Abstract
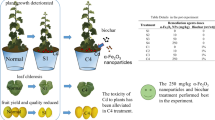
The severe cadmium (Cd) pollution of soil adversely affects food safety and human health. To prevent phytotoxicity and Cd accumulation in agricultural products, effective steps must be taken immediately. In the study, we added different concentrations (10, 50, and 250 mg/kg) of α-Fe2O3 nanoparticles (NPs) into Cd-contaminated soil, investigating the effects of α-Fe2O3 NPs on Cd toxicity. The various physiological indicators, such as antioxidant system, the distribution of Cd and the content of active ingredients in different parts of muskmelon were detected. The results revealed that α-Fe2O3 NPs greatly reduce Cd transport in plants and minimize Cd toxicity. Specifically, 250 mg/kg α-Fe2O3 NPs treatment reduced the root Cd content by 30.19% and 50 mg/kg α-Fe2O3 NPs treatment reduced the stem Cd content by 38.75%. For the content of active ingredients, 50 mg/kg α-Fe2O3 NPs treatment increased root polysaccharide content and leaf saponin content by 14.45 and 27.71%, respectively. Overall, α-Fe2O3 NPs can reduce Cd stress in muskmelon. The current work gave a unique notion for Cd remediation in soil using α-Fe2O3 NPs as amendments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
With the advancement of urbanization and industrialization, environmental issues have been emerged simultaneously. Heavy metals have emerged as one of the most serious environmental issues, impairing food security and human health significantly [1]. Cadmium (Cd) is a highly toxic heavy metal which is harmful to human and plants. Cd enters soil via plant roots and then enters the human body via the food chain, posing a serious threat to human health [2]. At present, many countries in the world have carried out various studies and taken many positive measures to restore soil contaminated by heavy metals. However, the physical method requiring large amount of engineering, the biological method taking long time and slow effect and the chemical leaching method causing large side effects [3]. In situ passivation, which freezes and stabilizes heavy metals, has the advantages of high efficiency and low energy consumption when compared to other processes.
Technology of in situ passivation is to reduce the uptake of Cd by crops by reducing the available state of soil (especially rhizosphere soil), so as to reduce the harm of Cd. The passivation mechanism of this method is that the passivator surfaces undergo a succession of chemical reactions, among which the fixation of Cd is realized through redox and surface complexation [4]. For in situ passivation, current studies indicate that there are many restoratives available, such as Cellulose-zeolitic imidazolate frameworks, nanoparticles, polylactic acid-hydroxyapatite composite [4–6]. As a new adsorption material, nanomaterials can repair contaminated soil and improve agricultural environment. This is because of its high adsorption capacity and diversified structure. Pollutants or harmful microorganisms can be degraded by nano iron oxide by adsorption or photocatalysis. Praveen et al. used nanoscale iron oxide as a nanoscale adsorbent to adsorb arsenic in soil to reduce its phytotoxicity, indicating that nano iron oxide can repair plant heavy metal toxicity by absorbing heavy metals in soil [7]. By treating Cd-contaminated soil with green synthesized iron oxide nanoparticles (GION) prepared by plant leaf extract, Lin et al. found that the exchangeable Cd fraction in the 1, 3, and 9% treatments had fallen by 14.2, 24.9, and 83.5%, respectively [8]. The findings showed that nano iron oxide can reduce Cd toxicity in exposed plants.
Muskmelon (Cucumis melo L.) is a Cucurbitaceae diploid plant (2n = 2x = 24) that is widely grown for its commercial and nutritional value around the world. Muskmelon is reported to include a number of antioxidant and radical-scavenging components. The abundance of phenolics, flavonoids and tocopherols in muskmelon fruit flesh and seeds, according to the study, makes them a strong source of antioxidants [9]. However, there are relatively few studies on the medicinal active components of other parts of muskmelon and the effect of heavy metals on active components.
At present, most studies on the repair of Cd toxicity in muskmelon are focused on the seedling stage, and there are relatively few studies on the systematic determination of Cd content and active ingredient contents in various parts of muskmelon. Hematite, α-Fe2O3, has a corundum structure and is therefore the most stable form of iron oxide. Therefore, in this study, α‑Fe2O3 nanoparticles (NPs) were used in the pot experiment of Cd containing soil planted with muskmelon. We hypothesized that α-Fe2O3 NPs could alleviate Cd toxicity, it could reduce Cd content in various parts of the plant, and it could reflect the alleviation of Cd toxicity in physiological indicators. Therefore, the effects of α-Fe2O3 NPs on the distribution of Cd, the activity of enzymes and active ingredients were explored in various parts of muskmelon. This study can serve as a theoretical foundation for the development of effective, efficient, and safe heavy metal cleanup solutions. The importance of fostering the in-depth development of soil pollution control is both theoretical and practical.
MATERIALS AND METHODS
Materials
The muskmelon (Cucumis melon L.) seeds were provided by Hubei Academy of Agricultural Sciences (China). CdCl2·2.5H2O (99% in purity) was purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. (China). The α-Fe2O3 nanoparticles (NPs) were purchased from Macklin Company. The hydrodynamic diameter and zeta potential of α-Fe2O3 NPs were examined (Supplementary Fig. S1). Scanning electron microscope S-4800 (SEM, Japan) was used to understand surface morphology and structure of the α-Fe2O3 NPs. The XRD (X-ray diffraction) diffractograms were recorded on powder X-ray diffractometer D8 ADVANCE (Bruker, Germany). The 20 was within range of 10–70°. Ni-filtered Cu K alpha radiation was used as the X-ray source with voltage and current were 40 kV and 40 mA, respectively. The SEM image and XRD pattern of α-Fe2O3 NPs are shown in Supplementary Fig. S2.
Greenhouse Experimental Design
The effects of varying concentrations of α-Fe2O3 NPs on muskmelon in Cd-contain soil were investigated in a greenhouse experiment. Briefly, muskmelon seeds were wrapped with moist gauze placed in an incubator at 30°C for 24 h, and then the germinated muskmelon seeds were sown in the orifice plate using acupoint seeding method. At about 25 days, the muskmelon seedlings reached the three-leaf stage and were transferred to potting soil pots with different designated treatment. Before that, the potting soil pots had stilled in the same environmental conditions for a month. There is one plant per one pot. The upper diameter of the pot is 34 cm, the lower diameter is 18.5 cm and the height is 22 cm. The potting media is matrix soil, and its physical and chemical properties are put in Supplementary Table S1. Setting this time as week-1 and two sampling times were set in this experiment, respectively at in the eleventh and the twenty-second week. The two periods sampling times were the flourishing period of muskmelon plant growth in the eleventh week and the mature period of muskmelon fruit at in the twenty-second week, respectively. In the eleventh week, we sampled the leaves of all plants at the 12th knot, which was non-destructive. At week-22, we sampled various parts of the plant, which was destructive. The physiological indicators of the leaves sampled (including basic physiological indicators and antioxidant system indicators) and plant growth indicators (including plant height, hypocotyl thickness, leaf length and width of plant) at week-11 were measured, and the other indicators were measured (including Cd content in the soil–plant system, active ingredients and enzymatic activity) at week-22. In the process of plant cultivation, drip irrigation was used for water and fertilizer, at a frequency of once a day (except in rainy days) and once 10–15 days, respectively.
Because muskmelon is a Cd-tolerant plant, as evidenced by our prior findings, the Cd exposure concentrations were raised [10]. Five treatments were designed and the details are listed in Table 1. A randomized complete block design (RCBD) was used in this experiment, and three independent replicates were applied in each treatment.
Analysis of Cd Content in the Soil–Plant System
In this experiment, the plant was divided into seven parts including root, stem, leaf, pedicel, peel, flesh and seed. The seven parts were dried and ground to fine powder. The Cd content in the seven parts was extracted by nitrolysis method [11]. The diethylenetriaminepentaacetate acid (DTPA) extraction method was used to extract the bioavailable Cd content in soil. Soil samples were oven-dried and ground to a fine powder, and 500 mg of the powder was added to 30 mL of DTPA extracting agent, oscillated at a speed of 180 ± 20 rpm at 25 ± 2°C for 2 h and filtered by filter paper, waiting for the test.
For subcellular separation, plant tissues were treated according to the method previously reported [12]. The fresh leaf and root samples were placed in 50 mL centrifugation tubes, respectively, and 15 mL of pre-cooled extraction buffer was added. The extraction buffer was composed of 50 mmol/L Tris-HCl (pH 7.5), 250 mmol/L sucrose and 1 mmol/L dithiol. After centrifugation at 3000 rpm for 15 min, the precipitate was the cell wall component (FI). The organelle component (FII) was precipitated after centrifugation at 10000 rpm for 30 min. The soluble component, containing macromolecule organic matter and inorganic ions in the cytoplasm and vacuole (FIII), was waiting for the test. At 4°C, all operations were carried out. The FI and FII collected components were extracted by nitrolysis.
Finally, the Avanta M atomic absorption spectrophotometer was used to determine all of the aforesaid Cd content (GBC, Australia). The following formula (1) was used to compute the transfer factor (TF) from portion ‘a’ to part ‘b’:
here ‘a’ and ‘b’ = (mg/kg Cd of dry weight (DW)).
Growth and Physiological Indicators Measurements
Growth indicators: growth indicators including plant height, hypocotyl thickness, leaf length and width of plant and the weight, vertical, transverse diameter and pulp thickness of fruit were measured using basic physical methods. The growth indicators of each plant were measured three times, averaged and recorded to avoid error.
Basic physiological indicators: soluble protein content was detected by colorimetric method of colorimetric blue G-250 [13]. A SPAD-502 Plus chlorophyll meter (Konica Minolta, USA) was used to determine the amount of chlorophyll presents [14].
Antioxidant system indicators were calculated per fresh weight (FW) [15]: superoxide dismutase (SOD) activity was determined by the nitrogen blue tetrazole photochemical reduction method, catalase (CAT) activity was determined by the hydrogen peroxide oxidation method and peroxidase (POD) activity was determined by the guaiacol oxidation method. Ascorbic acid peroxidase (APX) activity was determined by ascorbic acid oxidation method [16]. Malondialdehyde (MDA) content was determined by thiobarbituric acid colorimetry [16].
Measuring Active Ingredients
For active ingredients measurement, the plant was divided into six components: root, stem, leaf, peel, flesh and seed. The six parts were dried and ground into powder, and then the ethanol heating extraction method was used to extract them. The extraction procedure was as follows: the extraction temperature was 60°C, the solid/liquid ratio was 1 : 40, the ethanol concentration was 80%, the extraction time was 5 h, and the extract solution was obtained after filtration. The concentration of each active ingredient in the extract solution was determined. The ferrous tartrate spectrophotometry was used to assess the amount of polyphenols in the muskmelon pedicel [17]. The AlCl3 colorimetric method is used to determine flavonoids content [18]. Vanillin-glacial acetic acid was used to determine saponin content [19]. A spectrophotometric technique was used to determine the polysaccharide content [20].
Enzymatic Activity Measurements
Nanjing Jiancheng Bioengineering Institute (Nanjing, China) offered kits to evaluate soil urease and dehydrogenase activity. The indigo colorimetry method was used to assess urease activity [21] and the dehydrogenase activity was analyzed measured by the 2,3,5-triphenyte-trazoliumchloride reduction method [22].
Health Risk Assessment of Heavy Metals
The health risk models, developed by USEPA, assess health risk assessments for heavy metals (HMs) through vegetable ingestion. Threshold hazard quotient (THQ) is a recognized parameter which is used to determine the health risk of all HMs, and the THQ for HMs is calculated using Eq. (2). If THQ ≤ 1.0, it is considered as an acceptable exposure level. On the contrary, if greater than this value indicates potential health risk.
where C is the concentration of heavy metals in plant, IngRveg. is the ingestion rate (g/day), EF is the exposure frequency of vegetable (day/year), ED is the exposure duration of vegetable (year), BW is body weight of human (kg), AT is the average time of exposure, RfD is the reference dose (being 0.001 mg/kg day for Cd) [23]. Children are more at risk than adults, so the correlation parameters are based on children. All the values of above parameters values used in Eq. (1) are all from published scientific research papers, and the specific information are shown in Supplementary Table S2 [24–26].
Data Analysis
The results are presented as mean ± standard deviation (SD). To examine the significance at P < 0.05, oneway analysis of variance (ANOVA) was used, followed by Duncan’s multiple comparisons (IBM SPSS version 22). Principal component analysis (PCA) was used to analyze variables of physiological parameters in leaf and growth indicators of plant exhibiting natural variance under different treatments. Normalization procedure was achieved using z-score. Biplots were draw to further elucidate their relationships using R 4.0.0 in ggplot2 packages.
RESULTS
Cd Content in the Soil-Plant System of Muskmelon
As shown in Table 2, we determined the Cd content in various parts of the soil–plant system. The Cd content in the whole system is in the order of soil > root > stem > leaf > muskmelon parts. Soil DTPA-extractable Cd content in Cd exposure alone group was highest, and different concentrations of nano iron oxide treatments could reduce it by 4.6–8.9%. In the Cd-alone, 10Fe and 50Fe treatments, the root Cd content was lower slightly than soil DTPA-extractable Cd. However, root Cd content was lower remarkably than soil DTPA-extractable Cd content in the 250Fe treatment. As an important nutrient organ between root and leaf, the stem Cd content directly determines the amount of Cd transported in plant. In this experiment, the stem Cd content in the Cd treatment is the highest, which is significantly higher than that in the other treatments, indicating that different concentrations of nano iron oxide could reduce the transport of Cd from the underground part to the above-ground part. In the 10Fe and 250Fe treatments, the accumulation of Cd in leaves was also significantly lower than that in Cd treatment.
The fruit Cd content is intimately related with public health since it is the most important portion of the muskmelon plant for human food and medication. The fruit yield under the 10Fe treatment was poor this year because to the weather. Therefore, in order to ensure the accuracy of data, we decided to discard the indexes related to muskmelon in the 10Fe treatment. To further analyze the specific distribution of Cd in plants, we divided muskmelon fruit into pedicel, peel, flesh and seed. As shown in Table 2, both 50 and 250Fe groups could significantly reduce the Cd content in each part of muskmelon fruit. More surprisingly, compared to 50Fe group, 250Fe group can further reduce the distribution of Cd in muskmelon seed, thus reducing the harm that may bring to human.
Urease and Dehydrogenase Activity in Soil
Urease exists widely in soil and is a kind of enzyme which has been studied deeply. In the study, urease activity was significantly inhibited by 15.72% after the addition of Cd (Supplementary Table S3). When the concentration of nano iron oxide was 50 mg/kg, the urease activity in soil rose to the control level. Adding Cd alone had no significant effect on dehydrogenase activity. However, all repair groups could increase dehydrogenase activity.
Transfer Factor in the Soil–plant System of Muskmelon
The changes in Cd transfer factors of muskmelon under different treatments are shown in Table 3. The transfer factors from the soil to root (TFsoil–root) of Cd, 10Fe and 50Fe groups were 0.902, 0.950, and 0.927, respectively, showing little change. However, TFsoil–root of the 250Fe group changed significantly, indicating that 250 mg/kg α-Fe2O3 NPs could reduce Cd uptake by plant roots, which is consistent with the previous results (Table 2).
However, we were surprised to find that the transfer factor from the stem to pedicel (TFstem–pedicel) decreased evidently with 50Fe and 250Fe compared to the Cd-alone treatment, and the 250Fe treatment had a better effect. When Cd is transported into the muskmelon pedicle, it will be transported to the muskmelon peel, flesh and seed. Compared to Cd-alone group, under the treatment of nano iron oxide, Cd will be more assigned to the muskmelon peel and flesh, and less to seed part. This may be a plant protection mechanism of nano iron oxide excitation–as far as possible to reduce toxic substances to seed parts transportation, so as to avoid being passed on to the next generation.
Cd Distribution in Three Subcellular Fractions of Leaf and Root
Cd concentration of subcellular fractions of the roots and leaves of the different treatments is shown in Table 4. It showed the order of Cd accumulation in subcellular fractions of the roots and leaves of the different treatments: cell wall (F1) > cell soluble component (F3) > organelle (F2). It can be seen that most Cd is trapped in the cell wall, followed by the cell soluble component, and the least part is organelle. It should be noted that the total Cd content in roots of 250Fe amendment is the lowest, which is also consistent with Table 2. In addition, the cell wall (F1) proportion in leaves of 250Fe amendment is the highest. Interestingly, the proportion of Cd in organelle (F2) in 250Fe amendment in leaves was the lowest in all groups, and the Cd content of organelle (F2) in roots was also the lowest in all groups.
Growth Indicators of Muskmelon
The physiological state of the plant can be best visualized via growth markers. Cd stress can have an effect on the macroscopic markers of plants at any time [27]. In the study, the three indexes (plant height, leaf length and leaf width) were not significantly affected by Cd treatment compared to those in control (Fig. 1). The Cd could only inhibit hypocotyl thickness of muskmelon, but the addition of nano iron oxide could not improve it. The fruit harvest was poor this year due to the weather, so the fruit index was put in the supplemental materials (Supplementary Table S4). However, it can still be seen that all indexes of muskmelon are significantly decreased after Cd treatment. The pulp thickness and sugar content were increased in 50Fe and 250Fe group compared to Cd exposure alone. Consequently, it was discovered that α-Fe2O3 NPs have some positive effects on Cd toxicity.
Oxidative Stress in Muskmelon Leaves
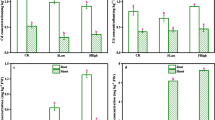
Antioxidant enzyme activity regulation is an intrinsic plant response to avoid oxidative stress generated by a variety of external biotic and abiotic factors [15]. As shown in Fig. 2, after adding Cd, the SOD and APX activities decreased significantly, indicating that excessive Cd poisoning impaired the antioxidant defense system’s ability to quench ROS in muskmelon. Under Cd stress, the 50Fe and 250Fe treatment boosted APX activity, indicating that α-Fe2O3 NPs have the potential to reduce Cd toxicity by boosting plant antioxidant capabilities. MDA is commonly employed as a marker of cell damage caused by free radicals since it is a secondary compound of lipid peroxidation processes. In this study, the Cd-alone treatment increased evidently the MDA content in plant leaves, and all α-Fe2O3 NPs-contain treatments could reduce the leaves MDA content. In addition, MDA content in the 50Fe group decreased significantly.
Chlorophyll and Soluble Protein Content in Muskmelon Leaves
In the study, the chlorophyll content was assessed using the SPAD values (a correlated index of chlorophyll content in leaves). After the addition of Cd, the chlorophyll content of plants decreased significantly. No matter which concentration of nano iron oxide was added, the chlorophyll content of plants increased to the same level as that of the control group (Supplementary Fig. S3). What’s more, 50Fe group saw the largest increase compared to Cd exposure alone, by 11.80%. Cd stress causes a variety of protective reactions, including the accumulation of proteins. After the addition of Cd, the protein content in muskmelon leaves significantly increased by 61.26% compared to the control group (Supplementary Fig. S4). However, 50 mg/kg α-Fe2O3 NPs treatment reduced the protein content to the control level.
DISCUSSION
Immobilization of Cadmium and Risk Assessment
The ability of muskmelon to absorb Cd from the soil, as well as its accumulation and distribution in different areas of the plant, were evaluated (Table 2). DTPA extraction is a popular and successful approach for determining heavy metal toxicity and accessibility [28]. The Cd concentration in the exposure systems was 400 mg/kg in the experiment. After four months of cultivation experiment, the soil DTPA-extractable Cd in the experimental treatment groups was 63.45–69.63% of the initial Cd content (400 mg/kg). In the study, the soil DTPA-extractable Cd was significantly lower under 50 mg/kg α-Fe2O3 treatment and 250 mg/kg α-Fe2O3 treatment than in the control group, which might be related to electrostatic adsorption, redox and coprecipitation [8].
Root Cd content is always close to that of the soil DTPA-extractable Cd. However, in this experiment, root Cd content was lower remarkably than soil DTPA-extractable Cd content in the 250Fe treatment. Kim et al. found that green synthesized iron oxide NPs (GION) could promote the formation of larger soil aggregates [29]. GION can absorb Cd and co-precipitate it under the action of soil microorganisms. In addition, with increasing GION dosage, more amorphous fractions were converted into stable crystalline fractions [8]. Hence, high concentration nano iron oxide treatment (250Fe group) might have converted more Cd to stable crystalline component, which is not conducive to plant absorption, resulting in less Cd transport to plant roots.
Pedicel and flesh are the most popular parts for human consumption, and muskmelon base (Pedicellus Melo.) has a long history as a Chinese traditional medicine (since the Ming Dynasty). Hence, the health risks of posed by Cd to children with the three parts were calculated (Table 2). The exposure frequency was set as 10 days/year. With α-Fe2O3 NPs application, a significant reduction of THQ for Cd was found for children. In the Cd-alone treatment, the THQ value of pedicel, flesh and seed is bigger than 1. It indicated that the edible parts of muskmelon treated with Cd alone have adverse health effect, and the risk of muskmelon seeds is the highest. When adding 50 g or 250 mg/kg α-Fe2O3 NPs, the THQ values of pedicel and flesh were 0.67, 0.67, 0.96, and 0.98. These THQ values are all less than 1, indicating that nano iron oxide could reduce potential health risk.
The objective of in situ immobilization of heavy metals is not to remove the metals, but by altering the form of occurrence of the sediments to stabilize it. In the experiment, the soil DTPA-extractable Cd content decreased after adding nano ferric oxide. In our previous research, we have shown that α-Fe2O3 NPs have a good adsorption effect on Cd [15]. There are active adsorptions sites on α-Fe2O3 NPs, Cd in soil will be desorbed by soil, and adsorbed by nano ferric oxide. α-Fe2O3 NPs in the soil will form secondary Fe minerals, and then re-adsorption and co-precipitation with Cd, forming more stable Fe minerals. Finally, the surface of Fe minerals is continuously covered by layers of newly formed iron oxides, forming stable soil aggregates that reduce Cd mobility [30]. After adding α-Fe2O3 NPs, Cd content in plants was also significantly reduced, reducing the potential risk of human consumption. Based on our results, using α-Fe2O3 NPs in production agriculture is an effective way to limit the risk of Cd to human health.
Effects of Nano Iron Oxide on Soil Enzyme Activity
Urease enzyme products, ammonia, are one of the nitrogen sources in plants, which is suitable for plant absorption [21]. Urease can also be used to determine the effects of heavy metals on soil function. Soil dehydrogenase belongs to the redox enzyme family, which can be used as an indicator of microbial redox ability and reflect the overall metabolic activity of soil microorganisms by oxidizing hydrogen or hydrogen donors from certain substrates [22]. Under Cd stress, Soil microorganisms will be in a dynamic equilibrium, and their enzyme activities can be suppressed and increased repeatedly [27]. In general, soil enzyme activities increased after adding α-Fe2O3 NPs. Various factors could be responsible for the improvement in soil enzymatic activities. Lin et al. found that iron oxide nanoparticles could improve the soil environment, as evidenced by changes in soil microorganisms [8]. It showed that microorganisms can fix Cd by altering the biogeochemical cycles of iron and nitrogen. So, Cd is more easily fixed when soil microbes are more active [31]. Combined with our experimental results, we believe that nano iron oxide has a certain remediation effect on the Cd-contaminated soil environment.
Effects of Nano Iron Oxide on Cd Subcellular Distribution
During the plant transport of Cd, Cd is distributed to different subcellular fractions. Evasion mechanism and tolerance mechanism are mainly two categories of Cd tolerance mechanisms in plants [32]. Retention of cell walls is one of evasion mechanism, which is also well reflected in our experiment. The majority of Cd was retained in the cell wall, demonstrating that the muskmelon cell wall bound Cd ions and inhibit their transmembrane transit, which can protect protoplasts from Cd toxicity. As a result, it plays a crucial role in Cd compartmentalization and resistance. That the highest cell wall (F1) proportion in leaves of 250Fe amendment indicated that 250 mg/kg α-Fe2O3 NPs could help plants to reduce Cd toxicity by using the evasion mechanism. However, the ability of cell walls to separate Cd is usually limited because polysaccharides (such as cellulose, hemicellulose and pectin) and proteins in cell walls lack enough functional bases to bind Cd ions [33]. Thereby, a part of Cd passes through the cell wall and enters the protoplast. Metal transporters in the protoplast can either pump metal ions into the vacuole or chelate the metal in the vacuole, keeping heavy metals out of metabolic processes and lowering their toxicity [34]. There also are high Cd levels in F3, and this is also consistent with the results of the experiment. In 250Fe group, the proportion of Cd in F1 was the highest and F2 was the lowest in leaves. Based on the subcellular distribution of Cd in plants, the 250Fe group could strengthen the self-defense mechanism of cells to reduce Cd toxicity.
Effects of Nano Iron Oxide on Physiological and Biochemical Indicators
SOD is the first line of defense against ROS. It converts superoxide anion radicals to H2O2 and O2. Under normal conditions, POD, APX and CAT can effectively remove H2O2. The activity of antioxidant enzymes is linked to plant resistance under stress [15]. Cd stress induced changes in antioxidant enzyme activities, including increased SOD and POD activities [15]. Under high Cd levels, Gowayed discovered that SOD and CAT activities reduced, revealing a threshold below which heavy metals enhance the antioxidant defense mechanism in plants [35]. In the experiment, α-Fe2O3 NPs can relieve Cd stress in plants by activating antioxidant enzymes. Thus, MDA content was reduced in all α-Fe2O3 NPs-contain treatments.
The chlorophyll content provides information on the physiological state of plants [13]. Under Cd toxicity, protochlorophyllide reductase and δ-aminolevulinic acid dehydrogenase are both inhibited by metal, and the chlorophyll content of plants will decrease significantly [36]. The chlorophyll content of plants increased most in 50Fe group, and protein accumulation caused by Cd was avoided. Combined with the results of chlorophyll and soluble protein, 50 mg/kg α-Fe2O3 NPs treatment showed the best repair effect.
Effects of Nano Iron Oxide on Active Ingredients
Plant polysaccharides are biodegradable and renewable primary metabolites that are widely used in food and pharmaceuticals. Some polysaccharides have been shown to have anti-cancer, hepatoprotective, antithrombotic, antidiabetic, and other properties. Compared to the control treatment, the Cd2+ significantly decreased polysaccharide content in the root, stem and flesh (Fig. 3a). Liu found that under the Cd stress of 4 nmg/kg, the polysaccharide content in Agaricus brasiliensis J77 was significantly lower than that of control group [20]. It might be that Cd inhibited the synthesis of polysaccharides. However, all α-Fe2O3 NPs-contain treatments could increase the root polysaccharide content by 10.18–27.46% and 50 mg/kg α‑Fe2O3 NPs treatment could increase the stem polysaccharide content by 11.19%. This indicates that nano iron oxide had a certain repairing effect.
Content of (a) polysaccharides, (b) polyphenols, (c) flavonoids, (d) saponins in different parts of muskmelon plants under the (1) CK treatment, (2) Cd treatment, (3) Cd + 10Fe treatment, (4) Cd + 50Fe treatment, (5) Cd + 250Fe treatment. The results are shown as mean ± SD (n = 3). Values preceded by various lowercase letters are statistically different at P < 0.05.
Polyphenols are a type of phytonutrient found largely in the vacuoles of plant cells in soluble form, considered to confer health benefits [17]. The addition of Cd alone decreased polyphenol content of stem, leaf, seed, pericarp and flesh compared to control (Fig. 3b). Misuzu et al. found that compared to the control treatment, phenolic compounds declines as Cd leaf concentrations increase [37]. The results reflected the toxicity of Cd to plants. Nonetheless 250Fe amendment could improve the root polysaccharide content of stem, seed, pericarp and flesh, which had a certain relieving effect on toxicity of Cd.
Many flavonoids have been proven to have antioxidative, free-radical scavenging, coronary heart disease prevention, and anticancer properties, and other flavonoids have the potential to have anti-HIV effects. In the study, in the Cd-alone treatment, flavonoid of root, leaf, seed and flesh decreased significantly compare to control group (Fig. 3c). Similarly, Zhao et al. found that the content of total flavonoids presented a decline trend in wild-type and the mutant snc1 following Cd exposure [38]. This was due to the toxic effect of Cd on plants. Nevertheless, all α-Fe2O3 NPs-contain treatments could improve the flavonoid content of root and flesh, indicating that these treatments have positive effects.
Saponins are amphipathic glycosides found in a variety of plant species [19]. They cut cholesterol, reducing hepatic steatosis and reduce inflammation, etc. Under Cd exposure, the flavonoid concentration in the root, leaf, pericarp and flesh was dramatically reduced compared to the control (Fig. 3d). It might be because Cd stress inhibited the synthesis of terpenoids precursors [39]. However, root saponin content of the 50Fe and 250Fe group, leaf saponin content of the 50Fe group and pericarp saponin content of the 250Fe group increased observably. It should be the effects caused by the α-Fe2O3 NPs repair treatments.
From the overall perspective of effective components, we found that effective component content decreased after the addition of Cd. This might be due to the toxic effect of Cd on plants. What’s more, α‑Fe2O3 NPs in various concentrations were able to alleviate Cd toxic in muskmelon fruit, and 250Fe group had the best restorative effect.
PCA of Physiological Indicators in Muskmelon
Principal component analysis (PCA) is used to analyze the main components of data. PCA can propose the main components from a large number of indicators by dimensionality reduction, so as to better understand some relationships between variables. In the experiment, four main components were extracted (Supplementary Fig. S5). Among them, the cumulative variance explanation rate of first and second principal components is about 80% (Fig. 4), indicting the total variations are nicely represented by the biplots. For growth index, hypocotyl thickness and plant height were negatively correlated with Cd content. For physiological indicators, Cd content was positively correlated with POD. APX and chlorophyll were negatively correlated with Cd content. Among them, POD was the index most affected by Cd. For active ingredients, Cd content was significantly negatively correlated with polyphenols and flavonoids.
The control group was far distant from the Cd exposure group in terms of the effects of several treatment groups. It was discovered that applying Cd to the muskmelon plant had a significant effect. The point of 50Fe group was closer to the control group’s point, showing that 50 mg/kg α-Fe2O3 NPs reduced Cd toxicity, as evidenced by a number of indicators in the results data, such as chlorophyll and MDA. The point of 250Fe group is also closer to CK group than Cd group. Therefore, it also has some restorative effects. It can also be seen from the quality of muskmelon (Supplementary Table S2), the effect of 50Fe group and 250Fe group are very similar. It may be because nano ferric oxide can not only reduce the availability of Cd in soil, but also act as iron fertilizer [40]. When a particular concentration is attained, further raising the concentration has little impact on plants [40]. It’s also possible that nano iron oxide has a somewhat harmful effect on plants at large concentration. As the concentration increases, the positive and negative effects cancel each other out, so that 50Fe group and 250Fe group had similar effects. Therefore, considering cost saving and plant-friendly, it is preferable to choose 50 mg/kg α-Fe2O3 NPs treatment. The worst repair effect was found in the 10Fe group, which might also be one of the reasons for the poor harvest of the group.
In conclusion, it can be found in the experiment that when plants are exposed to Cd stress, plant height and hypocotyl thickness will decrease observably. The synthesis of APX would be inhibited, and the plant would mainly activate POD to resist stress. Cd inhibited the synthesis of polyphenols and flavonoids. Compared with 10Fe and 50Fe group, a 250Fe group had the best repair effect.
CONCLUSION
In this study, a number of physiological and biochemical indicators were examined to study the effects of α-Fe2O3 NPs on Cd toxicity toward muskmelon plants. Our research found that Cd is easily absorbed and accumulated by muskmelon plants, and THQ values showed that there is a health risk in edible parts. After adding α-Fe2O3 NPs, it would adsorb Cd and co-precipitate to form more stable Fe minerals, which reduced its migration. Surprisingly, according to various indicators, α-Fe2O3 NPs minimized Cd transport in plants, lower human health risk, and alleviates plant Cd toxicity. α-Fe2O3 NPs could also increase the content of active ingredients in plants and enzyme activities in soil. The different concentrations of α-Fe2O3 NPs had different effects on Cd toxicity, but 50 mg/kg α-Fe2O3 NPs treatment was found best. According to the experimental results, using α-Fe2O3 NPs to repair Cd toxicity in soil is a great choice. Our findings suggest various agricultural production and environmental protection strategies in the face of Cd pollution.
REFERENCES
Zhou, D., Song, X., Zhao, F., and Gu, B., Soil environment and pollution remediation, Pedosphere, 2017, vol. 27, p. 387. https://doi.org/10.1016/s1002-0160(17)60359-x
Rizwan, M., Ali, S., Zia-ur-Rehman, M., Adrees, M., Arshad, M., Qayyum, M.F., Ali, L., Hussain A., Chatha, S., and Imran, M., Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil, Environ. Pollut., 2019, vol. 248, p. 358. https://doi.org/10.1016/j.envpol.2019.02.031
Kim, E.J., Jeon, E.K., and Baek, K., Role of reducing agent in extraction of arsenic and heavy metals fromsoils by use of EDTA, Chemosphere, 2016, vol. 152, p. 274. https://doi.org/10.1016/j.chemosphere.2016.03.005
Liu, X., Zeng, Z., Tie, B., Chen, Q., Chen, Z., and Zheng, Q., Effects of biochar and lime application on soluble Cd, Pb, As release and non-point loads of rice agroecosystem by in situ field experiment, Central Hunan Province Mining Area, Hamdard Medicus, 2013, vol. 32, p. 28.
Hu, J., Guo, H., Li, J., Gan, Q., and Xing, B., Comparative impacts of iron oxide nanoparticles and ferric ions on the growth of Citrus maxima, Environ. Pollut., 2017, vol. 221, p. 199. https://doi.org/10.1016/j.envpol.2016.11.064
Abdelhamid, H.N. and Mathew, A.P., Cellulose-zeolitic imidazolate frameworks (CelloZIFs) for multifunctional environmental remediation: Adsorption and catalytic degradation, Chem. Eng. J., 2021, vol. 426, p. 131733. https://doi.org/10.1016/j.cej.2021.131733
Praveen, A., Khan, E., Ngiimei D.S., Perwez, M., Sardar, M., and Gupta, M., Iron oxide nanoparticles as nano-adsorbents: a possible way to reduce arsenic phytotoxicity in indian mustard plant (Brassica juncea L.), J. Plant Growth Regul., 2017, vol. 37, p. 612. https://doi.org/10.1007/s00344-017-9760-0
Lin, J., He, F., Su, B., Sun, M., Owens, G., and Chen, Z., The stabilizing mechanism of cadmium in contaminated soil using green synthesized iron oxide nanoparticles under long-term incubation, J. Hazard. Mater., 2019, vol. 379, p. 120832. https://doi.org/10.1016/j.jhazmat.2019.120832
Silva, M.A., Albuquerque, T.G., Alves, R.C., Oliveira, M.B.P.P., and Costa, H.S., Melon (Cucumis melo L.) by-products: Potential food ingredients for novel functional foods? Trends Food Sci. Technol., 2018, vol. 98, p. 181. https://doi.org/10.1016/j.tifs.2018.07.005
Zou, Z., Wang, Y., Huang, J., Lei, Z., Wan, F., Dai, Z., Yi, L., and Li, J., A study on the mixture repairing effect of biochar and nano iron oxide on toxicity of Cd toward muskmelon, Environ. Pollut., 2020, vol. 266, p. 115371. https://doi.org/10.1016/j.envpol.2020.115371
Zhou, Y., Zou, Z., Wang, M., Wang, Y., Li, J., Qiu, L., Cheng, Y., and Dai, Z., Biochar and nano-ferric oxide synergistically alleviate cadmium toxicity of muskmelon, Environ. Res. Public Health, 2023, vol. 30, p. 57945. https://doi.org/10.1007/s11356-023-26369-8
Niu, H., Wang, Z., Song, J., Long, A., Cao, M., and Luo, J., Cadmium subcellular distribution and chemical form in Festuca arundinacea in different intercropping systems during phytoremediation, Chemosphere, 2021, vol. 276, p. 130137. https://doi.org/10.1016/j.chemosphere.2021.130137
Wan, Y., Li, J., Ren, H., Huang, J., and Yuan, H., Physiological investigation of gold nanorods toward watermelon, J. Nanosci. Nanotechnol., 2014, vol. 14, p. 6089. https://doi.org/10.1166/jnn.2014.8853
Wang, Y., Zou, Z., Su, X., Wan, F., Zhou, Y., Lei, Z., Yi, L., Dai, Z., and Li, J., Physiological of biochar and α-Fe2O3 nanoparticles as amendments of Cd accumulation and toxicity toward muskmelon grown in pots, J. Nanobiotechnol., 2021, vol. 19, p. 442. https://doi.org/10.1186/s12951-021-01187-7
Li, Z., Fu, J., Shi, D., and Peng, Y., Myo-inositol enhances drought tolerance in creeping bentgrass through alteration of osmotic adjustment, photosynthesis, and antioxidant defense, Crop Sci., 2020, vol. 60, p. 2149. https://doi.org/10.1002/csc2.20186
Niu, Y., Liu, Z., He, H., Han, X., Qi, Z., and Yang, Y., Gene expression and metabolic changes of Momordica charantia L. seedlings in response to low temperature stress, PLoS One, 2020, vol. 15, p. e0233130. https://doi.org/10.1371/journal.pone.0233130
Turkmen, N., Sari, F., and Velioglu, Y.S., Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods, Food Chem., 2006, vol. 99, p. 835. https://doi.org/10.1016/j.foodchem.2005.08.034
Shad, A.A., Ahmad, S., Ullah, R., AbdEl-Salam, N.M., Fouad, H., Rehman, N.U., Hussain, H., and Saeed, W., Phytochemical and biological activities of four wild medicinal plants, Sci. World J., 2014, vol. 2014, p. 857363. https://doi.org/10.1155/2014/857363
Dou, F., Xi, M., Wang, J., Tian, X., Hong, L., Tang, H., and Wen, A., α-Glucosidase and -amylase inhibitory activities of saponins from traditional Chinese medicines in the treatment of diabetes mellitus, Pharmazie, 2013, vol. 68, p. 300. https://doi.org/10.1691/ph.2013.2753
Liu, L., Chen, H., Yuan, J., Wang, Y., Weng, B., Liu, P., and Li, G., Effects of cadmium stress on physiological indexes and fruiting body nutritions of Agaricus brasiliensis, Sci. Rep., 2021, vol. 11, p. 8653. https://doi.org/10.21203/rs.3.rs-119919/v1
Chen, J., Yang, W., Li, J., Anwar, S., Wang, K., Yang, Z., and Gao, Z., Effects of herbicides on the microbial community and urease activity in the rhizosphere soil of maize at maturity stage, J. Sens., 2021, vol. 2021, p. 6649498. https://doi.org/10.1155/2021/6649498
Medo, J., Maková, J., Medová, J., Lipková, N., and Javoreková, S., Changes in soil microbial community and activity caused by application of dimethachlor and linuron, Sci. Rep., 2021, vol. 11, p. 12786. https://doi.org/10.1038/s41598-021-91755-6
Avigliano, E., Lozano, C., Plá, R.R., and Volpedo, A.V., Toxic element determination in fish from Paraná River Delta (Argentina) by neutron activation analysis: tissue distribution and accumulation and health risk assessment by direct consumption, J. Food Compos. Anal., 2016, vol. 54, p. 27. https://doi.org/10.1016/j.jfca.2016.09.011
Lu, M., Liang, Y., Lakshmanan, P., Guan, X., Liu, D., and Chen, X., Magnesium application reduced heavy metal-associated health risks and improved nutritional quality of field-grown Chinese cabbage, Environ. Pollut., 2021, vol. 4, p. 17881. https://doi.org/10.1016/j.envpol.2021.117881
Cao, S., Duan, X., Zhao, X., Ma, J., Dong, T., Huang, N., Sun, C., He, B., and Wei, F., Health risks from the exposure of children to As, Se, Pb and other heavy metals near the largest coking plant in China, Sci. Total Environ., 2014, vol. 472, p. 1001. https://doi.org/10.1016/j.scitotenv.2013.11.124
Guo, G., Lei, M., Wang, Y., Song, B., and Yang, J., Accumulation of As, Cd, and Pb in sixteen wheat cultivars grown in contaminated soils and associated health risk assessment, Int. J. Environ. Res. Public Health, 2018, vol. 15, p. 2601. https://doi.org/10.3390/ijerph15112601
Yu, B., Peng, Y., Xu, J., Qin, D., Gao, T., Zhu, H., Zuo, S., Song, H., and Dong, J., Phytoremediation potential of Youngia japonica (L.) DC: a newly discovered cadmium hyperaccumulator, Environ. Sci. Pollut. Res., 2021, vol. 28, p. 6044. https://doi.org/10.1007/s11356-020-10853-6
Liu, Q., Chen, Z., Tang, J., Luo, J., Huang, F., Wang, P., and Xiao, R., Cd and Pb immobilisation with iron oxide/lignin composite and the bacterial community response in soil, Sci. Total Environ., 2021, vol. 802, p. 149922. https://doi.org/10.1016/j.scitotenv.2021.149922
Kim, E.J., Jeon, E.K., and Baek, K., Role of reducing agent in extraction of arsenic and heavy metals from soils by use of EDTA, Chemosphere, 2016, vol. 152, p. 274. https://doi.org/10.1016/j.chemosphere.2016.03.005
Li, X.C., Yang, Z.Z., Zhang, C., Wei, J.J., Zhang, H.Q., Li, Z.H., Ma, Chi., Wang, M.S., Chen, J.Q., and Hu, J.W., Effects of different crystalline iron oxides on immobilization and bioavailability of Cd in contaminated sediment, Chem. Eng. J., 2019, vol. 373, p. 307. https://doi.org/10.1016/j.cej.2019.05.015
Liu, M., Wang, J., Xu, M., Tang, S., Zhou, J., Pan, W., Ma, Q., and Wu, L., Nano zero-valent iron-induced changes in soil iron species and soil bacterial communities contribute to the fate of Cd, J. Hazard. Mater., 2022, vol. 424, p. 127343. https://doi.org/10.1016/j.jhazmat.2021.127343
Su, H., Zou, T., Lin, R., Zheng, J., Jian, S., and Zhang, M., Characterization of a phytochelatin synthase gene from Ipomoea pes-caprae involved in cadmium tolerance and accumulation in yeast and plants, Plant Physiol. Biochem., 2020, vol. 155, p. 743. https://doi.org/10.1016/j.plaphy.2020.08.012
Fu, X., Dou, C., Chen, Y., Chen, X., Shi, J., Yu, M., and Xu, J., Subcellular distribution and chemical forms of cadmium in Phytolacca americana L., J. Hazard. Mater., 2011, vol. 186, p. 103. https://doi.org/10.1016/j.jhazmat.2010.10.122
Hu, X., Li, T., Xu, W., and Chai, Y., Distribution of cadmium in subcellular fraction and expression difference of its transport genes among three cultivars of pepper, Ecotoxicol. Environ. Saf., 2021, vol. 216, p. 112182. https://doi.org/10.1016/j.ecoenv.2021.112182
Gowayed, S., Impact of zinc oxide nanoparticles on germination and antioxidant system of maize (Zea mays L.) seedling under cadmium stress, Plant Prod. Sci., 2017, vol. 6, p. 1. https://doi.org/10.21608/jpps.2017.7389
Janeeshma, E., Kalaji, H.M., and Puthur, J.T., Differential responses in the photosynthetic efficiency of Oryza sativa and Zea mays on exposure to Cd and Zn toxicity, Acta Physiol. Plant., 2021, vol. 43, p. 12. https://doi.org/10.1007/s11738-020-03178-x
Sakurai, M., Tomioka, R., Hokura, A., Terada, Y., and Takenaka, C., Distributions of cadmium, zinc, and polyphenols in Gamblea innovans, Int. J. Phytorem., 2019, vol. 21, p. 217. https://doi.org/10.1080/15226514.2018.1524840
Zhao, Q., Gu, C., Sun, Y., Li, G., Li, L.L., and Hao, L., Root defense in salicylic acid-altering arabidopsis plants in responses to cadmium stress, J. Plant Growth Regul., 2020, vol. 40, p. 1764. https://doi.org/10.1007/s00344-020-10233-x
Liao, P., Shi, Y., Li, Z., Chen, Q., Xu, T.R., Cui, X., Guan, H., Guo, L., and Yang, Y., Impaired terpenoid backbone biosynthesis reduces saponin accumulation in Panax notoginseng under Cd stress, Funct. Plant Biol., 2019, vol. 46, p. 56. https://doi.org/10.1071/fp18003
Askary, M., Amirjani, M.R., and Saberi, T., Comparison of the effects of nano-iron fertilizer with iron-chelate on growth parameters and some biochemical properties of Catharanthus roseus, J. Plant Nutr., 2016, vol. 40, p. 974. https://doi.org/10.1080/01904167.2016.1262399
Funding
This work was supported by China Agriculture Research System of MOF and MARA (grant no. CARS-25), the Fundamental Research Funds for the Central Universities (grant no. 2019IB005, 2020IB029, 2021IA005), Hubei Provincial Natural Science Foundation of China (grant no. 2022CFB380).
Author information
Authors and Affiliations
Contributions
Z. Zou and Y. Zhou equally contributed to this work. Z. Zou and Y. Zhou performed investigation, formal analysis, writing original draft, writing review and editing. Y. Hao, Y. Wang, M. Qi, L. Qiu and Y. Cheng participated in investigation and formal analysis. Z. Dai contributed to supervision, conceptualization, validation, resources. J. Li contributed in supervision, conceptualization, resources, project administration, funding acquisition, writing review and editing.
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants as objects of research.
Additional information
Abbreviations: Cd—cadmium; GION—green synthesized iron oxide nanoparticles; NPs—nanoparticles; RCBD—randomized complete block design; DTPA—diethylenetriaminepentaacetate TF—wild type; SOD—superoxide dismutase; CAT—catalase; POD—peroxidase; APX—ascorbic acid peroxidase; HMs—heavy metals; PCA—principal component analysis.
Supplementary Information
Rights and permissions
About this article
Cite this article
Zou, Z., Zhou, Y., Hao, Y. et al. The Effect of α-Fe2O3 Nanoparticles on the Physiological Characteristics and Active Ingredients of Muskmelon on the Cadmium-Polluted Soil. Russ J Plant Physiol 70, 139 (2023). https://doi.org/10.1134/S1021443723601532
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443723601532