL. The importance of exercise in Alzheimer's disease and the minds in motion((R)) program: an editorial. J Funct Morphol Kinesiol. 2020;5(3)
Yu F, Salisbury D, Mathiason MA. Inter-individual differences in the responses to aerobic exercise in Alzheimer's disease: findings from the FIT-AD trial. J Sport Health Sci. 2021;10(1):65–72.
Yu F, Thomas W, Nelson NW, Bronas UG, Dysken M, Wyman JF. Impact of 6-month aerobic exercise on Alzheimer's symptoms. J Appl Gerontol. 2015;34(4):484–500.
Yu F, Vock DM, Barclay TR. Executive function: responses to aerobic exercise in Alzheimer's disease. Geriatr Nurs. 2018;39(2):219–24.
Yu F, Vock DM, Zhang L, Salisbury D, Nelson NW, Chow LS, et al. Cognitive effects of aerobic exercise in Alzheimer's disease: a pilot randomized controlled trial. J Alzheimers Dis. 2021;80(1):233–44.
Meng L, Li XY, Shen L, Ji HF. Type 2 diabetes mellitus drugs for Alzheimer's disease: current evidence and therapeutic opportunities. Trends Mol Med. 2020;26(6):597–614.
Thomas L, Eckhardt M, Langkopf E, Tadayyon M, Himmelsbach F, Mark M. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylm ethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors. J Pharmacol Exp Ther. 2008;325(1):175–82.
Chen S, Sun J, Zhao G, Guo A, Chen Y, Fu R, et al. Liraglutide improves water maze learning and memory performance while reduces hyperphosphorylation of tau and neurofilaments in APP/PS1/Tau triple transgenic mice. Neurochem Res. 2017;42(8):2326–35.
Egefjord L, Gejl M, Møller A, Brændgaard H, Gottrup H, Antropova O, et al. Effects of liraglutide on neurodegeneration, blood flow and cognition in Alzheimer's disease - protocol for a controlled, randomized double-blinded trial. Dan Med J. 2012;59(10)
Zhang G, Kim S, Gu X, Yu SP, Wei L. DPP-4 Inhibitor linagliptin is neuroprotective in hyperglycemic mice with stroke via the AKT/mTOR pathway and anti-apoptotic effects. Neurosci Bull. 2020;36(4):407–18.
Kosaraju J, Holsinger RMD, Guo L, Tam KY. Linagliptin, a dipeptidyl peptidase-4 inhibitor, mitigates cognitive deficits and pathology in the 3xTg-AD mouse model of Alzheimer's disease. Mol Neurobiol. 2017;54(8):6074–84.
Yang M, Zhang L, Wang C, Liu H, Boden G, Yang G, et al. Liraglutide increases FGF-21 activity and insulin sensitivity in high fat diet and adiponectin knockdown induced insulin resistance. PLoS One. 2012;7(11):e48392.
Zhou J, Poudel A, Li L. Liraglutide improves insulin sensitivity in diabetic mice through reduction of inflammation and induction of thermogenesis. FASEB J. 2018;32(51)
Bomba M, Ciavardelli D, Silvestri E, Canzoniero LM, Lattanzio R, Chiappini P, et al. Exenatide promotes cognitive enhancement and positive brain metabolic changes in PS1-KI mice but has no effects in 3xTg-AD animals. Cell Death Dis. 2013;4:e612.
Searcy JL, Phelps JT, Pancani T, Kadish I, Popovic J, Anderson KL, et al. Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2012;30(4):943–61.
El-Megiri N, Mostafa YM, Ahmed A, Mehanna ET, El-Azab MF, Alshehri F, et al. Pioglitazone ameliorates hippocampal neurodegeneration, disturbances in glucose metabolism and AKT/mTOR signaling pathways in pentyelenetetrazole-kindled mice. Pharmaceuticals (Basel). 2022;15(9)
Masciopinto F, Di Pietro N, Corona C, Bomba M, Pipino C, Curcio M, et al. Effects of long-term treatment with pioglitazone on cognition and glucose metabolism of PS1-KI, 3xTg-AD, and wild-type mice. Cell Death Dis. 2012;3:e448.
Yu Y, Li X, Blanchard J, Li Y, Iqbal K, Liu F, et al. Insulin sensitizers improve learning and attenuate tau hyperphosphorylation and neuroinflammation in 3xTg-AD mice. J Neural Transm (Vienna). 2015;122(4):593–606.
Finan B, Yang B, Ottaway N, Smiley DL, Ma T, Clemmensen C, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21(1):27–36.
Li T, Jiao JJ, Holscher C, Wu MN, Zhang J, Tong JQ, et al. A novel GLP-1/GIP/Gcg triagonist reduces cognitive deficits and pathology in the 3xTg mouse model of Alzheimer's disease. Hippocampus. 2018;28(5):358–72.
Li T, Jiao JJ, Su Q, Holscher C, Zhang J, Yan XD, et al. A GLP-1/GIP/Gcg receptor triagonist improves memory behavior, as well as synaptic transmission, neuronal excitability and Ca(2+) homeostasis in 3xTg-AD mice. Neuropharmacology. 2020;170:108042.
Vandal M, White PJ, Tremblay C, St-Amour I, Chevrier G, Emond V, et al. Insulin reverses the high-fat diet-induced increase in brain Abeta and improves memory in an animal model of Alzheimer disease. Diabetes. 2014;63(12):4291–301.
Chen Y, Zhao Y, Dai CL, Liang Z, Run X, Iqbal K, et al. Intranasal insulin restores insulin signaling, increases synaptic proteins, and reduces Abeta level and microglia activation in the brains of 3xTg-AD mice. Exp Neurol. 2014;261:610–9.
Claxton A, Baker LD, Wilkinson CW, Trittschuh EH, Chapman D, Watson GS, et al. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer's disease. J Alzheimers Dis. 2013;35(4):789–97.
Craft S, Raman R, Chow TW, Rafii MS, Sun CK, Rissman RA, et al. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical trial. JAMA Neurol. 2020;77(9):1099–109.
Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13(3):323–31.
Michailidis M, Tata DA, Moraitou D, Kavvadas D, Karachrysafi S, Papamitsou T, et al. Antidiabetic drugs in the treatment of Alzheimer's disease. Int J Mol Sci. 2022;23(9)
Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, et al. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. J Alzheimers Dis. 2015;44(3):897–906.
Brunden KR, Yao Y, Potuzak JS, Ferrer NI, Ballatore C, James MJ, et al. The characterization of microtubule-stabilizing drugs as possible therapeutic agents for Alzheimer's disease and related tauopathies. Pharmacol Res. 2011;63(4):341–51.
Fernandez-Valenzuela JJ, Sanchez-Varo R, Munoz-Castro C, De Castro V, Sanchez-Mejias E, Navarro V, et al. Enhancing microtubule stabilization rescues cognitive deficits and ameliorates pathological phenotype in an amyloidogenic Alzheimer's disease model. Sci Rep. 2020;10(1):14776.
Lou K, Yao Y, Hoye AT, James MJ, Cornec AS, Hyde E, et al. Brain-penetrant, orally bioavailable microtubule-stabilizing small molecules are potential candidate therapeutics for Alzheimer's disease and related tauopathies. J Med Chem. 2014;57(14):6116–27.
Cross DJ, Huber BR, Silverman MA, Cline MM, Gill TB, Cross CG, et al. Intranasal paclitaxel alters Alzheimer's disease phenotypic features in 3xTg-AD mice. J Alzheimers Dis. 2021;83(1):379–94.
Diaz JF, Barasoain I, Andreu JM. Fast kinetics of Taxol binding to microtubules. Effects of solution variables and microtubule-associated proteins. J Biol Chem. 2003;278(10):8407–19.
Zhang Y, Huang P, Liu X, Xiang Y, Zhang T, Wu Y, et al. Polyphyllin I inhibits growth and invasion of cisplatin-resistant gastric cancer cells by partially inhibiting CIP2A/PP2A/Akt signaling axis. J Pharmacol Sci. 2018;137(3):305–12.
Zhou Y, Yang D, Chen H, Zheng C, Jiang H, Liu X, et al. Polyphyllin I attenuates cognitive impairments and reduces AD-like pathology through CIP2A-PP2A signaling pathway in 3XTg-AD mice. FASEB J. 2020;34(12):16414–31.
Marsh JC, Gielda BT, Herskovic AM, Abrams RA. Cognitive sparing during the administration of whole brain radiotherapy and prophylactic cranial irradiation: current concepts and approaches. J Oncol. 2010;2010:198208.
Wilson GD, Wilson TG, Hanna A, Fontanesi G, Kulchycki J, Buelow K, et al. Low dose brain irradiation reduces amyloid-beta and Tau in 3xTg-AD mice. J Alzheimers Dis. 2020;75(1):15–21.
Kurrus JA, Hayes JK, Hoidal JR, Menendez MM, Elstad MR. Radiation therapy for tracheobronchial amyloidosis. Chest. 1998;114(5):1489–92.
Poovaneswaran S, Razak A, Lockman H, Bone M, Pollard K, Mazdai G. Tracheobronchial amyloidosis: utilization of radiotherapy as a treatment modality. Medscape J Med. 2008;10(2)
Cuttler JM, Moore ER, Hosfeld VD, Nadolski DL. Treatment of Alzheimer disease with CT scans: a case report. Dose-Response. 2016;14(2)
Cuttler JM, Abdellah E, Goldberg Y, Al-Shamaa S, Symons SP, Black SE, et al. Low doses of ionizing radiation as a treatment for Alzheimer's disease: a pilot study. J Alzheimers Dis. 2021;80(3):1119–28.
Ancidoni A, Bacigalupo I, Remoli G, Lacorte E, Piscopo P, Sarti G, et al. Anticancer drugs repurposed for Alzheimer's disease: a systematic review. Alzheimers Res Ther. 2021;13(1):96.
Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol. 2014;6(4)
Kim E, Bisson WH, Lohr CV, Williams DE, Ho E, Dashwood RH, et al. Histone and non-histone targets of dietary deacetylase inhibitors. Curr Top Med Chem. 2016;16(7):714–31.
Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, et al. Nicotinamide restores cognition in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci. 2008;28(45):11500–10.
Gardian G, Yang L, Cleren C, Calingasan NY, Klivenyi P, Beal MF. Neuroprotective effects of phenylbutyrate against MPTP neurotoxicity. NeuroMolecular Med. 2004;5(3):235–41.
Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447(7141)
Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120.
Janczura KJ, Volmar CH, Sartor GC, Rao SJ, Ricciardi NR, Lambert G, et al. Inhibition of HDAC3 reverses Alzheimer's disease-related pathologies in vitro and in the 3xTg-AD mouse model. Proc Natl Acad Sci U S A. 2018;115(47):E11148–E57.
Jia H, Wang Y, Morris CD, Jacques V, Gottesfeld JM, Rusche JR, et al. The effects of pharmacological inhibition of histone deacetylase 3 (HDAC3) in Huntington's disease mice. PLoS One. 2016;11(3):e0152498.
Leus NG, Zwinderman MR, Dekker FJ. Histone deacetylase 3 (HDAC 3) as emerging drug target in NF-kappaB-mediated inflammation. Curr Opin Chem Biol. 2016;33:160–8.
Salech F, Ponce DP, Paula-Lima AC, SanMartin CD, Behrens MI. Nicotinamide, a poly [ADP-Ribose] polymerase 1 (PARP-1) inhibitor, as an adjunctive therapy for the treatment of Alzheimer's disease. Front Aging Neurosci. 2020;12:255.
Maiese K. Nicotinamide: oversight of metabolic dysfunction through SIRT1, mTOR, and clock genes. Curr Neurovasc Res. 2020;17(5):765–83.
Grill J. Nicotinamide as an early Alzheimer's disease treatment (NEAT) ClinicalTrials.gov: University of California, Irvine 2017 [updated 12/19/2022. Available from: https://clinicaltrials.gov/ct2/show/NCT03061474.
Pascoal TA, Chamoun M, Lax E, Wey HY, Shin M, Ng KP, et al. [(11)C]Martinostat PET analysis reveals reduced HDAC I availability in Alzheimer's disease. Nat Commun. 2022;13(1):4171.
Xu K, Dai XL, Huang HC, Jiang ZF. Targeting HDACs: a promising therapy for Alzheimer's disease. Oxid Med Cell Longev. 2011;2011
Shukla S, Tekwani BL. Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front Pharmacol. 2020;11:537.
Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, et al. NAD(+) supplementation normalizes key Alzheimer's features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci U S A. 2018;115(8):E1876–E85.
Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, et al. Mitophagy and Alzheimer's disease: cellular and molecular mechanisms. Trends Neurosci. 2017;40(3):151–66.
Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD(+) and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17(11):679–90.
Zhao H, Tian Y, Zuo Y, Zhang X, Gao Y, Wang P, et al. Nicotinamide riboside ameliorates high-fructose-induced lipid metabolism disorder in mice via improving FGF21 resistance in the liver and white adipose tissue. Food Funct. 2022;13(23):12400–11.
Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506.
Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50(11):3337–40.
Corpas R, Grinan-Ferre C, Rodriguez-Farre E, Pallas M, Sanfeliu C. Resveratrol induces brain resilience against Alzheimer neurodegeneration through proteostasis enhancement. Mol Neurobiol. 2019;56(2):1502–16.
Malhotra A, Bath S, Elbarbry F. An organ system approach to explore the antioxidative, anti-inflammatory, and cytoprotective actions of resveratrol. Oxidative Med Cell Longev. 2015;2015:803971.
Novelle MG, Wahl D, Dieguez C, Bernier M, de Cabo R. Resveratrol supplementation: where are we now and where should we go? Ageing Res Rev. 2015;21:1–15.
Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J Neurochem. 2009;110(5):1445–56.
Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3(6):e2264.
Porquet D, Casadesus G, Bayod S, Vicente A, Canudas AM, Vilaplana J, et al. Dietary resveratrol prevents Alzheimer's markers and increases life span in SAMP8. Age (Dordr). 2013;35(5):1851–65.
Broderick TL, Rasool S, Li R, Zhang Y, Anderson M, Al-Nakkash L, et al. Neuroprotective effects of chronic resveratrol treatment and exercise training in the 3xTg-AD mouse model of Alzheimer’s disease. Int J Mol Sci. 2020;21(19)
Dennison JL, Volmar CH, Ke D, Wang J, Gravel E, Hammond-Vignini S, et al. JOTROL, a novel formulation of resveratrol, shows beneficial effects in the 3xTg-AD mouse model. J Alzheimers Dis. 2022;86(1):173–90.
Orr ME, Kotkowski E, Bair-Kelps D, Romo T, Espinoza S, Musi N, et al. Results from a pilot study: the effects of nicotinamide riboside on mild cognitive impairment. Alzheimers Dement. 2020;16(S9)
Sawda C, Moussa C, Turner RS. Resveratrol for Alzheimer's disease. Ann N Y Acad Sci. 2017;1403(1):142–9.
Chen W, Lu H, Yang J, Xiang H, Peng H. Sphingosine 1-phosphate in metabolic syndrome (Review). Int J Mol Med. 2016;38(4):1030–8.
Guitton J, Bandet CL, Mariko ML, Tan-Chen S, Bourron O, Benomar Y, et al. Sphingosine-1-phosphate metabolism in the regulation of obesity/type 2 diabetes. Cells. 2020;9(7)
Couttas TA, Kain N, Daniels B, Lim XY, Shepherd C, Kril J, et al. Loss of the neuroprotective factor Sphingosine 1-phosphate early in Alzheimer's disease pathogenesis. Acta Neuropathol Commun. 2014;2:9.
Fagan SG, Bechet S, Dev KK. Fingolimod rescues memory and improves pathological hallmarks in the 3xTg-AD model of Alzheimer's disease. Mol Neurobiol. 2022;59(3):1882–95.
Czubowicz K, Jesko H, Wencel P, Lukiw WJ, Strosznajder RP. The role of ceramide and sphingosine-1-phosphate in Alzheimer's disease and other neurodegenerative disorders. Mol Neurobiol. 2019;56(8):5436–55.
O'Sullivan S, Dev KK. Sphingosine-1-phosphate receptor therapies: advances in clinical trials for CNS-related diseases. Neuropharmacology. 2017;113(Pt B):597–607.
Angelopoulou E, Piperi C. Beneficial effects of fingolimod in Alzheimer's disease: molecular mechanisms and therapeutic potential. NeuroMolecular Med. 2019;21(3):227–38.
Fessel J. Reversing Alzheimer's disease dementia with clemastine, fingolimod, or rolipram, plus anti-amyloid therapy. Alzheimers Dement (N Y). 2022;8(1):e12242.
Medeiros R, Castello NA, Cheng D, Kitazawa M, Baglietto-Vargas D, Green KN, et al. alpha7 nicotinic receptor agonist enhances cognition in aged 3xTg-AD mice with robust plaques and tangles. Am J Pathol. 2014;184(2):520–9.
Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362(4):329–44.
D'Andrea MRD, Nagele RG. Targeting the alpha 7 nicotinic acetylcholine receptor to reduce amyloid accumulation in AD pyramidal neurons. Curr Pharm Des. 2006;12:677–84.
Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology. 2004;174(1):54–64.
Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–8.
Nagele RG, D'Andrea MRD, Anderson WJ, Wang HY. Intracellular accumulation of Beta-amyloid1-42 in neurons is facilitated by the Alpha-7 nicotinic acetylcholine receptor in Alzheimer's Disease. Neuroscience. 2002;110(2):199–211.
Wang HY, Lee DH, D'Andrea MR, Peterson PA, Shank RP, Reitz AB. beta-Amyloid(1-42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer's disease pathology. J Biol Chem. 2000;275(8):5626–32.
Wang HY, Lee DH, Davis CB, Shank RP. Amyloid peptide Abeta(1-42) binds selectively and with picomolar affinity to alpha7 nicotinic acetylcholine receptors. J Neurochem. 2000;75(3):1155–61.
Deardorff WJ, Shobassy A, Grossberg GT. Safety and clinical effects of EVP-6124 in subjects with Alzheimer's disease currently or previously receiving an acetylcholinesterase inhibitor medication. Expert Rev Neurother. 2015;15(1):7–17.
Rockwood K, Mintzer J, Truyen L, Wessel T, Wilkinson D. Effects of a flexible galantamine dose in Alzheimer's disease: a randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2001;71(5):589–95.
Aisen P. Memory improvement through nicotine dosing (MIND) study (MIND) ClinicalTrials.gov: University of Southern California 2016 [updated 02/13/2023.
Alzforum. Rivastigmine Alzforum2023 [Available from: https://www.alzforum.org/therapeutics/rivastigmine.
Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43(3):321–32.
Walls KC, Ager RR, Vasilevko V, Cheng D, Medeiros R, LaFerla FM. p-Tau immunotherapy reduces soluble and insoluble tau in aged 3xTg-AD mice. Neurosci Lett. 2014;575:96–100.
Guell-Bosch J, Lope-Piedrafita S, Esquerda-Canals G, Montoliu-Gaya L, Villegas S. Progression of Alzheimer's disease and effect of scFv-h3D6 immunotherapy in the 3xTg-AD mouse model: an in vivo longitudinal study using magnetic resonance imaging and spectroscopy. NMR Biomed. 2020;33(5):e4263.
Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, et al. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine - a novel immunotherapeutic strategy. PLoS One. 2008;3(5):e2124.
Rajamohamedsait H, Rasool S, Rajamohamedsait W, Lin Y, Sigurdsson EM. Prophylactic active tau immunization leads to sustained reduction in both tau and amyloid-beta pathologies in 3xTg mice. Sci Rep. 2017;7(1):17034.
Roda AR, Esquerda-Canals G, Marti-Clua J, Villegas S. Cognitive impairment in the 3xTg-AD mouse model of Alzheimer's disease is affected by abeta-immunotherapy and cognitive stimulation. Pharmaceutics. 2020;12(10)
Roda AR, Montoliu-Gaya L, Serra-Mir G, Villegas S. Both amyloid-beta peptide and tau protein are affected by an anti-amyloid-beta antibody fragment in elderly 3xTg-AD mice. Int J Mol Sci. 2020;21(18)
St-Amour I, Pare I, Tremblay C, Coulombe K, Bazin R, Calon F. IVIg protects the 3xTg-AD mouse model of Alzheimer's disease from memory deficit and Abeta pathology. J Neuroinflammation. 2014;11:54.
Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med. 2012;367(21):2015–25.
Dodel R, Neff F, Noelker C, Pul R, Du Y, Bacher M, et al. Intravenous immunoglobulins as a treatment for Alzheimer's disease: rationale and current evidence. Drugs. 2010;70(5):513–28.
Dodel RC, Du Y, Depboylu C, Hampel H, Frolich L, Haag A, et al. Intravenous immunoglobulins containing antibodies against beta-amyloid for the treatment of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75(10):1472–4.
Szabo P, Relkin N, Weksler ME. Natural human antibodies to amyloid beta peptide. Autoimmun Rev. 2008;7(6):415–20.
Song C, Shi J, Zhang P, Zhang Y, Xu J, Zhao L, et al. Immunotherapy for Alzheimer's disease: targeting beta-amyloid and beyond. Transl Neurodegener. 2022;11(1):18.
Valiukas Z, Ephraim R, Tangalakis K, Davidson M, Apostolopoulos V, Feehan J. Immunotherapies for Alzheimer's disease-a review. Vaccines (Basel). 2022;10(9)
Baglietto-Vargas D, Forner S, Cai L, Martini AC, Trujillo-Estrada L, Swarup V, et al. Generation of a humanized Abeta expressing mouse demonstrating aspects of Alzheimer's disease-like pathology. Nat Commun. 2021;12(1):2421.
Cho JD, Kim YA, Rafikian EE, Yang M, Santa-Maria I. Marked mild cognitive deficits in humanized mouse model of Alzheimer's-type tau pathology. Front Behav Neurosci. 2021;15:634157.
Kshirsagar S, Alvir RV, Hindle A, Kumar S, Vijayan M, Pradeepkiran JA, et al. Early cellular, molecular, morphological and behavioral changes in the humanized amyloid-beta-knock-in mouse model of late-onset Alzheimer's disease. Cells. 2022;11(4)
Souder DC, Dreischmeier IA, Smith AB, Wright S, Martin SA, Sagar MAK, et al. Rhesus monkeys as a translational model for late-onset Alzheimer's disease. Aging Cell. 2021;20(6):e13374.
Miller RA, Harrison DE, Allison DB, Bogue M, Debarba L, Diaz V, et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight. 2020;5(21)
Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–82.
Harrison DE, Strong R, Alavez S, Astle CM, DiGiovanni J, Fernandez E, et al. Acarbose improves health and lifespan in aging HET3 mice. Aging Cell. 2019;18(2):e12898.
Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192.
Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15(5):872–84.
Neuner SM, Heuer SE, Huentelman MJ, O'Connell KMS, Kaczorowski CC. Harnessing genetic complexity to enhance translatability of Alzheimer's disease mouse models: a path toward precision medicine. Neuron. 2019;101(3):399–411.
Acknowledgements
We would like to thank all members of the Lamming lab for their valuable insights and comments.
Funding
The Lamming Laboratory is supported in part by the NIH/National Institute on Aging (AG056771, AG062328 and AG061635 to D.W.L.), NIH/NIDDK (DK125859) and startup funds from the University of Wisconsin-Madison School of Medicine and Public Health and Department of Medicine to D.W.L. M.M.S. was supported in part by a Research Supplement to Promote Diversity in Health-Related Research (3RF1AG056771-06S1). The Lamming laboratory is supported in part by the U.S. Department of Veterans Affairs (I01-BX004031), and this work was supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Contributions
All of the authors contributed to all aspects of the article.
Corresponding author
Ethics declarations
Conflict of interest
D.W.L has received funding from, and is a scientific advisory board member of Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sonsalla, M.M., Lamming, D.W. Geroprotective interventions in the 3xTg mouse model of Alzheimer’s disease. GeroScience 45, 1343–1381 (2023). https://doi.org/10.1007/s11357-023-00782-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00782-w