Abstract
Cadmium is toxic to plants. The accumulation of cadmium in edible plants such as muskmelon may affect the safe production of crops and result in human health problem. Thus effective measures are urgently needed for soil remediation. This work aims to investigate the effects of nano-ferric oxide and biochar alone or mixture on muskmelon under cadmium stress. The results of growth and physiological indexes showed that compared with the application of cadmium alone, the composite treatment (biochar and nano-ferric oxide) decreased malondialdehyde content by 59.12% and ascorbate peroxidase activity increased by 276.6%. Their addition can increase the stress resistance of plants. The results of soil analysis and cadmium content determination in plants showed that the composite treatment was beneficial to reduce the cadmium content in various parts of muskmelon. In the presence of high concentration of cadmium, the Target Hazard Quotient value of peel and flesh of muskmelon in the composite treatment was less than 1, which means the edible risk was greatly reduced. Furthermore, the addition of composite treatment increased the content of effective components; the contents of polyphenols, flavonoids, and saponins in the flesh of the compound treatment were increased by 99.73%, 143.07%, and 18.78% compared with the cadmium treatment. The results provide a technical reference for the further application of biochar combined with nano-ferric oxide in the field of soil heavy metal remediation, and provide a theoretical basis for further research on reducing the toxicity of cadmium to plants and improving the edible quality of crops.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Muskmelon, as one of the most important types of fruit in the world, has both nutritional and medicinal value, and is in huge demand. With extensive research, it is found that muskmelons are edible not only in flesh, but also in peel and seeds, and there are high levels of bioactive components in the peels and seeds, such as polyphenols and flavonoids (Fundo et al. 2018). Therefore, as a source of compounds in food and health products, the value of muskmelon peel and seed has attracted attention and reuse has been encouraged. The rational utilization of flesh, peel, and seed resources of muskmelon fruit is of great significance for the sustainable development of related agricultural food and industrial sectors (Vella et al. 2019).
However, agricultural soil contamination with cadmium (Cd) is a serious environmental problem that poses a challenge to crop production and threatens human health. As the industrialization and urbanization processes accelerate (Bashir et al. 2018), the global annual Cd consumption has reached 25,000 metric tons. Currently, more than 280,000 ha of agricultural land in China is polluted by Cd (Wan et al. 2018). As a non-essential toxic element, Cd has a long biological half-life (more than 10 years in the human body) and is unbiodegradable (Freddie et al. 2018; Karadas and Kara 2012). It not only affects the normal growth of plants, but also easily enters the human body along the food chain, which poses a threat to human health (Das et al. 1997; Luo et al. 2011; Clemens and Ma 2016). Therefore, as a major source of Cd intake for humans, the accumulation of Cd in food crops has received widespread attention (Clemens and Ma 2016; Rasafi et al. 2020; Song et al. 2017). It is of great significance to study the mechanism of Cd absorption, transport and regulation in plants, especially the redistribution of Cd in the aboveground part of plants, and find appropriate strategies to ensure the normal survival of muskmelon plants exposed to Cd, reduce their food safety risk and produce high-quality fruits.
In recent years, the study of adding exogenous substances to alleviate the poisoning of plants by Cd has been extensive, including the addition of soil amendments, the application of plant hormones, the inoculation of strains, and so on (Zhang et al. 2019; Ran et al. 2020; Sangsuwan and Prapagdee 2021; Halim et al. 2020). Among them, the Cd toxicity mitigation methods based on soil improvement, especially the addition of nanomaterials, are highly feasible and have received extensive attention in the in situ remediation of heavy metal pollution in farmland (Wang et al. 2021a; Hao et al. 2021). As fertilizer, nanoparticles (NPs) can not only effectively reduce nutrient loss during fertilization, but also improve nutrient absorption of plants (Tan et al. 2020). For example, the application of ZnO NPs and Si NPs can significantly increase wheat yield and reduce the accumulation of Cd in wheat grains (Khan et al. 2019; Ali et al. 2019).
Nano-ferric oxide has the characteristics of high surface activity, which is widely used in alleviating heavy metal stress of plants in contaminated soil (Gillispie et al. 2019; Lin et al. 2019). Previous studies demonstrated that Fe2O3 NPs can replace traditional Ferrum (Fe) fertilizers in the cultivation of peanut (Arachis hypogaea) (Rui et al. 2016). A study by Wang et al. (2021b) revealed that nano-ferric oxide significantly reduced the soil available Cd content, and it has low metal toxicity and little effect on soil microorganisms and their related functions (Shah et al. 2022). Its potential environmental toxicities to bioluminescence activity, seed germination, and bacterial gene mutation are less than that of CuO NPs, NiO NPs, and ZnO NPs and so on (Kyung-Seok and In Chul 2014). However, in addition to these advantages, metal oxide nanoparticles have disadvantages such as poor stability, easy aggregation, and passivation, which reduce its reactivity and limit its application (Rodriguez et al. 2019). Solid-phase loading is a common modification method, and a solid matrix can prevent nanoparticle aggregation by masking dipole interactions (Magdalena et al. 2016). Biochar, a porous carbon material, is a common supported substrate, which is a highly aromatic carbonaceous material produced by the pyrolysis of biomass under complete or partial anoxic conditions. Biochar has low cost, low environmental risk, high stability (Kumar et al. 2018), and its large specific surface area is conducive to the effective loading of nanoparticles, and abundant surface functional groups (such as -OH, -COOH, C-O/C = O) can play a role in dispersion and stabilizing nanoparticles, thereby overcoming the defects of metal oxide nanoparticles aggregation (Liu et al. 2020, 2015). For example, the combination of zerovalent iron and biochar can improve the absorption efficiency of heavy metals (Zhu et al. 2017), the coupling of porous biochar with nano-zerovalent iron/nano-α-iron oxyhydroxide can improve the remediation efficiency of Cd in aqueous solution (Zhu et al. 2018). At the same time, both biochar and nano-ferric oxide can reduce the Cd toxicity of plants and promote plant growth (Michalkova et al. 2014; Lu et al. 2014; Zhou et al. 2022), so it is possible to synergistically reduce plant Cd stress.
Previous studies mostly applied a single material (Manori et al. 2021; Lu et al. 2014; Yu et al. 2019; Zheng et al. 2013; Zhen et al. 2018), and the research on the composite use of biochar and nano-ferric oxide is less. The research on the existing composites mainly focused on the interaction between remediation agents and their interaction with soil components or heavy metal pollutants, with limited attention to plant growth (Liu et al. 2020, 2015). The effects of biochar and nano-ferric oxide composites on plant growth under Cd stress have been neglected.
Most of the schemes to alleviate Cd stress are aimed at the effects of Cd accumulation and antioxidant activity in roots, stems, and leaves during plant growth, while there are few studies on the changes of active components in various parts during the whole growth period of plants and fruits (Ullah et al. 2019; Zhang et al. 2015). For muskmelon plants containing edible parts, the study of methods to improve their Cd tolerance should start from the changes of indicators in various parts of the whole growth process, and find feasible solutions without affecting or even increasing the content of active ingredients.
In this study, the changes of growth physiology, Cd content, active components in each part of muskmelon under different treatments were investigated. The mechanism of Cd uptake, transport, and accumulation in plants under extreme Cd stress was discussed, and the transport and mitigation mechanisms of Cd stress were revealed. The potentially feasible schemes to reduce Cd toxicity and improve the edible ability of muskmelon fruits was determined. It provides a theoretical basis and feasible way to promote the efficient utilization of muskmelon crops.
Materials and methods
Experimental materials and treatments applied
The muskmelon (Cucumis melon L.) seeds 2021CTB411 were purchased from Hubei Academy of Agricultural Sciences. CdCl2·2.5H2O (99.0% in purity) was purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd, (Shanghai, China). The nano-ferric oxide were purchased from Macklin Company. Biochar was prepared using rice straw at 500 ℃ for 5 h and then crushed to pass through a 40-mesh sieve. The scanning electron microscope (SEM) image and X-ray diffraction (XRD) pattern of nano-ferric oxide and biochar are shown in Figure S1 and S2.
The experiments were conducted in Hubei Academy of Agricultural Sciences from February 4, 2021, to June 29, 2021. The experiment lasted for the whole growth period of muskmelon. In order to make the experimental phenomenon more obvious and to explore the related mechanism of plant production under extreme Cd stress, we used a higher concentration of Cd in the experiment. Five experimental treatments were designed: CK, Cd (400 mg/kg Cd), B + Cd (1% biochar and 400 mg/kg Cd), Fe + Cd (250 mg/kg nano-ferric oxide and 400 mg/kg Cd), B + Fe + Cd (1% biochar, 250 mg/kg nano-ferric oxide and 400 mg/kg Cd). Physical agitation was used to distribute these treatments evenly in the soil. After treating, let it stand still for a week. Fifteen muskmelon seedlings were in each treatment with a total of 75 pots. The protective row was added around the planting to minimize experimental error.
The seeds germinate in deionized water. Then transferred to the orifice plate culture, in the greenhouse of Hubei Academy of Agricultural Sciences natural conditions to grow, water once every three days, until reached the three-leaf stage. Subsequently, the seedlings were individually transplanted in 2.5 kg of matrix soil in planting pots. After transplanting, the muskmelon plants will go through the process of seedling stage, vine elongation stage, blooming stage and fruiting stage. All the plants were cultivated through surface irrigation with day and night temperatures of 23 ~ 27 °C and 15 ~ 20 °C, respectively. During these stages, the soil moisture content was kept at 60 ~ 65% during the growing period and 70 ~ 80% after the blooming stage. The flowers were pollinated by artificial method in the blooming stage. Muskmelons need about 100 days from seedling to fruit maturity, which is called a whole growth period.
Sample collection and pretreatment
Collection of soil samples
The soil samples were collected by five-point sampling method. The details are as follows: after sufficient physical mixing of the soil, the center of the planting pots and the center of the four sides of the planting pot’s inner square are taken as sampling points. The soil samples were taken from 7.5 to 10 cm depth and stored in a 4 ℃ refrigerator.
Collection and pretreatment of plant samples (roots, stems, leaves, and fruits)
At the vine elongation stage, fresh leaves were collected at the 12th knot of each muskmelon plant for the detection of hysiological and biochemical indexes. At the fruiting stage, samples were collected from various parts of the muskmelon plant. Roots were washed with EDTA (ethylene diamine tetraacetic acid), and stems, leaves, and fruits were gently washed with deionized water to remove attachments and dry surfaces. Fresh samples of roots and leaves were divided into two parts. The fresh samples of roots and leaves were divided into two parts. One part was used for the determination of subcellular distribution of Cd, and the other part was dried in an oven at 80 ℃ to constant weight with the stems. Under laboratory conditions, fruit parts were manually divided into peels, seeds and flesh, sliced and dried to constant weight at 80 ℃. The dried samples (roots, stems, leaves, peels, seeds, and flesh) were ground into powder with a grinder, labeled, and stored in a refrigerator at − 20 ℃.
Determination of total Cd and effective Cd content
Soil samples were separated into 2 parts to extract the total Cd (acid-digested) and effective Cd (DTPA-extractable) according to the methods described by John M K and Faust (John et al. 1972; Faust et al. 2000).The total Cd and effective Cd concentrations were determined by Flame Atomic Absorption Spectroscopy (FAAS) with the detection limit of Cd at 0.01 mg/kg. The specific steps of acid-digestion are as follows: take 0.1 g powder sample into centrifugal tube, add 1 mL concentrated nitric acid, place at room temperature for 24 h. Heat at 120 ℃ for 1 h until the liquid is clear and free of floccules. Add 0.5 mL 30% peroxide hydrogen, heat at 120 ℃ for 0.5 h to decolorize.
Plant analysis
Measurement of morphological indexes
The plant height, the distance between the highest point reached by stems and the ground, was measured by rolling ruler. The length and width of the leaves (fully unfolded and undamaged) were measured with a ruler. Measure stem thickness with vernier caliper.
Detection of hysiological and biochemical indexes
The fresh muskmelon leaves were used to determine the following indexes. The chlorophyll content was determined by the SPAD-502 Plus chlorophyll meter (Konica Minolta). The malondialdehyde (MDA) content was determined to express oxidative damage to lipids by the method of nitro blue tetrazolium (NBT) photoreduction (Xu et al. 2008).The activity of superoxide dismutase (SOD) was detected by NBT method as Giannopolites and Ries (1977) et al. suggested. The activity of catalase (CAT) is indirectly determined by measuring the consumption of H2O2 when it exists (Aebi 1984). Peroxidase (POD) activity was analyzed according to Kochba et al. (1977). Ascorbate peroxidase (APX) activity was determined by ascorbic acid difference method (Arrigoni et al. 1992).
Determination of Cd and Fe content in plant
According to Turkekul et al. (2004), the Cd and Fe concentration in different parts of the plant was determined by FAAS after acid digestion. The Cd and Fe content in plants were calculated on dry weight (DW) basis.
Extraction and content detection of active ingredients
Active ingredients were extracted at 60 ℃, solid–liquid ratio 1: 40 (powder sample 0.4 g, 80% ethanol 16 mL) for 5 h, filtered to obtain the extract. The content of polysaccharides, flavonoids, saponins, and polyphenol was measured according to previous research (Guo et al. 2010; Cheng et al. 2013; Peng et al. 2006; Turkmen et al. 2006).
Determination of subcellular distribution of Cd
Subcellular components in plant tissues were separated in differential centrifugation technique (Wei et al. 2021). In the brief, fresh root and leaf samples (2.0 g) were cut and grinded with pre-cooling extraction buffer (50 mM Tris–HCl pH 7.5, 250 mM sucrose, 1 mM dithioerythritol), fixed volume to 20 mL, centrifuged at 3000 r / min for 15 min, and precipitated as cell wall component (FI). The supernatant was centrifuged at 10 000 r / min for 30 min, and precipitated as organelle component (FII). The supernatant was FIII, containing macromolecules and macromolecule organic matter and inorganic ions in cytoplasm and vacuole. All operations above are performed at 4 ℃. The FI, FII, and FIII components were dried in oven at 60 ℃ and digested to determine Cd content by atomic absorption spectroscopy.
Calculation of the bio-accumulation factor and the translocation factor
Cd contents in plant tissues and soil were used to calculate the bio-accumulation factor (BAF) and translocation factor (TF). The TF (from root to stem and from stem to leaf) is the ratio of Cd concentration in later tissue to that in former tissue, which can be expressed as TFa-b = Cb/Ca, where a and b represent muskmelon tissue (root, stem, leaf) (Mattina et al. 2003). The BAF is calculated by dividing Cd concentration in edible parts by the effective Cd concentration in the soil surrounding the plant root zone, i.e., BAF = Cplant/Csoil, which can be used to estimate the metal accumulation capacity of edible parts of vegetables (Khaokaew and Landrot 2015).
Health risk assessment
To assess the potential health risk of Cd intake during muskmelon consumption, we used the Target Hazard Quotient (THQ) model. The THQ was described in detail by the United States Environmental Protection Agency, and we have adjusted it to some extent (Storelli 2008; Wang et al. 2005). The THQ is given by the following formula:
- E F :
-
the exposure frequency (15 days/year);
- E D :
-
the exposure duration (70 years, equivalent to the average lifetime of the Chinese population);
- F IR :
-
the food ingestion rate (Taking into account the actual consumption of muskmelon, muskmelon consumption values was set at 150 g/person/day) (Wang et al. 2005);
- C :
-
the metal concentration in the edible parts of vegetables (mg/kg);
- R FD :
-
the oral reference dose (Cd value was 0.001 mg/kg/day) (Bortey-Sam 2015; Zhou et al. 2016);
- W AB :
-
the average body weight (55.9 kg for adults) (Wang et al. 2005);
- T A :
-
the average exposure time for non-carcinogens (EDˆ365 days/year).
If THQ < 1, it indicates that the health risk to the exposed population is acceptable; otherwise, the exposure is likely to cause obvious adverse effects.
Statistical analysis
In order to determine the significant differences between the treatments (p < 0.05), the method of Duncan test in IBM SPSS Statistics 23.0 was used for analysis of variance. The results are reported as mean ± standard deviation (SD) from data collected at least in triplicate. OriginPro 9.1 is used for drawing. Two principal components were extracted from the detection index results, and the principal component analysis (PCA) of the differences between treatments was performed, and its visualization was completed by R studio.
Results
Effects of different treatments on muskmelon growth
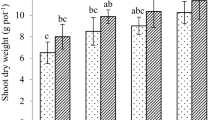
Regardless of whether it was treated with Cd alone or treated with additional biochar or/and nano-ferric oxide, the growth conditions of the muskmelon plants in the plant height, stem thickness, leaf length, leaf width, etc. were not significantly different from those of the control group (Table 1). From the observation of fruit characteristic indexes, compared with the control group, the weight, vertical diameter, transverse diameter and flesh thickness of the fruit in the Cd treatment were decreased. Although the combined treatment could not completely restore these characteristic indexes of the fruit to the level of the control group, it improved the vertical diameter and flesh thickness of the fruit than Cd treatment (Table 1).
Physiological and biochemical differences of muskmelon under different treatments
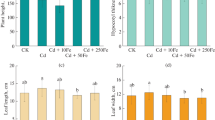
Decreased chlorophyll biosynthesis is one of the toxic manifestations of Cd. As shown in Fig. 1 A, Cd stress significantly reduced the chlorophyll content in muskmelon leaves compared to control. Relative to control, the chlorophyll content of the muskmelon treated with Cd alone was reduced by 14.21%. Compared with simple Cd stress, the chlorophyll content of the treatment added with biochar or/and nano-ferric oxide increased to a certain extent, but the effect was not significant.
As an important index of membrane lipid peroxidation, the content of MDA indirectly reflects the damage of cell membrane. As shown in Fig. 1 B, the MDA content in leaves of Cd treated group was the highest, which was 49.49% higher than that of control group. The presence of biochar decreased the MDA content to the same level as the control group. Compared with Cd group, the MDA content decreased by 59.12% after the addition of biochar and nano-ferric oxide. However, the increase of MDA under Cd stress was not significantly relieved by adding nano-ferric oxide alone.
It can be seen from Fig. 2 that compared with the control group, the SOD, APX, and POD activities of muskmelon leaves in the Cd treatment were decreased by 14.44%, 78.11%, and 55.29%, respectively. Compared with Cd-alone treatment, adding biochar or/and nano-ferric oxide had no significant effect on SOD activity in muskmelon leaves, but significantly increased their APX activity, which still did not restore to the level of the control group. The effects of different treatments on POD activity and CAT activity were significantly different. In the determination of POD activity, the lowest value appeared in the control group and the highest value appeared in the compound treatment. The addition of nano-ferric oxide reduced the POD activity significantly compared with the Cd treatment, while the compound treatment of nano-ferric oxide and biochar increased the POD activity in muskmelon leaves. Compared with Cd treatment, the CAT activity of leaves was not significantly affected by adding nano-ferric oxide, but decreased by 18.95% by adding biochar alone.
Response changes of related active ingredients
As shown in the Fig. 3, we studied the active components in various parts of muskmelon, and analyzed the content of active components in edible parts of muskmelon under different treatments. We found that the presence of Cd reduced the content of flavonoids in seed and the content of polyphenols in peel. It had no significant effect on the content of other active components in peel and seed, but had a significant effect on the content of active components in flesh. The content of polyphenols, flavonoids, saponins, and polysaccharides in flesh decreased significantly by 49.09%, 54.80%, 16.79%, and 8.19% over control.
Different treatments had different effects on the decrease of effective components caused by Cd stress. For example, the application of nano-ferric oxide alone increased the content of polyphenols and flavonoids in muskmelon flesh, but had no significant effect on the content of saponins, polysaccharides, and other active ingredients. What surprises us is compared with Cd-alone treatment, the contents of polyphenols, flavonoids, and saponins in flesh increased by 99.73%, 143.07%, and 18.78%, respectively, when the nano-ferric oxide was combined with biochar.
Transport, transfer, and accumulation of Cd
Figure 4 illustrates the total Cd and effective Cd content in soils under different treatments. The total Cd content in both the biochar treatment and the combined treatment was significantly higher than that in the single Cd treatment, indicating that the addition of biochar hindered the entry of Cd into plants to a certain extent, and combined treatment significantly reduced the ratio of effective Cd to the total Cd compared with Cd treatment.
As shown in Table 2, among all the treatments added with Cd, the muskmelon seeds had the highest Cd content in the edible parts of the muskmelon. Except for the treatment added with nano-ferric oxide, the stems of the muskmelon plants in the other treatments had the highest Cd content. Compared with the Cd content of Cd-alone-treated muskmelon plants, that in the flesh decreased by 10.66% only when the composite treatment of nano-ferric oxide and biochar was used. This is consistent with the increase of total Cd and effective Cd in soil. However, using one of biochar and nano-ferric oxide alone would cause the Cd content of flesh increases.
In addition, we conducted a study on the distribution of Cd in subcells, and found that when muskmelons are stressed by Cd, except the treatment nano-ferric oxide added alone, the Cd content in the cell wall components of roots and leaves is the highest, and that of the organelles is the least (Table 2). The application of biochar and/or nano-ferric oxide reduced the Cd content of the cell wall, organelle, and cytosolic components in the root, which was consistent with the aforementioned change in the Cd content of the muskmelon plant root.
Furthermore, Cd translocation from root to stem and stem to leaf was evaluated by calculating translocation factor. The TF from the root to the stem was bigger than 1 in all the treatments except the treatment with nano-ferric oxide, which indicated that the ability of transporting Cd from the root to the stem was very strong. Compared with the Cd treatment, the transport of Cd from stem to leaf in the composite treatment plants was reduced by 26.61%. Through BAF value calculation, we found that the addition of composite materials to reduce the accumulation of Cd in muskmelon fruit is the best, in muskmelon seeds, peel, and flesh are the same.
Health risk assessment
When Cd is added, regardless of whether biochar and other materials are applied, the THQ value of seed is much bigger than 1, and exposure is likely to cause obvious adverse effects. When the Cd treatment is performed alone and other exogenous substances are not used, the THQ value of peel and flesh is also bigger than 1. When the measures of applying biochar or nano-ferric oxide alone were adopted, the THQ values of flesh were 1.0256 and 1.1524, which were still bigger than 1. Only when the biochar and nano-ferric oxide composite materials were added, the THQ value of peel and flesh was less than 1, and the risk of adverse effects from exposure was less than that of the other treatments (Table 2).
Discussion
Mechanisms of Cd toxicity in plants
The harm of Cd to plants is manifested in the morphological, physiological, and biochemical levels (Muhammad et al. 2017). Studies have shown that Cd stress may reduce the total plant length and inhibit root elongation (Bae et al. 2016; Mani et al. 2012). However, in our experiment, the effect of Cd on plant morphology was not significant during the whole growth cycle, which may be due to the least significant negative effect (LOEC) of Cd on the muskmelon plants is high. Previous research found, compare the classic (germination and biomass) and biochemical (enzyme activity) endpoints to establish a sensitivity ranking, namely enzyme activity > biomass (Correa et al. 2006). So we conducted research on the more sensitive enzyme activity and other physiological aspects. In terms of plant physiological changes, when Cd entered the plant, photosynthetic pigment degradation enzyme activity increased, chlorophyll synthase activity decreased, resulting in chlorophyll content decreased (Grobelak and Napora 2015; Li et al. 2015). Compared with the control group, the chlorophyll content of muskmelon leaves in Cd treatment decreased by 14.21%. At the same time, the accumulation of Cd causes oxidative stress, excessive production of reactive oxygen species causes excessive free radicals, induces lipid peroxidation, and damages the rigidity of important structures such as membrane, protein and nucleic acid (Sarwar et al. 2017; Konate et al. 2017). The MDA content of muskmelon leaves exposed to Cd stress increased by 49.49%, and the cell membrane of plants was seriously damaged (Moradi et al. 2019). In order to resist the oxidative damage induced by reactive oxygen species, plants can defense through their own antioxidant system, antioxidant defense system removes free radicals and protects plant membrane structure and function (Fei et al. 2010). Therefore, Cd leads to the increase of reactive oxygen species (ROS) formation, and ROS as a signal not only causes toxic effects, but also induces the role of plant defense system (Kumar and Prasad 2018; Jalmi et al. 2018). This has been widely confirmed in previous studies (He et al. 2018; Baxter et al. 2014).
However, compared with the changes of plant morphology and physiology, whether the fruit quality is not affected by Cd stress environment is a more worthy of our attention. When 400 mg/kg Cd was added, the contents of polyphenols, flavonoids, saponins, polysaccharides, and other effective components in muskmelon flesh decreased significantly. Polyphenols and flavonoids are antioxidant substances, and they are widely related to the antioxidant defense of plants (Sakuraia et al. 2019; Corsol et al. 2018). Their accumulation in plant tissues can enhance plant tolerance to abiotic stress. Li et al. showed that flavonoids can improve plant tolerance and reduce the damage to cells caused by ultraviolet radiation (Li et al. 2018). Dong et al. tested Tartary buckwheat under UV-B stress and found that under UV-B treatment, the flavonoid content in the early stage would increase significantly, while the flavonoid content in the late stage would decrease significantly (Dong et al. 2006). This indicated that long-term stress can cause irreversible damage to plant cells, accelerate the degradation of secondary metabolites such as flavonoids and polyphenols, and make their synthesis difficult (Maleva et al. 2018). In addition, polyphenols and flavonoids can protect plants under stress by chemical quenching, so flavonoids and polyphenols are constantly consumed in the antioxidant process, which may be the reason why they are reduced in plants under Cd stress (Ferdinando et al. 2014).
The high mobility of Cd from soil to plants makes it possible to accumulate along the food chain, thus threatening human health. At present, some Cd transferred to plants is the main source of Cd intake by humans and animals (Bolan et al. 2013; Shahid et al. 2016). Through the calculation of TF and BAF, we found that muskmelon had a strong ability to transfer and accumulate Cd. In this study, the order of Cd content in different parts of muskmelon treated with Cd alone was stem > root > leaf > seed > peel > flesh, while the THQ value of flesh with the lowest Cd content was still bigger than 1, which was still a risk to the health of consumers.
Detoxification and tolerance of muskmelon to Cd
Cd is toxic to plants. For this reason, plants exposed to Cd must be able to prevent excessive absorption of Cd or detoxify it after absorption to grow normally. The detoxification mechanisms of plants to heavy metals include minimizing Cd absorption to avoid stress, and eliminating Cd effects in plants through various approaches to establish Cd tolerance (Da et al. 2010). In this experiment, under 400 mg/kg Cd treatment, the plant morphology of muskmelon did not change, and there was no significant difference in plant height, stem thickness, and leaf width, which may be because muskmelon has a certain Cd tolerance. Through the study on the transport, accumulation, and distribution of Cd in muskmelon and the activity of related enzymes in antioxidant enzyme system, we found that the transfer ability of Cd from root to stem in muskmelon plants was bigger than 1 when exposed to Cd, and the activity of POD in leaves increased significantly. It can be seen from Table 1 that in the subcellular structure of roots and leaves, the Cd content in FI component was the highest. The results showed that the tolerance of muskmelon to Cd is not due to the limited absorption of Cd by its roots, but through the mechanism of inducing detoxification, by inducing antioxidant enzymes, cell wall fixation, and vacuole separation to enhance tolerance of muskmelon plants to Cd. When the Cd concentration is too high, the plant defense system will be damaged. In our study, the enzyme activity of defense enzyme system in muskmelon plants decreased, the activity of SOD and APX decreased by 14.44% and 78.11%, and membrane lipid peroxidation damage occurred. This is consistent with the results of previous studies (Jaskulak and Grobelak 2019; Xu et al. 2017).
Effects of different treatments on Cd toxicity in muskmelon plants
Biochar is commonly used as a soil additive for heavy metal soil remediation. It is generally accepted that biochar materials are alkaline. The application of biochar can increase soil pH and change the distribution of heavy metal forms. With the increase of soil pH, the availability and mobility of heavy metals in soil decreased (Ding et al. 2011). Moreover, the addition of nano-ferric oxide can affect the mobility of Cd in the environment by providing a reactive surface for adsorption (Muehe et al. 2013). Chen et al. found that Fe concentration in roots affects Cd transport from roots to stems (Chen et al. 2017). Cd is transferred from root to stem through ion channel proteins, which may be affected by Fe concentration. In this experiment, compared with the Cd treated group, the addition of Fe enhanced the retention of Cd by muskmelon roots and reduced the transport of Cd from the root to the above-ground part (Table 2). In addition, the competition between Cd and Fe was also found in our experiments; the addition of Fe inhibited Cd accumulation in muskmelon (Fig. S3) In the combined treatment, the Fe content was higher than that of the Cd group, and the Cd content was lower than that of the Cd group. When biochar was added alone, the Fe content of roots and leaves was lower than that of the combined treatment, so the Cd content of roots and leaves was correspondingly higher than that of the combined treatment. It can be considered that there is a competition between Cd and Fe determined by the regulation of metal-associated transporters (Andresen and Küpper 2013).
Biochar and nano-ferric oxide might simultaneously have activation and passivation of Cd in soil (Li et al. 2017; Yin et al. 2017). In our study, the passivation effect was more predominant. Experiments showed (Table 2) that biochar and nano-ferric oxide had a synergistic effect on reducing plant Cd uptake, and when compounded, the Cd content of each part of muskmelon was significantly lower than that of Cd-treated muskmelon.
The synergistic effect of them in reducing plant Cd toxicity is also reflected in physiological aspects. SOD can transform superoxide anion into H2O2, CAT, POD, and APX can decompose H2O2 into water and oxygen, thus reducing the cytotoxicity of reactive oxygen species. Under normal conditions, the production and scavenging of reactive oxygen species in plants are in a dynamic balance. After Cd stress, plant homeostasis is disrupted, resulting in massive accumulation of reactive oxygen species. The compound treatment of nano-ferric oxide and biochar can increase the activity of SOD, APX, and POD, and reduce the accumulation of active oxygen in muskmelons. The accumulation of reactive oxygen species (ROS) leads to enhanced membrane lipid peroxidation, which is one of the important mechanisms by which heavy metal stress induces plant cell damage and death. Therefore, we can see that the compound treatment MDA content than Cd treatment decreased about 59.12%, the degree of membrane lipid peroxidation significantly reduced. The effect of the biochar or nano-ferric oxide treatment on the antioxidant enzyme system of muskmelon was weaker than that of the combined treatment.
In the experiment, we extracted two main components, covering more than 75% of all changes in all treatments and parameters. Through principal component analysis (Fig. 5), in terms of growth index, plant height was negatively correlated with Cd content, followed by stem diameter. In physiological indexes of muskmelon leaves, Cd content was negatively correlated with chlorophyll content, APX activity and SOD activity, and positively correlated with MDA content. From the perspective of active component content, Cd content was negatively correlated with the content of polyphenols and flavonoids. From the effects of different treatments, there are great differences between the Cd treatment and the control group, indicating that the addition of Cd has a significant impact on muskmelon, while there is little difference between other groups and the control group, indicating that single or combined treatment has alleviated the toxicity of Cd to a certain extent. The above results showed that when muskmelons were exposed to Cd, the content of chlorophyll decreased and photosynthesis weakened, resulting in a significant decrease in plant height and stem diameter. At the same time, the contents of APX and SOD in muskmelon leaves decreased, the membrane lipid peroxidation increased, and the cell membrane was damaged. Cd inhibited the formation of polyphenols and flavonoids, reduced the content of active components and quality of muskmelon. The three treatments had a certain mitigation effect on Cd toxicity. Among them, the repair effect of nano-ferric oxide group and composite group is better.
Evaluation of edible value of muskmelon
The nutritional value and edible risk of muskmelon fruit deserve our attention. Active ingredients such as polyphenols and flavonoids can directly eliminate reactive oxygen species in cells, which preventing the entry of toxic small molecules by interacting with membrane phospholipids of cell membranes, protecting the integrity of plant cell membranes, which plays an important role in the plant-environment interaction.
In our study, for edible parts, we found that the contents of polyphenols, flavonoids, and saponins in the flesh were significantly higher than those in the Cd treatment, and the risk of adverse reactions after eating the peel and flesh was also lower than that in other groups. The single treatment and combined treatment reduced the Cd content of muskmelon seeds to a certain extent, but through the calculation of THQ, we found that in the case of high concentrations of Cd pollution, muskmelon seeds still have some security risks, which need to be further explored.
Conclusion
Our study found that Cd pollution had toxic effects on muskmelon plants, which were manifested in the decrease of chlorophyll content, the aggravation of membrane damage, and the decrease of SOD and APX activity. At the same time, high concentration of Cd pollution led to the decrease of the effective component content in muskmelon flesh, and the presence of Cd leads to the increase of Cd content in edible parts of muskmelon, and there are food safety hazards. In addition, this study investigated the effects of biochar, nano-ferric oxide, and their complex on the growth, physiology, biochemistry, and fruit quality of muskmelon under Cd stress. Compared with biochar or nano-ferric oxide treated separately, the composite treatment has great potential in in situ remediation of Cd-contaminated soil, which can improve plant Cd tolerance and reduce fruit edible risk. The results of this study provide a theoretical basis for further research on alleviating Cd toxicity in plants and improving the edibility of fruits and vegetables.
Data availability
The data set used in the study can be obtained by a reasonable request from the corresponding author Junli Li.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105(105):121. https://doi.org/10.1016/S0076-6879(84)05016-3
Ali S, Rizwan M, Hussain A et al (2019) Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol Biochem 140:1–8. https://doi.org/10.1016/j.plaphy.2019.04.041
Andresen E, Küpper H (2013) Cadmium toxicity in plants. Met Ions Life Sci 11:395–413. https://doi.org/10.1007/978-94-007-5179-8_13
Arrigoni O, Gara LD, Tommasi F et al (1992) Changes in the ascorbate system during seed development of Vicia faba L. Plant Physiol 99(1):235–238. https://doi.org/10.1104/pp.99.1.235
Bae J, Benoit DL, Watson AK (2016) Effect of heavy metals on seed germination and seedling growth of common ragweed and roadside ground cover legumes. Environ Pollut 213:112–118. https://doi.org/10.1016/j.envpol.2015.11.041
Bashir S, Zhu J, Fu Q et al (2018) Cadmium mobility, uptake and anti-oxidative response of water spinach ( Ipomoea aquatic ) under rice straw biochar, zeolite and rock phosphate as amendments. Chemosphere 194:579–587. https://doi.org/10.1016/j.chemosphere.2017.11.162
Baxter A, Mittler et al (2014) ROS as key players in plant stress signalling. J Exp Bot 65(5):1229–1240. https://doi.org/10.1093/jxb/ert375
Bolan NS, Makino T, Kunhikrishnan A et al (2013) Cadmium contamination and its risk management in rice ecosystems. Adv Agron 119(47):183–273. https://doi.org/10.1016/B978-0-12-407247-3.00004-4
Bortey-Sam N, Nakayama S, Ikenaka Y et al (2015) Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: Estimation of the daily intakes and target hazard quotients (THQs). Ecotoxicol Environ Saf 111:160–167. https://doi.org/10.1016/j.ecoenv.2014.09.008
Chen Z, Tang et al (2017) Mechanisms of Fe biofortification and mitigation of Cd accumulation in rice (Oryza sativa L.) grown hydroponically with Fe chelate fertilization. Chemosphere 175:275–285. https://doi.org/10.1016/j.chemosphere.2017.02.053
Cheng A, Chen X, Jin Q et al (2013) Comparison of Phenolic content and antioxidant capacity of red and yellow onions. Czech J Food Sci 31(5):501–508. https://doi.org/10.1108/BFJ-11-2011-0284
Clemens S, Ma JF (2016) Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu Rev Plant Biol 67(1):489. https://doi.org/10.1146/annurev-arplant-043015-112301
Correa AXDR, Rorig LR, Verdinelli MA et al (2006) Cadmium phytotoxicity: quantitative sensitivity relationships between classical endpoints and antioxidative enzyme biomarkers. Sci Total Environ 357(1–3):120–127. https://doi.org/10.1016/j.scitotenv.2005.05.002
Corsol M, Schvartzman M, Guzzo F et al (2018) Contrasting cadmium resistance strategies in two metallicolous populations of Arabidopsis halleri. New Phytol 218(1):283–297. https://doi.org/10.1111/nph.14948
Da Lcorso G, Fadnati S, Maistd S et al (2010) How plants cope with cadmium: staking all on metabolism and gene expression. J Integr Plant Biol 50(10):1268–1280. https://doi.org/10.1111/j.1744-7909.2008.00737.x
Das P, Samantaray S, Rout GR (1997) Studies on cadmium toxicity in plants: a review. Environ Pollut 98(1):29–36. https://doi.org/10.1016/S0269-7491(97)00110-3
Ding W, Zhu Q, Zeng X et al (2011) Biochars from different pyrolytic temperature amending lead and cadmium contaminated soil. Sci Technol Rev 29:22–25. https://doi.org/10.1631/jzus.B1000275
Dong XC, Zhao SJ, Guo SS et al (2006) To enhance the relationship between flavonoids and Tartary buckwheat stress damage and antioxidant enzymes under UV-B condition. J Shandong Agric Univ (natural Science Edition) 02:157–162. https://doi.org/10.1016/j.ymben.2018.11.008
Faust MB, Christians et al (2000) Copper reduces shoot growth and root development of creeping bentgrass. Crop Sci 40:498–502. https://doi.org/10.2135/cropsci2000.402498x
Fei C, Wang F, Wu F et al (2010) Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol Biochem 48(8):663–672. https://doi.org/10.1016/j.plaphy.2010.05.001
Ferdinando MD, Brunetti C, Agati G et al (2014) Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environ Exp Bot 103:107–116. https://doi.org/10.1016/j.envexpbot.2013.09.012
Freddie B, Jacques et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Fundo JF et al (2018) Physicochemical characteristics, bioactive compounds and antioxidant activity in juice, pulp, peel and seeds of Cantaloupe muskmelon. J Food Meas Charact 12(1):292–300. https://doi.org/10.1007/s11694-017-9640-0
Giannopolites CN, Ries SK (1977) Superoxide dismutase occurrence in higher plants. Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Gillispie EC, Taylor SE, Qafoku NP et al (2019) Impact of iron and manganese nano-metal-oxides on contaminant interaction and fortification potential in agricultural systems – a review. Environ Chem 16(6):377–390. https://doi.org/10.1016/j.jwpe.2018.10.018
Grobelak A, Napora A (2015) The chemophytostabilisation process of heavy metal polluted soil. PLoS One 10(6):0129538. https://doi.org/10.1371/journal.pone.0129538
Guo XL, Zhu SC, Zhai XF et al (2010) Comparison of methods in determination of polysaccharide in Ganoderma Lucidum. Chinese Arch Tradit Chinese Med 8(9):2000–2002. https://doi.org/10.13193/j.archtcm.2010.09.210.guoxl.062
Halim MA, Rahman MM, Mallavarapu M et al (2020) Cadmium immobilization in the rhizosphere and plant cellular detoxification: role of plant-growth-promoting rhizobacteria as a sustainable solution. J Agric Food Chem 68(47):13497–13529. https://doi.org/10.1021/acs.jafc.0c04579
Hao Y, Lv R, Ma C et al (2021) Graphitic carbon nitride (g-C3N4) alleviates cadmium-induced phytotoxicity to rice (Oryza sativa L.). Enviro Sci Pollut Res 28(17):21276–21284. https://doi.org/10.1007/s11356-020-12027-w
He M, He CQ, Ding NZ (2018) Abiotic stresses: general defenses of land plants and chances for engineering multistress tolerance. Front Plant Sci 871:1–18. https://doi.org/10.3389/fpls.2018.01771
Jalmi SK, Bhagat PK, Verma D et al (2018) Traversing the links between heavy metal stress and plant signaling. Front Plant Sci 9:12–21. https://doi.org/10.3389/fpls.2018.00012
Jaskulak M, Grobelak A (2019) Cadmium phytotoxicity—biomarkers. Cadmium Tolerance Plants 177–191. https://doi.org/10.1016/B978-0-12-815794-7.00006-0
John MK, Hong HC, Vanlaerhoven CJ (1972) Cadmium contamination of soil and its uptake by oats. Environ Sci Technol 6(6):555–557. https://doi.org/10.1021/es60065a001
Karadas C, Kara D (2012) Chemometric approach to evaluate trace metal concentrations in some spices and herbs. Food Chem 130(1):196–202. https://doi.org/10.1016/j.foodchem.2011.07.006
Khan ZS, Rizwan M, Hafeez M et al (2019) The accumulation of cadmium in wheat ( Triticum aestivum ) as influenced by zinc oxide nanoparticles and soil moisture conditions[J]. Environ Sci Pollut Res 26:19859–19870. https://doi.org/10.1007/s11356-019-05333-5
Khaokaew S, Landrot G (2015) A field-scale study of cadmium phytoremediation in a contaminated agricultural soil at Mae Sot District, Tak Province, Thailand: (1) Determination of Cd-hyperaccumulating plants. Chemosphere 138:883–887. https://doi.org/10.1016/j.chemosphere.2014.09.108
Kochba J, Lavee S, Spiegel-Roy P (1977) Differences in peroxidase activity and isoenzymes in embryogenic ane non-embryogenic ‘Shamouti’ orange ovular callus lines. Plant Cell Physiol 18(2):463–467. https://doi.org/10.1093/oxfordjournals.pcp.a075455
Konate A, He X, Zhang Z et al (2017) Magnetic (Fe3O4) Nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability 9(5):790. https://doi.org/10.3390/su9050790
Kumar A, Prasad M (2018) Plant-lead interactions: transport, toxicity, tolerance, and detoxification mechanisms. Ecotoxicol Environ Saf 166:401–418. https://doi.org/10.1016/j.ecoenv.2018.09.113
Kumar A, Tsechansky L, Lew B et al (2018) Biochar alleviates phytotoxicity in Ficus elastica grown in Zn-contaminated soil. Sci Total Environ 618:188–198. https://doi.org/10.1016/j.scitotenv.2017.11.013
Kyung-Seok K, In Chul K (2014) Toxic effects of nanoparticles on bioluminescence activity, seed germination, and gene mutation. Appl Microbiol Biotechnol 98:3295–3303. https://doi.org/10.1007/s00253-013-5404-x
Li S, Yang W, Yang T et al (2015) Effects of cadmium stress on leaf chlorophyll fluorescence and photosynthesis of Elsholtzia argyi —a cadmium accumulating plant. Int J Phytorem 17(1):85–92. https://doi.org/10.1080/15226514.2013.828020
Li H, Dong X, Silva E et al (2017) Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 178:466–478. https://doi.org/10.1016/j.chemosphere.2017.03.072
Li J, Tian C, Xia Y et al (2018) Production of plant-specific flavones baicalein and scutellarein in an engineered E. coli from available phenylalanine and tyrosine. Metab Eng 52:124–133. https://doi.org/10.1016/j.ymben.2018.11.008
Lin J, He F, Su B et al (2019) The stabilizing mechanism of cadmium in contaminated soil using green synthesized iron oxide nanoparticles under long-term incubation. J Hazard Mater 379:120832. https://doi.org/10.1016/j.jhazmat.2019.120832
Liu WJ, Jiang H, Yu HQ (2015) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115(22):12251–12285. https://doi.org/10.1016/10.1021/acs.chemrev.5b00195
Liu J, Jiang J, Meng Y et al (2020) Preparation, environmental application and prospect of biochar-supported metal nanoparticles: a review. J Hazard Mater 388:122026. https://doi.org/10.1016/j.jhazmat.2020.122026
Lu K, Yang X, Shen J et al (2014) Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agr Ecosyst Environ 191:124–132. https://doi.org/10.1016/j.agee.2014.04.010
Luo C, Liu C, Wang Y et al (2011) Heavy metal contamination in soils and vegetables near an e-waste processing site, south China. J Hazard Mater 186(1):481–490. https://doi.org/10.1016/j.jhazmat.2010.11.024
Magdalena S, Oleszczuk P, Yong SO (2016) Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chem Eng J 287:618–632. https://doi.org/10.1016/j.cej.2015.11.046
Maleva M, Garmash E, Chukina N et al (2018) Effect of the exogenous anthocyanin extract on key metabolic pathways and antioxidant status of Brazilian elodea (Egeria densa (Planch.) Casp.) exposed to cadmium and manganese. Ecotoxicol Environ Saf 160:197–206. https://doi.org/10.1016/j.ecoenv.2018.05.031
Mani D, Bechan B, Bullet S et al (2012) Cadmium and lead bioaccumulation during growth stages alters sugar and vitamin C content in dietary vegetables. Proc Natl Acad Sci India 82(4):477–488. https://doi.org/10.1007/s40011-012-0057-6
Manori S, Shah V, Soni V et al (2021) Phytoremediation of cadmium-contaminated soil by Bidens pilosa L.: impact of pine needle biochar amendment. Environ Sci Pollut Res 28:58872–58884. https://doi.org/10.1007/s11356-021-12953-3
Mattina M, Lannucci-Berger W, Musante C et al (2003) Concurrent plant uptake of heavy metals and persistent organic pollutants from soil. Environ Pollut 124(3):375–378. https://doi.org/10.1016/S0269-7491(03)00060-5
Michalkova Z, Komarek M, Sillerova H et al (2014) Evaluating the potential of three Fe- and Mn-(nano)oxides for the stabilization of Cd, Cu and Pb in contaminated soils. J Environ Manag 146(15):226–234. https://doi.org/10.1016/j.jenvman.2014.08.004
Moradi R, Pourghasemian N, Naghizadeh M (2019) Effect of beeswax waste biochar on growth, physiology and cadmium uptake in saffron. J Clean Prod 229:1251–1261. https://doi.org/10.1016/j.jclepro.2019.05.047
Muehe EM, Obst M, Hitchcock A et al (2013) Fate of Cd during microbial Fe(III) mineral reduction by a novel and Cd-tolerant geobacter species. Environ Sci Technol 47(24):14099–14109. https://doi.org/10.1021/es403365w
Muhammad R, Shafaqat et al (2017) A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 182:90–105. https://doi.org/10.1016/j.chemosphere.2017.05.013
Peng AH, Xiong HJ, Yan YH (2006) Study on determination of the content of total saponins in radix pseudostellariae. J Sichuan Normal Univ (natural Science) 29(6):743–746. https://doi.org/10.1016/S1872-2059(06)60026-6
Ran J, Zheng W, Wang H et al (2020) Indole-3-acetic acid promotes cadmium (Cd) accumulation in a Cd hyperaccumulator and a non-hyperaccumulator by different physiological responses. Ecotoxicol Environ Safety 191:110213. https://doi.org/10.1016/j.ecoenv.2020.110213
Rasafi TE, Oukarroum A, Haddioui A et al (2020) Cadmium stress in plants: a critical review of the effects, mechanisms, and tolerance strategies. Crit Rev Environ Sci Technol 52(5):675–726. https://doi.org/10.1146/annurev-arplant-043015-112301
Rodriguez O, Peralta-Hernandez JM, Goonetilleke A et al (2019) Biochar-supported nanomaterials for environmental applications. J Ind Eng Chem 78:21–33. https://doi.org/10.1016/j.jiec.2019.06.008
Rui M, Ma C, Hao Y et al (2016) Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front Plant Sci 7:185. https://doi.org/10.3389/fpls.2016.00815
Sakuraia M, Tomiokaa R, Hokura A et al (2019) Distributions of cadmium, zinc, and polyphenols in Gamblea innovans. Int J Phytorem 21(3):217–223. https://doi.org/10.1080/15226514.2018.1524840
Sangsuwan P, Prapagdee B (2021) Cadmium phytoremediation performance of two species of Chlorophytum and enhancing their potentials by cadmium-resistant bacteria. Environ Technol Innov 21:101311. https://doi.org/10.1016/j.eti.2020.101311
Sarwar N, Imran M, Shaheen MR et al (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721. https://doi.org/10.1016/j.chemosphere.2016.12.116
Shah G, Amin M, Shahid M, et al (2022) Toxicity of ZnO and Fe2O3 nano-agro-chemicals to soil microbial activities, nitrogen utilization, and associated human health risks. Environ Sci Eur 34(1) Article number: 106. https://doi.org/10.1186/s12302-022-00687-z
Shahid M, Dumat C, Khalid S et al (2016) Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev Environ Contam Toxicol 241:73–137. https://doi.org/10.1007/398_2016_8
Song Y, Jin L, Wang X (2017) Cadmium absorption and transportation pathways in plants. Int J Phytorem 19(1–2):133–141. https://doi.org/10.1080/15226514.2016.1207598
Storelli MM (2008) Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem Toxicol 46(8):2782–2788. https://doi.org/10.1016/j.fct.2008.05.011
Tan L, Qu M, Zhu Y (2020) Zinc Transporter5 and Zinc Transporter9 function synergistically in zinc/Cadmium uptake. Plant Physiol 183(3):1235–1249. https://doi.org/10.1104/pp.19.01569
Turkekul I, Elmastas M, Tuezen M (2004) Determination of iron, copper, manganese, zinc, lead, and cadmium in mushroom samples from Tokat, Turkey. Food Chem 84(3):389–392. https://doi.org/10.1016/S0308-8146(03)00245-0
Turkmen N, Sari F, Velioglu YS (2006) Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem 99(4):835–841. https://doi.org/10.1016/j.foodchem.2005.08.034
Ullah I, Al-Johny BO, Al-Ghamdi K et al (2019) Endophytic bacteria isolated from Solanum nigrum L. alleviate cadmium (Cd) stress response by their antioxidant potentials, including SOD synthesis by sodA gene. Ecotoxicol Environ Saf 174:197–207. https://doi.org/10.1016/j.ecoenv.2019.02.074
Vella FM, Cautela D, Laratta B (2019) Characterization of polyphenolic compounds in cantaloupe muskmelon by-products. Foods 8(6):196. https://doi.org/10.3390/foods8060196
Wan XM, Yang J, Song W (2018) Pollution status of agricultural land in china: impact of land use and geographical position. Soil Water Res 13(4):234–242. https://doi.org/10.17221/211/2017-SWR
Wang X, Sato T, Xing B et al (2005) Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci Total Environ 350(1/3):28–37. https://doi.org/10.1016/j.scitotenv.2004.09.044
Wang Y, Zou Z, Su X, et al (2021a) Physiological of biochar and α-Fe2O3 nanoparticles as amendments of Cd accumulation and toxicity toward muskmelon grown in pots. J Nanobiotechnol 19(1). https://doi.org/10.1186/s12951-021-01187-7
Wang Y, Wang L, Ma C et al (2021b) Effects of cerium oxide on rice seedlings as affected by co-exposure of cadmium and salt. Environ Pollut 252:1087–1096. https://doi.org/10.1016/j.envpol.2019.06.007
Wei T, Sun Y, Yashir N et al (2021) Inoculation with Rhizobacteria enhanced tolerance of tomato (Solanum lycopersicum L.) plants in response to cadmium stress. J Plant Growth Regul 41:445–460. https://doi.org/10.1007/s00344-021-10315-4
Xu X, Yang F, Xiao X et al (2008) Sex-specific responses of Populus cathayanato drought and elevated temperatures. Plant Cell Environ 31(6):850–860. https://doi.org/10.1111/j.1365-3040.2008.01799.x
Xu X, Zhang S, Xian J et al (2017) Subcellular distribution, chemical forms and thiol synthesis involved in cadmium tolerance and detoxification in Siegesbeckia orientalis L. Int J Phytorem 20(10):973–980. https://doi.org/10.1080/15226514.2017.1365351
Yin D, Wang X, Peng B et al (2017) Effect of biochar and Fe-biochar on Cd and As mobility and transfer in soil-rice system. Chemosphere 186:928–937. https://doi.org/10.1016/j.chemosphere.2017.07.126
Yu H, Zou W, Chen J et al (2019) Biochar amendment improves crop production in problem soils: a review. J Environ Manag 232:8–21. https://doi.org/10.1016/j.jenvman.2018.10.117
Zhang Y, Shuang X, Yang S et al (2015) Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in two muskmelon cultivars (Cucumis melo L.). Protoplasma 252(3):911–924. https://doi.org/10.1007/s00709-014-0732-y
Zhang F, Liu M, Li Y et al (2019) Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa - ScienceDirect. Sci Total Environ 655:1150–1158. https://doi.org/10.1016/j.scitotenv.2018.11.317
Zhen Q, Jinhuan C, Jiawen T et al (2018) A study of cadmium remediation and mechanisms: Improvements in the stability of walnut shell-derived biochar. Sci Total Environ 636:80–84. https://doi.org/10.1016/j.scitotenv.2018.04.215
Zheng RL, Chen et al (2013) Effect of biochars from rice husk, bran, and straw on heavy metal uptake by pot-grown wheat seedling in a historically contaminated soil. Bioresources 8:5965–5988. https://doi.org/10.15376/biores.8.4.5965-5982
Zhou, Yang WT et al (2016) Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int J Environ Res Public Health 13(3):289. https://doi.org/10.3390/ijerph13030289
Zhou P, Adeel M, Guo M et al (2022) Characterisation of biochar produced from two types of chestnut shells for use in remediation of cadmium- and lead-contaminated soil. Crop Pasture Sci 10(8):1627. https://doi.org/10.1071/CP21297
Zhu S, Ho SH, Huang X et al (2017) Magnetic nanoscale zerovalent iron assisted biochar: interfacial chemical behaviors and heavy metals remediation performance. Acs Sustain Chem Eng 5(11):9673–9682. https://doi.org/10.1021/acssuschemeng.7b00542
Zhu L, Tong L, Zhao N et al (2018) Coupling interaction between porous biochar and nano zero valent iron/nano α-hydroxyl iron oxide improves the remediation efficiency of cadmium in aqueous solution. Chemosphere 219(MAR):493–503. https://doi.org/10.1016/j.chemosphere.2018.12.013
Funding
This work was supported by China Agriculture Research System of MOF and MARA [Grant No. CARS-25]; Key Research and Development Program of Hubei Province, China [Grant No. 2020BBA037]; and the Fundamental Research Funds for the Central Universities [Grant No. 2019IB005, 2020IB029, 2021IA005].
Author information
Authors and Affiliations
Contributions
YZ and ZZ: Performed experiments; JL: Conceptualized and supervised the work; MW, LQ, and YC: Help in data analysis; YW and ZD: Provided technical and helpful discussions; YZ, ZZ, YW, and JL wrote and edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors agreed with the content and that all gave explicit consent to submit. They obtained consent from the responsible authorities at the institute/organization, where the work has been carried out.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. The growth, physiology, active ingredients, and other indexes of muskmelon are studied.
2. Biochar and/or nano-ferric oxide can mitigate cadmium toxicity of muskmelon.
3. The edible risk of the composite treatment is less than in cadmium treatment.
4. The composite treatment had the best effect to increase the active ingredients content.
Ying Zhou and Zhengkang Zou are co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Zou, Z., Wang, M. et al. Biochar and nano-ferric oxide synergistically alleviate cadmium toxicity of muskmelon. Environ Sci Pollut Res 30, 57945–57959 (2023). https://doi.org/10.1007/s11356-023-26369-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26369-8