Abstract
Dipeptidyl peptidase 4 (DPP-4) inhibitors have been shown to have neuroprotective effects in diabetic patients suffering from stroke, but less research has focused on patients with mild hyperglycemia below the threshold for a diagnosis of diabetes. In this investigation, a hyperglycemic mouse model was generated by intraperitoneal injection of streptozotocin and then subjected to focal cerebral ischemia. We demonstrated that the DPP-4 inhibitor linagliptin significantly decreased the infarct volume, reduced neuronal cell death, decreased inflammation, and improved neurological deficit compared with control mice. Linagliptin up-regulated the expression of p-Akt and p-mTOR and regulated the apoptosis factors Bcl-2, Bax, and caspase 9. Taken together, these results suggest that linagliptin exerts a neuroprotective action likely through activation of the Akt/mTOR pathway along with anti-apoptotic and anti-inflammatory mechanisms. Therefore, linagliptin may be considered as a therapeutic treatment for stroke patients with mild hyperglycemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke is a global health concern that leads to lifelong disability or death [1]. In the USA, approximately 795,000 people experience acute first and recurrent ischemic strokes each year [2]. Despite declining stroke mortality rates, the global burden of stroke is increasing. As of 2010, the total expenditure on the disease was $53 billion/year in the USA and is expected to double by 2030 [3]. Over the past 20 years, the understanding of the pathophysiology, diagnosis, and treatment of acute ischemic stroke has improved significantly [4]. Many causes of stroke have been identified, such as high blood pressure, cardiovascular disease, high cholesterol, diabetes, cigarette smoking or exposure to secondhand smoke, and a personal or family history of stroke. More specifically, the prognosis of acute ischemic stroke is affected by multiple factors such as the patient’s glucose level on admission. Retrospective clinical studies have shown that hyperglycemia, an independent risk factor for stroke, is closely associated with the progression, treatment, and prognosis of stroke [5, 6]. Higher admission blood glucose levels are associated with increased morbidity and mortality [7, 8]. Studies have also shown that hyperglycemia increases the area of cerebral infarction in animal models [9,10,11]. This may occur because hyperglycemia contributes to damaging processes such as increased anaerobic metabolism, hyperosmolarity, lactic acidosis, and focal toxicity of ischemic vascular disease [12,13,14,15]. For these reasons, the management of blood glucose levels is especially important in patients with stroke and hyperglycemia.
Hyperglycemia is recognized as an important risk factor for cerebrovascular disease, especially ischemic stroke [16, 17]. Patients with hyperglycemia carry a 1.5–3 times higher risk of stroke compared with the general population [18]. Stroke mortality is also higher and post-stroke outcomes are poorer in patients with hyperglycemia [19]. Most notably, however, clinical studies have found that effective control of blood glucose effectively improves the prognosis of patients with diabetes complicated with stroke.
Inhibitors of dipeptidyl peptidase 4 (DPP-4) are widely used in clinical settings to treat type 2 diabetes [20, 21] due to their ability to effectively reduce blood sugar levels in diabetics. The effect of DPP-4 inhibitors on reducing stroke incidence tends to vary widely, with some studies suggesting no effect on stroke incidence [22,23,24] and others suggesting reduced incidence after DPP-4 inhibitor treatment [25]. Despite this, there is evidence that DPP-4 inhibitor treatment promotes neuroprotective effects in patients with diabetes complicated with stroke [26, 27], suggesting that DPP-4 inhibitors may be useful for stroke recovery in patients with conditions such as hyperglycemia. Thus, this investigation focused on markers of neuroprotection such as cell death and sensorimotor deficit, rather than rates of stroke incidence and death that tend to be the focus of clinical trials [28]. In addition, previous studies failed to recognize patients with hyperglycemia below the threshold for a diagnosis of diabetes. Whether DPP-4 inhibitors also promote neuroprotection in the patients that suffer a stroke is not clear. In this study, we established a hyperglycemic mouse model with stroke to determine the potential efficacy of the DPP-4 inhibitor linagliptin. We further investigated its neuroprotective mechanism.
Materials and Methods
Animals
Adult male C57BL/6 mice were housed in standard cages in 12-h light/12-h dark cycle in the Emory University animal facility where the room temperature was kept at 22°C ± 1°C. All experimental procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Emory University Institutional Animal Care and Use Committee.
Hyperglycemic Mouse Model
All animal experiments and surgical procedures were approved by the Institutional Animal Care and Use Committee at Emory University and met NIH standards. Hyperglycemia was induced in adult male C57BL/6 mice 10–12 weeks old weighing ~ 25 g. Mice were given an intraperitoneal (i.p.) injection of streptozotocin (STZ) dissolved in citrate buffer. All solutions were prepared immediately before use. Controls received i.p. vehicle. After the injections, mice were housed for 2–3 weeks and given food and water ad libitum. Every week, blood glucose levels were measured after a 4-h fast using samples from the tail vein with the FreeStyle glucose meter.
Focal Ischemic Stroke Model
The focal cerebral ischemic stroke model in adult male mice was created as previously described [29]. In summary, mice were anesthetized with 4% chloral hydrate. The distal branches of the right middle cerebral artery were permanently ligated, followed by bilateral occlusion of the common carotid arteries and subsequent reperfusion. During surgery and recovery, body temperature was maintained at 37.0 °C ± 0.5° C using a temperature-controlling ventilator and heating pads.
Quantification of Infarct Volume Using 2,3,5-triphenyltetrazolium Chloride (TTC) Staining
Infarct volume was assessed 3 days after ischemia via TTC staining. Brains were removed and cut into 1-mm coronal slices using a brain matrix. The slices were incubated in 2% TTC (Sigma) at 37°C for 5 min, then stored in 10% buffered formalin for 24 h. A flatbed scanner was then used to capture digital images of the caudal aspect of each slice. Ipsilateral and contralateral hemispheres and infarct areas were measured using ImageJ software (NIH, Bethesda, MD, USA) and infarct volume was calculated using the indirect method [30].
Cell Death Assay Using Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick-End Labeling (TUNEL)
A TUNEL assay kit (DeadEnd Fluorometric TUNEL system; Promega, Madison, WI, USA) was used to assess cell death in 10-μm brain sections according to manufacturer’s instructions. Briefly, sections were fixed in 10% buffered formalin for 10 min and then permeabilized in 0.2% Triton-X 100 after fixation for 5 min in ethanol:acetic acid (2:1). They were incubated in equilibration buffer for 10 min and then incubated in the dark at 37°C for 60 min in recombinant terminal deoxynucleotidyl transferase and nucleotide mixtures. The reaction was stopped by 2× SSC solution for 15 min, and nuclei were then counterstained with DAPI for 5 min.
Immunohistochemical Staining and Cell Counts
Brain cryosections 10 μm thick were dried on a slide warmer for 30 min, fixed in 10% buffered formalin for 10 min, washed with −20°C ethanol:acetic acid (2:1) for 10 min, permeabilized with 0.2% Triton-X 100 and phosphate-buffered saline (PBS) for 5 min, blocked with 1% fish gel (Sigma) and PBS for 60 min, and incubated with the following primary antibodies overnight at 4°C: mouse anti-NeuN (1:400; Chemicon, Temecula, CA) for mature neurons, rabbit anti-glucose transporter 1 (GLUT-1) (1:400, Chemicon, Millipore) for vessels, and rabbit anti-ionized calcium binding adaptor molecule-1(Iba-1) (1:200; Biocare Medical, CA). After rinsing with PBS, the sections were then exposed for 1 h at room temperature to the relevant secondary antibodies: secondary antibody Alexa Fluor 488 anti-rabbit, anti-goat, anti-mouse IgG (1:200; Invitrogen, Carlsbad, CA), cyanine-3 (Cy3)-conjugated anti-rat, anti-rabbit IgG (1:1000; Invitrogen), or Cy5-conjugated anti-goat IgG (1:400; Invitrogen). DAPI was applied at 1:25,000 for 5 min and washed with PBS. Slides were then mounted with Vectashield (Vector Laboratory, Burlingame, CA) for fluorescence microscopy analysis and imaging.
For systematic random sampling in design-based stereological cell counting, 9 sections, each 90 μm apart, in the region of interest were used. For multi-stage random sampling, 6 fields per section were randomly selected in the penumbra [31] at a magnification of 400× under a fluorescence microscope. This was repeated in 6 separate sections per brain.
Western Blotting
The peri-infarct region was defined by a 500-mm boundary extending from the edge of the infarct core, medial and lateral to the infarct, as previously described [32]. Peri-infarct tissue samples of cortex were collected from each group 3 days after ischemia. Proteins were extracted from the tissue by homogenization in protein lysis buffer (in mmol/L: 25 Tris-HCl [pH 7.6], 150 NaCl, 5 EDTA, 2 sodium orthovanadate, 100 NaF, 0.1% SDS, 1% Triton, leupeptin, aprotinin, and pepstatin). Protein from each sample was loaded into the gradient gel and run at a constant current until the protein markers were sufficiently separated. Protein was transferred onto polyvinyl difluoride membranes that were then probed using a standard protocol [33]. The membranes were then blocked with 5% bovine serum albumin for 1 h and incubated overnight at 4°C with primary antibodies. The primary antibodies used and the dilutions for each were mouse β-actin (1: 5000; Sigma-Aldrich, St. Louis, MO), rabbit mTOR (1:500; Santa Cruz Biotech, Inc., Dallas, TX), rabbit AKT (1:1000; Santa Cruz), rabbit TNF-α (1:1000; Cell Signaling, Danvers, MA), rabbit IL-1β (1:1000; Cell Signaling), rabbit IL-6 (1:1000; Cell Signaling), rabbit Bcl-2 (1:1000; Cell Signaling), rabbit Bax (1:1000; Cell Signaling), cleaved caspase-3 (1:1000; Cell Signaling), p-mTOR (1:1000; Santa Cruz) and p-AKT (1:1000; Santa Cruz). After washing in TBST, the membranes were incubated with ammonium persulfate (AP)-conjugated secondary antibodies (GE Healthcare, Piscataway, NJ) for 1 h at room temperature. The signal was developed using nitro-blue tetrazolium and 5-bromo-4-chloro-3’indolyphophate solution. The band intensities were analyzed using ImageJ. The expression of each target protein was then normalized to β-actin.
Local Cerebral Blood Flow (LCBF) Measurement
Laser scanning imaging was used to measure LCBF at 0 day for baseline and at 21 days after ischemia as previously described. Mice were anesthetized and an incision was made to expose the skull above the right middle cerebral artery. The laser was centered on the right coronal suture. Unlike conventional laser Doppler probes which measure small blood flow points, the scanner method used a laser Doppler perfusion imaging system (PeriFlux System 5000-PF5010 LDPM unit, Perimed, Stockholm, Sweden) to measure a square area of 2.4 mm × 2.4 mm [2]. This measurement largely avoided inaccuracies caused by inconsistent positioning of the laser. Laser scanning imaging measurements and analyses of the area were performed using the PeriScans system and LDPIWin programs (Perimed).
Adhesive Removal Test
The adhesive removal test was used to assess sensorimotor deficits after focal cerebral ischemia in mice. In summary, a small adhesive dot was placed on one forepaw, and the number of times to contact and remove the adhesive dot were recorded. Mice were trained in three sessions before surgery to ensure normal sensorimotor function. A recording was discontinued after 120 s of no contact with the adhesive dot. Three trials were performed by the mice in different groups at 3, 7, and 14 days after ischemia and the mean time was calculated for data analysis.
Statistical Analysis
The mice were randomly separated into sham, stroke, hyperglycemia stroke, and linagliptin treatment + hyperglycemia groups. The behavioral tests and data analyses were done double-blind by 2–3 people without knowledge of grouping. GraphPad Prism 6 (GraphPad Software, San Diego, CA) was used for statistical analysis and graphic presentation, and sample sizes were tested with G-Power Analysis (University of Dusseldorf, Dusseldorf, Germany) to yield sufficient statistical power. Student’s t-test was used to compare two groups. Multiple comparisons were done using one-way analysis of variance (ANOVA) followed by Tukey’s test for multiple comparisons. One-way analysis followed by Bonferroni selective comparisons was performed for pairwise comparisons. Data with P ≤ 0.05 were considered significantly different. Data are expressed as the mean ± SEM.
Results
Assessment of the Hyperglycemic Mouse Model
A hyperglycemic mouse model was created in adult male mice by injection of STZ. Two weeks after the injection, animals exhibited significantly elevated levels of blood glucose with respect to age-matched vehicle control mice (Fig. 1A). This hyperglycemic state lasted for at least 3 weeks before we performed ischemic surgery. The results showed that linagliptin treatment (10 mg/kg by gavage) decreased the fasting blood glucose levels (Fig. 1B). However, we did not find that the blood glucose levels were related to the body weight, and linagliptin treatment did not affect the body weight (Fig. 1C and D).
Changes in body weight and fasting blood glucose. A Levels of fasting blood glucose after STZ injection. B Levels of fasting blood glucose after daily linagliptin administration after stroke. C Body weight after STZ injection. D Body weight after daily linagliptin administration after stroke. Values are expressed as the mean ± SEM. *P < 0.05, **P < 0.01.
Linagliptin Reduces Infarct Volume after Ischemic Stroke in Hyperglycemic Mice
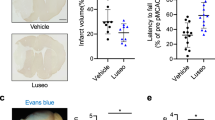
Compared with animals with stroke and normal blood glucose, the hyperglycemic stroke mice showed a significantly larger brain infarct volume (Fig. 2A–C). Linagliptin treatment significantly reduced the infarct volume compared with the stroke control group (Fig. 2A–C).
Linagliptin reduces infarct volume after ischemic stroke in hyperglycemic mice. A TTC staining 3 days after stroke to evaluate infarct formation after stroke. B Linagliptin treatment significantly reduced the indirect infarct volume. C Linagliptin treatment significantly reduced the indirect infarct ratio. Values are expressed as the mean ± SEM. *P < 0.05, **P < 0.01.
Linagliptin Reduces Neuronal Cell Death in Hyperglycemic Mice
To understand the protective mechanism of linagliptin at the cellular level, TUNEL staining in brain tissues harvested 3 days after ischemia was used to determine whether linagliptin treatment affected cell death. Compared with the normal blood glucose group, the hyperglycemic group showed significantly increased numbers of TUNEL-positive cells and TUNEL +/NeuN + co-labeled cells in the penumbra. However, linagliptin treatment significantly reduced the neuronal cell death, as evidenced by a decrease in the number of TUNEL-positive cells double-stained with NeuN, compared with the hyperglycemic stroke control group (P < 0.05) (Fig. 3A–C). In addition, we assessed the protein expression of cleaved caspase-3 in the penumbra 3 days after stroke. The data showed that cleaved caspase-3 significantly increased after stroke. Linagliptin treatment decreased the caspase-3 activation (P < 0.001) (Fig. 3D).
Linagliptin reduces neuronal cell death in hyperglycemic mice. A TUNEL (green), DAPI (blue), and the neuronal marker NeuN (red) staining 3 days after stroke. TUNEL +/NeuN + co-labeled cells indicate dead neurons; TUNEL +/DAPI + co-labeled cells indicate dead cells. B The ratio of TUNEL-positive cells to DAPI-positive (blue) cells. C The ratio of TUNEL +/NeuN + co-labeled cells to total NeuN + cells. D Representative western blots of the protein expression of cleaved caspase-3 in the penumbra 3 days after stroke. Values are expressed as the mean ± SEM. *P < 0.05, **P < 0.01.
Linagliptin Treatment Decreases Apoptotic Cell Death via the Akt/mTOR Pathway
In Western blotting analysis, there were no significant differences in the protein levels of Akt and mTOR between the normal blood glucose and hyperglycemic stroke groups (Fig. 4A–C). The phosphorylated Akt (p-Akt) and phosphorylated mTOR (p-mTOR) proteins, however, were lower in the hyperglycemic stroke group. After treatment with linagliptin, the protein levels were significantly higher (Fig. 4D–F). Compared with the normal blood glucose with stroke group, the Bcl-2 expression in the hyperglycemic group was decreased, and this was prevented by linagliptin treatment (P < 0.05) (Fig. 4G–I). On the other hand, compared with the normal blood glucose and stroke group, a higher level of the pro-apoptotic Bax protein was seen in the hyperglycemic group (P < 0.05). Consistent with an anti-apoptotic action, the Bax expression was suppressed by linagliptin treatment (P < 0.05) (Fig. 4G–I).
Linagliptin treatment affects apoptotic cell death via the Akt/mTOR pathway. A–C The protein expression levels of Akt and mTOR did not significantly differ among groups. D–F Representative western blot images and quantification of the protein expression of p-Akt and p-mTOR in the penumbra region. The levels of p-Akt and p-mTOR significantly increased after treatment with linagliptin. G–I Representative western blot images and quantification of the protein expression of Bcl-2 and Bax. Bcl-2 levels significantly increased and Bax significantly decreased after treatment with linagliptin. In A, D and G, lane 1, sham group; lane 2, stroke + euglycemia group; lane 3, stroke + hyperglycemia group; and lane 4, stroke + hyperglycemia + linagliptin group. Values are expressed as the mean ± SEM. *P < 0.05, **P < 0.01.
Linagliptin Decreases Inflammation in Hyperglycemic Mice
Inflammation and microglial activation play important roles in the pathogenesis of stroke. To measure whether linagliptin influenced the activation of microglia/macrophages, we detected the expression of Iba-1 in the penumbra 3 days after stroke. Compared with the sham group, microglia were significantly activated in the stroke groups. In addition, the number of Iba-1-positive cells was significantly higher in the hyperglycemic group. After treatment with linagliptin, the number of Iba-1-positive cells was significantly decreased (Fig. 5A, B). Some studies have shown that inflammatory factor precursors are released by activated microglia in stroke [34, 35], so we measured the protein expression of inflammatory factors in the penumbra extra 3 days after stroke using western blot. The results showed that the protein levels of the inflammatory factors IL-1β, IL-6, and TNF-α were significantly higher in the stroke groups than in the sham group. After treatment with linagliptin, the IL-1β and IL-6 levels were increased compared with the hyperglycemia group (P < 0.05) (Fig. 5C–E).
Linagliptin reduces inflammation in hyperglycemic mice. A Iba-1 (red) staining shows microglial activation in the penumbra 3 days after stroke. Nuclei were stained using DAPI (blue). B The ratio of Iba-1+/DAPI+ cells in each group. C–E Representative western blot images of the protein expression of IL-1β, IL-6, and TNF-α in the penumbra 3 days after stroke. Inflammatory factors significantly increased after stroke, and this was significantly ameliorated by linagliptin. Values are expressed as the mean ± SEM. *P < 0.05, **P < 0.01.
Linagliptin Promotes Recovery of LCBF after Stroke in Hyperglycemic Mice
Recovery of local blood flow is important for cell survival and tissue repair after stroke. To demonstrate that linagliptin promoted angiogenesis and the production of functional blood vessels, we measured the LCBF using a laser Doppler scanner 21 days after stroke. Scanning imaging showed that mice with stroke that received linagliptin treatment had significantly higher LCBF than the stroke-saline group. At the same time, we found that LCBF in the hyperglycemic group was relatively lower than that of the normal blood glucose group (P < 0.01) (Fig. 6A, B).
Linagliptin promotes LCBF recovery after stroke in hyperglycemic mice. A Local cerebral blood flow (LCBF) in the penumbra 21 days after stroke measured by laser Doppler in each group. B Quantified data showing that animals with stroke that received linagliptin exhibited better LCBF recovery than stroke hyperglycemic animals that did not receive linagliptin. Values are expressed as the mean ± SEM. **P < 0.01.
Linagliptin Promotes Functional Recovery after Stroke in Hyperglycemia Mice
We also evaluated whether linagliptin can improve functional deficits after acute stroke. To specifically assess sensorimotor function, we used the adhesive removal test at 0, 3, 7, and 14 days after stroke. The results showed that both normal blood glucose and hyperglycemic mice took significantly more time to detect and remove sticky dots attached to the left paw after ischemic damage to the sensorimotor cortex in the right hemisphere. Linagliptin-treated animals took less time to detect the dot than stroke control hyperglycemic mice at 7 and 14 days after stroke (P < 0.05) (Fig. 7A). Linagliptin-treated animals were significantly faster in removing the sticky dot than stroke control hyperglycemic animals at 7 and 14 days after stroke (P < 0.05) (Fig. 7B). In addition, there was no significant difference in right paw function.
Linagliptin promotes functional recovery after stroke in hyperglycemic mice. A Linagliptin-treated animals took less time to detect the adhesive than stroke hyperglycemic mice that did not receive linagliptin at 7 and 14 days in the adhesive removal test. B Linagliptin-treated animals were significantly faster in removing the sticky dot than stroke hyperglycemic animals that did not receive linagliptin at 7 and 14 days. Values are expressed as the mean ± SEM. *P < 0.05.
Discussion
In this study, we investigated whether DPP-4 inhibitors are neuroprotective in a rodent stroke model with hyperglycemia. Although previous large studies have shown that DPP-4 inhibitor treatment does not reduce the incidence of stroke in diabetic patients [22,23,24], studying factors that promote functional recovery after stroke remains directly relevant to clinical treatment and therapy. Our study demonstrated the neuroprotective effects of acute linagliptin treatment, explored possible mediating mechanisms via anti-apoptotic and anti-inflammatory actions, and demonstrated the ability of linagliptin to improve functional sensorimotor recovery in a hyperglycemic stroke mouse model. Previous studies have suggested that DPP-4 inhibitors such as alogliptin and linagliptin have beneficial effects after stroke in normal mice [36, 37] and diabetic patients who suffer strokes [26, 27]. However, we investigated the neuroprotective effects of linagliptin on less-studied hyperglycemic mice and explored the unclear mechanism of the Akt/mTOR pathway.
DPP-4 inhibitors block the enzymatic action of dipeptidyl peptidase-4 and increase the levels of glucagon-like peptide-1 (GLP-1), a peptide hormone secreted by cells in the distal gut and central nervous system (CNS), by preventing the degradation of GLP-1 by DPP-4. GLP-1 increases blood sugar levels via direct stimulation of pancreatic β-cells to potentiate insulin secretion [38]. In addition, GLP-1 leads to the feeling of satiety by transmitting signals to the CNS via the gut-to-brain axis [39]. GLP-1 receptors have been found in pancreatic tissue and have also been discovered in tissues outside the pancreas, including the heart, kidneys, and brain [40]. They are widely distributed throughout the CNS, with expression in the thalamus, hypothalamus, cerebellum, hippocampus, cortex, and brainstem [41]. Interestingly, GLP-1 has also been identified as a neuropeptide which can cross the blood-brain barrier [42,43,44].
Recently, the neuroprotective effect of GLP-1 was reported in animal models and clinical work [45,46,47]. The anti-inflammatory effects of GLP-1 have been found in endothelial islets and pancreatic cells [48,49,50]. A previous study showed that stimulating GLP-1 receptors reduces the inflammatory response in a rat model of type 1 diabetes [51]. Here, we evaluated the expression of Iba-1 in the penumbra 3 days after stroke. Immunostaining revealed that the number of Iba-1-positive cells was significantly higher in the penumbra than in a sham group. Compared with the normal blood glucose group, the hyperglycemic group showed that Iba-1-positive cells were significantly increased. However, linagliptin treatment significantly reduced the number of Iba-1-positive cells. In addition, we found that the expression of the inflammatory protein was lower in the linagliptin treatment group than in the hyperglycemic group. A previous study has also shown that GLP-1 might be a modulator of inflammation in the CNS by preventing LPS-induced IL-1β mRNA expression [52]. GLP-1 plays a neuroprotective role by alleviating the inflammatory response in neurodegenerative diseases such as Alzheimer’s and Parkinson’s Disease [45, 53]. Thus, it has been suggested that GLP-1 may be a therapeutic option for targeting inflammation in the brain, although further clinical research is necessary [54, 55]. In addition, we demonstrated that linagliptin improved the recovery of blood flow after stroke, suggesting the mediation of angiogenesis and the preservation of functional blood vessels by DPP-4 inhibitors. This is in line with previous studies demonstrating that linagliptin improves cerebrovascular function and reverses diabetes-mediated cerebral artery remodeling in diabetic rats [56, 57] and provides another important avenue for future investigations of the use of linagliptin treatment for hyperglycemic stroke patients.
In this study, we observed a significant reduction in neuronal apoptosis in the linagliptin-treated group, suggesting that linagliptin may be involved in regulating the apoptotic pathway. A previous study in a mouse model of stroke with diabetes showed significantly increased expression levels of GLP-1 in peripheral blood and significantly reduced ischemic area of brain injury after linagliptin treatment [58]. In vitro experiments revealed that GLP-1 promoted the survival and proliferation of neurons and prevented apoptosis and oxidative stress-related cell death in hippocampal cells and glia [59]. GLP-1 mediates neuronal proliferation through the activation of protein kinase A and phosphoinositide 3-kinase, and regulates the apoptotic signaling pathway through decreased Bax and caspase-3 and increased Bcl-2 to enhance cell survival [60,61,62,63]. In our study, we found that linagliptin reduced neuronal death in hyperglycemic mice. It is possible that linagliptin might play a neuroprotective role by suppressing serum GLP-1 degradation and activating GLP-1-induced Akt/mTOR signaling pathways. Previous studies have also reported that GLP-1 protects against amyloid-induced neuronal apoptosis via the cAMP signaling pathway and oxidative stress-induced apoptosis via the MAPK pathway [64, 65]. Our data showed that linagliptin upregulated the anti-apoptotic protein Bcl-2 and downregulated the pro-apoptotic protein Bax. Linagliptin had an inhibitory effect on the cleavage of caspase-3. The Bcl-2 family contains two classes of protein: pro-apoptotic (such as Bax, Bak, Bok, and BAD) and anti-apoptotic proteins (such as Bcl-w, Bcl-2, and Bcl-xL) [66]. The Bcl-2 protein family constitutes a complex of interactions involved in the regulation of apoptosis. The response of cells to apoptotic signals is determined by the relative expression levels of apoptosis-inducing genes and apoptosis-inhibiting genes. When the expression of Bcl-2 is excessive, apoptosis is inhibited; when Bax is overexpressed, apoptosis is promoted; thus, the ratio of Bcl-2 to Bax is very important. In general, a higher ratio of Bcl-2:Bax protein expression is essential for cell survival and the reverse is essential for apoptosis. For example, after cerebral ischemia, both Bax and Bcl-2 mRNA levels are increased in the penumbra. However, Bax is more abundant than Bcl-2, so the former is the key cause of ischemia-induced cell death [67]. Our further experiments demonstrated that the effects of linagliptin might be mediated through the upregulation of AKT/mTOR signal pathway proteins. Although mTOR is a key regulator of cell growth and proliferation [68, 69], it is also involved in the regulation of apoptosis in a variety of cells [70,71,72]. Many factors lead to apoptosis, such as oxidative stress. Previous studies by Holville and Green suggest that the oxidative stress-induced apoptosis pathways are the Fas, P53/MPTP, NF-kB, and JNK pathways [73, 74]. In this investigation, we simply explored a possible mechanism in one pathway, which leaves the remaining pathways as interesting targets for future investigations.
In summary, the DPP-4 inhibitor linagliptin reduced neuronal death in hyperglycemic mice, and this may be mediated via suppression of the degradation of serum GLP-1 and the activation of GLP-1-induced Akt/mTOR signaling pathways. We also demonstrated that linagliptin improves tolerance to inflammatory processes. With regard to long-term recovery, linagliptin induced significantly higher LCBF and also promoted functional recovery after stroke in hyperglycemic mice compared to the stroke-saline group. As a result, the DPP-4 inhibitor linagliptin holds future translational potential in stroke patients with hyperglycemia by promoting functional recovery in stroke survivors.
References
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019, 139: e56–e66.
Guzik A, Bushnell C. Stroke epidemiology and risk factor management. Continuum (Minneap Minn) 2017, 23: 15–39.
Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011, 123: 933–944.
National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995, 333: 1581–1587.
Jeremitsky E, Omert LA, Dunham CM, Wilberger J, Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J Trauma 2005, 58: 47–50.
Juvela S, Siironen J, Kuhmonen J. Hyperglycemia, excess weight, and history of hypertension as risk factors for poor outcome and cerebral infarction after aneurysmal subarachnoid hemorrhage. J Neurosurg 2005, 102: 998–1003.
Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001, 32: 2426–2432.
Demchuk AM, Tanne D, Hill MD, Kasner SE, Hanson S, Grond M, et al. Predictors of good outcome after intravenous tPA for acute ischemic stroke. Neurology 2001, 57: 474–480.
de Courten-Myers G, Myers RE, Schoolfield L. Hyperglycemia enlarges infarct size in cerebrovascular occlusion in cats. Stroke 1988, 19: 623–630.
Nedergaard M. Transient focal ischemia in hyperglycemic rats is associated with increased cerebral infarction. Brain Res 1987, 408: 79–85.
Prado R, Ginsberg MD, Dietrich WD, Watson BD, Busto R. Hyperglycemia increases infarct size in collaterally perfused but not end-arterial vascular territories. J Cereb Blood Flow Metab 1988, 8: 186–192.
Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol 2002, 52: 20–28.
Els T, Klisch J, Orszagh M, Hetzel A, Schulte-Monting J, Schumacher M, et al. Hyperglycemia in patients with focal cerebral ischemia after intravenous thrombolysis: influence on clinical outcome and infarct size. Cerebrovasc Dis 2002, 13: 89–94.
Ribo M, Molina CA, Delgado P, Rubiera M, Delgado-Mederos R, Rovira A, et al. Hyperglycemia during ischemia rapidly accelerates brain damage in stroke patients treated with tPA. J Cereb Blood Flow Metab 2007, 27: 1616–1622.
Baird TA, Parsons MW, Phan T, Butcher KS, Desmond PM, Tress BM, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke 2003, 34: 2208–2214.
Gray CS, Hildreth AJ, Sandercock PA, O’Connell JE, Johnston DE, Cartlidge NE, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol 2007, 6: 397–406.
Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab 2007, 27: 435–451.
Sander D, Kearney MT. Reducing the risk of stroke in type 2 diabetes: pathophysiological and therapeutic perspectives. J Neurol 2009, 256: 1603–1619.
Idris I, Thomson GA, Sharma JC. Diabetes mellitus and stroke. Int J Clin Pract 2006, 60: 48–56.
Yehya A, Sadhu AR. New therapeutic strategies for type 2 diabetes (CME). Methodist Debakey Cardiovasc J 2018, 14: 281–288.
Cahn A, Cernea S, Raz I. An update on DPP-4 inhibitors in the management of type 2 diabetes. Expert Opin Emerg Drugs 2016, 21: 409–419.
Zhang Z, Chen X, Lu P, Zhang J, Xu Y, He W, et al. Incretin-based agents in type 2 diabetic patients at cardiovascular risk: compare the effect of GLP-1 agonists and DPP-4 inhibitors on cardiovascular and pancreatic outcomes. Cardiovasc Diabetol 2017, 16: 31.
Li YR, Tsai SS, Chen DY, Chen ST, Sun JH, Chang HY, et al. Linagliptin and cardiovascular outcomes in type 2 diabetes after acute coronary syndrome or acute ischemic stroke. Cardiovasc Diabetol 2018, 17: 2.
Barkas F, Elisaf M, Tsimihodimos V, Milionis H. Dipeptidyl peptidase-4 inhibitors and protection against stroke: A systematic review and meta-analysis. Diabetes Metab 2017, 43: 1–8.
Gallwitz B. Emerging DPP-4 inhibitors: focus on linagliptin for type 2 diabetes. Diabetes Metab Syndr Obes 2013, 6: 1–9.
Darsalia V, Klein T, Nystrom T, Patrone C. Glucagon-like receptor 1 agonists and DPP-4 inhibitors: Anti-diabetic drugs with anti-stroke potential. Neuropharmacology 2018, 136: 280–286.
Magkou D, Tziomalos K. Antidiabetic treatment, stroke severity and outcome. World J Diabetes 2014, 5: 84–88.
Darsalia V, Larsson M, Klein T, Patrone C. The high need for trials assessing functional outcome after stroke rather than stroke prevention with GLP-1 agonists and DPP-4 inhibitors. Cardiovasc Diabetol 2018, 17: 32.
Wei L, Cui L, Snider BJ, Rivkin M, Yu SS, Lee CS, et al. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis 2005, 19: 183–193.
Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 1990, 10: 290–293.
Whitaker VR, Cui L, Miller S, Yu SP, Wei L. Whisker stimulation enhances angiogenesis in the barrel cortex following focal ischemia in mice. J Cereb Blood Flow Metab 2007, 27: 57–68.
Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci 2006, 26: 13007–13016.
Choi KE, Hall CL, Sun JM, Wei L, Mohamad O, Dix TA, et al. A novel stroke therapy of pharmacologically induced hypothermia after focal cerebral ischemia in mice. FASEB J 2012, 26: 2799–2810.
Zhao SC, Ma LS, Chu ZH, Xu H, Wu WQ, Liu F. Regulation of microglial activation in stroke. Acta Pharmacol Sin 2017, 38: 445–458.
Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol 2006, 147 Suppl 1: S232–240.
Chiazza F, Tammen H, Pintana H, Lietzau G, Collino M, Nystrom T, et al. The effect of DPP-4 inhibition to improve functional outcome after stroke is mediated by the SDF-1alpha/CXCR4 pathway. Cardiovasc Diabetol 2018, 17: 60.
Yang D, Nakajo Y, Iihara K, Kataoka H, Yanamoto H. Alogliptin, a dipeptidylpeptidase-4 inhibitor, for patients with diabetes mellitus type 2, induces tolerance to focal cerebral ischemia in non-diabetic, normal mice. Brain Res 2013, 1517: 104–113.
Drucker DJ. The biology of incretin hormones. Cell Metab 2006, 3: 153–165.
Burcelin R, Serino M, Cabou C. A role for the gut-to-brain GLP-1-dependent axis in the control of metabolism. Curr Opin Pharmacol 2009, 9: 744–752.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007, 87: 1409–1439.
Alvarez E, Martinez MD, Roncero I, Chowen JA, Garcia-Cuartero B, Gispert JD, et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem 2005, 92: 798–806.
Larsen PJ, Holst JJ. Glucagon-related peptide 1 (GLP-1): hormone and neurotransmitter. Regul Pept 2005, 128: 97–107.
Trapp S, Richards JE. The gut hormone glucagon-like peptide-1 produced in brain: is this physiologically relevant? Curr Opin Pharmacol 2013, 13: 964–969.
Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci 2002, 18: 7–14.
Zhang L, Li L, Holscher C. Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson’s disease mouse model. Neuropeptides 2018, 71: 70–80.
Yang JL, Chen WY, Chen YP, Kuo CY, Chen SD. Activation of GLP-1 receptor enhances neuronal base excision repair via PI3 K-AKT-induced expression of apurinic/apyrimidinic endonuclease 1. Theranostics 2016, 6: 2015–2027.
Chen F, Wang W, Ding H, Yang Q, Dong Q, Cui M. The glucagon-like peptide-1 receptor agonist exendin-4 ameliorates warfarin-associated hemorrhagic transformation after cerebral ischemia. J Neuroinflammation 2016, 13: 204.
Cechin SR, Perez-Alvarez I, Fenjves E, Molano RD, Pileggi A, Berggren PO, et al. Anti-inflammatory properties of exenatide in human pancreatic islets. Cell Transplant 2012, 21: 633–648.
Krasner NM, Ido Y, Ruderman NB, Cacicedo JM. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS One 2014, 9: e97554.
Ceriello A, Novials A, Ortega E, Canivell S, La Sala L, Pujadas G, et al. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care 2013, 36: 2346–2350.
Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011, 54: 965–978.
Iwai T, Ito S, Tanimitsu K, Udagawa S, Oka J. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res 2006, 55: 352–360.
Holscher C. Novel dual GLP-1/GIP receptor agonists show neuroprotective effects in Alzheimer’s and Parkinson’s disease models. Neuropharmacology 2018, 136: 251–259.
Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today 2016, 21: 802–818.
Candeias EM, Sebastiao IC, Cardoso SM, Correia SC, Carvalho CI, Placido AI, et al. Gut-brain connection: The neuroprotective effects of the anti-diabetic drug liraglutide. World J Diabetes 2015, 6: 807–827.
Hardigan T, Yasir A, Abdelsaid M, Coucha M, El-Shaffey S, Li W, et al. Linagliptin treatment improves cerebrovascular function and remodeling and restores reduced cerebral perfusion in Type 2 diabetes. Am J Physiol Regul Integr Comp Physiol 2016, 311: R466–477.
Yasir A, Hardigan T, Ergul A. Diabetes-mediated middle cerebral artery remodeling is restored by linagliptin: Interaction with the vascular smooth muscle cell endothelin system. Life Sci 2016, 159: 76–82.
Darsalia V, Ortsater H, Olverling A, Darlof E, Wolbert P, Nystrom T, et al. The DPP-4 inhibitor linagliptin counteracts stroke in the normal and diabetic mouse brain: a comparison with glimepiride. Diabetes 2013, 62: 1289–1296.
Velmurugan K, Bouchard R, Mahaffey G, Pugazhenthi S. Neuroprotective actions of glucagon-like peptide-1 in differentiated human neuroprogenitor cells. J Neurochem 2012, 123: 919–931.
Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther 2002, 302: 881–888.
Holst JJ, Burcelin R, Nathanson E. Neuroprotective properties of GLP-1: theoretical and practical applications. Curr Med Res Opin 2011, 27: 547–558.
Liu WJ, Jin HY, Lee KA, Xie SH, Baek HS, Park TS. Neuroprotective effect of the glucagon-like peptide-1 receptor agonist, synthetic exendin-4, in streptozotocin-induced diabetic rats. Br J Pharmacol 2011, 164: 1410–1420.
Li Y, Tweedie D, Mattson MP, Holloway HW, Greig NH. Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J Neurochem 2010, 113: 1621–1631.
Qin Z, Sun Z, Huang J, Hu Y, Wu Z, Mei B. Mutated recombinant human glucagon-like peptide-1 protects SH-SY5Y cells from apoptosis induced by amyloid-beta peptide (1-42). Neurosci Lett 2008, 444: 217–221.
Liu J, Yin F, Zheng X, Jing J, Hu Y. Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway. Neurochem Int 2007, 51: 361–369.
Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 2009, 122: 437–441.
Lin CH, Lu YZ, Cheng FC, Chu LF, Hsueh CM. Bax-regulated mitochondria-mediated apoptosis is responsible for the in vitro ischemia induced neuronal cell death of Sprague Dawley rat. Neurosci Lett 2005, 387: 22–27.
Carter AJ. TOR of the cell cycle: Are there important implications for diabetics in the era of the drug-eluting stent? Catheter Cardiovasc Interv 2004, 61: 233–236.
Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol 2005, 17: 596–603.
Lieberthal W, Fuhro R, Andry CC, Rennke H, Abernathy VE, Koh JS, et al. Rapamycin impairs recovery from acute renal failure: role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Renal Physiol 2001, 281: F693–706.
Woltman AM, de Fijter JW, Kamerling SW, van Der Kooij SW, Paul LC, Daha MR, et al. Rapamycin induces apoptosis in monocyte- and CD34-derived dendritic cells but not in monocytes and macrophages. Blood 2001, 98: 174–180.
Wu X, Reiter CE, Antonetti DA, Kimball SR, Jefferson LS, Gardner TW. Insulin promotes rat retinal neuronal cell survival in a p70S6 K-dependent manner. J Biol Chem 2004, 279: 9167–9175.
Hollville E, Romero SE, Deshmukh M. Apoptotic cell death regulation in neurons. FEBS J 2019, 286: 3276–3298.
Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol 2015. https://doi.org/10.1101/cshperspect.a006080
Acknowledgements
This work was supported by the John E. Steinhaus Endowment fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Rights and permissions
About this article
Cite this article
Zhang, G., Kim, S., Gu, X. et al. DPP-4 Inhibitor Linagliptin is Neuroprotective in Hyperglycemic Mice with Stroke via the AKT/mTOR Pathway and Anti-apoptotic Effects. Neurosci. Bull. 36, 407–418 (2020). https://doi.org/10.1007/s12264-019-00446-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-019-00446-w