Abstract
Polyhydroxyalkanoates (PHAs) are one of the most promising, degradable and eco-friendly alternatives to fossil fuel-based plastic. Nevertheless, PHA derived from edible sources is a relatively easier process than using other resources. Cost of raw material is being considered as a significant constraint for sustainability of industrial bioplastic production. In recent days, lignocellulosic biomass (LCB) in the form of waste residue generated from agriculture, forestry, energy crop system, marine biomass, industrial and municipal solid waste has gained great attention as it is the most abundant feedstock worldwide and supports the sustainable production of PHA. However, the conversion efficiency and PHA yield vary significantly based on the source and nature of LCB due to their content distinction. The complex structure of LCB, mainly composed of cellulose, hemicellulose, and lignin, makes it challenging to be depolymerized. Therefore, the processes required to utilize LCB for production of PHA are covered in this review including pretreatment, hydrolysis, fermentation, and the associated difficulties during the process development. In addition, several attempts made to exploit LCB as a feedstock for PHA production were also discussed in order to improve the overall conversion process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The biodegradable plastics received considerable attention in recent years due to several environmental issues raised around the world related to the accumulation of the non-degradable petrochemical plastic wastes (Khanna and Srivastava 2005a) and fossil fuel resource depletion (Harding et al. 2007). Polyhydroxyalkanoates (PHAs) are considered as an efficient degradable biopolymer alternative to the conventional plastic due to its similar material properties to thermoplastics and elastomers (Verlinden et al. 2007; Pandian et al. 2010; Annamalai et al. 2018a).
PHAs are classified as both biodegradable and biocompatible polyesters of 3-, 4-, 5- and 6-hydroxyalkanoic acids (Chen and Wu 2005; Khanna and Srivastava 2005b). They are widely synthesized by a variety of bacterial species as a storage material for energy and carbon. PHA sythesis occurs under unfavorable growth conditions such as excess carbon with an environmental stress of at least one nutrient essential for growth (nitrogen, phosphate or oxygen limitation) (Valappil et al. 2007; Annamalai and Sivakumar 2016). PHAs are accumulated as intracellular granules up to 90% of the cellular dry weight to survive under stress conditions (Gouda et al. 2001). The PHAs are classified into short chain length PHAs (scl-PHAs) and medium chain length PHAs (mcl-PHAs) based on its monomeric composition (Solaiman et al. 2006). The scl-PHAs are composed of hydroxy fatty acids (HFAs) with three to five carbons, whereas the mcl-PHAs contain HFAs with six to fourteen carbon atoms. In general, scl-PHAs compete in the market with polyethylene or polypropylene to be used for packaging or everyday plastic commodities owing to its thermoplastic nature and unique physical properties (Koller and Muhr 2014). Contrarily, characteristic of mcl-PHAs resemble those of elastomers, latex, and resins with potential applications in drug delivery systems, tissue engineering, smart latexes and thermo-sensitive adhesives (Muhr et al. 2013). And also, the low glass transition temperature of mcl-PHAs is accountable for their rigidity which makes them suitable to be used as biological rubber materials (Cerrone et al. 2014).
PHAs are recognized as entirely biodegradable with zero toxic waste and recyclable into organic waste (Avérous and Pollet 2012). PHAs are synthesized and accumulated intracellularly by various gram-positive and negative bacteria, some fungi and archaea (Bhuwal et al. 2013). Several studies have been made on isolation of PHAs producing bacteria from a wide range of sources such as soils from terrestrial and marine, sludge, water and polluted environments (Table 1). However, only a few bacteria have been used at industrial scales such as Cupriavidus necator, Azohydromonas lata and recombinant Escherichia coli (Peña et al. 2014).
The investigations on the production of PHA have received much attention continuously in the past few decades due to its potential applications in packaging, medical, agricultural and fisheries (Rehm 2006). The total production of PHA was estimated as 54 kt in 2014 and is expected to increase fivefold dramatically by 2020 (Annamalai et al. 2018a). In 2017, the global (PHAs) market reported that PHA market is growing at a compound annual growth rate (CAGR) of about 6.3% over the next decade and reaching almost $119.15 million by 2025 because of the increasing demand from the healthcare industry and market for renewable materials, and recent improvements in PHAs manufacturing technologies (Fig. 1) (https://www.researchandmarkets.com/reports/4375504/global-polyhydroxyalkanoate-pha-market-analysis). Among the PHAs, poly-3-hydroxybutyrate (PHB) which is a linear polyester of D(-) 3-hydroxybutyrate is the most common, widely produced and the best-characterized homopolymer (Valappil et al. 2007; Hamieh et al. 2013).
However, the primary limiting factor for its commercial success is the cost of sugar used for production, and it was estimated that 3 tons of glucose are required to produce a ton of PHA, especially PHB (Collins 1987; Annamalai et al. 2018a). About 45% of the total production cost is attributed to the raw materials where the carbon source could account for 70–80% of the total expense (Choi and Lee 1997; Du et al. 2012). Regardless of the production costs of PHA declined considerably over the last several decades, the cost of production is still considered as highly expensive compared to those petrochemical plastics (Ferreira and Schlottbom 2016). In 2011, the prices for PHAs were in the range of 4.3–5.26 $/kg, while the conventional polyolefins such as polyethylene terephthalate and polystyrene were about 1.6–1.9 $/kg (Cesário et al. 2014a). Production of PHAs by microbial fermentation using simple and pure carbon source leads to increases the price as these processes required multiple steps for large-scale production and industrialization (Sawant et al. 2016).
Adequate knowledge in LCB derived plastic helps the researchers to continue building on and solve the obstacles, leading toward sustainable commercialization of LCB based plastic. The present article attempts to survey the current trends in PHAs production including the sources, pretreatment, conversion process, and fermentation strategies using LCB. Major constraints happened during biomass processing were discussed along with the pretreatment and hydrolysis considerations. Up to date studies about the status of different LCB exploitation for PHA production were covered in this review. Finally, different technological process issues which must be considered during PHA production implementation on an industrial scale were discussed.
2 LCB biomass: source and chemical structure
2.1 Availability of LCB
The LCB is classified into three main groups based on the resources such as (1) forest residues (2) crop residues and (3) municipal solid wastes (MSW) (Taherzadeh and Karimi 2008). These biomass resources appear to be the largest, abundant and worldwide available feedstock. Meanwhile, LCB does not require extra cultivation land, therefore the production of food and fiber crop is not affected. The plant biomasses which contain 90% of lignocellulosic materials are amounted to be about 200 × 109 tons/year, of which about 8–20 × 109 tons is potentially utilized (Saini et al. 2015). Most of the global agriculture residues come from four crops which are corn, rice, wheat, and sugarcane, while the rest of agro-wastes constitute a small part of the total world biomass. Asia is the leading producer of rice and wheat straws, while sugarcane bagasse and corn stover are mainly generated in America. Furthermore, energy crops, particularly C3 and C4 plants, such as perennial grasses and aquatic plants are the most promising source among other LCB. Of which C4 plants are the more favorable source since they possess an effective photosynthetic pathway. Hence, they produce more than two times of biomass annually in warm and temperate regions (Zabed et al. 2016). Forest wastes mainly consist of pure forestry and forestry by-products such as sawdust, wood chips from top and branches dead tree, pruning, barks in the form of hardwood (e.g., willow and poplar) and softwood (e.g., pine and spruce) (Cai et al. 2017). In addition, in EU countries, more than 250 × 106 tons of MSW is being generated annually, with 3% increase per year (Hadar 2013). Organic fraction of MSW in the form of food waste and waste paper are considered as an alternative and potential source. However, wide variation in their composition and presence of contamination hinder their exploitation in biorefinery applications. (Zabed et al. 2016).
2.2 Chemical structure and basic components of LCB
The exact composition of LCB varies from source to source depending on the plant species, growth conditions and types of plant tissue, and it usually comprises of 35–50% cellulose, 20–35% hemicellulose and 10–25% lignin (Table 2) (Sawant et al. 2016). Cellulose composed of d-glucose subunits, linked by β-1, 4 glycosidic bonds, whereas the hemicellulose that consists of different polymers like pentoses (e.g., xylose and arabinose), hexoses (e.g., mannose, glucose, and galactose) and sugar acids. The major sugar of hemicelluloses is mannose in case of softwoods, whereas xylose in agriculture residues and hardwoods (Persson et al. 2006). Hemicellulose serves as a connecting link between lignin and cellulose fibers which makes the whole cellulose-hemicellulose-lignin network more rigid (Hendriks and Zeeman 2009). Lignin is an amorphous molecule which is constructed of phenylpropane units linked in large three-dimensional structures, and it is extremely resistant to chemical and enzymatic hydrolysis due to its molecular configuration (Mussatto and Teixeira 2010). Thus, it remains a significant limiting factor for efficient hydrolysis where it shields the cellulose and adsorbs the enzyme (Sethi et al. 2013). Moreover, the degradation of the phenylpropanoid matrix leads to release the toxic metabolites which greatly affect the enzymatic hydrolysis (Studer et al. 2011). Although the lignocellulosic material seems to be potential and alternative feedstock for PHA production owing to the composition and massive availability, its economic feasibility mainly depends on several factors including pretreatment, hydrolysis and fermentation methods.
3 Conventional processes of PHA production from LCB
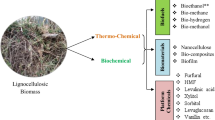
The Fig. 2 described the conventional production process of PHAs from LCB. Though several studies have been made on the production of PHAs from LCB, its effective utilization remains as a challenge due to its recalcitrance structure which complicates the conversion process into monomer sugars from cellulose and hemicellulose (Du et al. 2012).
3.1 Pretreatment of LCB
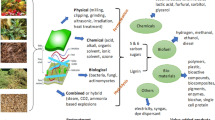
Several factors influence the conversion of LCB such as cellulose crystallinity, its accessible surface area, protection by lignin and hemicellulose, the degree of cellulose polymerization, and degree of acetylation of hemicelluloses (Taherzadeh and Karimi 2008). Thus, pretreatment becomes a crucial step before enzymatic hydrolysis process since it accelerates the degradation of the lignin-carbohydrate complex to produce sugars (Verardi et al. 2012; Jia et al. 2015). The pretreatment and delignification are considered as essential steps which cause significant changes in size, assembly of biomass and chemical composition which leads to more efficient hydrolysis and high yield of sugars (Davis et al. 2013). However, the lignin and xylooligomers released during the pretreatment inhibit the enzymatic digestion by binding irreversibly to the enzymes or blocking the accessibility of substrate to enzymes (Kumar et al. 2013). Hence, it is critical that the pretreatment process should be a feedstock nonspecific, utilizing less chemical, water, and energy. The resulting biomass should be extremely reactive at both macro and micro-accessible sites to increase the rate of enzymatic hydrolysis and sugar yield.
Several pretreatment methods have been exploited such as (1) physico-mechanical comminution and ultrasound (2) chemical-hydrogen peroxide, diluted and concentrated acid or alkali and sodium chlorite (3) physico-chemical—steam explosion, liquid hot water, ammonia fiber expansion (AFEX) pretreatment, and (4) biological pretreatment—microbial enzymes for delignification (lignin-modifying enzymes and lignin-degrading auxiliary enzymes) (Hendriks and Zeeman 2009; FitzPatrick et al. 2010; Kim et al. 2011; Dubey et al. 2012; Cesário et al. 2014a; Annamalai and Sivakumar 2016; Ravindran and Jaiswal 2016; Annamalai et al. 2018a, b). Wang et al. (2012) reported that pretreatment of office and newspaper led to a significant increasing of the glucose yield. Office paper pretreated with diluted acid showed an increase in glucose yield from 69 to 91%. Whereas, AFEX pretreatment of newspaper caused a 13% increase in glucose concentration and more than 50% using oxidative lime compared with untreated paper. Among them, the physicochemical pretreatments such as steam explosion, AFEX, and supercritical CO2 helps to increase the accessible area; whereas, alkali, acid, steam explosion and hydrothermal pretreatments remove the hemicelluloses, and the lignin could be removed by alkali, oxidative delignification, and biological pretreatment (Sun et al. 2016). Among the various pretreatments, AFEX is an ideal pretreatment technology to accomplish effective pretreatment. It is crucial to increase the surface accessibility for enzymatic hydrolysis, reduce the lignin content and remove the hemicellulose while de-crystallizing cellulose. Moreover, AFEX has several unique benefits such as (1) complete recovery of pretreated chemical (ammonia), (2) the remaining ammonia in pretreated biomass is an excellent nutrient source of microbial growth, and (3) washing step during the process is not needed which enables high solid loading hydrolysis (Balan et al. 2009).
In recent days, the usage of ionic liquids (ILs) has received great attention in lignocellulose biorefinery concept as it can fractionate the biomass into carbohydrate-rich materials (CRMs), lignin-rich materials (LRMs) and hemicellulose (An et al. 2015). The ILs are a type of molten salts with melting points below 100 °C, were proposed for LCB pretreatment (An et al. 2015). Unlike traditional organic solvents, ILs possesses unique properties including chemical and thermal stability, negligible vapor pressure, non-flammability and excellent solubility in many organic compounds (An et al. 2015; Li et al. 2016). Additionally, ILs are recyclable materials with minimal loss of activity and the raw materials used for their synthesis are renewable and readily existing (An et al. 2015; Wu et al. 2013). Wu et al. (2014) reported that the IL pretreatment of poplar wood using 1-ethyl-3-methylimidazolium acetate ([EMIM]-OAC) improved the enzymatic digestibility, decrease the cellulose crystallinity which led the complete hydrolysis of cellulose at very low enzyme loadings (4 FPU/g). While, pretreatment of pine wood by dimethyl sulfoxide/1-allyl-3-methylimidazolium chloride (AmimCl) using microwave, caused a significant increase in the enzymatic hydrolysis reaching 85.4% (Liu et al. 2014).
3.1.1 Pretreatment inhibition
Despite the pretreatment enhances the cellulose and hemicellulose and decreases the lignin, it may cause hydrolysis inhibition through inhibitory compounds produced during pretreatment (Fig. 2). The dilute sulfuric acid pretreatment was considered as a potential method in removing the hemicellulose from the LCB; however, it lead to the production of toxic byproducts such as furfural, 5-hydroxymethylfurfural (HMF), and phenolics, which greatly affects the production of any byproduct (Kuhad et al. 2010). The furfural and HMF are the degradation products of pentose and hexose sugars, respectively. Whereas, lignin ends up with phenolic compounds which suppress the activity of several enzymes such as alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase which are required for PHA fermentation. The furfural has more severe consequences than HMF since they compete with acetaldehyde which is the natural substrate of aldehyde dehydrogenase causing microbial growth inhibition (Modig et al. 2002).
The moderate conditions of AFEX treatment suited for herbaceous and agricultural residues which minimize the formation of inhibitors like furaldehydes, organic acids and aromatic compounds (Cesário et al. 2014a). Bals et al. (2010) reported that almost 90% hydrolysis of cellulose and hemicellulose was obtained after AFEX pretreatment of bermudagrass (5% lignin), switchgrass (15% lignin) and bagasse (15% lignin). However, the AFEX process was not very efficient for biomass with higher lignin content (> 25%) such as aspen chips, newspaper, woods, and nut shells (Balan et al. 2009).
3.2 Hydrolysis of LCB
Cellulose and hemicellulose in the LCB could be hydrolyzed either by enzymatically or chemically into sugars, such as glucose, xylose, etc., which are potential carbon sources for the production of value-added chemicals (Table 2) (Maitan-Alfenas et al. 2015). Cellulose hydrolysis into fermentable sugars is a biorefining area that has invested enormous research efforts, as it is essential for the consequent production of bioproducts (Kumar et al. 2008). In general, acidic and enzymatic hydrolysis of biomasses is the conventional and widely used methods in practice.
3.2.1 Acid hydrolysis
Among the various hydrolysis methods, acid hydrolysis is well known and widely used for LCB where it can penetrate lignin and break down both cellulose and hemicellulose to produce simple sugar without any pretreatments (Sun et al. 2016). In general, acid hydrolysis can be done using either concentrated (10–30%) or diluted (2–5%) sulfuric acid, hydrochloric acid, mineral acids (phosphoric and nitric acid) and some organic acids (formic acid) (Lenihan et al. 2010). The concentrated acid hydrolysis requires moderate temperature producing high glucose yield (90%), but it causes severe corrosion and instrumentation damage. Contrarily, hydrolysis using diluted acid requires high temperatures to reach acceptable sugar yield and cellulose degradation. Although the biomass hydrolysis using concentrated and diluted acid is being considered as an appropriate method for production of sugars, it leads to the production of sugar degradation products such as furfural and HMF (Verardi et al. 2012) which greatly inhibits the cell growth and product yield. Lenihan et al. (2010) suggested that the phosphoric acid is comparatively less aggressive than other acids and produced fewer growth inhibitors.
3.2.2 Enzymatic hydrolysis
Due to the several limitations in acid hydrolysis such as degradation of sugars and formation of fermentation inhibitors, enzymatic hydrolysis is considered as the most promising approach as it is a mild and eco-friendly process and needs less energy (Maitan-Alfenas et al. 2015). In general, the LCB intended for PHA production is commonly pretreated using either alkali or acid process to increase the exposure of cellulose to enzymatic hydrolysis (Amache et al. 2013; Jönsson et al. 2013; Lee et al. 2014; Li et al. 2016; Heng et al. 2017). Figure 3 illustrates the comparison of different reported investigation of LCB based PHA yield (g/L) from acidic and enzymatic hydrolysis (Silva et al. 2007; Yu and Stahl 2008; Sathesh Prabu and Murugesan 2010; Pan et al. 2012; Radhika and Murugesan 2012; Davis et al. 2013; Narayanan et al. 2013; Zhang et al. 2013; Cesário et al. 2014a; Follonier et al. 2014; Gowda and Shivakumar 2014; Obruca et al. 2015a, b; Sawant et al. 2015; Alkotaini et al. 2016; Annamalai and Sivakumar 2016; Kim et al. 2016; Annamalai et al. 2018a), and it is suggested that the yield is varied within and among two groups. Mostly, the average sugar yield achieved from enzymatic hydrolysis is more than double the amount of sugar produced with acid based hydrolysis and the PHA yield also higher than the average, where mcl-PHA yield of the enzymatic hydrolyzed LCB caused overall average reduction. The comparison of PHA yield from independent studies limited our conclusion; however, it would provide a proper evaluation of kind of process required to achieve the higher yield. A comparison of a wide range of LCB with different experimental factors is required to finalize the best LCB and conversion process.
3.2.2.1 Cellulose and hemicellulose hydrolysis
Besides the application of cellulase in several industries, it has been widely used to convert lignocellulosic wastes into high-value products (bioplastic, bioethanol, biodiesel, etc.). The complete hydrolysis of cellulosic part requires the synergistic action of three different types of cellulases; endoglucanase (1,4-β-d-glucan-4-glucanohydrolase; carboxymethylcellulase), exocellobiohydrolase (1,4-β-d-glucanglucohydrolase; avicelase), and β-glucosidase (β-d-glucosideglucohydrolase) (Bals et al. 2010; Annamalai et al. 2012, 2014; Verardi et al. 2012; AL-Kharousi et al. 2015). The endoglucanase randomly cleaves the β-1, 4 linkages of cellulose at exposed positions to produce new reducing ends; whereas, the exocellobiohydrolase specifically hydrolyzing cellobiosyl units from non-reducing ends (Pérez et al. 2002). Finally, the β-glucosidase converts the cellobiose into glucose (Maitan-Alfenas et al. 2015).
Unlike cellulose, hydrolysis of hemicellulose is much complicated as it requires several enzymes with diverse modes of action and specificity to get complete hydrolysis into monosaccharides (Azam 2010). The xylanolytic enzyme system for degradation of xylan comprises of endoxylanase, exoxylanase, β-d-xylosidase, acetyl xylan esterase, α-glucuronidase, α-arabinofuranosidase and ferulic acid esterase. These enzymes carry out a combination of action where endo-enzymes cleave the internal bonds, exo-enzymes cut the end of the oligosaccharides and disaccharides, and remaining enzymes hydrolyze glucuronoyl and the acetyl residues (Balat 2011).
3.2.3 Hydrolysis inhibition
Although enzymatic hydrolysis is the most potential method in biorefinery, hydrolysis conditions and end-product inhibition of this process obstructs its efficiency (Alvira et al. 2010; Sun et al. 2016). During the hydrolysis, deactivation of cellulase caused by specific phenolic compounds derived from lignin such as vanillin, syringaldehyde, transcinnamic acid and hydroxybenzoic acid and also furfural, HMF, xylooligomers, and xylose, which are released from the lignocellulosic material upon hydrolysis (Follonier et al. 2014).
The end product inhibition of cellulases by glucose and cellobiose significantly delay the rates of enzyme-catalyzed cellulose hydrolysis (Andrić et al. 2010). The activities of both β-glucosidase and cellulase are substantially inhibited by the high content of glucose in the hydrolysate. Furthermore, the cellobiose combined with tryptophan which occurs near the active site of cellobiohydrolase forming a steric hindrance to prevent cellulose chains reaching the active sites of cellulase which could be resolved by the addition of β-glucosidase to decrease the formation of cellobiose (Sun et al. 2016). Although enzymatic hydrolysis is eco-friendly, the reaction is slow and highly expensive process due to the high cost of cellulase enzyme which considered as a significant obstacle for industrial scale application (Verardi et al. 2012).
3.3 Detoxification of hydrolysate
Several inhibitory compounds formed during acid hydrolysis or pretreatment step before hydrolysis greatly inhibits the enzymatic hydrolysis as well as microbial fermentation (Kumar et al. 2008; Jönsson et al. 2013). The effect of the these compounds varied depending on their concentration, type of microorganism and fermentation conditions used, which subsequently affects the microbial growth and PHAs content (Castilho et al. 2009; Chandel et al. 2011; Obruca et al. 2015a, b). Hence, the hydrolysates should be detoxified to improve their fermentability and these processes are considered as cost-effective for PHA production (Chandel et al. 2011; Cavka and Jönsson 2013; Jönsson et al. 2013).
Various chemical, physical, and biological methods were used to detoxify the LCB hydrolysate. Most of these methods have been reviewed in details including overliming, extraction (liquid–liquid extration and solid–liquid extraction), vacuum, heating, evaporation, and treatment with microbial and enzymatic biocatalysts (Jönsson et al. 2013; Wang et al. 2018). However, these techniques possess several drawbacks such as high production input, complexity, time consuming process, extensive loss of fermentable sugar and waste accumulation. As a result, these techniques are unsuitable for industrial applications. Therefore, recent studies focused on separation methods based on pressure-derived membrane processes in form of nanofiltration and reverse osmosis membranes. Wang et al. (2018) reported that reverse osmosis membranes possess more ability to retain fermentable sugars under various conditions (pH, pressure, temperature and elute concentration) and led to remove inhibitory compounds as well as increasing the concentration of the sugar in the hydrolysate simultaneously. On the other hand, recovery of these non-sugar inhibitory compounds without a significant loss of fermentable sugar to be exploited as platform chemicals in multiple industries is a prospective strategy. Flocculant in form of organic salts or organic polyelectrolytes showed promising results in detoxification of LCB hydrolysates (Carter et al. 2011). The flocculant polyethylenimine (PEI) was able to remove 100% furan, 73% total phenolic compounds, and 43% organic acids with minimal reducing sugar loss from diluted ammonia pretreated bagasse with a potential flocculant recyclability (Fang and Aita 2018).
However, Yu and Stahl (2008) suggested that the effect of inhibitors on microbial activity could be overcome efficiently by (1) a large inoculum, (2) a diluted hydrolysate solution, and (3) a tolerant strain, or a combination of the three for the production PHAs. Several other reports also suggested using resistant strains to overcome the HMF inhibitory effects for the cost-effective production of PHA from LCB hydrolysate (Nduko et al. 2012; Dietrich et al. 2013; Weissgram et al. 2015).
3.4 PHA fermentation
PHAs are formed intracellularly as an inclusion bodies, and its production mainly depends on cell densities (Ienczak et al. 2013). Several researchers have been working extensively on better fermentation strategy to increase the yield of PHAs through batch, fed-batch and continuous fermentation (Peña et al. 2011; Amache et al. 2013). However, the selection of operation strategy mainly relies on several factors such as the type of culture (pure or mixed), carbon source [simple pure sugars or complex waste materials) and bioreactor type (air-lift reactor and continuously stirred tank reactor (CSTR)] (Peña et al. 2011; Amache et al. 2013). Nevertheless, most of the studies using LCB hydrolysates for PHA production have been implemented using batch culture, and only a few were conducted using fed-batch fermentation (Table 3). Furthermore, the fermentation could be done by a single stage or multi-stages through sequencing batch system (SBR) (Peña et al. 2014).
3.4.1 Batch fermentation
Batch fermentation is an appropriate process and widely used for PHA production because of low cost and flexibility. However, the productivity of batch fermentation is low compared to other fermentations due to the degradation of the accumulated PHAs after complete utilization of the carbon source caused a reduction in PHAs content. Addition of high concentration of substrate in batch culture to overcome exhausting of carbon leads to inhibit the growth as well as production yield (Amache et al. 2013). Studies suggested that the concentration of glucose in bioreactor should be between 0 and 20 g/L (Ryu et al. 1997) and 10 and 20 g/L (Kim et al. 1994). However, Ienczak et al. (2013) suggested that carbon source pulse during fermentation should not be performed upon carbon source exhaustion as it leads to the degradation of PHA due to PHA depolymerase activity.
3.4.2 Fed-batch fermentation
Fed-batch fermentation is industrially preferred and also a most effective method to reach high cell density cultivation (HCDC), high yield and productivity. Consequently, it is used widely in microbial fermentation for PHA synthesis (Ibrahim and Steinbüchel 2010). In fed-batch fermentation, cells are initially grown under a batch regime until it reaches the end of the exponential phase and then under famine condition of essential nutrients (nitrogen, phosphorus, and oxygen) with supplementation of carbon source and other needs into the bioreactor (Shamala et al. 2014). Till date, many studies have been made with fed-batch fermentation for production of PHA by Burkholderia sacchari, Burkholderia cepacia, Pseudomonas resinovorans and Bacillus megaterium utilizing hydrolysates of lignocellulosic biomass (Pan et al. 2012; Cesário et al. 2014a; Follonier et al. 2014; Alkotaini et al. 2016). Cesário et al. (2014a) achieved very high biomass and polymer accumulation (135.8 and 105.0 g/L) from wheat straw hydrolysate as a source for PHB production using fed-batch fermentation strategy. Since good feeding strategy is critical for high cell density, PHA content, and PHA productivity, several attempts of carbon feeding strategies is continuously tried.
3.4.3 Continuous fermentation
Continuous fermentation is known as chemostat cultivation, is another operation strategy broadly employed for PHA production, in which the substrate, culture medium, and other requirements are being pumped continuously into bioreactor system (Balat 2011). In this, the substrate (carbon source) is continuously fed in excess while one or more nutrient such as nitrogen and phosphorous are kept in famine condition. It is highly controllable and dependent on specific growth rate which can be adjustable through dilution rate. The cultivation using continuous strategy can advantageously maximize the PHA accumulation, yield, and productivity; however, there is a higher risk of contamination (Amache et al. 2013).
3.4.4 Carbon catabolite repression phenomenon
Carbon catabolite repression (CCR) is found to be a major bottleneck in PHA production utilizing LCB as an alternative feedstock due to the selective consumption of specific sugar in the hydrolysate as it mainly composed of glucose and xylose (Zhou et al. 2013). The consumption of mixed sugars achieved sequentially in many PHAs producing bacteria, whereby the utilization of glucose represses xylose utilization due to CCR and makes the fermentation process more complex with reduced productivity (Cesário et al. 2014a). Hence, few studies have been made on native xylose utilizing bacteria such as E. coli and B. cepacia to utilize xylose for PHA production through different feeding strategies with glucose and xylose to overcome the CCR phenomenon. Lopes et al. (2011) studied the CCR using B. sacchari IPT10 which utilizes xylose after the complete consumption of glucose, while the UV PTS mutant consumed both glucose and xylose simultaneously. Likewise, Li et al. (2007) used E. coli phosphotransferase system (PTS) mutants which harboring phaC (Re) and phaAB (Re) genes from Ralstonia eutropha (known as C. necator) could utilize glucose and xylose simultaneously and produced scl-PHA, while E. coli LR1120 and LR1110 containing phaC1 gene from Pseudomonas aeruginosa were able to accumulate mcl-PHA.
4 Lignocellulosic materials as a potential raw material for PHA production
In recent days, several researchers are in search of renewable and economic resources as feedstock to be implemented in the PHAs production. Of which, LCB is considered as one of the major feedstock sources due to its high cellulose content and its abundant availability. In order to maximize the value derived from the biomass feedstock, it is better to take advantage of the different component and even intermediate of the biomass (Rabelo et al. 2011). There are several investigations have been made continuously on the production of PHAs utilizing various LCB (Table 3).
4.1 Woody biomass
Approximately, out of 180 billion tons of total plant matter generated annually, 80 billion tons is woody biomass, which represents a massive reservoir of renewable source. Most of the cellulosic constituent is efficiently exploited by the paper/pulp industry resulting in the generation of a variety of solid waste materials, for instance, sawdust, shavings or bark (Kucera et al. 2017). Wood waste in the form of hemicellulose and lignin fractions are enormously underutilized process streams, which hold potential as platform intermediates in the production of value-added products, such as PHAs (Obruca et al. 2015a, b; Kucera et al. 2017). The woody biomass requires very harsh conditions to be degraded into fermentable sugars which leads to the production of several types of inhibitors that affected the later fermentation process. The concentration of xylose content of acid hydrolysed sugar maple hemicellulosic biomass was about 160.7 g/L; however, the detoxification steps caused about 50% loss of xylose. The fed-batch fermentation of B. cepacia using woody hydrolysate exhibited maximum PHAs production after 96 h resulted in 8.72 g/L and 51.4% of PHA content and dry cell weight, respectively (Pan et al. 2012). Likewise, Silva et al. (2007) reported that the Brevundimonas vesicularis or Sphingopyxis macrogoltabida produced the significantly high amount of PHA with the acid hydrolyzed Pinus radiata sawdust used without any detoxification. Furthermore, the PHAs-producing bacterium, Sphingobium scionense WP01 was grown on pretreated P. radiata hydrolysate produced the PHAs content and yield of 32% (w/w) and 0.22 (g/g), respectively which was comparatively lower than the yield of pure glucose due to the inhibitors in the hydrolysate.
4.2 Grass biomass
The grass biomass comprises of 25–40% cellulose, 15–50% hemicellulose, 10–30% lignin with free sugars (10–26%) varied based on the species, the maturity of the plant and the environmental conditions (Nizami et al. 2009; Ellis et al. 2012). The grass biomass is considered as an important feedstock for PHA production as it contains water-soluble carbohydrates (WSC) which are directly available substrates for bacterial fermentation and also insoluble carbohydrates in the form of cellulose and hemicellulose (Mandl 2010). The strains, B. sacchari IPT101 and Pseudomonas chlororaphis IMD555, produced the cell mass and PHB content utilizing the mannitol-rich ensiled grass press juice (EGPJ) of perennial ryegrass was 44.5 g/L and 33%, and 37 g/L and 10%, respectively (Cerrone et al. 2015). Further, the grass biomass anaerobically used to produce 15.3 g/L of volatile fatty acids (VFAs) and the strain, Pseudomonas putida CA-3 grown on VFA produced the biomass and PHA content of 1.56 g/L and 39%, respectively (Cerrone et al. 2014).
4.3 Bagasse
Sugarcane bagasse is the major byproduct of sugar mills, representing 30% of the processed sugarcane (Alva Munoz and Riley 2008). The usage of bagasse to produce fuels, chemicals, and other value-added chemicals have been increased continuously due to its abundant availability for bioenergy production. Acidic hydrolysis of sugarcane bagasse resulted in 16.9 g/L xylose, and 9.7 g/L glucose was utilized by xylose-utilising B. sacchari IPT 101 and B. cepacia IPT 048 and accumulated PHA about 62 and 53%, respectively (Silva et al. 2004). Whereas PHB content reached to 65% while using sugarcane bagasse hydrolysates by R. eutropha under nutrient-limiting conditions at high cell density culture, but xylose remains unutilized (Yu and Stahl 2008). Alva Munoz and Riley (2008) reported that the Saccharophagus degradans directly converted the tequila bagasse and other the cellulosic materials into PHAs without any hydrolysis which greatly reduces the cost limitation of the production of bioplastics; however, the process still needs to be optimized for final PHAs concentration and productivity.
4.4 Corn stover
Utilization of corn stover for production of PHAs seems to be a good option as it is one of the most promising renewable feedstocks for the biological conversion to fuels and chemicals (Kim et al. 2003). The major advantage of corn stover holds over alternative crops, such as switchgrass, it is already produced with grain as the target and does not require dedicated land. Recently, corn stover hydrolysate prepared by cellulase of Trichoderma reesei and Aspergillus niger was used for PHA production by Paracoccus sp. LL1 without any detoxification and the resulting PHAs production was 9.71 g/L suggests that the corn stover is one of the favorable substrates for the PHA production (Sawant et al. 2015).
4.5 Rice husk
About 22% of paddy weight is rice husk which is the result of rice milling, while rice straw to paddy ratio is 1–4.3. Although rice straw and husk are the readily available agro-industrial waste, rice husk is much preferable as a renewable feedstock because of easy procurement; however, rice straw is less available and difficult to collect (Zafar 2015). The production of PHB and its copolymer poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (P(3HB-co-3HV) ranged between 19 and 34.5% (w/w) from acid hydrolyzed rice husk which mainly consist of pentose sugars: xylose (5.5–7.1 g/L), arabinose (1.19–1.36 g/L) and glucose and galactose (0.5–0.7 g/L) (Narayanan et al. 2013). However, alkaline pretreatment increased the sugar yield to 87% (20 g/L), and the PHA production was 50% by B. cepacia which is more efficient compared with C. necator (Heng et al. 2017). Saratale and Oh (2015) suggested that the alkaline pretreated rice straw was utilized for PHB production after two-steps of hydrolysis and the PHB content and PHB yield was about 75.45% and 11.42 g/L, respectively.
4.6 Wheat
Global wheat production was estimated as 660 million tons in 2012–2013, of which 15–20% is the straw which is an abundant feedstock with low economic value and is typically used as cattle feed (Cesário et al. 2014a). Wheat straw contains approximately 25% hemicellulose, mainly pentoses (80%) which could be used as a potential source of carbohydrates to use as a source for PHA production (Lee et al. 2007; Doan and Nguyen 2012; Le Meur et al. 2012; Cesário et al. 2014a; Cesário and de Almeida 2015). The PHB producer, B. sacchari IPT has been used for PHB production utilizing wheat straw hydrolysate and produced 60% of PHB (Cesário et al. 2014a).
Similarly, wheat bran also used as a renewable resource where it is produced in massive amount as a byproduct of the cereal industry (Martel et al. 2010). Recently, Annamalai and Sivakumar (2016) attempted to produce PHB by R. eutropha utilizing wheat bran hydrolysate (62.91 g/L sugars) and the cell dry weight (CDW), PHB content and yield achieved were 24.5 g/L, 62.5%, and 0.319 g/g sugar, respectively with a productivity of 0.255 g/L/h.
4.7 Spent coffee
Spent coffee ground (SCG) is the major end residue of coffee industry which has been used beyond the limited traditional usage as livestock food and fertilizers, and it is mainly composed of carbohydrates, lipids, and proteins (Couto et al. 2009). One ton of green coffee generated 330–650 kg of dry SCG and it is about 2.7 billion tons of SCG waste were produced worldwide in 2014 (Obruca et al. 2014a). In recent days, oil extracted from SCG (15%) was used for the production of PHAs by employing C. necator H16 (Obruca et al. 2014b). However, the residues remained were hydrolysed into fermentable sugar and further utilized by B. megaterium and B. cepacia to produce about 51 and 56% of PHA, respectively (Obruca et al. 2014a, 2015a, b).
4.8 Sunflower meal
Sunflower is considered as one of the most important sources for oilseed extraction for both food and biodiesel production which ends up with the production of 15 × 106 tons of oilseed meal during the production of biodiesel is corresponding to generate 3 × 106 tons of crude glycerol (Kachrimanidou et al. 2014). In recent days, several studies were made to use the oilseed biorefineries to produce other chemical and value-added products. The sunflower meal (SFM) hydrolysate was used for P(3HB-co-3HV) production using C. necator DSM 545, and the yield achieved was 9.9 g/L containing 3 mol% of 3HV (Kachrimanidou et al. 2013). Recently, the recombinant R. eutropha NCIMB11599 expressing E. coli xylAB genes produced the PHB, content, yield, and productivity of 8.79 g/L, 88.69%, 42.0%, and 0.15 g/L/h, respectively using sunflower stalk hydrolysate which consist of 6.15 g/L of xylose and 16.94 g/L of glucose (Kim et al. 2016). Furthermore, SFM was also used to produce nutrient-rich fermentation supplements after antioxidant extraction with crude glycerol from the biodiesel byproduct waste using fed-batch bioreactor fermentation which caused to maximize profit and the efficiency of resource exploitation (Kachrimanidou et al. 2015).
4.9 Oil palm
Among the LCB resources, palm oil has been spotlighted as a valuable source for PHA production. Due to the demands of oil palm, there is an increase of palm oil plantations in many countries which lead to generate a significant amount of biomass in dry basis. The amount of solid and liquid biomass is expected to rise by 2020 is 85–110 million dry Mg, generating about 70–110 × 109 kg of palm oil mill effluent (POME) (Hassan et al. 2013). Lokesh et al. investigated the potential of sap extracted from chopped oil palm trunk (OPT) for PHA production using B. megaterium MC1 (Lokesh et al. 2012). Likewise, Zhang et al. (2013) studied the PHA production using oil palm empty fruit bunch (OPEFB) by B. megaterium R11. The PHB content and production were 58.5% and 9.32 g/L, and 51.6% and 12.48 g/L from the OPEFB hydrolysate with 45 and 60 g/L sugar, respectively.
4.10 Coir pith
Coir pith, the byproduct of the coir industry, contains about 42% cellulose, 43% lignin and a negligible amount of hemicellulose (Asha et al. 2016). Utilization of coir pith solved not only the environmental problem but also reduced the PHA production cost. Sathesh Prabu and Murugesan (2010) reported that 30% reducing sugar produced by enzymatic hydrolysis of the pretreated coir pith was converted to 48.19% of PHB by Azotobacter beijerinickii.
4.11 Fruit pomace
The pomaces derived after processing grape is mainly composed of fruit skins, pulp, stalks, and seeds, which contains high quantity of cellulose, hemicellulose, pectin and starch that could be used as raw material for the production of reducing sugars (Korkie et al. 2002). The hydrolysate of apricot and cherries pomace contains about 45 and 106 g/L sugar and the PHAs production was 1.4 and 21.3 g/L, respectively (Follonier et al. 2014).
4.12 Macrophyte
Macrophytes which are dominating most of aquatic and freshwater wetlands in form of emergent, submergent or floating plants, influencing directly or indirectly the structure and function of the ecosystems (Thomaz and da Cunha 2010; Jiang et al. 2018). Utilization of the aquatic crop has been used as a carbon source for the production of other eco-friendly bioproducts including ethanol, biogases, methane, levulonic acid and PHAs (Cheng et al. 2010; Radhika and Murugesan 2012). Of which, water hyacinth (Eichhornia crassipes) is one of the an aquatic weed which consists of 25, 35 and 10% of cellulose, hemicellulose, and lignin, respectively. Recently, Pradhan et al. (2017) achieved the CDW and PHB of 3.70 and 0.30, and 4.44 and 0.96 (g/L) from pentose and hexose rich hydrolysate of Water hyacinth, respectively. However, production reached the maximum (12 g/L CDW and 7 g/L PHB) when water hyacinth hydrolysate (35 g/L reducing sugar) was utilized by C. necator MTCC-1472 under the optimized physical and nutrient condition through central composite design methods in laboratory scale fermenter (Radhika and Murugesan 2012).
4.13 Macroalgae
Macroalgal biomass is considered as a potential feedstock for biorefinery products due to its low lignin and also the simple process required to convert it into sugars (Azizi et al. 2017). The acid pretreated red algae Gelidium amansii consist of galactose (25.5 g/L), glucose (3.6 g/L) and levulinic acid (1.05 g/L) were used for PHA production by B. megaterium and the yield achieved was comparatively higher than that of other lignocellulosic materials (Table 3) (Alkotaini et al. 2016). Azizi et al. (2017) reported the highest cell dry weight (5.36 g/L) and PHB (3.93 g/L) by C. necator with PHB yield of 0.54 g/g reducing sugar using the brown algae (Sargassum sp.) after a series of optimization with NaCl as an external stress factor. Thus, the macroalgae could be an excellent future feedstock not only because of their availability but also for their easy and forward use without any requirement for enzymatic hydrolysis and inhibitor removal which lead to reduce the cost of the production process.
4.14 Waste paper
The waste paper which accounts more than 35% of the total lignocellulosic waste of the MSW and industrial solid wastes is a potential feedstock for PHA production due to its richness in cellulose and abundant at low cost (average $52/ton) (Dubey et al. 2012; Annamalai et al. 2018a, b). More than 400 million tons of waste paper is generated worldwide where only 50–65% is being recycled because of the limitations in the recycling of paper fibers which turned into low-quality paper products (Wang et al. 2012; Al Azkawi et al. 2018; Nair et al. 2018). Utilization of waste paper for PHA production is much valuable and an alternative route for waste management. Recently, a suitable and feasible bioprocess has been developed to use waste office paper (WOP) for production of PHAs. The hydrolysate of WOP consist of 24.48 g/L reducing sugar (91.5%) used for PHB production using C. necator without any detoxification and the resulted cell dry weight (CDW), PHB production and PHB content achieved was 7.74 g/L, 4.45 g/L and 57.52% of, respectively, suggested that WOP could be a potential alternative feedstock for the biorefinery production of bioplastics (Annamalai et al. 2018a).
5 Technological progress in lignocellulosic derived PHA
5.1 Process integration
Although utilization of LCB for PHA production is a promising alternative to reduce the production cost, the different technological process required including pretreatment, hydrolysis, and fermentation affected their economic competition with petrochemical-based plastic. Nevertheless, scale-up of PHA production requires further research on integration steps to reduce cost, process, energy consumption and process time.
The utilization of LCB for production of PHA mostly depends on separate hydrolysis and fermentation (SHF) and the major bottleneck is the end-point inhibition which greatly inhibits the microbial growth and then hinders PHA accumulation. Hence, it is essential to develop a suitable process and fermentation strategy for one-step process such as simultaneous saccharification and fermentation (SSF) to overcome the troubles in reduction of energy consumption in SHF, and the investigations were recently started to study the feasibility of SSF in LCB derived PHA from wheat straw and cereal mash (Dahman and Ugwu 2014; García-Torreiro et al. 2016). Hence, investigation, assessment and any future improvement of the technological process should be assured to improve the productivity of PHA derived from LCB which would be a critical point to increase the attraction towards the industrial scale implementation.
5.2 Third generation feedstock
Though the second-generation LCBs have been used intensively as feedstock in PHA research, there are still many untapped biomass resources. The marine biomasses, especially marine wastes (fish and other shell wastes) and microalgae which identified as third-generation raw materials, needs further investigation to assess its application in larger scale since it is considered as full nutrients medium containing for instance carbohydrates and lipids and easy to grow. The utilization of microalgae could be an appropriate option because of the most effective renewable source (Demirbas 2011).
5.3 Complete utilization of hydrolysate—hexose and pentose sugar fermentation
The hydrolysate obtained from LCB containing mixed of sugars, hexose and pentose sugars due to the complex structure of the LCB. The PHA producers tend to discriminatingly consume the desired sugar mainly glucose with the known CCR phenomenon and are considered as a challenging bottleneck in LCB biorefinery. In addition to the wild-type bacteria which gained more interest industrially, genetically modified bacteria were also developed by incorporation of E. coli xylAB genes encoding xylose isomerase and xylulokinase in R. eutropha for PHA production to utilize both hexose and pentose sugars (Kim et al. 2016) which required sterile condition and substrate-specific growth nutrient causing high production cost. Hence, it is necessary to find out novel, efficient, powerful PHA-producing strains which could able to utilize both hexose and pentose sugars to be incorporated into PHA polymer (Fig. 4). Research should be focused on microbes from extreme environments such marine microbes (halophile) and/or utilize unusual substrates would be a feasible tool due to low risk of contamination, and thus, production can be carried out under unsterile/open process. In recent days, production of PHA by mixed microbial culture (MMC) using activated sludge under non-sterile and the microbial consortia accumulated PHA were enriched in aerobic SBR under feast and famine phase cycles (Koller 2016).
6 Summary and future perspectives
PHAs received great attention in the biodegradable polymer market since it represents a potential sustainable replacement of the harmful fossil-fuel based plastics. However, the price is considered the main constraint in its commercialization since carbon source constitutes half the production cost. Therefore, it is crucial to explore cheap, renewable, sustainable and alternative feedstock making bioplastic compete with its counterpart. Hence, LCB has a few points of interest over regular sugar and starch-based crude biomass and has been anticipated to be one of the fundamental bio-resources of biorefinery. Unlike other sources, LCB-based PHA production needs several processing steps (pretreatment, hydrolysis and detoxification) prior the fermentation. Due to the distinction of their composition and structure, pretreatment ranged from simple to complicated process. After pretreatment, optimization is required to achieve maximum hydrolysis of each type of LCB. While enzymatic hydrolysis represents an ideal route resulted in higher sugar and PHA yield despite the use of expensive enzymes. However, the cost of enzyme will be negligible in case of integration of enzyme production in PHA production system. To improve the feasibility of PHA production utilizing LCB hydrolysate, prior detoxification step is used to remove undesired inhibitory compounds as well as retaining the maximum sugar. An additional advantage is recovering these compounds to be exploited as platform chemicals in multiple industries. Fermentation of highly prospective LCB hydrolysate opens the door for PHA market leading to a dual benefit of reducing the cost of both PHA and waste disposal.
Despite LCB-based PHA is still at the investigation phase; however, the results are promising. The recent advances in PHA production utilizing LCB involve several new strategies to enhance the yield, productivity and overcome the process difficulties to reach the industrial production level. Development of an efficient biorefining system could be achieved through process integration like SSF as well as the integration of PHA sustainable processing stream in LCB waste producing conventional industrial plants. In addition, more efforts are needed towards the product quality improvement and efficient recovery process, which would result in high yield. Moreover, several attempts are being made continuously to find out strains capable of utilizing both C5 and C6 sugars to produce a high amount of PHAs from the inexpensive renewable sources. Zero waste policies by efficient utilization of these resources are essential for PHA sustainable markets.
In conclusion, LCB received more attention in the last decades as a feedstock for PHA. However, the investigation is continuing to improve the process and encounter the challenges before turning to large-scale implementation. Therefore, future innovation to reduce PHA production price in the existing industries is an urgent need.
References
Al Azkawi AS, Sivakumar N, Al Bahry S (2018) Bioprocessing of cardboard waste for cellulase production. Biomass Conv Bioref 8:597–606. https://doi.org/10.1007/s13399-018-0309-7
Alias Z, Tan IKP (2005) Isolation of palm oil-utilising, polyhydroxyalkanoate (PHA)-producing bacteria by an enrichment technique. Bioresour Technol 96:1229–1234. https://doi.org/10.1016/j.biortech.2004.10.012
AL-Kharousi MM, Sivakumar N, Elshafie A (2015) Characterization of cellulase enzyme produced by Chaetomium sp. isolated from books and archives. EurAsian J Biosci 9:52–60
Alkotaini B, Koo H, Kim BS (2016) Production of polyhydroxyalkanoates by batch and fed-batch cultivations of Bacillus megaterium from acid-treated red algae. Korean J Chem Eng 33:1669–1673. https://doi.org/10.1007/s11814-015-0293-6
Alva Munoz LE, Riley MR (2008) Utilization of cellulosic waste from tequila bagasse and production of polyhydroxyalkanoate (PHA) bioplastics by Saccharophagus degradans. Biotechnol Bioeng 100:882–888. https://doi.org/10.1002/bit.21854
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861. https://doi.org/10.1016/j.biortech.2009.11.093
Amache R, Sukan A, Safari M et al (2013) Advances in PHAs production. Chem Eng Trans 32:931–936. https://doi.org/10.3303/CET1332156
An Y-X, Zong M-H, Wu H, Li N (2015) Pretreatment of lignocellulosic biomass with renewable cholinium ionic liquids: biomass fractionation, enzymatic digestion and ionic liquid reuse. Bioresour Technol 192:165–171. https://doi.org/10.1016/j.biortech.2015.05.064
Andrić P, Meyer AS, Jensen PA, Dam-Johansen K (2010) Reactor design for minimizing product inhibition during enzymatic lignocellulose hydrolysis: II. Quantification of inhibition and suitability of membrane reactors. Biotechnol Adv 28:407–425. https://doi.org/10.1016/j.biotechadv.2010.02.005
Annamalai N, Sivakumar N (2016) Production of polyhydroxybutyrate from wheat bran hydrolysate using Ralstonia eutropha through microbial fermentation. J Biotechnol 237:13–17. https://doi.org/10.1016/j.jbiotec.2016.09.001
Annamalai N, Rajeswari MV, Elayaraja S et al (2012) Purification and characterization of thermostable alkaline cellulase from marine bacterium Bacillus licheniformis AU01 by utilizing cellulosic wastes. Waste Biomass Valoriz 3:305–310
Annamalai N, Rajeswari MV, Balasubramanian T (2014) Enzymatic saccharification of pretreated rice straw by cellulase produced from Bacillus carboniphilus CAS 3 utilizing lignocellulosic wastes through statistical optimization. Biomass Bioenergy 68:151–160. https://doi.org/10.1016/j.biombioe.2014.06.018
Annamalai N, Al-Battashi H, Al-Bahry S, Sivakumar N (2018a) Biorefinery production of poly-3-hydroxybutyrate using waste office paper hydrolysate as feedstock for microbial fermentation. J Biotechnol 265:25–30. https://doi.org/10.1016/j.jbiotec.2017.11.002
Annamalai N, Sivakumar N, Oleskowicz-Popiel P (2018b) Enhanced production of microbial lipids from waste office paper by the oleaginous yeast Cryptococcus curvatus. Fuel 217:420–426. https://doi.org/10.1016/j.fuel.2017.12.108
Asha P, Divya J, Bright Singh IS (2016) Purification and characterisation of processive-type endoglucanase and β-glucosidase from Aspergillus ochraceus MTCC 1810 through saccharification of delignified coir pith to glucose. Bioresour Technol 213:245–248. https://doi.org/10.1016/j.biortech.2016.03.013
Avérous L, Pollet E (2012) Biodegradable Polymers. In: Avérous L, Pollet E (eds) Environmental silicate nano-biocomposites. Springer London, London, pp 13–39
Azam M (2010) Hydrolysis of hemicellulose by commercial enzyme mixtures. Master, Luleå University of Technology
Azizi N, Najafpour G, Younesi H (2017) Acid pretreatment and enzymatic saccharification of brown seaweed for polyhydroxybutyrate (PHB) production using Cupriavidus necator. Int J Biol Macromol 101:1029–1040. https://doi.org/10.1016/j.ijbiomac.2017.03.184
Balan V, Bals B, Chundawat SPS et al (2009) Lignocellulosic biomass pretreatment using AFEX. Methods Mol Biol Clifton NJ 581:61–77. https://doi.org/10.1007/978-1-60761-214-8_5
Balat M (2011) Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manag 52:858–875. https://doi.org/10.1016/j.enconman.2010.08.013
Bals B, Rogers C, Jin M et al (2010) Evaluation of ammonia fibre expansion (AFEX) pretreatment for enzymatic hydrolysis of switchgrass harvested in different seasons and locations. Biotechnol Biofuels 3:1. https://doi.org/10.1186/1754-6834-3-1
Berlanga M, Montero MT, Hernández-Borrell J, Guerrero R (2006) Rapid spectrofluorometric screening of poly-hydroxyalkanoate-producing bacteria from microbial mats. Int Microbiol 9:95–102
Bhuwal AK, Singh G, Aggarwal NK et al (2013) Isolation and screening of polyhydroxyalkanoates producing bacteria from pulp, paper, and cardboard industry wastes. Int J Biomater 2013:e752821. https://doi.org/10.1155/2013/752821
Cai J, He Y, Yu X et al (2017) Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew Sustain Energy Rev 76:309–322. https://doi.org/10.1016/j.rser.2017.03.072
Carter B, Gilcrease PC, Menkhaus TJ (2011) Removal and recovery of furfural, 5-hydroxymethylfurfural, and acetic acid from aqueous solutions using a soluble polyelectrolyte. Biotechnol Bioeng 108:2046–2052. https://doi.org/10.1002/bit.23153
Castilho LR, Mitchell DA, Freire DMG (2009) Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour Technol 100:5996–6009. https://doi.org/10.1016/j.biortech.2009.03.088
Cavka A, Jönsson LJ (2013) Detoxification of lignocellulosic hydrolysates using sodium borohydride. Bioresour Technol 136:368–376. https://doi.org/10.1016/j.biortech.2013.03.014
Cerrone F, Choudhari SK, Davis R et al (2014) Medium chain length polyhydroxyalkanoate (mcl-PHA) production from volatile fatty acids derived from the anaerobic digestion of grass. Appl Microbiol Biotechnol 98:611–620. https://doi.org/10.1007/s00253-013-5323-x
Cerrone F, Davis R, Kenny ST et al (2015) Use of a mannitol rich ensiled grass press juice (EGPJ) as a sole carbon source for polyhydroxyalkanoates (PHAs) production through high cell density cultivation. Bioresour Technol 191:45–52. https://doi.org/10.1016/j.biortech.2015.04.128
Cesário MTF, de Almeida MCMD (2015) Lignocellulosic hydrolysates for the production of polyhydroxyalkanoates. In: Kamm B (ed) Microorganisms in biorefineries. Springer, Berlin, pp 79–104
Cesário MT, Raposo RS, de Almeida MCMD et al (2014a) Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Burkholderia sacchari using wheat straw hydrolysates and gamma-butyrolactone. Int J Biol Macromol 71:59–67. https://doi.org/10.1016/j.ijbiomac.2014.04.054
Cesário MT, Raposo RS, de Almeida MCMD et al (2014b) Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol 31:104–113. https://doi.org/10.1016/j.nbt.2013.10.004
Chandel AK, da Silva SS, Singh OV (2011) Detoxification of lignocellulosic hydrolysates for improved bioethanol production. In: Bernardes MADS (ed) Biofuel production-recent developments and prospects. INTECH Open Access Publisher, pp 225–246
Chen G-Q, Wu Q (2005) The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26:6565–6578. https://doi.org/10.1016/j.biomaterials.2005.04.036
Cheng J, Xie B, Zhou J et al (2010) Cogeneration of H2 and CH4 from water hyacinth by two-step anaerobic fermentation. Int J Hydrog Energy 35:3029–3035. https://doi.org/10.1016/j.ijhydene.2009.07.012
Choi J, Lee SY (1997) Process analysis and economic evaluation for poly(3-hydroxybutyrate) production by fermentation. Bioprocess Eng 17:335–342. https://doi.org/10.1007/s004490050394
Collins SH (1987) Choice of substrate in polyhydroxybutyrate synthesis. In: Stowell JD, Beardsmore AJ, Keevil CW, Woodward JR (eds) Carbon substrates in biotechnology. Irl Press, Washington, DC
Couto RM, Fernandes J, da Silva MDRG, Simões PC (2009) Supercritical fluid extraction of lipids from spent coffee grounds. J Supercrit Fluids 51:159–166. https://doi.org/10.1016/j.supflu.2009.09.009
Dahman Y, Ugwu CU (2014) Production of green biodegradable plastics of poly(3-hydroxybutyrate) from renewable resources of agricultural residues. Bioprocess Biosyst Eng 37:1561–1568. https://doi.org/10.1007/s00449-014-1128-2
Davis R, Kataria R, Cerrone F et al (2013) Conversion of grass biomass into fermentable sugars and its utilization for medium chain length polyhydroxyalkanoate (mcl-PHA) production by Pseudomonas strains. Bioresour Technol 150:202–209. https://doi.org/10.1016/j.biortech.2013.10.001
Demirbas MF (2011) Biofuels from algae for sustainable development. Appl Energy 88:3473–3480. https://doi.org/10.1016/j.apenergy.2011.01.059
Dietrich D, Illman B, Crooks C (2013) Differential sensitivity of polyhydroxyalkanoate producing bacteria to fermentation inhibitors and comparison of polyhydroxybutyrate production from Burkholderia cepacia and Pseudomonas pseudoflava. BMC Res Notes 6:219. https://doi.org/10.1186/1756-0500-6-219
Doan TV, Nguyen BT (2012) Polyhydroxyalkanoates production by a bacte-rium isolated from mangrove soil samples collected from Quang Ninh province. J Vietnam Environ 3:76–79
Du C, Sabirova J, Soetaert W, Ki Carol Lin S (2012) Polyhydroxyalkanoates production from low-cost sustainable raw materials. Curr Chem Biol 6:14–25
Dubey AK, Gupta PK, Garg N, Naithani S (2012) Bioethanol production from waste paper acid pretreated hydrolyzate with xylose fermenting Pichia stipitis. Carbohydr Polym 88:825–829. https://doi.org/10.1016/j.carbpol.2012.01.004
Ellis JL, Dijkstra J, France J et al (2012) Effect of high-sugar grasses on methane emissions simulated using a dynamic model. J Dairy Sci 95:272–285. https://doi.org/10.3168/jds.2011-4385
Fang D, Aita GM (2018) Detoxification of dilute ammonia pretreated energy cane bagasse enzymatic hydrolysate by soluble polyelectrolyte flocculants. Ind Crops Prod 112:681–690
Ferreira BS, Schlottbom C (2016) Production of polyhydroxybutyrate from lignocellulosic hydrolysates—optimization of Bacillus sacchari fermentation and scale up from 2 to 200 L. Eppendrof 302:1–6
FitzPatrick M, Champagne P, Cunningham MF, Whitney RA (2010) A biorefinery processing perspective: treatment of lignocellulosic materials for the production of value-added products. Bioresour Technol 101:8915–8922. https://doi.org/10.1016/j.biortech.2010.06.125
Follonier S, Goyder MS, Silvestri A-C et al (2014) Fruit pomace and waste frying oil as sustainable resources for the bioproduction of medium-chain-length polyhydroxyalkanoates. Int J Biol Macromol 71:42–52. https://doi.org/10.1016/j.ijbiomac.2014.05.061
García-Torreiro M, López-Abelairas M, Lu-Chau TA, Lema JM (2016) Production of poly(3-hydroxybutyrate) by simultaneous saccharification and fermentation of cereal mash using Halomonas boliviensis. Biochem Eng J 114:140–146. https://doi.org/10.1016/j.bej.2016.07.002
Gouda MK, Swellam AE, Omar SH (2001) Production of PHB by a Bacillus megaterium strain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen sources. Microbiol Res 156:201–207. https://doi.org/10.1078/0944-5013-00104
Gowda V, Shivakumar S (2014) Agrowaste-based polyhydroxyalkanoate (PHA) production using hydrolytic potential of Bacillus thuringiensis IAM 12077. Braz Arch Biol Technol 57:55–61. https://doi.org/10.1590/S1516-89132014000100009
Hadar Y (2013) Sources for lignocellulosic raw materials for the production of ethanol. In: Faraco V (ed) Lignocellulose conversion. Springer, Berlin, pp 21–38
Hamieh A, Olama Z, Holail H (2013) Microbial production of polyhydroxybutyrate, a biodegradable plastic using agro-industrial waste products. Glob Adv Res J Microbiol 2:54–64
Harding KG, Dennis JS, von Blottnitz H, Harrison STL (2007) Environmental analysis of plastic production processes: comparing petroleum-based polypropylene and polyethylene with biologically-based poly-β-hydroxybutyric acid using life cycle analysis. J Biotechnol 130:57–66. https://doi.org/10.1016/j.jbiotec.2007.02.012
Hassan MA, Yee L-N, Yee PL et al (2013) Sustainable production of polyhydroxyalkanoates from renewable oil-palm biomass. Biomass Bioenergy 50:1–9. https://doi.org/10.1016/j.biombioe.2012.10.014
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18. https://doi.org/10.1016/j.biortech.2008.05.027
Heng K-S, Hatti-Kaul R, Adam F et al (2017) Conversion of rice husks to polyhydroxyalkanoates (PHA) via a three-step process: optimized alkaline pretreatment, enzymatic hydrolysis, and biosynthesis by Burkholderia cepaciaUSM (JCM 15050). J Chem Technol Biotechnol 92:100–108. https://doi.org/10.1002/jctb.4993
Ibrahim MHA, Steinbüchel A (2010) High-cell-density cyclic fed-batch fermentation of a poly(3-Hydroxybutyrate)-accumulating thermophile, Chelatococcus sp. Strain MW10. Appl Environ Microbiol 76:7890–7895. https://doi.org/10.1128/AEM.01488-10
Ienczak JL, Schmidell W, de Aragão GMF (2013) High-cell-density culture strategies for polyhydroxyalkanoate production: a review. J Ind Microbiol Biotechnol 40:275–286. https://doi.org/10.1007/s10295-013-1236-z
Jia L, Gonçalves GAL, Takasugi Y et al (2015) Effect of pretreatment methods on the synergism of cellulase and xylanase during the hydrolysis of bagasse. Bioresour Technol 185:158–164. https://doi.org/10.1016/j.biortech.2015.02.041
Jiang Y, Marang L, Tamis J et al (2012) Waste to resource: converting paper mill wastewater to bioplastic. Water Res 46:5517–5530. https://doi.org/10.1016/j.watres.2012.07.028
Jiang HS, Zhang Y, Yin L et al (2018) Diurnal changes in photosynthesis by six submerged macrophytes measured using fluorescence. Aquat Bot 149:33–39. https://doi.org/10.1016/j.aquabot.2018.05.003
Jönsson LJ, Alriksson B, Nilvebrant N-O (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:16. https://doi.org/10.1186/1754-6834-6-16
Kachrimanidou V, Kopsahelis N, Chatzifragkou A et al (2013) Utilisation of by-products from sunflower-based biodiesel production processes for the production of fermentation feedstock. Waste Biomass Valoriz 4:529. https://doi.org/10.1007/s12649-012-9191-x
Kachrimanidou V, Kopsahelis N, Papanikolaou S et al (2014) Sunflower-based biorefinery: poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production from crude glycerol, sunflower meal and levulinic acid. Bioresour Technol 172:121–130. https://doi.org/10.1016/j.biortech.2014.08.044
Kachrimanidou V, Kopsahelis N, Alexandri M et al (2015) Integrated sunflower-based biorefinery for the production of antioxidants, protein isolate and poly(3-hydroxybutyrate). Ind Crops Prod 71:106–113. https://doi.org/10.1016/j.indcrop.2015.03.003
Keenan TM, Nakas JP, Tanenbaum SW (2006) Polyhydroxyalkanoate copolymers from forest biomass. J Ind Microbiol Biotechnol 33:616–626. https://doi.org/10.1007/s10295-006-0131-2
Khanna S, Srivastava AK (2005a) Recent advances in microbial polyhydroxyalkanoates. Process Biochem 40:607–619. https://doi.org/10.1016/j.procbio.2004.01.053
Khanna S, Srivastava AK (2005b) Statistical media optimization studies for growth and PHB production by Ralstonia eutropha. Process Biochem 40:2173–2182. https://doi.org/10.1016/j.procbio.2004.08.011
Kim BS, Lee SC, Lee SY et al (1994) Production of poly(3-hydroxybutyric acid) by fed-batch culture of Alcaligenes eutrophus with glucose concentration control. Biotechnol Bioeng 43:892–898. https://doi.org/10.1002/bit.260430908
Kim TH, Kim JS, Sunwoo C, Lee YY (2003) Pretreatment of corn stover by aqueous ammonia. Bioresour Technol 90:39–47. https://doi.org/10.1016/S0960-8524(03)00097-X
Kim Y, Mosier NS, Ladisch MR et al (2011) Comparative study on enzymatic digestibility of switchgrass varieties and harvests processed by leading pretreatment technologies. Bioresour Technol 102:11089–11096. https://doi.org/10.1016/j.biortech.2011.06.054
Kim HS, Oh YH, Jang Y-A et al (2016) Recombinant Ralstonia eutropha engineered to utilize xylose and its use for the production of poly(3-hydroxybutyrate) from sunflower stalk hydrolysate solution. Microb Cell Factories 15:95. https://doi.org/10.1186/s12934-016-0495-6
Koller M (2016) Recent advances in biotechnology volume, 1: microbial biopolyester production, performance and processing microbiology, feedstocks, and metabolism. Bentham Science Publishers, Sharjah
Koller M, Muhr A (2014) Continuous production mode as a viable process-engineering tool for efficient poly (hydroxyalkanoate)(PHA) bio-production. Chem Biochem Eng Q 28:65–77
Korkie LJ, Janse BJH, Viljoen-Bloom M (2002) Utilising grape pomace for ethanol production. Afr J Enol Vitic 23:31–36
Kucera D, Benesova P, Ladicky P et al (2017) Production of polyhydroxyalkanoates using hydrolyzates of spruce sawdust: comparison of hydrolyzates detoxification by application of overliming, active carbon, and lignite. Bioengineering 4:53. https://doi.org/10.3390/bioengineering4020053
Kuhad RC, Gupta R, Khasa YP, Singh A (2010) Bioethanol production from Lantana camara (red sage): pretreatment, saccharification and fermentation. Bioresour Technol 101:8348–8354. https://doi.org/10.1016/j.biortech.2010.06.043
Kumar R, Singh S, Singh OV (2008) Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. J Ind Microbiol Biotechnol 35:377–391. https://doi.org/10.1007/s10295-008-0327-8
Kumar R, Hu F, Hubbell CA et al (2013) Comparison of laboratory delignification methods, their selectivity, and impacts on physiochemical characteristics of cellulosic biomass. Bioresour Technol 130:372–381. https://doi.org/10.1016/j.biortech.2012.12.028
Kung S-S, Chuang Y-C, Chen C-H, Chien C-C (2007) Isolation of polyhydroxyalkanoates-producing bacteria using a combination of phenotypic and genotypic approach. Lett Appl Microbiol 44:364–371. https://doi.org/10.1111/j.1472-765X.2006.02090.x
Le Meur S, Zinn M, Egli T et al (2012) Production of medium-chain-length polyhydroxyalkanoates by sequential feeding of xylose and octanoic acid in engineered Pseudomonas putida KT2440. BMC Biotechnol 12:53. https://doi.org/10.1186/1472-6750-12-53
Lee D, Owens VN, Boe A, Jeranyama P (2007) Composition of herbaceous biomass feedstocks. North Central Sun Grant Center, South Dakota State University, Brookings
Lee HV, Hamid SBA, Zain SK (2014) Conversion of lignocellulosic biomass to nanocellulose: structure and chemical process. Sci World J 2014:e631013. https://doi.org/10.1155/2014/631013
Lenihan P, Orozco A, O’Neill E et al (2010) Dilute acid hydrolysis of lignocellulosic biomass. Chem Eng J 156:395–403. https://doi.org/10.1016/j.cej.2009.10.061
Li R, Chen Q, Wang PG, Qi Q (2007) A novel-designed Escherichia coli for the production of various polyhydroxyalkanoates from inexpensive substrate mixture. Appl Microbiol Biotechnol 75:1103–1109. https://doi.org/10.1007/s00253-007-0903-2
Li H-Y, Chen X, Wang C-Z et al (2016) Evaluation of the two-step treatment with ionic liquids and alkali for enhancing enzymatic hydrolysis of Eucalyptus: chemical and anatomical changes. Biotechnol Biofuels 9:166. https://doi.org/10.1186/s13068-016-0578-y
Liu J-F, Cao Y, Yang M-H et al (2014) Enhanced saccharification of lignocellulosic biomass with 1-allyl-3-methylimidazolium chloride (AmimCl) pretreatment. Chin Chem Lett 25:1485–1488. https://doi.org/10.1016/j.cclet.2014.06.001
Lokesh BE, Hamid ZAA, Arai T et al (2012) Potential of oil palm trunk sap as a novel inexpensive renewable carbon feedstock for polyhydroxyalkanoate biosynthesis and as a bacterial growth medium. CLEAN Soil Air Water 40:310–317. https://doi.org/10.1002/clen.201000598
Lopes MSG, Gosset G, Rocha RCS et al (2011) PHB biosynthesis in catabolite repression mutant of Burkholderia sacchari. Curr Microbiol 63:319–326. https://doi.org/10.1007/s00284-011-9981-6
López-Cortés A, Lanz-Landázuri A, García-Maldonado JQ (2008) Screening and isolation of PHB-producing bacteria in a polluted marine microbial mat. Microb Ecol 56:112–120. https://doi.org/10.1007/s00248-007-9329-8
Maitan-Alfenas GP, Visser EM, Guimarães VM (2015) Enzymatic hydrolysis of lignocellulosic biomass: converting food waste in valuable products. Curr Opin Food Sci 1:44–49. https://doi.org/10.1016/j.cofs.2014.10.001
Mandl MG (2010) Status of green biorefining in Europe. Biofuels Bioprod Biorefining 4:268–274. https://doi.org/10.1002/bbb.219
Marjadi D, Dharaiya N (2011) Isolation, screening and characterization of polyhydroxyalkanoates producing bacteria utilizing edible oil as carbon source. J Environ Res Dev 5:764–772
Martel F, Estrine B, Plantier-Royon R et al (2010) Development of agriculture left-overs: fine organic chemicals from wheat hemicellulose-derived pentoses. In: Rauter AP, Vogel P, Queneau Y (eds) Carbohydrates in sustainable development I. Springer, Berlin, pp 79–115
Michael P, Loganayagi R, Nancy D et al (2012) Isolation and characterization of indigenous Ralstonia strain, YRF1 for high Polyhydroxy Alkanoates (PHA) production. Elixir Int J 48:424–9427
Mizuno K, Ohta A, Hyakutake M et al (2010) Isolation of polyhydroxyalkanoate-producing bacteria from a polluted soil and characterization of the isolated strain Bacillus cereus YB-4. Polym Degrad Stab 95:1335–1339. https://doi.org/10.1016/j.polymdegradstab.2010.01.033
Modig T, Lidén G, Taherzadeh MJ (2002) Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem J 363:769–776
Motamedi H, Ardakani MR, Mayeli N (2015) Isolation and screening of native polyhydroxyalkanoate producing bacteria from oil contaminated soils of Abadan refinery. Biol J Microorg 3:93–104
Muhr A, Rechberger EM, Salerno A et al (2013) Novel description of mcl-PHA biosynthesis by Pseudomonas chlororaphis from animal-derived waste. J Biotechnol 165:45–51. https://doi.org/10.1016/j.jbiotec.2013.02.003
Mussatto SI, Teixeira JA (2010) Lignocellulose as raw material in fermentation processes. Curr Res Technol Educ Top Appl Microbiol Microb Biotechnol Méndez-Vilas Ed 2:897–907
Nair AS, Al-Battashi H, Al-Akzawi A et al (2018) Waste office paper: a potential feedstock for cellulase production by a novel strain Bacillus velezensis ASN1. Waste Manag 79:491–500. https://doi.org/10.1016/j.wasman.2018.08.014
Narayanan A, Kumar VAS, Ramana KV (2013) Production and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from Bacillus mycoides DFC1 using rice husk hydrolyzate. Waste Biomass Valoriz 5:109–118. https://doi.org/10.1007/s12649-013-9213-3
Nduko JM, Suzuki W, Matsumoto K et al (2012) Polyhydroxyalkanoates production from cellulose hydrolysate in Escherichia coli LS5218 with superior resistance to 5-hydroxymethylfurfural. J Biosci Bioeng 113:70–72. https://doi.org/10.1016/j.jbiosc.2011.08.021
Ng L-M, Sudesh K (2016) Identification of a new polyhydroxyalkanoate (PHA) producer Aquitalea sp. USM4 (JCM 19919) and characterization of its PHA synthase. J Biosci Bioeng. https://doi.org/10.1016/j.jbiosc.2016.03.024
Nizami A-S, Korres NE, Murphy JD (2009) Review of the integrated process for the production of grass biomethane. Environ Sci Technol 43:8496–8508. https://doi.org/10.1021/es901533j
Obruca S, Benesova P, Petrik S et al (2014a) Production of polyhydroxyalkanoates using hydrolysate of spent coffee grounds. Process Biochem 49:1409–1414. https://doi.org/10.1016/j.procbio.2014.05.013
Obruca S, Petrik S, Benesova P et al (2014b) Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl Microbiol Biotechnol 98:5883–5890. https://doi.org/10.1007/s00253-014-5653-3
Obruca S, Benesova P, Kucera D et al (2015a) Biotechnological conversion of spent coffee grounds into polyhydroxyalkanoates and carotenoids. New Biotechnol 32:569–574. https://doi.org/10.1016/j.nbt.2015.02.008
Obruca S, Benesova P, Marsalek L, Marova I (2015b) Use of lignocellulosic materials for PHA production. Chem Biochem Eng Q 29:135–144. https://doi.org/10.15255/CABEQ.2014.2253
Pan W, Perrotta JA, Stipanovic AJ et al (2012) Production of polyhydroxyalkanoates by Burkholderia cepacia ATCC 17759 using a detoxified sugar maple hemicellulosic hydrolysate. J Ind Microbiol Biotechnol 39:459–469. https://doi.org/10.1007/s10295-011-1040-6
Pandian SR, Deepak V, Kalishwaralal K et al (2010) Optimization and fed-batch production of PHB utilizing dairy waste and sea water as nutrient sources by Bacillus megaterium SRKP-3. Bioresour Technol 101:705–711. https://doi.org/10.1016/j.biortech.2009.08.040
Peña C, Castillo T, Núñez C, Segura D (2011) Bioprocess design: fermentation strategies for improving the production of alginate and poly-β-hydroxyalkanoates (PHAs) by Azotobacter vinelandii. In: Progress in molecular and environmental bioengineering-from analysis and modeling to technology applications. INTECH Open Access Publisher, Rijeka, Croatia, pp 217–242
Peña C, Castillo T, García A et al (2014) Biotechnological strategies to improve production of microbial poly-(3-hydroxybutyrate): a review of recent research work. Microb Biotechnol 7:278–293. https://doi.org/10.1111/1751-7915.12129
Pérez J, Muñoz-Dorado J, de la Rubia T, Martínez J (2002) Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int Microbiol 5:53–63. https://doi.org/10.1007/s10123-002-0062-3
Persson T, Matusiak M, Zacchi G, Jönsson A-S (2006) Extraction of hemicelluloses from process water from the production of masonite. Desalination 199:411–412
Pradhan S, Borah AJ, Poddar MK et al (2017) Microbial production, ultrasound-assisted extraction and characterization of biopolymer polyhydroxybutyrate (PHB) from terrestrial (P. hysterophorus) and aquatic (E. crassipes) invasive weeds. Bioresour Technol 242:304–310. https://doi.org/10.1016/j.biortech.2017.03.117
Rabelo SC, Carrere H, Maciel Filho R, Costa AC (2011) Production of bioethanol, methane and heat from sugarcane bagasse in a biorefinery concept. Bioresour Technol 102:7887–7895. https://doi.org/10.1016/j.biortech.2011.05.081
Radhika D, Murugesan AG (2012) Bioproduction, statistical optimization and characterization of microbial plastic (poly 3-hydroxy butyrate) employing various hydrolysates of water hyacinth (Eichhornia crassipes) as sole carbon source. Bioresour Technol 121:83–92. https://doi.org/10.1016/j.biortech.2012.06.107
Ravindran R, Jaiswal AK (2016) A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: challenges and opportunities. Bioresour Technol 199:92–102. https://doi.org/10.1016/j.biortech.2015.07.106
Rehm BHA (2006) Genetics and biochemistry of polyhydroxyalkanoate granule self-assembly: the key role of polyester synthases. Biotechnol Lett 28:207–213. https://doi.org/10.1007/s10529-005-5521-4
Ryu HW, Hahn SK, Chang YK, Chang HN (1997) Production of poly(3-hydroxybutyrate) by high cell density fed-batch culture of Alcaligenes eutrophus with phospate limitation. Biotechnol Bioeng 55:28–32. https://doi.org/10.1002/(SICI)1097-0290(19970705)55:1<28:AID-BIT4>3.0.CO;2-Z
Saini JK, Saini R, Tewari L (2015) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech 5:337–353. https://doi.org/10.1007/s13205-014-0246-5
Saratale GD, Oh M-K (2015) Characterization of poly-3-hydroxybutyrate (PHB) produced from Ralstonia eutropha using an alkali-pretreated biomass feedstock. Int J Biol Macromol 80:627–635. https://doi.org/10.1016/j.ijbiomac.2015.07.034
Sathesh Prabu C, Murugesan AG (2010) Effective utilization and management of coir industrial waste for the production of poly-Î2-hydroxybutyrate (PHB) using the Bacterium Azotobacter Beijerinickii. Int J Environ Res 4:519–524
Sawant SS, Salunke BK, Kim BS (2015) Degradation of corn stover by fungal cellulase cocktail for production of polyhydroxyalkanoates by moderate halophile Paracoccus sp. LL1. Bioresour Technol 194:247–255. https://doi.org/10.1016/j.biortech.2015.07.019
Sawant SS, Salunke BK, Tran TK, Kim BS (2016) Lignocellulosic and marine biomass as resource for production of polyhydroxyalkanoates. Korean J Chem Eng 33:1505–1513. https://doi.org/10.1007/s11814-016-0019-4
Sethi A, Slack JM, Kovaleva ES et al (2013) Lignin-associated metagene expression in a lignocellulose-digesting termite. Insect Biochem Mol Biol 43:91–101. https://doi.org/10.1016/j.ibmb.2012.10.001
Shamala TR, Rohinishree YS, Vijayendra SVN (2014) Biosynthesis of multiple biopolymers by Sinorhizobium meliloti CFR 14 in high cell density cultures through fed batch fermentation. Biocatal Agric Biotechnol 3:316–322. https://doi.org/10.1016/j.bcab.2014.05.004
Shrivastav A, Mishra SK, Shethia B et al (2010) Isolation of promising bacterial strains from soil and marine environment for polyhydroxyalkanoates (PHAs) production utilizing Jatropha biodiesel byproduct. Int J Biol Macromol 47:283–287. https://doi.org/10.1016/j.ijbiomac.2010.04.007
Silva LF, Taciro MK, Ramos MEM et al (2004) Poly-3-hydroxybutyrate (P3HB) production by bacteria from xylose, glucose and sugarcane bagasse hydrolysate. J Ind Microbiol Biotechnol 31:245–254. https://doi.org/10.1007/s10295-004-0136-7
Silva JA, Tobella LM, Becerra J et al (2007) Biosynthesis of poly-beta-hydroxyalkanoate by Brevundimonas vesicularis LMG P-23615 and Sphingopyxis macrogoltabida LMG 17324 using acid-hydrolyzed sawdust as carbon source. J Biosci Bioeng 103:542–546. https://doi.org/10.1263/jbb.103.542
Singh P, Parmar N (2011) Isolation and characterization of two novel polyhydroxybutyrate (PHB)-producing bacteria. Afr J Biotechnol 10:4907–4919. https://doi.org/10.5897/AJB10.1737
Solaiman DKY, Ashby RD, Foglia TA, Marmer WN (2006) Conversion of agricultural feedstock and coproducts into poly(hydroxyalkanoates). Appl Microbiol Biotechnol 71:783–789. https://doi.org/10.1007/s00253-006-0451-1
Studer MH, DeMartini JD, Davis MF et al (2011) Lignin content in natural populus variants affects sugar release. Proc Natl Acad Sci U S A 108:6300–6305. https://doi.org/10.1073/pnas.1009252108
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199:49–58. https://doi.org/10.1016/j.biortech.2015.08.061
Taherzadeh MJ, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9:1621–1651. https://doi.org/10.3390/ijms9091621
Thomaz SM, da Cunha ER (2010) The role of macrophytes in habitat structuring in aquatic ecosystems: methods of measurement, causes and consequences on animal assemblages’ composition and biodiversity. Acta Limnol Bras 22:218–236. https://doi.org/10.4322/actalb.02202011
Vaithanomsat P, Chuichulcherm S, Apiwatanapiwat W (2009) Bioethanol production from enzymatically saccharified sunflower stalks using steam explosion as pretreatment. Proc World Acad Sci Eng Technol 49:140–143
Valappil SP, Misra SK, Boccaccini AR et al (2007) Large-scale production and efficient recovery of PHB with desirable material properties, from the newly characterised Bacillus cereus SPV. J Biotechnol 132:251–258. https://doi.org/10.1016/j.jbiotec.2007.03.013
Verardi A, Ricca E, De Bari I, Calabrò V (2012) Hydrolysis of lignocellulosic biomass: current status of processes and technologies and future perspectives. INTECH Open Access Publisher, Rijeka
Verlinden RAJ, Hill DJ, Kenward MA et al (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol 102:1437–1449. https://doi.org/10.1111/j.1365-2672.2007.03335.x
Wang L, Templer R, Murphy RJ (2012) High-solids loading enzymatic hydrolysis of waste papers for biofuel production. Appl Energy 99:23–31. https://doi.org/10.1016/j.apenergy.2012.03.045
Wang T, Meng Y, Qin Y et al (2018) Removal of furfural and HMF from monosaccharides by nanofiltration and reverse osmosis membranes. J Energy Inst 91:473–480. https://doi.org/10.1016/j.joei.2017.01.005
Weissgram M, Gstöttner J, Lorantfy B et al (2015) Generation of PHB from spent sulfite liquor using halophilic microorganisms. Microorganisms 3:268–289
Wu L, Lee S-H, Endo T (2013) Effect of dimethyl sulfoxide on ionic liquid 1-ethyl-3-methylimidazolium acetate pretreatment of eucalyptus wood for enzymatic hydrolysis. Bioresour Technol 140:90–96. https://doi.org/10.1016/j.biortech.2013.04.072
Wu L, Kumagai A, Lee S-H, Endo T (2014) Synergistic effect of delignification and treatment with the ionic liquid 1-ethyl-3-methylimidazolium acetate on enzymatic digestibility of poplar wood. Bioresour Technol 162:207–212. https://doi.org/10.1016/j.biortech.2014.03.144
Yu J, Stahl H (2008) Microbial utilization and biopolyester synthesis of bagasse hydrolysates. Bioresour Technol 99:8042–8048. https://doi.org/10.1016/j.biortech.2008.03.071
Zabed H, Sahu JN, Boyce AN, Faruq G (2016) Fuel ethanol production from lignocellulosic biomass: an overview on feedstocks and technological approaches. Renew Sustain Energy Rev 66:751–774. https://doi.org/10.1016/j.rser.2016.08.038
Zafar S (2015) Rice straw as bioenergy resource. In: BioEnergy Consult. http://www.bioenergyconsult.com/rice-straw-as-bioenergy-resource/. Accessed 25 June 2016
Zhang Y, Sun W, Wang H, Geng A (2013) Polyhydroxybutyrate production from oil palm empty fruit bunch using Bacillus megaterium R11. Bioresour Technol 147:307–314. https://doi.org/10.1016/j.biortech.2013.08.029
Zhou Y, Vazquez A, Wise A et al (2013) Carbon catabolite repression correlates with the maintenance of near invariant molecular crowding in proliferating E. coli cells. BMC Syst Biol 7:138. https://doi.org/10.1186/1752-0509-7-138
Funding
This work was supported by The Research Council (TRC), Oman under Grant (ORG/EBR/14/003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Al-Battashi, H.S., Annamalai, N., Sivakumar, N. et al. Lignocellulosic biomass (LCB): a potential alternative biorefinery feedstock for polyhydroxyalkanoates production. Rev Environ Sci Biotechnol 18, 183–205 (2019). https://doi.org/10.1007/s11157-018-09488-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-018-09488-4