Abstract
Strategic valorization of readily available sugarcane bagasse (SB) is very important for waste management and sustainable biorefinery. Conventional SB pretreatment methods are ineffective to meet the requirement for industrial adaptation. Several past studies have highlighted different pretreatment procedures which are lacking environmentally benign characteristics and effective SB bioconversion. This article provides an in-depth review of a variety of environmentally acceptable thermochemical and biological pretreatment techniques for SB. Advancements in the conversion processes such as pyrolysis, liquefaction, gasification, cogeneration, lignin conversion, and cellulose conversion via fermentation processes are critically reviewed for the formation of an extensive array of industrially relevant products such as biofuels, bioelectricity, bioplastics, bio adsorbents, and organic acids. This article would provide comprehensive insights into several crucial aspects of thermochemical and biological conversion processes, including systematic perceptions and scientific developments for value-added products from SB valorization. Moreover, it would lead to determining efficient pretreatment and/or conversion processes for sustainable development of industrial-scale sugarcane-based biorefinery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, the major problem faced by humankind is mainly raised from the shortage of conventional forms of energy and the subsequent impact on environmental sustainability. Therefore, global humankind has started to retrieve the alternative form of energy from lignocellulosic biomass such as agricultural and forest residues. The wastes generated from the agricultural lands and industries frequently pose a major challenge as per as their disposal is concerned. However, such agricultural wastes also emerged as appealing substrates for the fermentation process due to their accessibility and promising compositional contents (Ng et al. 2020). The strategical valorization of agricultural residues is a crucial aspect that assists to establish environment-friendly processes for the production of value-added commodities (Yaashikaa et al. 2021). In addition, the wastes generated through excessive agricultural practices are ultimately utilized for a variety of manufacturing chains (Debnath et al. 2021a, b). Biomass is a high-energy substance and its use potentially helps to alleviate the looming energy problem (Debnath et al. 2021a, b). Sugarcane is one of the most widely produced biomass on the planet, and scientific utilization of its waste part which is unanimously famous as bagasse might be a solution to such energy-related problems. In the very beginning, Southeast Asia and Western India were the first to cultivate sugarcane and now the global sugarcane cultivation is 1.6 billion tonnes per year, resulting in 279 million metric tonnes of biomass leftovers (Jugwanth et al. 2020). Primarily, bagasse accounts for around 35% of the entire weight of the cane, and only a minimum of 50% is hardly utilized to generate electricity and heat for thermochemical processing of biomass (Sarker et al. 2017a, b). The most difficult aspects of utilizing bagasse are the conversion processes which facilitate the transformation of biomass into value-added products (Conag et al. 2017; Haldar and Purkait 2020a, b). This can be accomplished using pretreatments, hydrolysis, and fermentation processes which are responsible to determine the effectiveness of the biomass (Zabed et al. 2019). As per pretreatment methods are concerned, the thermochemical processes are more appealing due to the process efficiencies, higher versatility, and quicker conversion rates. On the other side, the biological methods are time-consuming due to extended hydrolysis periods (Sarker et al. 2021). Now, owing to its intrinsic features, such as high water content, huge quantity, lower density, diversity, and reduced energy content, SB offers several obstacles to thermochemical conversion (Okolie et al. 2021). The recalcitrance nature of SB with the restricted degradation of lignin makes biological conversion of biomass highly difficult (Yoo et al. 2020). As a result, most of the studies attempted several thermochemical pretreatment methods not only to separate the lignin from biomass but also for an effective breakdown of SB. However, the consumption of heat energy during such pretreatment processes necessitates further attention to reduce the substantial intake of energy (Bala and Singh 2019). So the development/designing of an optimal reaction state is of utmost importance to achieve the best results from SB. Furthermore, the addition of several chemicals to the reaction mixture, such as organic solvents, alkali, acids, or a liquid combination of ions, considerably reduces the reaction time and results in faster and more effective thermochemical processes (Tu and Hallett 2019; Haldar and Purkait 2020a, b). The pretreatment efficiency of the SB was significantly improved by the introduction of exogenetic mineral acids such as phosphoric acid and sulfuric acid which catalyzed the reaction by shortening the time and temperature for the pretreatment of biomass (Santo et al. 2020). It was also reported that during an organosolv process, SB pretreated with 1.2% H2SO4 and 90% glycerol at 130 °C for 30 min resulted in 77% digestibility of glucan with a delignification efficiency of 57% (Zhao et al. 2017). However, acid addition must be carefully monitored to avoid the breakdown of pentoses into furfural and hexoses into 5-(hydroxymethyl)-furfural (HMF), whereas in the case of the organosolv process, a higher temperature is required for the utilization of alcohols with low boiling points (< 290 °C) such as ethyl glycol. Similarly, the incorporation of biological elements such as microorganisms or enzymes facilitates the biological pretreatment of lignocellulosic biomass (Vu et al. 2020). For instance, the biological pretreatment of SB with Sporotrichum thermophile BJAMDU5 at 45 °C for 96 h resulted in 76.4% delignification (Bala and Singh 2019). Likewise, a biological pretreatment utilizing the fungi Pycnoporus sanguineus considerably reduced the lignin content to 6.5% from an initial concentration of 12.9% (Hernández et al. 2017). Due to the mass and heat transport phenomena occurring in the gas–solid-liquid interphases, the scale-up effect is a common drawback of the biological pretreatment processes.
The thermochemical conversion processes are advocated as the viable conversion method for SB because of its indiscriminate mechanism for converting solids to fluids which may be further refined to create biofuels, fine chemicals, green solvents, and petrochemical feedstock, among others. Gasification, thermal liquefaction, pyrolysis, and combustion are the main thermochemical conversion processes for the valorization of SB into biofuels. For instance, it was reported that at 500 °C with a heating rate of 20 °C min−1, the pyrolysis of SB yielded 49.7 wt% of bio-oil against 42.1 wt% of pure SB in which 38.2% accounted for the organic phase and the remaining 11.5% was the aqueous phase (Ahmed et al. 2018). The downside of thermochemical processes is that their products must be further upgraded and refined before they can be utilized as fuel. The biological processing technique utilized for the valorization of SB to biofuel, bioplastics, and organic acids is fermentation. For instance, Clostridium acetobutylicum DSM 6228 fermented the hydrolysate of SB, yielding final concentrations of 9.1 g L−1 of biobutanol and 0.8 g L−1 of ethanol (da Conceição Gomes et al. 2019). The major drawback of the biological conversion process is that it is very time-consuming and results in a low yield of the product.
Therefore, the current review paper briefly discusses the traditional pretreatment methods to shed light on their efficacy before the processing of SB into value-added products. Given that, progress in a few thermochemical and biological pretreatment techniques, such as dilute acid, alkaline, organosolv, steam explosion, ammonia fiber expansion, and enzymatic delignification processes, is comprehensively analyzed to investigate the significance of the reaction parameters for process developments. Thereafter, based on the latest investigations, the strategical processing techniques are critically reviewed for product valorization of SB into different value-added products like bioelectricity, bioplastics, bio-adsorbents, and biofuels. Finally, the article is concluded by discussing the issues that are typically faced in a sugarcane-based biorefinery with the enlightenment on the technical ways to overcome the problems from a futuristic perspective. As a result, the readers of this paper will be able to learn about all of the major aspects of the emerging thermochemical and biological pretreatments methods before the processing technologies are adopted for the conversion of SB into several value-added products.
Overview of the structural composition of the sugarcane bagasse and its characteristics
The primary components of the SB are cellulose, hemicellulose, and lignin, which are structured in a three-dimensional non-uniform pattern of varying degrees and relative compositions (Machineni 2020). The SB comprises cellulose in the range of 35–50%, hemicellulose in 20–35%, and lignin in the range of 10–25%. The chemical composition of different portions of sugarcane obtained from various parts of the world is shown in Table 1. As inferred from Table 1, the chemical composition of cellulose, hemicellulose, and lignin of the sugarcane residues obtained from different parts of the world varied vastly. However, in all the regions, the cellulose content of the SB was predominant over the hemicellulose and the lignin content. The carbohydrates namely the hemicelluloses and celluloses, on hydrolysis, produce fermentable sugars (Dharmaraja et al. 2020).
Cellulose
Cellulose is among the pivotal structural constituents present in the cell wall of the plant, and it contributes to around 35–50% of the dry mass of the entire primary cell wall. Cellulose is a polysaccharide with a huge molecular weight consisting of repeated monomer units of glucose. Cellulose, in comparison with hemicellulose, is strong, crystalline, and resilient to hydrolysis, whereas hemicelluloses consist of an arbitrary and amorphous arrangement with minimal sturdiness (Akhtar et al. 2016). A parallel cell wall structure of a diameter of 10 to 25 nm is formed by the microfibrils of cellulose, and the diameter of the basic fibrils is between 2 and 4 nm. The consistent periodic arrangement of the cellulose fibers in micro-fibrils is known as a micelle (Khaire et al. 2021). Cellulose is an organic molecule at room temperature and is insoluble in water as well as alkali and acid solutions. The cellulose polymer consists of thousands of D-glucose units interconnected in a straight chain consisting of β-1,4 linkages. The positioning of the cellulose chain is brought about by the 180° turn of each repeating unit of the β-D-glucopyranose to the next unit of β-D-glucopyranose (Yadav et al. 2021). The microfibrils of cellulose are formed by the further aggregation or crosslinking through hydrogen bonds and by the development of intermolecular and intramolecular van der Waals bonds (Ji et al. 2021). The typical amount of polymerization in the primary cell wall is around 6000 units, whereas the secondary cell wall contains up to 14,000 units of glucose. The hydrophobic and crystalline nature of the cellulose microfibrils significantly contributes to biomass recalcitrance (Bhat et al. 2017).
Hemicellulose
Hemicellulose otherwise known as polyose consists of numerous heteropolysaccharides like arabinoxylans that are found in the cell wall of the plants in addition to cellulose. Xylan, glucuronoxylan, arabinoxylan, xyloglucan, and glucomannan are the polymers of hemicellulose that comprise a variety of monomers of sugar (Pournou 2020). The other sugar monosaccharides that are present in the hemicellulose are rhamnose, arabinose, xylose, galactose, and mannose (Becker et al. 2021). The hemicellulose has a large quantity of D-C5 sugars and occasionally tiny amounts of L-C5 sugars. Although mannose might be the predominant sugar in softwoods, xylose is the most common monomeric sugar found in hemicellulose polymer in most circumstances. Apart from the usual sugar residues, sugars that are acidified, such as galacturonic acids and glucuronic acid, are also detected in the hemicelluloses. Various enzymes, bases, or dilute acids can easily hydrolyze the hemicelluloses (Okolie et al. 2021). Hemicelluloses account for 15–35% of total the lignocellulosic biomass and they are amorphous heteropolysaccharides that can either be branched or form as a single chain (Chen et al. 2019). Hemicelluloses are comprised of monomeric sugars with a degree of polymerization of 500–3000 monomeric units. The monomeric sugar units are pentoses such as arabinose, xylose, and uronic acids, and hexoses such as acetylated sugars, glucose, and mannose (Yang and Lü 2021). The pyranol sugars, such as xylose, mannose, and glucose, are linked at the O4 position by a β-(1,4) glycosidic bond (Khaire et al. 2021). The hemicellulose is non-crystalline as the side chains of hemicelluloses are short, and the amount of various functional groups present in branches varies depending on the kind of biomass. Xyloglucans, arabinans, mannans, and xylans are the four principal groups of hemicelluloses based on the β-(1,4) linked monosaccharides found in the primary chain of polysaccharides (de Lucas et al. 2020). The most prevalent hemicellulose is xylan, which varies in composition between species (Zhou et al. 2017). The primary function of hemicellulose is to reinforce the plant cell wall by interacting with cellulose and lignin molecules.
Lignin
The tissues of algae and vascular plants consist of a group of intricate organic polymers known as lignin which serves an important function of providing structural support to the plants. Lignin is implicated in the production of woody bark of the plant cell wall and therefore contributes to the rigidity and stiffness of the plant (Armah et al. 2022). Lignins are phenolic crosslinked polymers with heterogenous, extremely branched, and amorphous structures. Lignins are made by the repetition of three essential phenylpropane monomers namely the p-coumaryl alcohol, sinapyl alcohol, and coniferyl alcohol, which are together known as monolignols (Naseem et al. 2016). Coumaryl alcohol makes up the majority of softwood lignin and coniferyl alcohols and sinapyl alcohol make up hardwood lignin and grass lignin (Ponnusamy et al. 2019). Lignin is found in the secondary cell wall of plants and helps vascular plants transfer nutrients and water to the xylem by providing mechanical sustenance to the plant cells. Plant pathogens are resistant to it because of its recalcitrance. The composition of lignin varies from species to species, and it constitutes 43.7% carbon, 46.5% oxygen, 5.1% hydrogen, and 4.0% ash (Santos et al. 2020). Due to its variability and deficit of clarity in its fundamental structures, lignin is a unique compound.
Physico-chemical properties of SB
Bagasse is made up of 50% cellulose and 25% hemicellulose and lignin, respectively. It consists of around 50% α-cellulose, 30% pentosans, and 2.4% ash chemically. Because of its low ash level, bagasse has several benefits over other agricultural leftovers such as wheat straw and rice straw, as they contain 11.5% ash and 17.5% ash, respectively (Sindhu et al. 2016a, b). Sugarcane processing procedures, such as harvesting, sugarcane transport, and biomass storage, are all linked to biomass ash concentration. Even after industrial grinding, bagasse contains modest amounts of waxes, as well as residual sugarcane juice, which must be thoroughly removed for appropriate biomass chemical characterization (Philippini et al. 2019). For removing hemicellulose and lignin, acid and alkaline pretreatments were found to be quite successful. The amount of ash (0.32%) in the alkali-pretreated samples was significantly less than what had been found in the native samples (0.68%) (Philippini et al. 2019).
Thermochemical strategies involved in the pretreatment of the sugarcane bagasse
A significant aspect of the supply chain is the employment of suitable technology to convert solid biomass into value-added products. Second-generation valorization processes have hurdles due to the heterogeneous content of the biomass, as opposed to the first-generation biofuel production. The successful extraction of sugar for energy-rich merchandise requires many pretreatment procedures for the disruption of the complex structures of lignin, cellulose, and hemicellulose (Singhvi and Gokhale 2019). Due to its indiscriminate process for converting solids biomass into liquid fuels, thermochemical processes are marketed as viable pretreatment methods for SB (Ascencio et al. 2020). The general principle behind the thermochemical pretreatment is the structural infringement of the lignin present in the bulky biomass molecules (Haldar et al. 2022). The content of heat required to break the bonds is provided by the high temperature at which the reactions occur (Cybulska et al. 2019). The operating conditions and key findings of the pretreatment methods which were adopted for SB by various authors are shown in Table 2. From the table, it is inferred that all the thermochemical pretreatment methods removed lignin effectively. The incorporation of heat in the below-stated process reduced the reaction time considerably.
Dilute acid pretreatment process
Among the others, dilute acid pretreatment is observed as the most effective method for SB (Kumari and Singh 2018). Apart from sulfuric acid, phosphoric acid, hydrochloric acid, and nitric acids are frequently utilized for the pretreatment of SB (Dai et al. 2021). Pretreatment in the presence of diluted acid is considered an economic technique as the reaction can be performed under milder reaction conditions. It was observed that inhibitors like furfural from pentoses and 5-hydroxymethylfurfural (HMF) from hexoses are usually generated as a result of acid hydrolysis of biomass which ultimately results in a loss of fermentable sugar (Rajendran et al. 2018). In addition, dilute acid pretreatment is also observed as a preferred method for enzymatic hydrolysis of bagasse of sugarcane before the formation of XOS (xylooligosaccharides) (Zhang et al. 2017). Primarily, the organic acids facilitate the formation of XOS with a reduction in furfural concentration, whereas mineral acids accelerate the production of xyloses and furfural, which slightly hinders XOS yield. For instance, it was reported that the pretreatment of SB with 2% sulphuric acid at 121 °C for 2 h resulted in the solubilization of 85% of the hemicelluloses and also eliminated 16% of the lignin content (Zhao et al. 2018). Similarly, Ascencio et al. observed that the SB on pretreatment with 1% nitric acid at 121 °C for 30 min resulted in the elimination of 55.5% hemicellulose (Ascencio et al. 2020). Hence, high-temperature acid-catalyzed pretreatment of SB results in the recovery of dissolved sugars from hemicellulose, thereby increasing the enzymatic digestibility of the solid component of cellulose into sugar monomers (Sasmal and Mohanty 2018).
Alkaline pretreatment process
Primarily, alkaline pretreatment is regarded as a cost-effective process for milder reaction conditions that leads to generating less amount of inhibitors (Raud et al. 2019). Alkaline pretreatment is usually conducted at lower temperatures and pressure for the removal of lignin and various other uronic acid–associated products of hemicellulose of SB. An alkaline pretreatment is preferable for delignifying the bagasse as it eliminates the uronic acid substitutions and other acetyl groups in the hemicellulose thereby making the cellulose and the hemicellulose more easily accessible (Khoo et al. 2018). Alkaline conditions typically cause the bagasse to swell its structural components with the reduction in cellulose crystallinity. Yu et al. treated the bagasse of sugarcane with 1.0% NaOH at 120 °C for 10 min and reported a delignification efficiency of 67.5% (Yu et al. 2018). Likewise, Zhang et al. employed various batches of NaOH and hydrogen peroxide with varying concentrations from 1.3 to 6.3% at temperatures ranging from 60 to 160 °C to successfully break down the hemicellulose as well as the lignin content to improve the enzymatic digestibility of biomass (Zhang et al. 2019). Nosratpour et al. observed that 0.5 mol L−1 sodium carbonate on reacting with the SB at 140 °C for 60 min removed lignin efficiently by 89.6% (Nosratpour et al. 2018). Similarly, it was also reported that the SB treated with 0.55 g of calcium hydroxide g−1 of dry biomass at 60 °C for 24 h resulted in the removal of hemicellulose as well as lignin by 41% and 30% respectively (Vaz et al. 2021). Therefore, alkaline pretreatment is well efficient for SB as per as the delignification and subsequent improvement in biomass digestibility are concerned.
Steam explosion process
Steam explosion is an ecologically friendly pretreatment technique that can be ideally utilized to pretreat SB (Sun et al. 2016). It involved exposing the bagasse to high-pressure steam accompanied by combustible decompression of the bagasse, resulting in hemicellulose and lignin hydrolysis and solubilization, respectively (Raud et al. 2016). The process generally changes the crystalline nature of cellulose and the polymerization degree, thus facilitating subsequent enzymatic hydrolysis. The steam explosion process is performed either with the presence of a catalyst like acid or alkali or in the absence of the catalyst. In a study, the biomass of SB was treated with saturated steam at high pressure ranging from 0.69 to 4.83 MPa and temperatures ranging from 160 to 260 °C. Thereafter, the sudden release of pressure resulted in the decompression of the material (Arenas-Cárdenas et al. 2017). Due to the appliance of high temperature and pressure, the technique caused the structural disarray of the substance, destruction of hemicellulose, and transformation of lignin, enabling the eventual hydrolysis of cellulose. For an increase in hydrolysis, Silveira et al. used a 65-L steam gun reactor to perform the steam explosion for pretreatment of the bagasse of sugarcane with a moisture content of 50% (w/w) (Silveira et al. 2018). Similarly, Pitarelo et al. conducted phosphoric acid–catalyzed steam pretreatment at varying temperatures ranging from 180 to 220 °C for different residence times, varying from 5 to 7.5 min and the optimum reaction condition was observed at 180 °C for 5 min for the biomass (Pitarelo et al. 2016). Thus, the steam explosion process has negligible recycling costs due to its low environmental impact and also promotes a significant digestibility of the SB.
Ammonia fiber expansion process
Ammonia fiber expansion (AFEX) is a favored thermochemical delignification approach that uses liquid ammonia for delignification at high pressure and temperature before the sudden reduction in the pressure (Shirkavand et al. 2016). The hemicellulose was efficiently dissolved with depolymerization of lignin, which ultimately improves the hydrolysis potential of biomass. In some of the studies, this process of pretreatment is tactically used to improve the delignification and saccharification rate of SB. It was reported that in AFEX (ammonia fiber expansion) under optimal circumstances such as temperature, ammonia loading, and pressure resulted in the conversion of more than 90% of the cellulose and hemicellulose to fermentable sugars due to the breakage of the ester and lignin-carbohydrates connections (Kumar and Sharma 2017). Pretreatment of SB with 15% ammonia at 170 °C for 60 min improved the delignification and saccharification of biomass (Ajala et al. 2021). Similarly, Bala and Singh also recommended an ammonia fiber expansion process for the pretreatment of SB as it removed about 75–85% of the lignin effectively (Bala and Singh 2019). Therefore, the AFEX (ammonia fiber expansion) process is an efficient pretreatment process for the delignification of SB and reduction of biomass recalcitrance.
Organosolv pretreatment
The organosolv pretreatment process is hailed as a constructive procedure for fractionating bagasse into its components, namely the hemicellulose, lignin, and cellulose, in comparatively unadulterated form while maximizing solvent reclamation and reutilization. Mostly, such a type of pretreatment employs an organic solvent with or without a catalyst for SB (Zhang et al. 2016). During pretreatment, most of the cellulose is recovered as solids in organic solvents, while hemicellulose and lignin are dissolved. As a result, improved enzymatic availability of carbohydrates is attained while reducing the recalcitrance of biomass. In acidic and non-acidic environments, it often employs liquids with low boiling points such as methanol or ethanol and solvents with high boiling points such as glycerol or glycol. Alcohols with low boiling points are often used as organo-solvents due to their economy and ease of retrieval throughout the process (Sidiras et al. 2021). However, the procedure is costly due to the requirement of specialized instruments to operate at high pressure. Various organic solvents, such as acetone, alcohols, chloro-ethanol, propionic acid, phenol, formaldehyde, and amines, are well reported for delignification of SB with or without the presence of catalysts. Several studies are reported on the usefulness of organosolvs for converting the bagasse of sugarcane into cellulose and comparatively unadulterated lignin, which increases bioethanol production’s feasibility (Santo et al. 2018). It mainly uses an amalgamation of organic solvents, like water and ethanol, to pretreat bagasse for substantial solubilization of lignin. Zhang et al. reported that the delignification process of the bagasse of sugarcane at 160 °C with 60% ethanol and FeCl3 at 0.03% for 72 h improved the lignin solubilization efficiency of the biomass (Zhang et al. 2018a, b). Schmatz and Brienzo reported that the pretreatment of SB at 121 °C with 50% ethanol in the presence of butylated hydroxytoluene resulted in the effective removal of 72.5% hemicellulose and 45.3% lignin (Schmatz and Brienzo 2021). Likewise, it was reported that the organosolv pretreatment process of SB at 180 °C with 60% ethanol and 5% NaOH for 45 min resulted in the removal of 75.5% lignin (Zhang et al. 2021). Thus, the organosolv pretreatment process for the SB is very effective in the removal of lignin thereby enhancing the conversion of cellulose.
The thermochemical pretreatment processes of SB involved the utilization of chemicals at high temperatures for a short duration to provide an effective delignification of the SB. The shorter residence time of the chemicals decreases the corrosion rate of the vessel and prevents the formation of inhibitory compounds such as furfural and HMF (Mankar et al. 2021). Therefore, the above-mentioned thermochemical pretreatment strategies are very effective for delignifying the lignin and liberating the celluloses of the SB.
Other approaches
Autohydrolysis employs hot, compressed water that is generally between 150 and 230 °C. This separation approach, like dilute acid pretreatment, employs hydronium ion to accelerate the hemicellulose extraction process. When the hydronium ion is released due to a temperature rise, O-glycosidic bonds and acetyl groups break, resulting in partial depolymerization. The acetate group cleaves to some amount, and the pH drops, causing further acetic acid to develop. Oligosaccharides and polysaccharides are found in the liquor produced by the hot water pretreatment. The autohydrolysis process yielded 55 to 84% hemicellulose. The solids left over after pretreatment mostly comprise cellulose and lignin, both of which may be transformed into valuable compounds (Dulie et al. 2021). For instance, the acetyl-assisted autohydrolysis of SB with water at a solid to liquid ratio of 1:10 at 160 °C for 70 min yielded 0.8 g L−1 xylose (Zhang et al. 2018a, b).
Electromagnetic waves with frequencies ranging from 0.3 to 300 GHz can be used to heat dielectric materials having a high microwave absorption potential. Furthermore, as compared to traditional heating, microwave heating provides advantages such as energy efficiency, faster response time, and lower reaction temperature. By interacting with electromagnetic fields at the molecular level, it directly delivers energy to substances. It also saves money by lowering the quantity of chemicals and solvents necessary to fractionate the biomass components, reducing the creation of by-products, and boosting yield. The degree of biomass fractionation is determined by the microwave intensity and duration of irradiation. For example, up to 50% (w/w) xylan and 6% (w/w) xylose were observed from sugarcane garbage at temperatures over 170 °C, as well as up to 70% (w/w) hemicellulose was obtained from sugarcane bagasse at 185 °C in 20 min (Dulie et al. 2021).
Biological strategies involved in the pretreatment of the sugarcane bagasse
Primarily, biological pretreatment is an environmentally welcoming method that significantly improves polysaccharide accessibility through enzymatic or microbial delignification (Tsegaye et al. 2019). This type of pretreatment is based on finding a suitable enzyme or microorganism to degrade the intricate network of biomass. Researchers are inclined toward such pretreatments due to their potential in saccharification and fermentation efficiency with the enhancement in the recovery of biobased products (Liguori and Faraco 2016). The biological pretreatment is mostly preferred over the physical and chemical pretreatments due to the requirement of less energy and the generation of fewer inhibitors (Malhotra and Suman 2021). Most fungi improved the polysaccharide digestibility, while merely a couple of microorganisms show the potential to decompose the lignin entirely while retaining the hemicelluloses, and the celluloses (Masran et al. 2016) of SB. Machado and Ferraz used Ceriporiopsis submervispora for biological pretreatment which was performed at 27 ± 2 °C for 60 days and achieved the recovery of around 47% of glucose as a sugar-rich syrup (da Silva Machado and Ferraz 2017). Laccase from Pycnoporus cinnabarinus was also utilized to delignify SB effectively by 27% (Rencoret et al. 2017). Thereafter, an enhancing effect in the subsequent product recovery from SB was recorded once the biomass was treated with Pleurotus pulmonarius PS2001 and Trametes villosa 82I6 which promoted selective modifications in the lignin content (Hartmann et al. 2021). Therefore, biological pretreatments are observed as effective in deconstructing the structure of SB particularly, the content of lignin. However, several attempts of delignification are reported to compensate for the slow process associated with the biological pretreatment.
Enzymatic delignification
According to previous studies on the biological delignification of the bagasse of sugarcane, the choice of microbes relies on the kind of biomass employed in pretreatment. For the breakdown of lignin and hemicellulose in SB, soft-rot, white-rot, and brown-rot fungi are commonly used (Akhtar et al. 2016). On the other hand, the commonly chosen procedure for the effective delignification process of the bagasse of sugarcane is the utilization of white-rot fungi in combination with the ligninolytic enzymes, such as peroxidases and laccases (Sindhu et al. 2016a, b; Haldar et al. 2018). Ligninolytic enzymes improve cellulose accessibility while recovering essential phenolic chemicals generated during lignin decomposition (Bilal and Iqbal 2020). With adequate delignification, white-rot fungus disintegrates lignocellulosic cell wall components. For improved biofuel generation, biological pretreatment was followed by an efficient saccharification process. The fungus, SK 7 isolated from Phanerochaete sordid of white-rot fungus was utilized by Jiraprasertwong et al. at 30 °C and pH 7.2 for 20 days which resulted in the increase of the crystallinity index of the SB by 1.1% thereby proving the efficient biological delignification of the bagasse of sugarcane (Sabiha-Hanim and Abd Halim 2018). Machado and Ferraz used Ceriporiopsis submervispora in a biological pretreatment for 60 days at 27 °C and observed a maximum lignin removal of 48% (da Silva Machado and Ferraz 2017). Laccase produced by Pycnoporus cinnabarinus was similarly utilized in the presence of 1-hydroxybenzotriazole as a mediator to decrease the lignin content of the SB effectively by 27% (Rencoret et al. 2017).
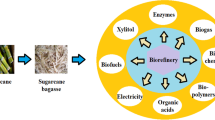
Processing of sugarcane bagasse for value-added products
Bagasse decomposition technologies are similar to those used in the agro-industry for biomass degradation. In most cases, the degradation procedures are determined by the biomass type, the treatment’s final objective, and other factors. Generally, the SB is processed for its conversion into energy products and non-energy products. The energy-related processing is focused to generate power in various ways, including synthetic biofuel, heat, steam, electricity, charcoal, biogases, methanol, ethanol, methane, and biodiesel (Ghosh 2016). Primarily, combustion, pyrolysis, and gasification are the widely acknowledged thermochemical conversion techniques for energy generation (Patel et al. 2016). The most common method of employing the bagasse of sugarcane for the generation of energy is direct combustion (a solid-state procedure) (Kumar et al. 2021). Moreover, fermentation techniques are used to produce bioethanol, while the concept of anaerobic digestion (AD) is introduced to yield biogas for the generation of energy through the conversion of SB (Baêta et al. 2016).
Thermochemical processing for the production of biofuel
In contrast to other biofuels, bioethanol is now the most advantageous biofuel due to its sparse carbon dioxide emissions, high density of energy, and heat of vaporization (Balat and Balat 2009). Bioethanol, an inebriating drink, is used as an essential chemical in producing numerous organic compounds such as diethyl ether, ethyl esters, and acetic acid. Over the last few years, SB has been attracting researchers for bioethanol synthesis due to its ample availability and huge content of carbohydrates (Niju and Swathika 2019). Most importantly, it has lower carbon intensity than fossil fuels resulting in less air contamination (Iram et al. 2018). Among the other factors, bagasse quality and bioethanol production technology significantly impact bioethanol yield.
Pyrolysis
Pyrolysis is a technique that has been around for over a century and gets its name after the Greek words “pyro,” meaning fire, and “lysis,” meaning decomposition or degradation of different parts. Primarily, the process of pyrolysis degrades organic substances at 350–700 °C without oxygen or at a dosage that prevents total combustion within reactors, known as pyrolyzers (Saber et al. 2016; Ordonez-Loza et al. 2021). Based on the process operating conditions of pyrolysis, namely the solid residence time, heating rate, pyrolysis temperature, and the size of the particle, it is categorized into three categories: traditional or slow, rapid, and flash (Kundu et al. 2018). Slow pyrolysis creates a lot of solids, whereas rapid pyrolysis concentrates on liquids and a lot of gases are formed by flash pyrolysis (Fahmy et al. 2020). As the process of pyrolysis may yield gas, solid, and liquid products, every one of them has unique applications to bagasse. Pyrolysis causes thermal decomposition of bagasse that began at 200 °C in moments. The rudimentary chemical reactions from the pyrolysis reactions were recognized to be prototypes of the gasification and combustion processes (Gollakota et al. 2016). Pyrolysis is among the most effective biomass transformation technologies as it produced a large number of fuels that can be utilized for multiple engines and exhibited a greater recovery of energy efficiency than the other thermo-chemical routes (Nanda et al. 2016). The pyrolyzer forms bio-oil as vapor, subsequently converted to a liquid by condensation. Catalytic and split-up techniques can also transform bio-oil into fuels and chemicals of greater significance. During pyrolysis, biomass drying is observed as an endothermic process that occurred at temperatures up to 150 °C. The primary biomass components started to break down when the moisture is removed from the biomass. Then, between 220 and 315 °C, the breakdown of hemicellulose occurred with the formation of two distinct peaks, and roughly 20% of solid leftovers were reported at or above 900 °C for SB. The hemicellulose is then depolymerized, resulting in oligosaccharides and other compounds, as well as furfural and 1,4-anhydrous-D-xylopyranose (Ghodake et al. 2021). Next, at 315 °C, cellulose began to disintegrate, resulting in an endothermic peak between 355 and 400 °C. As a result, cellulose is the constituent that generated the fewest solid residues at 6.50 wt%. Depolymerization events occurred during cellulose degradation, resulting in the formation of oligosaccharides. D-glucopyranose and levoglucosan were produced as a result of their glucosidic linkages. Finally, in addition to volatiles, the latter creates additional small-molecular-weight compounds, transforming all of these constituents into the products of liquid pyrolysis (Zanatta et al. 2016). Conclusively, due to its exceedingly complicated structure, lignin shows the sluggish and the utmost difficulty in the thermal breakdown procedure. The decomposition started from room temperature to 900 °C, with an exothermic peak at 365 °C. Furthermore, lignin breakdown yielded the most solid residues at around 45.7 wt% (Miranda et al. 2021). Varma and Mondal reported that the pyrolysis of the SB at 500 C with a heating rate of 50 °C min−1 resulted in the production of 45.2% bio-oil (Varma and Mondal 2017). Treedet and Suntivarakorn reported that fast pyrolysis of SB at 480 °C with a feed rate of 30 kg h−1 resulted in the production yield of 78.1% bio-oil (Treedet and Suntivarakorn 2018). Likewise, it was also reported that at 500 °C the pyrolysis of SB yielded 56.6% bio-oil (Stegen and Kaparaju 2020). As a result, if gasification is utilized, biomass with a huge lignin concentration inclines to perform more in terms of total breakdown.
Gasification
For lignocellulosic biomass conversion to bioenergy, biomass gasification (BG) is presumed to be one of the cost-effective and efficacious methods. Gasification is the process of thermally degrading lignocellulose at a high temperature to produce intermediary substances like bio-oil and syngas. Instead of the transformation of syngas and bio-oil to a large assortment of possible biofuels, the bio-oil is frequently combined with a catalytic and/or a chemical advancement unit. Primarily, gasification is the thermochemical conversion of liquid or solid biomass into an assortment of water vapor, hydrogen, methane, carbon monoxide, hydrogen sulfide, tar, CO2, and additional trace species, which are defined by the operational variables like pressure and temperature inside the gasifier, raw material properties, catalytic agents, and gasifying media like the carbon dioxide, steam, oxygen, and air (Kundu et al. 2018). Gasification is made up of several intersecting sub-procedures, including pyrolysis, drying, and partial oxidation, hence involving a complex set of reactions (La Villetta et al. 2017). The feedstocks are dried at around 120 °C and as a result, all the unstable species are generated below 500 °C. Char gasification commenced at a temperature of roughly 350 °C. The heat might be generated internally through exothermic combustion events or obtained from outside sources. Equation (1) is utilized to illustrate a simplified gasification response as follows:
According to the temperature ranges and reaction chemistry, the complete gasification process is divided into main, secondary, and tertiary reaction phases (Molino et al. 2016). SB was transformed into oxygenated vapor and liquid species with the formation of H2O and carbon dioxide in the main reaction regime. The molecular weight of the vapors from primary pyrolysis was low due to the vapors of monomers. Hydrogen, carbon monoxide, phenols, olefins, CO2, water vapor, and aromatics were formed from the primary vapor and liquid species in the secondary reaction regime at temperatures stretching from 700 to 850 °C (Sikarwar et al. 2017). Polynuclear aromatic hydrocarbons, mixed oxygenates, phenolic ethers, alkylphenols, and heterocyclic ethers were among the tars formed during this step. Furthermore, steam reforming, methanation, cracking reactions, and water gas shift occurred with the residual tars and gases (Natte et al. 2020). The tertiary reaction phase produced carbon dioxide, hydrogen, and carbon monoxide, as well as water vapor, polynuclear aromatics, and liquid tar from 850 to 1000 °C. With increasing temperature, the transitions occurred from mixed oxygenates to phenolic species to polyaromatic species. It is worth noting that coke was generated by the thermolysis of liquid and organic vapors, whereas soot was formed by the homogenous nucleation of intermediates formed at high temperatures. Until 400 °C, the quantity of char formed was conversely correlated to the growing temperature. The loss of hydroxyl and aliphatic C—H bonds, as well as carbonyl and olefinic carbon groups, decreased the carbon concentration of char with the temperature rise (Sikarwar et al. 2017). Fixed-bed (FXB) and fluidized-bed (FB) gasifiers remain the most common categories of gasifiers used to gasify biomass. Established on the course of air movement, the FXB gasifier is divided into three types: updraft, downdraft, and cross-draft. The design of the movement of air in the updraft fixed-bed gasifiers and downdraft fixed-bed gasifiers is shown in Fig. 1. From Fig. 1, it can be seen that in a downdraft fixed-bed gasifier system, both the gasifying agent and the fuel breeze penetrates from the bottom whereas in an updraft fixed-bed gasifier system, the gasifying agent infiltrates from the bottom and the fuel penetrates from the top. The three focal types of FB gasifiers are the DFB (dual fluidized-bed) gasifiers, CFB (circulating fluidized-bed), and BFB (bubbling fluidized-bed) (Anukam et al. 2016). Raheem et al. reported that the gasification of SB at 900 °C for 30 min produced 42.9 mol% of syngas (Raheem et al. 2019). Jahromi et al. observed that optimum operating parameters of 1500 K as the preheating temperature and an inlet velocity of 20 m s−1 resulted in conversion efficiency of 69.1% (Jahromi et al. 2021).
Comparative design of updraft and downdraft fixed-bed gasifiers. Reprinted with permission from (Sikarwar et al. 2016)
Similarly, it was reported that at 1000 °C the gasifier generated gas up to 4 Nm3 kg−1 of SB and the high operating temperature of the gasifier resulted in a low yield of tar at 50–100 mg m−3 and high production of gas from the SB (Dogru and Erdem 2019). Therefore, the gasification process of SB is very effective in the high production of syngas.
Liquefaction
Thermal liquefaction, which is successful in converting biomass with a high measure of H2O to liquid products is a more adaptable thermochemical method (Pang 2019). The liquefaction process produced liquid, gaseous, and solid products from the degradation of biological polymer compounds present in the feedstocks at a pressure range from 4 to 40 MPa and temperature ranging from 200 to 370 °C (Isa et al. 2018). The liquid organic product is of special importance since it comprises both unsaturated and saturated hydrocarbons which are highly influenced by the feedstock composition (Duarah et al. 2022). The use of a solvent in the liquefaction of biomass is required to derive the benefit of the reduced mass and heat transfer resistances as well as the better characteristics in the supercritical regions (Hrnčič et al. 2016). The liquefaction section includes the liquefaction reactor, slurry preparation phase, heat recovery from hot liquefaction product streams, filtration, and phase separation processes. The liquefaction section usually received bagasse with 48% moisture at a rate of 10 tonnes h−1 (Ramirez and Rainey 2019). The particle size of bagasse ranged from larger than 1.25 mm to less than 4 mm. The size profile is expected to vary from one mill to another; nevertheless, as the particle size of the biomass has no bearing on biocrude production, bagasse in its “as received” state was sent straight to the slurry preparation tank, in which ethanol was combined with the biomass at a solvent to biomass ratio of 19:1. As the slurry travels to the liquefaction reactor, it was expected to have a continuous solids loading of 5%. The slurry was warmed in the liquefaction reactor using the heat recycled from the products of liquefaction. In the reactor, the hot slurry was heated at a temperature of 300 °C and transformed into liquefaction products (Arturi et al. 2017). The reactor size was determined by the retention duration, which was set at 0.5 h, whereas the amount of ethanol lost during this procedure was assumed as negligible. As the yields of the product were generally expressed as a ratio between the mass of the product and the mass of the biomass feed, with an aggregate of 100%, ethanol was excluded from the total product mass calculation. The product mixture was allowed to cool in heat exchangers to retrieve heat before being split into gas, liquid, and solid fractions in flash separators (Nielsen et al. 2017). The solid residue was segregated from the liquid product by passing the denser portion through a filter (Purkait et al. 2020). The filtrate was then sent to the distillation phase, whereas the filter cake was discarded as a waste by-product of the process. The moisture quantity of filter cakes identical to the solid products in this route was utilized to compute the liquid retention on the filter cake employed in the prototype. Due to the characteristic of the liquid yields, 2% from the entire liquid products was utilized while the separation of gases and the recovery of ethanol were anticipated once the gas product was chilled to room temperature at 25 °C. The unreacted ethanol was separated and recovered from the liquid products in the solvent recovery stage. Flash separators, condensers, and distillation columns made up the solvent recovery section, which concentrates the biological crude and recovered the ethanol for recycling. The biocrude-ethanol combination was distilled in a five-stage column with a reflux ratio of 1.5 and a 0.78 distillate to feed ratio for the extraction of dissolved gases and extremely light volatile compounds (Ramirez et al. 2018). Steam was utilized to separate ethanol from biocrude and decrease the risk of cracking. The distillate was sent to the recycling stream, where it was mainly the mass of the ethanol. The bottom stream of the distillation was sent through a separator to remove water, then through a second distillation to additionally lower the ethanol concentration of the bio-crude and improve retrieval of ethanol for reutilization. A reflux ratio of 1.5 and a distillate to feed ratio of 0.7 were maintained as it reached the subsequent column with fifteen stages, and the second distillation feed was heated to flash 45% of the feed. The flashing feed made it easier to distinguish between light and heavy components. The recycle stream received the second column distillate while the hydrodeoxygenation section received the second distillation bottoms with extremely lower ethanol concentration. The biocrude stream was pressurized and preheated when it moved in the HDO (hydrodeoxygenation) portion before entering the HDO reactor with pressurized hydrogen. The hydrodeoxygenation processes took place at 300 °C and 80 bar pressure (Kosinkova et al. 2017). The biocrude was moderately deoxygenated and hydrogenated in the HDO reactor. The water, hydrodeoxygenated components, and coke were the end products of the reaction. Baloch et al. reported that the liquefaction process of the SB at 280 °C and in the presence of water as a solvent yields 51.8% of bio-oil (Ahmed Baloch et al. 2018). Likewise, it was reported that the liquefaction of SB at 300 °C for 30 min in the presence of fuel oil showed 91% conversion of the bagasse (Araújo et al. 2021).
Therefore, the above-described thermochemical conversion processes could also potentially manufacture biofuels from sugarcane bagasse, resulting in cleaner fuels and a reduction in solid waste from sugar production.
Biological processing for the production of biofuel
Apart from the thermochemical conversion methods utilized in the production of biofuel, biological conversion techniques also play an important role in the production of biofuels such as biodiesel, bioethanol, and biobutanol (Santosh et al. 2017; Abo et al. 2019). The main biological process utilized for the production of biofuel is the fermentation process. Li et al. reported that SB on fermentation with the microbial strain Clostridium acetobutylicum CH 02 at 37 °C and pH 6.5 for 120 h produced 4.6 g L−1 of butanol (Li et al. 2017). Méndez et al. reported that the hydrolysate of the SB during fermentation produced 33 g L−1 ethanol corresponding to a fermentation efficiency of 89% (Méndez et al. 2021). Similarly, it was reported that Saccharomyces cerevisiae on simultaneous saccharification and fermentation process with the SB at 39 °C produced a concentration of 4.8 g L−1 of ethanol (Jugwanth et al. 2020). Likewise, it was reported that Saccharomyces cerevisiae PE-2 on SSF with SB resulted in an ethanol yield of 4.9 g/100 g of SB and it was also observed that there was an 18.7% increase in the ethanol production by Saccharomyces cerevisiae PE-2 when compared to the Kluyveromyces marxianus ATCC 36,907 yeast strains (de Araujo Guilherme et al. 2019).
Bioelectricity
Countries will continue to look for renewable energy resources to provide power in the foreseeable future. In contrast to different biomass wastes for the generation of energy, the bagasse of sugarcane is among the extensively utilized lignocellulosic biomass. The utilization of the bagasse of sugarcane as a biofuel is a worthy idea for providing electricity to the nationwide network, both environmentally and economically. The energy generation using bagasse is very much cost-effective and replenishable since it meets all of the energy necessities of the ethanol and sugar manufacturing units (de Souza et al. 2018). At the moment, new mill developments are helping to create surplus power through cogeneration facilities. Cogeneration is a method of creating both thermal and electrical energy with high energy output (Molina-Guerrero et al. 2020). More efficient cogeneration systems are employed to generate more bioenergy from bagasse (Gongora and Villafranco 2018). Physical, biological, and thermochemical techniques are employed to turn bagasse into bioenergy. Direct combustion is the most basic and ancient thermochemical method and it is widely utilized in the cogeneration of energy in systems (Patel et al. 2016). Owing to the surge in the cost of power, bagasse-based cogeneration plants can save a lot of money. Cogeneration using bagasse is another option for conserving water in hydroelectric power plant reservoirs.
Cogeneration process
High-pressure boilers and specific steam turbines are frequently used to generate electricity from bagasse. For example, 1000 kg of dry bagasse produces approximately 0.450 MWh of energy (Arshad and Ahmed 2016). The conventional technique requires 3 kg of dry bagasse for the generation of 1 kWh of energy. A kilogram of bagasse may generate the same amount of power (Hiloidhari et al. 2021). About 2 kg of bagasse was necessary for the generation of 1 kWh of energy. From 1 tonne of bagasse, conventional boilers produce 2.2 t of steam at a temperature of 350 °C and a pressure of 23 bars; 11 kg of steam was used to generate 1 kWh of energy (Arshad and Ahmed 2016). High-pressure boilers can produce 2.40 t of 65 bar steam at temperatures over 500 °C. In the case of cogeneration, only 5 kg of steam was used to produce 1 kWh of electricity. The thermal energy from the direct burning of 1 tonne of bagasse in the boiler was utilized to produce 2.2 tonnes of steam at a pressure of 20–22 bars and a temperature of 350 °C (Singh et al. 2021). The steam produced was further utilized to control a backpressure steam turbine, thereby generating electricity (Sanaye et al. 2020). Co-generation is the method of creating combined steam utilizing a boiler and electricity in a steam turbine. The sugar crystals and other derivatives are usually obtained using the bioelectricity generated through such a co-generation method.
Bioplastics
Bioplastic markets are steadily expanding despite the 2% of overall plastic sales. It is critical to include additional greener components into lucrative biomass products to achieve technological, commercial, and ecological viability (Al-Battashi et al. 2019). The structure and content of lignocellulosic biomasses are complex and diverse. Primarily, pretreatment, saccharification, liquid detoxification, fermentation, purification, and development of biocomposites are the techniques that are frequently used to prepare bioplastics from biomass feedstocks (Brodin et al. 2017). Given that, various processing techniques are aimed to break down natural lignocellulosic biomass into simple components such as lactic acid and cellulose (Oonkhanond et al. 2017). Several different paths lead to the production of bioplastics with a range of properties that are appropriate for packing, biomedical applications, water treatment, and other valuable products (Machado et al. 2020). A schematic diagram representing the conversion of SB into bioplastics is shown in Fig. 2. Primarily, three factors are considered before the production of bioplastics from lignocellulosic resources: (1) revolutionary financially viable methodology, (2) resolution of political and environmental issues related to bioplastics manufacturing, and finally (3) development of business models in a bioeconomy framework (Brodin et al. 2017).
Cellulose conversion route
Depolymerization to obtain the target-specific monomers is one of the most challenging issues in the production of bioplastics from cellulose (Middleton et al. 2019; Singhania et al. 2022). Solvothermal liquefaction, pretreatments, gasification, pyrolysis, and hydrogenolysis are used for lignin depolymerization (Tran and Lee 2018). It was observed that the production of biofuels received priority over bioplastic manufacture as per as the utilization of monomers derived from biomass is concerned (Reshmy et al. 2021). The processing techniques like fermentative process, transformation, and segregation are involved in the production of bioplastics through the conversion of cellulose.
Saccharification
Among the conversion processes, saccharification is one of the most important stages and different approaches are used to accomplish the process of sugar production. The pretreated biomass can be directly saccharified by simultaneous saccharification and co-fermentation (SSCF), or by simultaneous saccharification and fermentation (SSF) processes. Quicker processing times, fewer external contaminants, less inhibitory impact, and cheaper costs are the advantages of such techniques. However, several inhibitor-like compounds generated from cellulose and hemicelluloses, such as acetaldehyde, furfuryl, and hydroxymethylfurfural, as well as p-hydroxybenzaldehyde, cinnamic acid, hydroxybenzoic acid, coniferyl, syringic acid, sinapyl alcohol and syringaldehyde from lignin, necessitate the process of detoxification before the fermentation process. Enzymatic hydrolysis is a cost-effective and environmentally acceptable method of saccharification that includes the breakdown of pretreated biomass utilizing enzymes isolated from cellulosic or hemicellulosic degrading microorganisms (Sharma et al. 2019). Kumar et al. reported that there was a 66% increase in the production of the PHA from lignin by the application of a bacterial strain Pandoraea sp. ISTKB on the biomass (Kumar et al. 2017). Similarly, it was observed that the lignin obtained from the sugarcane waste acted as a substrate for the bacterial strain Bacillus subtilis RS1 and on saccharification yielded 71% PHA (Li and Wilkins 2020). Following saccharification, the fermentable sugars were fermented with different types of microbes to produce a variety of fragments that are utilized for making bioplastics.
Fermentation
Fermentation is a straightforward and efficient processing method for producing building blocks for bioplastic manufacturing mutually in laboratories as well as industries. Lactic acid, for instance, is among the difficult constructing ingredients for polylactic acid (PLA), a popular bioplastic now on demand (Jem and Tan 2020). Submerged fermentation is the most commonly used technique among the other available fermentation techniques such as surface fermentation, simultaneous fermentation, and solid-state fermentation. Simultaneous saccharification and fermentation occur when the fermentative process and enzymatic hydrolysis take place in the parallel period. When enzymes and microorganisms were added to the same unit in SSF, it resulted in better fermentation than previous fermentation techniques. However, the several restrictions associated with SSF were that only an extremely dilute medium can be utilized and it requires more enzymes, increased production costs, and requires ideal operating conditions for both the microbe and the enzyme (Reshmy et al. 2021). It was reported that in an SSCF process at pH 5.5, the microorganism Lactobacillus brevis effectively produced lactic acid with a yield of 0.52 g g−1 of the SB (Grewal and Khare 2018). Similarly, it was reported that using an optimal fed-batch technique for black liquor, a residual lignin-rich effluent from alkaline-processed SB can generate biomass and PHA concentrations of 1.85 g L−1 and 238 mg L−1, respectively (Unrean et al. 2021). Sartale et al. observed that the bacterial strain Lysinibacillus sp. RGS at 37 °C and pH 7.0 effectively synthesized polyhydroxy butyrate (PHB) by the fermentation of SB and also accounted for the 61.5% accumulation of the PHB (Saratale et al. 2021).
Lignocellulosic biomass may be used to extract cellulose, which in turn is used for bioplastic production (Govil et al. 2020). The amount of cellulose in a product is determined by the origin from which it is derived. Delignification, hemicellulose removal, cellulose extraction, surface changes, and bioplastic composite manufacture are parts of such conversion processes. The advantages of this technique over the lignin conversion route include the incorporation of surface-modified cellulose with increased crystallinity, induced transparency, and improved mechanical and optical properties. Succinic and maleic acid groups are precisely inserted into the surface of the cellulose through monolayer deposition, which was achieved by a reaction between their anhydrides and the hydroxyl groups of cellulose (Wang et al. 2018). Bioplastics with cellulose in them provided excellent mechanical and barrier qualities. It was reported that for 50 g of SB, the cellulose conversion process yielded 6.18 g of carboxymethyl cellulose which was used in the formulation of various bioplastics (Yaradoddi et al. 2020). Similarly, the cellulose from the SB was utilized for the synthesis of cellulose nanocrystals which in turn improved the functional property of the bioplastics (Sothornvit 2019). Thus, SB containing a high concentration of cellulose proved to be one of the best raw materials for the production of bioplastics through the cellulose conversion route.
Wastewater treatment
Although the processing of sugarcane bagasse for wastewater treatment is not complicated, it still plays a humongous part in the treatment of wastewater. For the elimination of nonbiodegradable harmful compounds, several physicochemical techniques such as membrane filtration, coagulation, sedimentation, and ion-exchange methods were hugely applied in numerous cases (Azimi et al. 2017). Nowadays, bagasse-based adsorptive substances are of high research priority due to their year-wise availability, highly absorbent ability, and ease of manipulation throughout the treatment course. SB is a cheap, plentiful agro residue that was employed as a bio adsorbent in the adsorption of contaminants from polluted water (do Carmo Ramos et al. 2016). It was observed that bagasse-based bio adsorbent is made up of macromolecules such as hemicellulose, fulvic acid, cellulose, humic acids, lignin, and proteins that include a variety of functional groups such as –COOH, –NH2, –OH, –CONH2, –SH, and – OCH3 that act as adsorptive sites (Bagotia et al. 2021). By donating electron pairs, adsorption, or substituting pollutant ions with hydrogen ions, such bio adsorbent sites impasse and draw pollutant ions. With respect to that temperature, initial contaminant concentration, pH, biosorbent dose, adsorbent particle size, and contact duration are the key parameters that affect the adsorption efficacy of bagasse. For instance, it was observed that the bagasse-based bio adsorbents eliminated a maximum of 96% concentration of Cd(II) at pH 7.0 in 25 min of residence time (Alokika et al. 2021). Although raw SB is utilized to remove pollutants, it was well appropriate as a biosorbent after being physically and chemically processed. Given that, steaming, milling, grinding, and autoclaving are reported as physical treatments, whereas chemical treatments include the usage of mercerization, chemicals, chelating agents, and surfactants. Unmodified SB exhibited less adsorption ability for Cr(VI) and Zn(II) compared to the EDTA-modified or activated SB (Sarker et al. 2017a, b). SB shows a great perspective for metal biosorption due to its high cellulose concentration. SB is also used to biosorb different metals such as nickel, chromium, lead, copper, cadmium, and mercury (Milani et al. 2018). In a similar study, do Carmo Ramos et al. showed that carboxylate-functionalized bagasse of sugarcane was able to remove substantial ions of nickels (do Carmo Ramos et al. 2016). Therefore, SB is a very effective bio-adsorbent for the removal of heavy metals and other organic contaminants from the wastewater thereby purifying the water source (do Carmo Ramos et al. 2016).
Other applications
At an industrial scale, SB was extensively used to produce numerous organic compounds such as xylitol, succinic acid, lactic acid, and others utilizing microbial strains such as bacteria and fungi mainly through SSF (solid-state fermentation) and SMF (submerged fermentation) (Singhania et al. 2008). The organic compounds generated by microorganisms using SB as a substrate are listed in Table 3. From the table, it can be seen that the Lactobacillus pentosus yielded the highest concentration of lactic acid at 72.7 g L−1 in the fed-batch SSF process of the SB. The fed-batch fermentation of SB by Actinobacillus succinogenes produced 70.8 g L−1 succinic acid and the batch fermentation of SB by Candida guilliermondii FTI20037 produced xylitol at a concentration of 36.1 g L−1.
Challenges and future prospects
In a combined biorefinery, a C6 sugar such as glucose from SB may be used for the synthesis of lactic acid or ethanol synthesis, while C5 sugar such as xylose in hydrolysate can be used for the synthesis of succinic acid or xylitol or biogas generation through anaerobic digestion. An amalgamated strategy for the combined synthesis of ethanol from C6 and xylitol from C5 from sugarcane biomass is a potential thought (Felipe Hernández-Pérez et al. 2019). Nevertheless, more research is needed before such approaches can be implemented on a larger scale. To enhance the bioconversion of lignocellulosic sugars, it is crucial to utilize microorganisms that can metabolize both C6 and C5 sugars. Unlike glucose, biological xylose valorization is typically overlooked since most industrial microorganisms lack an effective metabolic pathway for their digestion. CCR (carbon catabolite repression) prevents xylose usage during co-fermentation (Prabhu et al. 2020). As a result, greater emphasis must be directed to rewiring metabolic systems of microorganisms to process different carbon sources from the feedstock, particularly enriched with glucose and xylose, which will be critical for ensuring the economic viability of bioprocesses. Efforts to valorize xylose have increased during the previous two decades. To increase the effectiveness of the xylose-based fermentative process, efficient xylose-using in addition to non-xylose and ineffective xylose-using strains are orchestrated to broaden the substrate range and facilitate faster xylose adaptation and eradicate CCR for the production of chemicals and fuels from LCB-based feedstock (de Paula et al. 2019). The development of highly efficient catalysts is crucial for achieving selective valorization of lignin value-added goods. Because of the high reactivity of the degradation intermediates/products, which are prone to repolymerization and condensation, using acid or base alone to produce lignin monomers is deemed useless. Several approaches may be utilized to address this problem. (1) Combining additional techniques, such as hydrodeoxygenation, to further change unstable primary products into stable products such as benzenes, alkyl benzenes, and hydrocarbons is a frequently utilized methodology. (2) Another technique is to utilize trapping agents to stabilize reactive species and decrease char formation, such as boric acid, diols, phenol, 2-naphthol, and p-cresol. (3) The use of appropriate solvents, such as formic acid and alcohols, can enhance product yield and prevent char formation since these organic solvents not only have a higher solubility for lignin and its depolymerized products, but also can function as H-donors. (4) Another extensively tried strategy is the screening/development of more active catalysts (e.g., bifunctional or bimetallic catalysts) that allow selective degradation/conversion of lignin under mild circumstances, particularly at low temperatures. (5) Furthermore, using a multi-phase reaction system for lignin monomer synthesis is advantageous because the produced monomers could be removed from acid or basic environment before being subjected to undesirable reactions (Wang et al. 2019). The existing advanced procedures aimed at converting SB into the valorized goods outlined here are not yet cost-effective (Kumar et al. 2018). Sustainable and renewable energy sources, comprehensive growth of the economy to save foreign exchange, less dependent on imported crude petroleum, reduced carbon footprint, and green infrastructure are all major motivations for the implementation of biorefineries (Alokika et al. 2021). Despite its enormous potential, establishing a biorefinery is fraught with difficulties. For instance, biorefineries are concerned about the availability of low-cost lignocellulosic feedstock throughout the year. It is indeed critical to put in place a system for collecting, transporting, and managing biomass feedstock. Then, 2G biorefinery projects are capital comprehensive, associated with significant threats, and require an extended period to reach market readiness (Felipe Hernández-Pérez et al. 2019). Lastly, up to 40% of overall operational expenses might be attributed to pretreatment and enzymatic hydrolysis. In terms of the feedstock and supply chain for the sugar industry in India, the current sugar mills have a well-recognized stock network for sugarcane and therefore easily access the SB on site. A sugar unit, an ethanol production unit, a cogeneration plant for steam and power, and an anaerobic digestion unit are all part of a typical sugar factory when a distillery is attached. These devices make it easier to utilize on-site extra steam, energy, and water. Anaerobic digesters (AD) also have one of the minimal investment needs among rival technology platforms (Felipe Hernández-Pérez et al. 2019). The fundamental transformation mechanisms in AD are often sluggish because they need a longer residence period, resulting in greater digester volumes. One of the future possibilities is to use AD not only as a bioprocess step for the recovery of energy but also as one of the effective pretreatment processes (Konde et al. 2021). It will not only improve energy in the form of biogas-biomethane, but also expose the lignocellulosic matrix of SB.
Conclusion
A substantial quantity of the bagasse of sugarcane is produced as a by-product in sugarcane companies throughout the world as a result of sugarcane processing. Sugar mills burn a considerable portion of bagasse in the open, resulting in contamination of the environment. Bagasse management strategies that are environmentally friendly prevent open burning and pollution. Urbanization and industrialization unleash a variety of dangerous compounds into the environment, posing a threat to plants and animals owing to an upsurge in the pollution created by the chemicals. The global energy and environmental issues are driving people to reconsider the most competent and cost-effective ways to use lignocellulosic biomass, such as bagasse. The degree of polymerization, cellulose and hemicellulose concentration, and moisture level of lignocellulosic biomass, as well as biomass availability, thickness, lignin content, and other factors, all influence long-term and effective usage. SB contains all of the necessary qualities to be used as sustainable alternative biomass for biofuels, generation of energy, sugars, enzymes, biosorbent, organic acids, and other valorized products. Bagasse from sugarcane contains a lot of cellulose and hemicellulose, as well as lignin. As lignin is present, the arrangement becomes extra stiff and resistive. The recalcitrance of biomass necessitates an appropriate pretreatment to increase efficiency while lowering the cost of microbes and enzymes to achieve large titers of the desired output. As a result, pretreatment of bagasse is a prerequisite for overcoming enzymatic hydrolysis obstacles. The article above discusses several thermochemical and biochemical pretreatment methods and procedures for the efficient valorization of SB. Despite the availability of numerous pretreatment procedures, none of them can be utilized as a standard approach for the pretreatment of sugarcane crop residues or sugar industrial by-products. The optimum pretreatment procedure must be chosen based on the intended product. Modern technology has greatly improved pretreatment operations and the accompanying release of bio-products from sugarcane solid by-products, yet there are still challenges to overcome. Establishing highly proficient pretreatment approaches and fabrication technologies, bioprospecting and establishing highly consistent genetically modified microorganisms, upgrading gene cloning and sequencing processes, improving yield at a large scale, and economically converting biomass into added-value products are among the challenges. This evaluation effort will aid in the discovery of solutions to the difficulties described above, as well as serve as a valuable resource for a wide variety of experts and innovators working in relevant fields.
Abbreviations
- AD:
-
Anaerobic digestion
- AFEX:
-
Ammonia fiber explosion
- BG:
-
Biomass gasification
- Ca(OH)2 :
-
Calcium hydroxide
- CCR:
-
Carbon catabolite repression
- HDO:
-
Hydrodeoxygenation
- HMF:
-
Hydroxy methyl furfural
- kWh:
-
Kilowatt-hour
- LCB:
-
Lignocellulosic biomass
- MWh:
-
Megawatt-hour
- NaOH:
-
Sodium hydroxide
- PLA:
-
Polylactic acid
- SB:
-
Sugarcane bagasse
- SSCF:
-
Simultaneous saccharification and co-fermentation
- SSF:
-
Simultaneous saccharification and fermentation
- XOS:
-
Xylooligosaccharide
References
Abo BO, Gao M, Wang Y, Wu C, Wang Q, Ma H (2019) Production of butanol from biomass: recent advances and future prospects. Environ Sci Pollut Res 20:20164–20182. https://doi.org/10.1007/s11356-019-05437-y
Ahmed Baloch H, Nizamuddin S, Siddiqui MTH, Mubarak NM, Dumbre DK, Srinivasan MP, Griffin GJ (2018) Sub-supercritical liquefaction of sugarcane bagasse for production of bio-oil and char: effect of two solvents. J Environ Chem Eng 5:6589–6601. https://doi.org/10.1016/j.jece.2018.10.017
Ahmed N, Zeeshan M, Iqbal N, Farooq MZ, Shah SA (2018) Investigation on bio-oil yield and quality with scrap tire addition in sugarcane bagasse pyrolysis. J Cleaner Prod 927-934. https://doi.org/10.1016/j.jclepro.2018.06.142
Ajala EO, Ighalo JO, Ajala MA, Adeniyi AG, Ayanshola AM (2021) Sugarcane bagasse: a biomass sufficiently applied for improving global energy, environment and economic sustainability. Bioresour Bioprocess 1:87. https://doi.org/10.1186/s40643-021-00440-z
Akhtar N, Gupta K, Goyal D, Goyal A (2016) Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ Prog Sustain Energy 2:489–511. https://doi.org/10.1002/ep.12257
Al-Battashi HS, Annamalai N, Sivakumar N, Al-Bahry S, Tripathi BN, Nguyen QD, Gupta VK (2019) Lignocellulosic biomass (LCB): a potential alternative biorefinery feedstock for polyhydroxyalkanoates production. Rev Environ Sci Biotechnol 1:183–205. https://doi.org/10.1007/s11157-018-09488-4
Alokika, Anu, Kumar A, Kumar V, Singh B (2021) Cellulosic and hemicellulosic fractions of sugarcane bagasse: potential, challenges and future perspective. Int J Biol Macromol 564-582. https://doi.org/10.1016/j.ijbiomac.2020.12.175
Anukam A, Mamphweli S, Reddy P, Meyer E, Okoh O (2016) Pre-processing of sugarcane bagasse for gasification in a downdraft biomass gasifier system: a comprehensive review. Renew Sustain Energy Rev 775-801. https://doi.org/10.1016/j.rser.2016.08.046
Araújo MFRS, Cardoso PL, Souza GLR, Cardoso CC, Pasa VMD (2021) Simultaneous thermal liquefaction of sugarcane bagasse and esterification with ethanol and fusel oil: one-step process for biofuel production. Chem Eng J 127432. https://doi.org/10.1016/j.cej.2020.127432
Arenas-Cárdenas P, López-López A, Moeller-Chávez GE, León-Becerril E (2017) Current pretreatments of lignocellulosic residues in the production of bioethanol. Waste Biomass Valorization 1:161–181. https://doi.org/10.1007/s12649-016-9559-4
Armah EK, Chetty M, Rathilal S, Asante-Sackey D, Tetteh EK (2022) Lignin: value addition is key to profitable biomass biorefinery, Academic Press, pp 233–247
Arshad M, Ahmed S (2016) Cogeneration through bagasse: a renewable strategy to meet the future energy needs. Renew Sustain Energy Rev. 732-737. https://doi.org/10.1016/j.rser.2015.10.145
Arturi KR, Strandgaard M, Nielsen RP, Søgaard EG, Maschietti M (2017) Hydrothermal liquefaction of lignin in near-critical water in a new batch reactor: influence of phenol and temperature. J Supercrit Fluids 28-39. https://doi.org/10.1016/j.supflu.2016.12.015
Ascencio JJ, Chandel AK, Philippini RR, da Silva SS (2020) Comparative study of cellulosic sugars production from sugarcane bagasse after dilute nitric acid, dilute sodium hydroxide and sequential nitric acid-sodium hydroxide pretreatment. Biomass Convers Biorefin 4:813–822. https://doi.org/10.1007/s13399-019-00547-6
Azimi A, Azari A, Rezakazemi M, Ansarpour M (2017) Removal of heavy metals from industrial wastewaters: a review. ChemBioEng Reviews 1:37–59. https://doi.org/10.1002/cben.201600010
Baêta BEL, Lima DRS, Filho JGB, Adarme OFH, Gurgel LVA, Aquino SFd (2016) Evaluation of hydrogen and methane production from sugarcane bagasse hemicellulose hydrolysates by two-stage anaerobic digestion process. Bioresour Technol 436-446. https://doi.org/10.1016/j.biortech.2016.06.113
Bagotia N, Sharma AK, Kumar S (2021) A review on modified sugarcane bagasse biosorbent for removal of dyes. Chemosphere 129309. https://doi.org/10.1016/j.chemosphere.2020.129309
Bala A, Singh B (2019) Development of an environmental-benign process for efficient pretreatment and saccharification of Saccharum biomasses for bioethanol production. Renew Energy 12-24. https://doi.org/10.1016/j.renene.2018.06.033
Balat M, Balat H (2009) Recent trends in global production and utilization of bio-ethanol fuel. Appl Energy 11:2273–2282. https://doi.org/10.1016/j.apenergy.2009.03.015
Becker M, Ahn K, Bacher M, Xu C, Sundberg A, Willför S, Rosenau T, Potthast A (2021) Comparative hydrolysis analysis of cellulose samples and aspects of its application in conservation science. Cellulose 13:8719–8734. https://doi.org/10.1007/s10570-021-04048-6
Bhardwaj NK, Kaur D, Chaudhry S, Sharma M, Arya S (2019) Approaches for converting sugarcane trash, a promising agro residue, into pulp and paper using soda pulping and elemental chlorine-free bleaching. J Cleaner Prod 225-233. https://doi.org/10.1016/j.jclepro.2019.01.223
Bhat A, Dasan Y, Khan I, Jawaid M (2017) Cellulosic biocomposites: potential materials for future, Springer, 69–100
Bilal M, Iqbal HM (2020) Ligninolytic enzymes mediated ligninolysis: an untapped biocatalytic potential to deconstruct lignocellulosic molecules in a sustainable manner. Catal Lett 2:524–543. https://doi.org/10.1007/s10562-019-03096-9
Brodin M, Vallejos M, Opedal MT, Area MC, Chinga-Carrasco G (2017) Lignocellulosics as sustainable resources for production of bioplastics–a review. J Cleaner Prod 646-664. https://doi.org/10.1016/j.jclepro.2017.05.209
Chambon CL, Mkhize TY, Reddy P, Brandt-Talbot A, Deenadayalu N, Fennell PS, Hallett JP (2018) Pretreatment of South African sugarcane bagasse using a low-cost protic ionic liquid: a comparison of whole, depithed, fibrous and pith bagasse fractions. Biotechnol Biofuels 1:247. https://doi.org/10.1186/s13068-018-1247-0
Chen P, Tao S, Zheng P (2016) Efficient and repeated production of succinic acid by turning sugarcane bagasse into sugar and support. Bioresour Technol 406-413. https://doi.org/10.1016/j.biortech.2016.03.108
Chen X, Che Q, Li S, Liu Z, Yang H, Chen Y, Wang X, Shao J, Chen H (2019) Recent developments in lignocellulosic biomass catalytic fast pyrolysis: strategies for the optimization of bio-oil quality and yield. Fuel Process Technol 106180. https://doi.org/10.1016/j.fuproc.2019.106180
Chotirotsukon C, Raita M, Champreda V, Laosiripojana N (2019) Fractionation of sugarcane trash by oxalic-acid catalyzed glycerol-based organosolv followed by mild solvent delignification. Ind Crops Prod 111753. https://doi.org/10.1016/j.indcrop.2019.111753
Conag AT, Villahermosa JER, Cabatingan LK, Go AW (2017) Energy densification of sugarcane bagasse through torrefaction under minimized oxidative atmosphere. J Environ Chem Eng 6:5411–5419. https://doi.org/10.1016/j.jece.2017.10.032
Cruz G, Santiago PA, Braz CEM, Seleghim P, Crnkovic PM (2018) Investigation into the physical–chemical properties of chemically pretreated sugarcane bagasse. J Therm Anal Calorim 2:1039–1053. https://doi.org/10.1007/s10973-018-7041-1
Cybulska I, Chaturvedi T, Thomsen MH (2019) Lignocellulosic thermochemical pretreatment processes, Springer, Cham, pp 153–165
da Conceição Gomes A, Rodrigues MI, de França Passos D, Machado de Castro A, Maria Mello Santa Anna L, Pereira N (2019) Acetone–butanol–ethanol fermentation from sugarcane bagasse hydrolysates: utilization of C5 and C6 sugars. Electron J Biotechnol 16-22. https://doi.org/10.1016/j.ejbt.2019.10.004
da Silva Machado A, Ferraz A (2017) Biological pretreatment of sugarcane bagasse with basidiomycetes producing varied patterns of biodegradation. Bioresour Technol 17-22. https://doi.org/10.1016/j.biortech.2016.11.053
Dai L, Huang T, Jiang K, Zhou X, Xu Y (2021) A novel recyclable furoic acid-assisted pretreatment for sugarcane bagasse biorefinery in co-production of xylooligosaccharides and glucose. Biotechnol Biofuels 1:1–8. https://doi.org/10.1186/s13068-021-01884-3
Dasgupta D, Ghosh D, Bandhu S, Adhikari DK (2017) Lignocellulosic sugar management for xylitol and ethanol fermentation with multiple cell recycling by Kluyveromyces marxianus IIPE453. Microbiol Res 64-72. https://doi.org/10.1016/j.micres.2017.04.002
de Araujo Guilherme A, Dantas PVF, Padilha CEdA, dos Santos ES, de Macedo GR (2019) Ethanol production from sugarcane bagasse: use of different fermentation strategies to enhance an environmental-friendly process. J Environ Manage 44-51. https://doi.org/10.1016/j.jenvman.2018.12.102
de Carvalho DM, Colodette JL (2017) Comparative study of acid hydrolysis of lignin and polysaccharides in biomasses. BioResources 4:6907–6923
de Lucas RC, de Oliveira TB, Lima MS, Pasin TM, de Almeida Scarcella AS, Prade RA, Segato F, de Moraes MdLT (2022) Effect of enzymatic pretreatment of sugarcane bagasse with recombinant hemicellulases and esterase prior to the application of the cellobiohydrolase CBH I Megazyme®. Biomass Convers Biorefin 1–9. https://doi.org/10.1007/s13399-020-00719-9
de Paula RG, Antoniêto ACC, Ribeiro LFC, Srivastava N, O’Donovan A, Mishra PK, Gupta VK, Silva RN (2019) Engineered microbial host selection for value-added bioproducts from lignocellulose. Biotechnol Adv 6:107347. https://doi.org/10.1016/j.biotechadv.2019.02.003
de Souza CC, Leandro JP, dos Reis Neto JF, Frainer DM, Castelão RA (2018) Cogeneration of electricity in sugar-alcohol plant: perspectives and viability. Renew Sustain Energy Rev 832-837. https://doi.org/10.1016/j.rser.2018.04.047
Debnath B, Haldar D, Purkait MK (2021a) A critical review on the techniques used for the synthesis and applications of crystalline cellulose derived from agricultural wastes and forest residues. Carbohydr Polym 118537. https://doi.org/10.1016/j.carbpol.2021a.118537
Debnath B, Haldar D, Purkait MK (2021b) Potential and sustainable utilization of tea waste: a review on present status and future trends. J Environ Chem Eng 5:106179. https://doi.org/10.1016/j.jece.2021.106179
Dharmaraja J, Shobana S, Arvindnarayan S, Vadivel M, Atabani A, Pugazhendhi A, Kumar G (2020) Biobutanol from lignocellulosic biomass: bioprocess strategies, Academic Press, pp 169–193
do Carmo Ramos SN, Xavier ALP, Teodoro FS, Gil LF, Gurgel LVA (2016) Removal of cobalt (II), copper (II), and nickel (II) ions from aqueous solutions using phthalate-functionalized sugarcane bagasse: mono-and multicomponent adsorption in batch mode. Ind Crops Prod 116-130. https://doi.org/10.1016/j.indcrop.2015.10.035
Dogru M, Erdem A (2019) Process intensification and miniaturization in gasification technology: downdraft gasification of sugarcane bagasse. Energy Fuels 1:340–347. https://doi.org/10.1021/acs.energyfuels.8b03460
Duarah P, Haldar D, Patel AK, Dong C-D, Singhania RR, Purkait MK (2022) A review on global perspectives of sustainable development in bioenergy generation. Bioresour Technol 126791. https://doi.org/10.1016/j.biortech.2022.126791
Dulie NW, Woldeyes B, Demsash HD, Jabasingh AS (2021) An insight into the valorization of hemicellulose fraction of biomass into furfural: catalytic conversion and product separation. Waste Biomass Valorization 2:531–552. https://doi.org/10.1007/S12649-020-00946-1
Espirito Santo M, Rezende CA, Bernardinelli OD, Pereira N, Curvelo AAS, deAzevedo ER, Guimarães FEG, Polikarpov I (2018) Structural and compositional changes in sugarcane bagasse subjected to hydrothermal and organosolv pretreatments and their impacts on enzymatic hydrolysis. Ind Crops Prod 64-74. https://doi.org/10.1016/j.indcrop.2018.01.014
Fahmy TY, Fahmy Y, Mobarak F, El-Sakhawy M, Abou-Zeid RE (2020) Biomass pyrolysis: past, present, and future. Environ Dev Sustain 1:17–32. https://doi.org/10.1007/s10668-018-0200-5
Felipe Hernández-Pérez A, de Arruda PV, Sene L, da Silva SS, Kumar Chandel A, de Almeida Felipe MdG (2019) Xylitol bioproduction: state-of-the-art, industrial paradigm shift, and opportunities for integrated biorefineries. Crit Rev Biotechnol 7:924–943. https://doi.org/10.1080/07388551.2019.1640658
Ghodake GS, Shinde SK, Kadam AA, Saratale RG, Saratale GD, Kumar M, Palem RR, AL-Shwaiman HA, Elgorban AM, Syed A (2021) Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: state-of-the-art framework to speed up vision of circular bioeconomy. J Cleaner Prod 126645. https://doi.org/10.1016/j.jclepro.2021.126645
Ghosh SK (2016) Biomass & bio-waste supply chain sustainability for bio-energy and bio-fuel production. Procedia Environ Sci 31-39. https://doi.org/10.1016/j.proenv.2016.02.005
Gollakota AR, Reddy M, Subramanyam MD, Kishore N (2016) A review on the upgradation techniques of pyrolysis oil. Renewable Sustainable Energy Rev 1543-1568. https://doi.org/10.1016/j.rser.2015.12.180
Gomes MG, Gurgel LVA, Baffi MA, Pasquini D (2020) Pretreatment of sugarcane bagasse using citric acid and its use in enzymatic hydrolysis. Renewable Energy 332-341. https://doi.org/10.1016/j.renene.2020.05.002
Gongora A, Villafranco D (2018) Sugarcane bagasse cogeneration in Belize: a review. Renewable Sustainable Energy Rev 58-63. https://doi.org/10.1016/j.rser.2018.07.034
Govil T, Wang J, Samanta D, David A, Tripathi A, Rauniyar S, Salem DR, Sani RK (2020) Lignocellulosic feedstock: a review of a sustainable platform for cleaner production of nature’s plastics. J Cleaner Prod 122521. https://doi.org/10.1016/j.jclepro.2020.122521
Grewal J, Khare SK (2018) One-pot bioprocess for lactic acid production from lignocellulosic agro-wastes by using ionic liquid stable Lactobacillus brevis. Bioresour Technol 268-273. https://doi.org/10.1016/j.biortech.2017.12.056
Haldar D, Dey P, Patel AK, Dong C-D, Singhania RR (2022) A critical review on the effect of lignin redeposition on biomass in controlling the process of enzymatic hydrolysis. Bioenergy Res. https://doi.org/10.1007/s12155-021-10374-1
Haldar D, Purkait MK (2020a) Lignocellulosic conversion into value-added products: a review. Process Biochem 110-133. https://doi.org/10.1016/j.procbio.2019.10.001
Haldar D, Purkait MK (2020b) Thermochemical pretreatment enhanced bioconversion of elephant grass (Pennisetum purpureum): insight on the production of sugars and lignin. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-00689-y
Haldar D, Sen D, Gayen K (2018) Enzymatic hydrolysis of banana stems (Musa acuminata): optimization of process parameters and inhibition characterization. Int J Green Energy 6:406–413. https://doi.org/10.1080/15435075.2018.1467834
Hartmann C, Fontana RC, de Siqueira FG, Camassola M (2021) Fungal pretreatment of sugarcane bagasse: a green pathway to improve saccharification and ethanol production. Bioenergy Res. https://doi.org/10.1007/s12155-021-10329-6
Hernández-Pérez AF, Costa IAL, Silva DDV, Dussán KJ, Villela TR, Canettieri EV, Carvalho JA, Soares Neto TG, Felipe MGA (2016) Biochemical conversion of sugarcane straw hemicellulosic hydrolyzate supplemented with co-substrates for xylitol production. Bioresour Technol 1085-1088. https://doi.org/10.1016/j.biortech.2015.11.036
Hernández CA, Ziarelli F, Gaime I, Farnet Da Silva AM, García G, García-Pérez JA, Gutiérrez-Rivera B, Alarcón E (2017) Chemical and biological pretreatments on sugarcane bagasse to enhance its enzymatic hydrolysis. ChemistrySelect 15:4213–4218. https://doi.org/10.1002/slct.201700425
Hiloidhari M, Vijay V, Banerjee R, Baruah D, Rao AB (2021) Energy-carbon-water footprint of sugarcane bioenergy: a district-level life cycle assessment in the state of Maharashtra, India. Renew Sustain Energy Rev 111583. https://doi.org/10.1007/s12155-021-10329-6
Hrnčič MK, Kravanja G, Knez Ž (2016) Hydrothermal treatment of biomass for energy and chemicals. Energy 1312-1322.https://doi.org/10.1016/j.energy.2016.06.148
Iram M, Asghar U, Irfan M, Huma Z, Jamil S, Nadeem M, Syed Q (2018) Production of bioethanol from sugarcane bagasse using yeast strains: a kinetic study. Energy Sources Part A 3:364–372. https://doi.org/10.1080/15567036.2017.1422056
Isa KM, Abdullah TAT, Ali UFM (2018) Hydrogen donor solvents in liquefaction of biomass: a review. Renew Sustain Energy Rev 1259-1268. https://doi.org/10.1016/j.rser.2017.04.006
Jahromi R, Rezaei M, Hashem Samadi S, Jahromi H (2021) Biomass gasification in a downdraft fixed-bed gasifier: optimization of operating conditions. Chem Eng Sci 116249. https://doi.org/10.1016/j.ces.2020.116249
Jem KJ, Tan B (2020) The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv Ind Eng Polym Res 2:60–70. https://doi.org/10.1016/j.aiepr.2020.01.002
Ji Q, Yu X, Yagoub AE-GA, Chen L, Zhou C (2021) Efficient cleavage of strong hydrogen bonds in sugarcane bagasse by ternary acidic deep eutectic solvent and ultrasonication to facile fabrication of cellulose nanofibers. Cellulose 1-24. https://doi.org/10.1007/s10570-021-03876-w
Jugwanth Y, Sewsynker-Sukai Y, Gueguim Kana EB (2020) Valorization of sugarcane bagasse for bioethanol production through simultaneous saccharification and fermentation: optimization and kinetic studies. Fuel 116552. https://doi.org/10.1016/j.fuel.2019.116552
Kanwal S, Chaudhry N, Munir S, Sana H (2019) Effect of torrefaction conditions on the physicochemical characterization of agricultural waste (sugarcane bagasse). Waste Manage (Oxford) 280-290. https://doi.org/10.1016/j.wasman.2019.03.053
Khaire KC, Moholkar VS, Goyal A (2021) Bioconversion of sugarcane tops to bioethanol and other value added products: an overview. Mater Sci Energy Technol 54-68. https://doi.org/10.1016/j.mset.2020.12.004
Khoo RZ, Chow WS, Ismail H (2018) Sugarcane bagasse fiber and its cellulose nanocrystals for polymer reinforcement and heavy metal adsorbent: a review. Cellulose 8:4303–4330. https://doi.org/10.1007/s10570-018-1879-z
Konde KS, Nagarajan S, Kumar V, Patil SV, Ranade VV (2021) Sugarcane bagasse based biorefineries in India: potential and challenges. Sustain Energy Fuels 1:52–78. https://doi.org/10.1039/D0SE01332C
Kosinkova J, Ramirez JA, Jablonský M, Ristovski ZD, Brown R, Rainey TJ (2017) Energy and chemical conversion of five Australian lignocellulosic feedstocks into bio-crude through liquefaction. RSC Adv 44:27707–27717. https://doi.org/10.1039/C7RA02335A
Kumar A, Kumar V, Singh B (2021) Cellulosic and hemicellulosic fractions of sugarcane bagasse: potential, challenges and future perspective. Int J Biol Macromol 564-582. https://doi.org/10.1016/j.ijbiomac.2020.12.175
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 1:7. https://doi.org/10.1186/s40643-017-0137-9
Kumar M, Singhal A, Verma PK, Thakur IS (2017) Production and characterization of polyhydroxyalkanoate from lignin derivatives by Pandoraea sp. ISTKB ACS Omega 12:9156–9163. https://doi.org/10.1021/acsomega.7b01615
Kumar V, Binod P, Sindhu R, Gnansounou E, Ahluwalia V (2018) Bioconversion of pentose sugars to value added chemicals and fuels: recent trends, challenges and possibilities. Bioresour Technol 443-451. https://doi.org/10.1016/j.biortech.2018.08.042
Kumari D, Singh R (2018) Pretreatment of lignocellulosic wastes for biofuel production: a critical review. Renew Sustain Energy Rev 877-891. https://doi.org/10.1016/j.rser.2018.03.111
Kundu K, Chatterjee A, Bhattacharyya T, Roy M, Kaur A (2018) Thermochemical conversion of biomass to bioenergy: a review. Prospects of alternative transportation fuels 235-268. https://doi.org/10.1007/978-981-10-7518-6_11
La Villetta M, Costa M, Massarotti N (2017) Modelling approaches to biomass gasification: a review with emphasis on the stoichiometric method. Renew Sustain Energy Rev 71-88. https://doi.org/10.1016/j.rser.2017.02.027
Laluce C, Roldan IU, Pecoraro E, Igbojionu LI, Ribeiro CA (2019) Effects of pretreatment applied to sugarcane bagasse on composition and morphology of cellulosic fractions. Biomass Bioenergy 231-238. https://doi.org/10.1016/j.biombioe.2019.03.002
Li H, Xiong L, Chen X, Wang C, Qi G, Huang C, Luo M, Chen X (2017) Enhanced enzymatic hydrolysis and acetone-butanol-ethanol fermentation of sugarcane bagasse by combined diluted acid with oxidate ammonolysis pretreatment. Bioresour Technol 257-263. https://doi.org/10.1016/j.biortech.2016.12.119
Li M, Wilkins MR (2020) Recent advances in polyhydroxyalkanoate production: feedstocks, strains and process developments. Int J Biol Macromol 691-703. https://doi.org/10.1016/j.ijbiomac.2020.04.082
Liguori R, Faraco V (2016) Biological processes for advancing lignocellulosic waste biorefinery by advocating circular economy. Bioresour Technol 13-20. https://doi.org/10.1016/j.biortech.2016.04.054
Liu ZJ, Lan TQ, Li H, Gao X, Zhang H (2017) Effect of bisulfite treatment on composition, structure, enzymatic hydrolysis and cellulase adsorption profiles of sugarcane bagasse. Bioresour Technol 27-33. https://doi.org/10.1016/j.biortech.2016.10.029
Machado G, Santos F, Lourega R, Mattia J, Faria D, Eichler P, Auler A (2020) Biopolymers from lignocellulosic biomass: feedstocks, production processes, and applications. Lignocellulosic Biorefining Technologies 125-158. https://doi.org/10.1002/9781119568858.ch7
Machineni L (2020) Lignocellulosic biofuel production: review of alternatives. Biomass Convers Biorefin 3:779–791. https://doi.org/10.1007/s13399-019-00445-x
Malhotra M, Suman SK (2021) Laccase-mediated delignification and detoxification of lignocellulosic biomass: removing obstacles in energy generation. Environ Sci Pollut Res 42:58929–58944. https://doi.org/10.1007/s11356-021-13283-0
Mankar AR, Pandey A, Modak A, Pant KK (2021) Pretreatment of lignocellulosic biomass: a review on recent advances. Bioresour Technol 125235. https://doi.org/10.1016/j.biortech.2021.125235
Masran R, Zanirun Z, Bahrin EK, Ibrahim MF, Lai Yee P, Abd-Aziz S (2016) Harnessing the potential of ligninolytic enzymes for lignocellulosic biomass pretreatment. Appl Microbiol Biotechnol 12:5231–5246. https://doi.org/10.1007/s00253-016-7545-1
Méndez J, Passos DdF, Wischral D, Modesto LF, Pereira N (2021) Second-generation ethanol production by separate hydrolysis and fermentation from sugarcane bagasse with cellulose hydrolysis using a customized enzyme cocktail. Biofuels 10:1225–1231. https://doi.org/10.1080/17597269.2019.1608034
Middleton DR, Watts MJ, Polya DA (2019) A comparative assessment of dilution correction methods for spot urinary analyte concentrations in a UK population exposed to arsenic in drinking water. Environ Int 104721. https://doi.org/10.1016/j.envint.2019.03.069
Milani PA, Consonni JL, Labuto G, Carrilho ENVM (2018) Agricultural solid waste for sorption of metal ions, part II: competitive assessment in multielemental solution and lake water. Environ Sci Pollut Res 36:35906–35914. https://doi.org/10.1007/s11356-018-1726-7
Miranda NT, Motta IL, Maciel Filho R, Maciel MRW (2021) Sugarcane bagasse pyrolysis: a review of operating conditions and products properties. Renew Sustain Energy Rev 111394. https://doi.org/10.1016/j.rser.2021.111394
Molina-Guerrero CE, Sanchez A, Vázquez-Núñez E (2020) Energy potential of agricultural residues generated in Mexico and their use for butanol and electricity production under a biorefinery configuration. Environ Sci Pollut Res 23:28607–28622. https://doi.org/10.1007/s11356-020-08430-y
Molino A, Chianese S, Musmarra D (2016) Biomass gasification technology: the state of the art overview. J Energy Chem 1:10–25. https://doi.org/10.1016/j.jechem.2015.11.005
Nanda S, Kozinski AJ, Dalai KA (2016) Lignocellulosic biomass: a review of conversion technologies and fuel products. Curr Biochem Eng 1:24–36
Naseem A, Tabasum S, Zia KM, Zuber M, Ali M, Noreen A (2016) Lignin-derivatives based polymers, blends and composites: a review. Int J Biol Macromol 296-313. https://doi.org/10.1016/j.ijbiomac.2016.08.030
Natte K, Narani A, Goyal V, Sarki N, Jagadeesh RV (2020) Synthesis of functional chemicals from lignin-derived monomers by selective organic transformations. Adv Synth Catal 23:5143–5169. https://doi.org/10.1002/adsc.202000634
Ng HS, Kee PE, Yim HS, Chen P-T, Wei Y-H, Chi-Wei Lan J (2020) Recent advances on the sustainable approaches for conversion and reutilization of food wastes to valuable bioproducts. Bioresour Technol 122889. https://doi.org/10.1016/j.biortech.2020.122889
Nielsen JB, Jensen A, Madsen LR, Larsen FH, Felby C, Jensen AD (2017) Noncatalytic direct liquefaction of biorefinery lignin by ethanol. Energy Fuels 7:7223–7233. https://doi.org/10.1021/acs.energyfuels.7b00968
Niju S, Swathika M (2019) Delignification of sugarcane bagasse using pretreatment strategies for bioethanol production. Biocatal Agric Biotechnol 101263. https://doi.org/10.1016/j.bcab.2019.101263
Nosratpour MJ, Karimi K, Sadeghi M (2018) Improvement of ethanol and biogas production from sugarcane bagasse using sodium alkaline pretreatments. J Environ Manage 329-339. https://doi.org/10.1016/j.jenvman.2018.08.058
Okolie JA, Nanda S, Dalai AK, Kozinski JA (2021) Chemistry and specialty industrial applications of lignocellulosic biomass. Waste and Biomass Valorization 5:2145–2169
Ong KL, Li C, Li X, Zhang Y, Xu J, Lin CSK (2019) Co-fermentation of glucose and xylose from sugarcane bagasse into succinic acid by Yarrowia lipolytica. Biochem Eng J 108-115. https://doi.org/10.1016/j.bej.2019.05.004
Oonkhanond B, Jonglertjunya W, Srimarut N, Bunpachart P, Tantinukul S, Nasongkla N, Sakdaronnarong C (2017) Lactic acid production from sugarcane bagasse by an integrated system of lignocellulose fractionation, saccharification, fermentation, and ex-situ nanofiltration. J Environ Chem Eng 3:2533–2541. https://doi.org/10.1016/j.jece.2017.05.004
Ordonez-Loza J, Chejne F, Jameel AGA, Telalovic S, Arrieta AA, Sarathy SM (2021) An investigation into the pyrolysis and oxidation of bio-oil from sugarcane bagasse: kinetics and evolved gases using TGA-FTIR. J Environ Chem Eng 5:106144. https://doi.org/10.1016/j.jece.2021.106144
Pang S (2019) Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol Adv 4:589–597. https://doi.org/10.1016/j.biotechadv.2018.11.004
Patel H, Chapla D, Shah A (2017) Bioconversion of pretreated sugarcane bagasse using enzymatic and acid followed by enzymatic hydrolysis approaches for bioethanol production. Renew Energy 323-331. https://doi.org/10.1016/j.renene.2017.03.057
Patel M, Zhang X, Kumar A (2016) Techno-economic and life cycle assessment on lignocellulosic biomass thermochemical conversion technologies: a review. Renew Sustain Energy Rev 1486-1499. https://doi.org/10.1016/j.rser.2015.09.070
Philippini RR, Martiniano SE, Chandel AK, de Carvalho W, da Silva SS (2019) Pretreatment of sugarcane bagasse from cane hybrids: effects on chemical composition and 2G sugars recovery. Waste Biomass Valorization 6:1561–1570. https://doi.org/10.1007/s12649-017-0162-0
Pitarelo AP, Fonseca CSd, Chiarello LM, Gírio FM, Ramos LP (2016) Ethanol production from sugarcane bagasse using phosphoric acid-catalyzed steam explosion. J Braz Chem Soc 1889-1898. https://doi.org/10.5935/0103-5053.20160075
Ponnusamy VK, Nguyen DD, Dharmaraja J, Shobana S, Banu JR, Saratale RG, Chang SW, Kumar G (2019) A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour Technol 462-472. https://doi.org/10.1016/j.biortech.2018.09.070
Pournou A (2020) Wood anatomy, chemistry and physical properties, Springer, Cham, pp 1–41
Prabhu AA, Thomas DJ, Ledesma-Amaro R, Leeke GA, Medina A, Verheecke-Vaessen C, Coulon F, Agrawal D, Kumar V (2020) Biovalorisation of crude glycerol and xylose into xylitol by oleaginous yeast Yarrowia lipolytica. Microb Cell Fact 1:121. https://doi.org/10.1186/s12934-020-01378-1
Purkait MK, Singh R, Haldar D, Mondal P (2020) Thermal induced membrane separation processes. Elsevier
Raheem A, Zhao M, Dastyar W, Channa AQ, Ji G, Zhang Y (2019) Parametric gasification process of sugarcane bagasse for syngas production. Int J Hydrogen Energy 31:16234–16247. https://doi.org/10.1016/j.ijhydene.2019.04.127
Rajendran K, Drielak E, Varma VS, Muthusamy S, Kumar G (2018) Updates on the pretreatment of lignocellulosic feedstocks for bioenergy production–a review. Biomass Convers Biorefin 2:471–483. https://doi.org/10.1007/s13399-017-0269-3
Ramirez JA, Brown R, Rainey TJ (2018) Techno-economic analysis of the thermal liquefaction of sugarcane bagasse in ethanol to produce liquid fuels. Appl Energy 184-193. https://doi.org/10.1016/j.apenergy.2018.04.127
Ramirez JA, Rainey TJ (2019) Comparative techno-economic analysis of biofuel production through gasification, thermal liquefaction and pyrolysis of sugarcane bagasse. J Cleaner Prod 513-527. https://doi.org/10.1016/j.jclepro.2019.05.017
Raud M, Kikas T, Sippula O, Shurpali N (2019) Potentials and challenges in lignocellulosic biofuel production technology. Renew Sustain Energy Rev 44-56. https://doi.org/10.1016/j.rser.2019.05.020
Raud M, Olt J, Kikas T (2016) N2 explosive decompression pretreatment of biomass for lignocellulosic ethanol production. Biomass Bioenergy 1-6. https://doi.org/10.1016/j.biombioe.2016.03.034
Rencoret J, Pereira A, del Río JC, Martínez ÁT, Gutiérrez A (2017) Delignification and saccharification enhancement of sugarcane byproducts by a laccase-based pretreatment. ACS Sustainable Chem Eng 8:7145–7154. https://doi.org/10.1021/acssuschemeng.7b01332
Reshmy R, Thomas D, Philip E, Paul SA, Madhavan A, Sindhu R, Sirohi R, Varjani S, Pugazhendhi A, Pandey A (2021) Bioplastic production from renewable lignocellulosic feedstocks: a review. Rev Environ Sci Biotechnol 1-21. https://doi.org/10.1007/s11157-021-09565-1
Saber M, Nakhshiniev B, Yoshikawa K (2016) A review of production and upgrading of algal bio-oil. Renew Sustain Energy Rev 918-930. https://doi.org/10.1016/j.rser.2015.12.342
Sabiha-Hanim S, Abd Halim NA (2018) Sugarcane bagasse pretreatment methods for ethanol production. Fuel Ethanol Production from Sugarcane, IntechOpen, London, pp 63–80
Sanaye S, Khakpaay N, Chitsaz A (2020) Thermo-economic and environmental multi-objective optimization of a novel arranged biomass-fueled gas engine and backpressure steam turbine combined system for pulp and paper mills. Sustain Energy Technol Assess 100778. https://doi.org/10.1016/j.seta.2020.100778
Santo MCE, Fockink DH, Pellegrini VO, Guimaraes FE, deAzevedo ER, Ramos LP, Polikarpov I (2020) Physical techniques shed light on the differences in sugarcane bagasse structure subjected to steam explosion pretreatments at equivalent combined severity factors. Ind Crops Prod 113003. https://doi.org/10.1016/j.indcrop.2020.113003
Santo ME, Rezende CA, Bernardinelli OD, Pereira Jr N, Curvelo AA, Deazevedo ER, Guimarães FE, Polikarpov I (2018) Structural and compositional changes in sugarcane bagasse subjected to hydrothermal and organosolv pretreatments and their impacts on enzymatic hydrolysis. Ind Crops Prod 64-74. https://doi.org/10.1016/j.indcrop.2018.01.014
Santos J, Ouadi M, Jahangiri H, Hornung A (2020) Valorisation of lignocellulosic biomass investigating different pyrolysis temperatures. J Energy Inst 5:1960–1969. https://doi.org/10.1016/j.joei.2020.04.011
Santosh I, Ashtavinayak P, Amol D, Sanjay P (2017) Enhanced bioethanol production from different sugarcane bagasse cultivars using co-culture of Saccharomyces cerevisiae and Scheffersomyces (Pichia) stipitis. J Environ Chem Eng 3:2861–2868. https://doi.org/10.1016/j.jece.2017.05.045
Saratale RG, Cho SK, Saratale GD, Ghodake GS, Bharagava RN, Kim DS, Nair S, Shin HS (2021) Efficient bioconversion of sugarcane bagasse into polyhydroxybutyrate (PHB) by Lysinibacillus sp. and its characterization. Bioresour Technol 124673. https://doi.org/10.1016/j.biortech.2021.124673
Sarker TC, Azam SMGG, Abd El-Gawad AM, Gaglione SA, Bonanomi G (2017a) Sugarcane bagasse: a potential low-cost biosorbent for the removal of hazardous materials. Clean Technol Environ Policy 10:2343–2362. https://doi.org/10.1007/s10098-017-1429-7
Sarker TC, Azam SMGG, Bonanomi G (2017b) Recent advances in sugarcane industry solid by-products valorization. Waste Biomass Valorization 2:241–266. https://doi.org/10.1007/s12649-016-9665-3
Sarker TR, Pattnaik F, Nanda S, Dalai AK, Meda V, Naik S (2021) Hydrothermal pretreatment technologies for lignocellulosic biomass: a review of steam explosion and subcritical water hydrolysis. Chemosphere 131372. https://doi.org/10.1016/j.chemosphere.2021.131372
Sasmal S, Mohanty K (2018) Pretreatment of lignocellulosic biomass toward biofuel production. Springer International Publishing, Cham, pp 203–221
Savou V, Grause G, Kumagai S, Saito Y, Kameda T, Yoshioka T (2019) Pyrolysis of sugarcane bagasse pretreated with sulfuric acid. J Energy Inst 4:1149–1157. https://doi.org/10.1016/j.joei.2018.06.003
Schmatz AA, Brienzo M (2021) Butylated hydroxytoluene improves lignin removal by organosolv pretreatment of sugarcane bagasse. Bioenergy Res. https://doi.org/10.1007/s12155-021-10317-w
Schmatz AA, Tyhoda L, Brienzo M (2020) Sugarcane biomass conversion influenced by lignin. Biofuels, Bioprod Biorefin 2:469–480. https://doi.org/10.1002/bbb.2070
Sharma HK, Xu C, Qin W (2019) Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: an overview. Waste Biomass Valorization 2:235–251. https://doi.org/10.1007/s12649-017-0059-y
Shirkavand E, Baroutian S, Gapes DJ, Young BR (2016) Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment – a review. Renew Sustain Energy Rev 217-234. https://doi.org/10.1016/j.rser.2015.10.003
Sidiras D, Politi D, Giakoumakis G, Salapa I (2021) Simulation and optimization of organosolv based lignocellulosic biomass refinery: a review. Bioresour Technol 126158. https://doi.org/10.1016/j.biortech.2021.126158
Sikarwar VS, Zhao M, Clough P, Yao J, Zhong X, Memon MZ, Shah N, Anthony EJ, Fennell PS (2016) An overview of advances in biomass gasification. Energy Environ Sci 10:2939–2977. https://doi.org/10.1039/C6EE00935B
Sikarwar VS, Zhao M, Fennell PS, Shah N, Anthony EJ (2017) Progress in biofuel production from gasification. Prog Energy Combust Sci 189-248. https://doi.org/10.1016/j.pecs.2017.04.001
Silva TAL, Zamora HDZ, Varão LHR, Prado NS, Baffi MA, Pasquini D (2018) Effect of steam explosion pretreatment catalysed by organic acid and alkali on chemical and structural properties and enzymatic hydrolysis of sugarcane bagasse. Waste Biomass Valorization 11:2191–2201. https://doi.org/10.1007/s12649-017-9989-7
Silveira MHL, Chandel AK, Vanelli BA, Sacilotto KS, Cardoso EB (2018) Production of hemicellulosic sugars from sugarcane bagasse via steam explosion employing industrially feasible conditions: pilot scale study. Bioresour Technol Reports 138-146. https://doi.org/10.1016/j.biteb.2018.07.011
Sindhu R, Binod P, Pandey A (2016a) Biological pretreatment of lignocellulosic biomass–an overview. Bioresour Technol 76-82. https://doi.org/10.1016/j.biortech.2015.08.030
Sindhu R, Gnansounou E, Binod P, Pandey A (2016b) Bioconversion of sugarcane crop residue for value added products – an overview. Renew Energy 203-215. https://doi.org/10.1016/j.renene.2016b.02.057
Singh SP, Jawaid M, Chandrasekar M, Senthilkumar K, Yadav B, Saba N, Siengchin S (2021) Sugarcane wastes into commercial products: processing methods, production optimization and challenges. J Cleaner Prod 129453. https://doi.org/10.1016/j.jclepro.2021.129453
Singhania RR, Patel AK, Raj T, Chen C-W, Ponnusamy VK, Tahir N, Kim S-H, Dong C-D (2022) Lignin valorisation via enzymes: a sustainable approach. Fuel 122608. https://doi.org/10.1016/j.fuel.2021.122608
Singhania RR, Soccol CR, Pandey A (2008) Application of tropical agro-industrial residues as substrate for solid-state fermentation processes. Springer, New York, pp 412–442
Singhvi MS, Gokhale DV (2019) Lignocellulosic biomass: hurdles and challenges in its valorization. Appl Microbiol Biotechnol 23:9305–9320. https://doi.org/10.1007/s00253-019-10212-7
Sothornvit R (2019) Nanostructured materials for food packaging systems: new functional properties. Curr Opin Food Sci 82-87. https://doi.org/10.1016/j.cofs.2019.03.001
Sritrakul N, Nitisinprasert S, Keawsompong S (2017) Evaluation of dilute acid pretreatment for bioethanol fermentation from sugarcane bagasse pith. Agric Nat Resour 6:512–519. https://doi.org/10.1016/j.anres.2017.12.006
Stegen S, Kaparaju P (2020) Effect of temperature on oil quality obtained through pyrolysis of sugarcane bagasse. Fuel 118112. https://doi.org/10.1016/j.fuel.2020.118112
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 49-58. https://doi.org/10.1016/j.biortech.2015.08.061
Terán-Hilares R, Reséndiz A, Martínez R, Silva S, Santos J (2016) Successive pretreatment and enzymatic saccharification of sugarcane bagasse in a packed bed flow-through column reactor aiming to support biorefineries. Bioresour Technol 42-49. https://doi.org/10.1016/j.biortech.2015.12.026
Tondro H, Musivand S, Zilouei H, Bazarganipour M, Zargoosh K (2020) Biological production of hydrogen and acetone- butanol-ethanol from sugarcane bagasse and rice straw using co-culture of Enterobacter aerogenes and Clostridium acetobutylicum. Biomass Bioenergy 105818. https://doi.org/10.1016/j.biombioe.2020.105818
Tran MH, Lee EY (2018) Green preparation of bioplastics based on degradation and chemical modification of lignin residue. J Wood Chem Technol 6:460–478. https://doi.org/10.1080/02773813.2018.1533978
Treedet W, Suntivarakorn R (2018) Design and operation of a low cost bio-oil fast pyrolysis from sugarcane bagasse on circulating fluidized bed reactor in a pilot plant. Fuel Process Technol 17-31. https://doi.org/10.1016/j.fuproc.2018.06.006
Tsegaye B, Balomajumder C, Roy P (2019) Microbial delignification and hydrolysis of lignocellulosic biomass to enhance biofuel production: an overview and future prospect. Bull Natl Res Cent 1:1–16. https://doi.org/10.1186/s42269-019-0094-x
Tu W-C, Hallett JP (2019) Recent advances in the pretreatment of lignocellulosic biomass. Curr Opin Green Sustain Chem 11-17. https://doi.org/10.1016/j.cogsc.2019.07.004
Unrean P (2018) Optimized feeding schemes of simultaneous saccharification and fermentation process for high lactic acid titer from sugarcane bagasse. Ind Crops Prod 660-666. https://doi.org/10.1016/j.indcrop.2017.11.043
Unrean P, Ketsub N (2018) Integrated lignocellulosic bioprocess for co-production of ethanol and xylitol from sugarcane bagasse. Ind Crops Prod 238-246. https://doi.org/10.1016/j.indcrop.2018.06.071
Unrean P, Napathorn SC, Tee KL, Wong TS, Champreda V (2021) Lignin to polyhydroxyalkanoate bioprocessing by novel strain of Pseudomonas monteilii. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01525-7
van der Pol EC, Eggink G, Weusthuis RA (2016) Production of l(+)-lactic acid from acid pretreated sugarcane bagasse using Bacillus coagulans DSM2314 in a simultaneous saccharification and fermentation strategy. Biotechnol Biofuels 1:248. https://doi.org/10.1186/s13068-016-0646-3
Varma AK, Mondal P (2017) Pyrolysis of sugarcane bagasse in semi batch reactor: effects of process parameters on product yields and characterization of products. Ind Crops Prod 704-717. https://doi.org/10.1016/j.indcrop.2016.11.039
Vaz FL, da Rocha Lins J, Alves Alencar BR, Silva de Abreu ÍB, Vidal EE, Ribeiro E, Valadares de Sá Barretto Sampaio E, Cezar Menezes RS, Dutra ED (2021) Chemical pretreatment of sugarcane bagasse with liquid fraction recycling. Renew Energy 666-673. https://doi.org/10.1016/j.renene.2021.04.087
Vieira S, Barros MV, Sydney ACN, Piekarski CM, de Francisco AC, de Souza Vandenberghe LP, Sydney EB (2020) Sustainability of sugarcane lignocellulosic biomass pretreatment for the production of bioethanol. Bioresour Technol 122635. https://doi.org/10.1016/j.biortech.2019.122635
Vu HP, Nguyen LN, Vu MT, Johir MAH, McLaughlan R, Nghiem LD (2020) A comprehensive review on the framework to valorise lignocellulosic biomass as biorefinery feedstocks. Sci Total Environ 140630. https://doi.org/10.1016/j.scitotenv.2020.140630
Wang H, Pu Y, Ragauskas A, Yang B (2019) From lignin to valuable products–strategies, challenges, and prospects. Bioresour Technol 449-461. https://doi.org/10.1016/j.biortech.2018.09.072
Wang Y, Wang X, Xie Y, Zhang K (2018) Functional nanomaterials through esterification of cellulose: a review of chemistry and application. Cellulose 7:3703–3731. https://doi.org/10.1007/s10570-018-1830-3
Wischral D, Arias JM, Modesto LF, de França PD, Pereira N Jr (2019) Lactic acid production from sugarcane bagasse hydrolysates by Lactobacillus pentosus: integrating xylose and glucose fermentation. Biotechnol Progr 1:e2718. https://doi.org/10.1002/btpr.2718
Yaashikaa P, Kumar PS, Varjani S (2022) Valorization of agro-industrial wastes for biorefinery process and circular bioeconomy: a critical review. Bioresour Technol 126126. https://doi.org/10.1016/j.biortech.2021.126126
Yadav C, Saini A, Zhang W, You X, Chauhan I, Mohanty P, Li X (2021) Plant-based nanocellulose: a review of routine and recent preparation methods with current progress in its applications as rheology modifier and 3D bioprinting. Int J Biol Macromol 1586-1616. https://doi.org/10.1016/j.ijbiomac.2020.11.038
Yang C, Lü X (2021) Composition of plant biomass and its impact on pretreatment, Woodhead Publishing, pp 71–85
Yaradoddi JS, Banapurmath NR, Ganachari SV, Soudagar MEM, Mubarak NM, Hallad S, Hugar S, Fayaz H (2020) Biodegradable carboxymethyl cellulose based material for sustainable packaging application. Sci Rep 1:21960. https://doi.org/10.1038/s41598-020-78912-z
Yoo CG, Meng X, Pu Y, Ragauskas AJ (2020) The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: a comprehensive review. Bioresour Technol 122784. https://doi.org/10.1016/j.biortech.2020.122784
Yu N, Tan L, Sun Z-Y, Nishimura H, Takei S, Tang Y-Q, Kida K (2018) Bioethanol from sugarcane bagasse: focused on optimum of lignin content and reduction of enzyme addition. Waste Manage (Oxford) 404-413. https://doi.org/10.1016/j.wasman.2018.03.047
Zabed HM, Akter S, Yun J, Zhang G, Awad FN, Qi X, Sahu J (2019) Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew Sustain Energy Rev 105–128. https://doi.org/10.1016/j.rser.2019.01.048
Zanatta ER, Reinehr TO, Awadallak JA, Kleinübing SJ, dos Santos JBO, Bariccatti RA, Arroyo PA, da Silva EA (2016) Kinetic studies of thermal decomposition of sugarcane bagasse and cassava bagasse. J Therm Anal Calorim 1:437–445. https://doi.org/10.1007/s10973-016-5378-x
Zhang H, Huang S, Wei W, Zhang J, Xie J (2019) Investigation of alkaline hydrogen peroxide pretreatment and Tween 80 to enhance enzymatic hydrolysis of sugarcane bagasse. Biotechnol Biofuels 1:1–9. https://doi.org/10.1186/s13068-019-1454-3
Zhang H, Xu Y, Yu S (2017) Co-production of functional xylooligosaccharides and fermentable sugars from corncob with effective acetic acid prehydrolysis. Bioresour Technol 343-349. https://doi.org/10.1016/j.biortech.2017.02.094
Zhang H, Zhang S, Yuan H, Lyu G, Xie J (2018a) FeCl3-catalyzed ethanol pretreatment of sugarcane bagasse boosts sugar yields with low enzyme loadings and short hydrolysis time. Bioresour Technol 395-401. https://doi.org/10.1016/j.biortech.2017.10.053
Zhang J, Xie J, Zhang H (2021) Sodium hydroxide catalytic ethanol pretreatment and surfactant on the enzymatic saccharification of sugarcane bagasse. Bioresour Technol 124171. https://doi.org/10.1016/j.biortech.2020.124171
Zhang K, Pei Z, Wang D (2016) Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: a review. Bioresour Technol 21-33. https://doi.org/10.1016/j.biortech.2015.08.102
Zhang W, You Y, Lei F, Li P, Jiang J (2018b) Acetyl-assisted autohydrolysis of sugarcane bagasse for the production of xylo-oligosaccharides without additional chemicals. Bioresour Technol 387-393. https://doi.org/10.1016/j.biortech.2018b.06.039
Zhao X, Li S, Wu R, Liu D (2017) Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: chemistry, kinetics, and substrate structures. Biofuels, Bioprod Biorefin 3:567–590. https://doi.org/10.1002/bbb.1768
Zhao X, Wen J, Chen H, Liu D (2018) The fate of lignin during atmospheric acetic acid pretreatment of sugarcane bagasse and the impacts on cellulose enzymatic hydrolyzability for bioethanol production. Renew Energy 200-209. https://doi.org/10.1016/j.renene.2018.05.071
Zhou X, Li W, Mabon R, Broadbelt LJ (2017) A critical review on hemicellulose pyrolysis. Energy Technol 1:52–79. https://doi.org/10.1002/ente.201600327
Zhu Z, Rezende CA, Simister R, McQueen-Mason SJ, Macquarrie DJ, Polikarpov I, Gomez LD (2016) Efficient sugar production from sugarcane bagasse by microwave assisted acid and alkali pretreatment. Biomass Bioenergy 269-278. https://doi.org/10.1016/j.biombioe.2016.06.017
Acknowledgements
The authors Asma Musfira Shabbirahmed, Dibyajyoti Haldar, and Pinaki Dey are thankful to Karunya Institute of Technology and Sciences (Deemed to be University), Coimbatore, India, for providing support for this study and to develop knowledge towards subsequent research works.
Author information
Authors and Affiliations
Contributions
All authors contributed to the review paper and design. The idea for the article was conceived by Dibyajyoti Haldar and Reeta Rani Singhania; Dibyajyoti Haldar and Asma Musfira Shabbirahmed performed the literature search and data analysis; the first draft was prepared by Asma Musfira Shabbirahmed and Dibyajyoti Haldar; Pinaki Dey, Reeta Rani Singhania, and Anil Kumar Patel are also involved in draft preparation and critically revised the work; overall, Cheng-Di Dong and Mihir Kumar Purkait supervised the review work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shabbirahmed, A.M., Haldar, D., Dey, P. et al. Sugarcane bagasse into value-added products: a review. Environ Sci Pollut Res 29, 62785–62806 (2022). https://doi.org/10.1007/s11356-022-21889-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21889-1