Abstract
Autism spectrum disorder (ASD) is a type of neurodevelopmental disorder characterized mainly by qualitative deficiencies in social communication skills, accompanied by repetitive and restricted behavior patterns. This study was conducted to investigate the associations between the risk of ASD development in children and exposure to trace elements (arsenic (As), cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), lead (Pb), nickel (Ni), and zinc (Zn)). Two groups of children, including 44 ASD and 35 typically developing (TD) children, were selected, and their fasting urine samples were obtained. The concentration levels of trace elements were assayed using ICP-MS. The results showed that as compared to the TD group, the concentration levels of As (p = 0.002) and Pb (p < 0.001) and also Cr (p < 0.001), Cu (p = 0.001), and Ni (p < 0.001) were significantly higher among ASD children. In terms of gender, boys with ASD showed elevated levels of Cr, Cu, Ni, and Pb, whereas the urine levels of As, Cr, Cu, Ni, and Pb were markedly higher among girls when compared to the non-ASD children. Under the logistic regression model, the risk difference for As, Co, Cr, Cu, Ni, Pb, and Zn remained significant when adjustment was applied for age and gender confounders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is one of the neurodevelopmental disorders characterized mainly by qualitative deficiencies in social communication skills, accompanied by repetitive and restricted behavior patterns (Nevison 2014; Ousley and Cermak 2014). In recent decades, the prevalence of ASD has increased worldwide by up to 30% (Calabrese et al. 2016). In 2002, of 150 newborn children, one child was identified with ASD. This figure changed to one of every 110 infants in 2006 and one of every 88 children in 2008. In 2014, of 68 births, one was identified with ASD (Bhat et al. 2014; Vakilizadeh et al. 2017; Weitlauf et al. 2014).
The prevalence of ASD among boys is four to five times more common than among girls. However, ASD is usually associated with more severe mental retardation in girls (Weitlauf et al. 2014). The prevalence of ASD in Iran is estimated to be 1 of 1000 births and more common among boys than girls with a 2:1 ratio (Mohammadi et al. 2019).
Although the exact causes of ASD have remained unknown, multiple factors are associated with the pathogenesis of ASD including obstetric complications, gestational diabetes, fetal hypoxia, advanced maternal or paternal age, type of diet and medication during the prenatal period, and child iron deficiency anemia (Meguid et al. 2017). Moreover, air pollution seems to play a key role in the etiology of ASD. Increasing numbers of research suggest that early-life exposure to environmental pollutants can be one of the most influential factors involved in the onset of ASD (Bjørklund et al. 2018; Bölte et al. 2019; Imbriani et al. 2021). The sensitivity of the developing nervous system to toxic metals such as arsenic (As), cadmium (Cd), lead (Pb), and mercury (Hg) is well-recognized and may cause several disorders, including ASD (El-Ansary et al. 2017; Mostafa et al. 2016). Children with ASD are at the risk of zinc (Zn) deficiency and subsequent metallothionein (MT) dysfunction which in turn make them particularly susceptible to metal accumulation during their life (Bjørklund 2013; Crăciun et al. 2016). It is of special importance as environmental stressor exposure during later childhood stages modulate ASD progression and treatment efficacy (Santos et al. 2021).
Many studies have been conducted to establish a potential association between ASD development and toxic metal exposure using hair, whole blood, blood serum, blood plasma, red blood cells, and urine samples (Dickerson et al. 2016; Fuentes-Albero et al. 2015; Roberts et al. 2013; Saghazadeh and Rezaei 2017). However, studies using the Iranian population are rarer. Therefore, this study was conducted to investigate the association between the risk of ASD development in children and exposure to trace elements in Eastern Iran.
Materials and methods
Design and study population
This case–control study was conducted in Birjand, the capital city of South Khorasan province at East of Iran, and approved by the Ethics Committee of Birjand University of Medical Sciences (no. IR.BUMS.REC.1397.204). Children with ASD (ASD group, n = 44) were recruited from different autism care units where they were clinically diagnosed with ASD by child psychiatrists using the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria. Exclusion criteria were physical disabilities, progressive neurological disorders, unstable epilepsy, and taking regular medications. TD children (control group, n = 35) were enrolled from schools of the same locations where cases came from. The informed consent for all children in the study was obtained from their parents. The collection of urine samples took place from February to August 2020. The necessary sample size at each group was obtained using the following formula:
where n1 denotes the sample size of the smaller group, r the ratio of the sample size at the larger group to the smaller group, α and β denote type I and type II errors, respectively, and σ is the common standard deviation of concentration levels. The ratio d/σ is the so-called Cohen effect size which can be pre-defined as 0.2, 0.5, and .8, representing small, medium, and large differences between two groups to detect. Setting effect size to a small value, say 0.2, will require a larger sample size to detect the differences between the mean of two groups and vice versa. By assuming r = 1, α = 0.05, β = 0.2, and an effect size equal to 0.66 which represents detecting medium toward large differences, the minimum number of samples at each group was obtained equal to 35:
Urine sample collection and trace element analysis
To avoid any metal contamination, sealed plastic containers were used for collecting early morning urine samples (10 mL) and kept at −20 °C. For the mineralization, the urine samples were mixed with 2 ml of nitric acid (HNO3, 65%, Merck, Germany) and 1 ml of perchloric acid (HClO4, 70%, Merck, Germany) and mineralized on a water bath (TW12, Julabo GmbH, Germany) at 100 °C. Mineralized samples were diluted with ultrapure water (18.2 MΩ−cm at 25 °C, Fistreem, WSC044, UK) up to 25 mL. Concentrations levels of As, Cd, Co, Cr, Cu, Ni, Pb, and Zn were measured using inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7900, Santa Clara, CA, USA). After every eight analyses, spikes and control samples were implemented. The recovery of As, Cd, Co, Cr, Cu, Ni, Pb, and Zn were 97%, 102%, 97%, 96%, 101%, 98%, 99%, and 98%, respectively.
Statistical analysis
The D’Agostino-Pearson test was used to check normality assumption, and the Levene test was used to test the homogeneity of variances. Univariate analysis (t test or Mann–Whitney, as appropriate) was used to check the differences in metal levels between the two groups. To assess whether the levels of metals were different between sexes and also between groups, a rank-based regression analysis that is robust against outliers was used and fitted using the Rfit package in R (Kloke and McKean 2012). The association between elements was evaluated using the Spearman correlation coefficient (R). Since metal concentrations are usually skewed, element levels were normalized by subtracting the mean levels of each element and dividing by its quartile range. A logistic regression model on the standardized element values was used to estimate the risk differences (RDs) between ASD and the control group. All analyses were performed using R 3.3.1 statistical software (R Core Team 2016).
Results
General comparisons
ASD children were 29 boys (65.9%) and 15 girls (34.1%), while the TD children were 18 boys (51.4%) and 17 girls (48.6%). The mean age of ASD children was 11.1 years and 10.4 years for TD children (Table 1). Both groups were comparable according to children’s age (t test, p = 0.208) and gender (χ2 test, p = 0.284). Figure 1 displays the density plot of the concentration levels of all 9 trace elements for ASD and TD children. It can be clearly seen that the concentration levels were not normally distributed at both groups, mainly due to the presence of outliers. Therefore, to retain outliers, median and range were used to report summaries, and non-parametric analyses were used for comparisons between groups. Table 2 reports the median ± intermediate quantile range (IQR) as well as the range of concentration levels of trace elements for both ASD and TD children. ASD group showed significantly higher levels of As (17.92 vs. 12.18 μg/L at TD group), Cr (3.62 vs. 1.53 μg/L), Cu (11.29 vs. 9.79 μg/L), Ni (10.92 vs. 3.68 μg/L), and Pb (6.19 vs. 2.1 μg/L). Moreover, lower levels of Co (0.69 μg/L) were observed among ASD children as compared to the TD group (0.88 μg/L). There were no significant differences in the concentration levels of Cd (p = 0.066) and Zn (p = 104) between the two groups (Table 2).
Assessment of gender and group effects
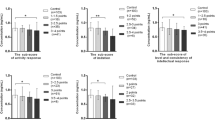
The effect of gender, groups, and also their interaction effect was investigated using robust regression analysis (Table 3). Results showed that (i) for all trace elements, no significant interaction effects of gender and groups were presented, (ii) Cu was the only element with different concentration levels between boys and girls (p = 0.01), and (iii) the concentration levels of most elements (with the exception of Cd and Zn) have significantly differed between the ASD and TD children (Table 3). Using the box and whisker plot, Figs. 2 and 3 compare graphically the differences in concentration levels of trace elements between ASD and TD for both girls and boys (Figs. 2 and 3). In addition to these figures, pairwise comparisons were also made between two groups using the Mann–Whitney test (Table 4).
The mean age of TD and ASD boys was 11.7 and 10.1 years, respectively. The order of concentration levels of trace elements for ASD and TD boys was similar: Cd < Co < Cr < Pb < Ni < As < Zn < Cu. Boys with ASD were characterized by significantly elevated level of Cr (+109%; p < 0.001), Cu (+14%; p = 0.014), Ni (+231%; p < 0.001), and Pb (+274%; p < 0.001). The mean age of TD and ASD girls was 10.1 and 11.7 years, respectively. The order of concentration levels of trace elements for ASD and TD girls was similar: Cd < Co < Cr < Pb < Ni < As < Zn < Cu. Girls with ASD presented significantly elevated level of As (+56%; p = 0.008), Cr (+128%; p = 0.004), Cu (+29%; p = 0.007), Ni (+321%; p < 0.001), and Pb (+260%; p = 0.003).
Relationships between elements
For the ASD group, the metal–metal Spearman, rs, correlation showed that the associations between As–Zn (−0.53), Cd–Co (0.37), Cd–Cu (−0.37), and Pb–Ni (0.36) were moderate and significant at a 5% significance level. At TD group, the moderate correlation between Ni–Pb (0.54), Cd–Cr (0.46), Fe–Pb (−0.43), As–Cd (0.40), Zn–Co (0.40), Cr–Fe (−0.37), and Ni-Fe (−0.35) was significant at 5% significance level. The association between other pairs (89% at case group and 80% at control group) was either statistically or clinically (rs < 0.30) non-significant. Since the correlation between trace elements was mainly non-significant, we didn’t carry out any further multivariate analysis.
Relationships between elements and ASD
Under a logistic regression model, for each element, both adjusted and unadjusted risk differences (RD) were calculated (Table 5). The unadjusted RDs suggest that all trace elements were the risk factor of ASD, but when adjusting for age and gender, the effect of Cd was ruled out (RD = −0.156; 95% CI, −0.323, 0.009). The risk difference for As, Co, Cr, Cu, Ni, Pb, and Zn remained significant when adjustment was applied for age and gender confounders. A positive RD value means increased risk, and a negative one means decreased risk by the exposure. RD describes the actual difference in the observed risk due to an element between ASD and TD children. The positive sign of the RD denotes excessive risk for ASD children when element levels are elevated. For instance, RD = −0.141 for As indicates that there is a lower risk of developing autism for a TD who had higher levels of As.
Discussion
Simultaneous exposure to heavy metals may have more adverse effects on children’s neurodevelopment than the effects of each metal alone. So in this study, we aimed to assess the levels of some essential and toxic elements using the ICP-MS method in autistic children compared to healthy ones. Our results showed that As, Co, Cr, Cu, Ni, Pb, and Zn in biological samples (urine) were associated with the ASD risk.
As is a toxic heavy metal that may be damaging even at low levels of exposure (Akyuzlu et al. 2014). It has the ability to cross the blood–brain barrier (BBB) and damage directly the central nervous system of neonates (Eqani et al. 2020). Similar to the results of the current study, previous literature described a significant association between As exposure and ASD (Obrenovich et al. 2011; Yasuda et al. 2013). Eqani et al. (2020) found that As levels in urine and hair were differed significantly between ASD children and controls (Eqani et al. 2020). Significantly higher urinary concentration of As in patients with ASD may be due to specialized dietary preferences (Eqani et al. 2020). More intake of As-rich foods such as preserved juices and rice by ASD children compared to healthy controls has been reported previously (Eqani et al. 2020).
Cr has two oxidation states: +3 and +6. Cr (+3) is considered an essential trace element that has an important effect on the metabolism of glucose and cholesterol (Baj et al. 2021). Cytotoxic Cr (+6) can lead to lipid peroxidation, protein degradation, damage of membrane integrity, and cell death (Baj et al. 2021). Yorbik et al. (2002) reported that the antioxidant activity is much lower in patients with ASD, providing the hypothesis that oxidative stress can play an important role in the development of ASD (Yorbik et al. 2002). Other independent literature has also supported this hypothesis (Yao et al. 2006). Metallomics studies in ASD vary depending on the type of tissue studied. Some studies showed lower hair levels of Cr among ASD children as compared to controls. However, urinary Cr levels were generally reported at higher levels in children with autism (Baj et al. 2021; Yorbik et al. 2010).
Cigarette smoking, air, and food pollution can be considered as the main sources of Cd in the general population (Akyuzlu et al. 2014). It was found that Cd is associated with learning difficulties and intelligence levels of children. In addition, this neurotoxic metal can affect motor, visual, and cognitive abilities and neurodegenerative diseases (Akyuzlu et al. 2014). There is still much to understand about the mechanisms of its cellular toxicity. It may interfere with the mechanism of DNA repair as well as the production of reactive oxygen species (ROS) (Sulaiman et al. 2020). Findings on cadmium levels in autistic children are mixed. While some studies have reported higher urinary levels of Cd (3.24 μg/L vs. 0.53 μg/L) in ASD children as compared to controls (Akyuzlu et al. 2014), other studies either show no significant association between Cd and ASD occurrence (Adams et al. 2017; Adams et al. 2013; Albizzati et al. 2012; Blaurock-Busch et al. 2011) or a negative association between urinary Cd levels and ASD (Sulaiman et al. 2020; Yorbik et al. 2010). Yorbik et al. (2010) reported significantly lower urinary levels of Cd and Pb in children with ASD compared to healthy subjects (Yorbik et al. 2010). They suggested that autistic children may have difficulties in heavy metal detoxification mechanism which may lead to the body burden of toxic metals (Yorbik et al. 2010). Apparently, more studies are needed to examine the potential body load of different toxic metals. Urine samples may be considered a good indicator for the long-term body burden of cadmium. It tends to accumulate in the kidneys with a half-life of 30 years. Cd levels may also increase after acute exposure (Sulaiman et al. 2020).
For element Ni, some studies report similar concentration levels of Ni in urinary samples of cases and controls (Adams et al. 2013; Blaurock-Busch et al. 2011), while some studies suggest slightly lower Ni concentration levels in hair of ASD children when compared to TD subjects (Blaurock-Busch et al. 2011; Skalny et al. 2017). One study in Oman, however, claimed higher hair Ni levels in cases compared to their controls (Al-Farsi et al. 2013). There is also some evidence on the increased risk of ASD in children born in areas with higher concentrations of Ni in the air (Roberts et al. 2013). Additionally, Roberts et al. (2013) found positive relationships between some elements including Ni (OR = 1.9) and developing ASD among boys (Roberts et al. 2013).
Pb is a toxic metal that has a long half-life in the body. Lower IQ, decreased frustration tolerance, attention deficit hyperactivity disorder, poor reaction control, cognitive and social disorders are some of the possible consequences of Pb exposure (Akyuzlu et al. 2014). In the growing brain, elevated levels of Pb may induce the inappropriate release of neurotransmitters and impair brain function (Baj et al. 2021). The urinary determination of Pb is considered to reflect the exerted lead through the kidneys which count for about two-thirds of total excretion (Abd Wahil 2021). Short- and long-term exposure to Pb, from a few days to prolonged exposure, can be identified from urinary levels of Pb (Abd Wahil 2021). However, findings of the connection between Pb levels and ASD are greatly inconsistent. Some studies report lowered urinary Pb levels in ASD children as compared to TD subjects (Abd Wahil 2021; Rahbar et al. 2020; Yorbik et al. 2010), whereas elevated Pb levels in hair samples have been reported in ASD children (Fiłon et al. 2020). It has been hypothesized that ASD children may have a reduced ability for heavy metal excretion (including Pb) compared to TD children (Abd Wahil 2021). The relatively inconsistent reports on Pb levels in ASD children may be attributed to ASD heterogeneity, different geographical locations, or methodological variations suggesting more comprehensive future studies. Previous literature suggested that some factors such as magnesium or calcium deficiency can increase the toxic effect of Pb on cognitive functions and developmental behavior of children (Baj et al. 2021). The Pb toxicity mechanism may also be mediated by Zn (Baj et al. 2021). The presence of essential elements in the body, mainly Zn and Fe, is essential to counteract the neurological effects of Pb (Abd Wahil 2021).
Essential trace elements (such as Cu and Zn) play an important role as antioxidant agents. These trace elements also reduce teratogenic effects through the formation of insoluble metal–mineral complexes (Abd Wahil 2021; Jan et al. 2015). They also reduce the gastrointestinal absorption of toxic metals through competitive mechanisms (Abd Wahil 2021, Jan et al. 2015). A crucial element in CNS development and both deficiency and the excess levels of Cu may cause health problems (Baj et al. 2021). Cu overload may cause synaptic damage detected in ASD children (Baj et al. 2021). It may also block dopamine beta-hydroxylase function that is known to alter the noradrenaline synthesis mechanism (Li et al. 2014) and reduce the production of serotonin (Priya and Geetha 2011).
Limitations
In this study, toxic and essential elements were assessed only in the urine samples, which may not fully clarify the complex pathological mechanism that occurs due to these elements in the brain. Another limitation was the lack of control over participants’ diets. Therefore, it is not clear that the differences in urinary levels of elements observed were due to the diet and/or metabolism. Further studies are recommended to assess how this behavior affects nutritional deficiencies.
Conclusion
In summary, we observed ASD children presented significantly higher levels of As, Cr, Cu, Ni, and Pb and simultaneously significantly lower levels of Co. There were no significant differences in Cd and Zn levels between ASD and TD children. Further studies are necessary to shed light on the possible role of heavy metals in autism.
References
Abd Wahil MS, Ja’afar MH, Isa ZM (2021): Assessment of urinary lead (Pb) and essential trace elements in autism spectrum disorder: a case-control study among preschool children in Malaysia. Biol Trace Element Res 200, 97-121
Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, Gehn E, Loresto M, Mitchell J, Atwood S (2013) Toxicological status of children with autism vs. neurotypical children and the association with autism severity. Biol Trace Element Res 151:171–180
Adams J, Howsmon DP, Kruger U, Geis E, Gehn E, Fimbres V, Pollard E, Mitchell J, Ingram J, Hellmers R (2017) Significant association of urinary toxic metals and autism-related symptoms—a nonlinear statistical analysis with cross validation. PloS one 12:e0169526
Akyuzlu DK, Kayaalti Z, Soylemez E, Soylemezoglu T (2014) Association between autism and arsenic, lead, cadmium, manganese levels in hair and urine. J Pharm Pharmacol 2:140
Albizzati A, More L, Di Candia D, Saccani M, Lenti C (2012) Normal concentrations of heavy metals in autistic spectrum disorders. Minerva Pediatr 64:27–31
Al-Farsi YM, Waly MI, Al-Sharbati MM, Al-Shafaee MA, Al-Farsi OA, Al-Khaduri MM, Gupta I, Ouhtit A, Al-Adawi S, Al-Said MF (2013) Levels of heavy metals and essential minerals in hair samples of children with autism in Oman: a case–control study. Biol Trace Element Res 151:181–186
Baj J, Flieger W, Flieger M, Forma A, Sitarz E, Skórzyńska-Dziduszko K, Grochowski C, Maciejewski R, Karakuła-Juchnowicz H (2021) Autism spectrum disorder: trace elements imbalances and the pathogenesis and severity of autistic symptoms. Neurosci Biobehav Rev 1:117–132
Bhat S, Acharya UR, Adeli H, Bairy GM, Adeli A (2014) Autism: cause factors, early diagnosis and therapies. Rev Neurosci 25:841–850
Bjørklund G (2013) The role of zinc and copper in autism spectrum disorders. Acta Neurobiol Exp (Wars) 73:225–236
Bjørklund G, Skalny AV, Rahman MM, Dadar M, Yassa HA, Aaseth J, Chirumbolo S, Skalnaya MG, Tinkov AA (2018) Toxic metal (loid)-based pollutants and their possible role in autism spectrum disorder. Environ Res 166:234–250
Blaurock-Busch E, Amin OR, Rabah T (2011) Heavy metals and trace elements in hair and urine of a sample of Arab children with autistic spectrum disorder. Maedica 6:247
Bölte S, Girdler S, Marschik PB (2019) The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci 76:1275–1297
Calabrese V, Giordano J, Ruggieri M, Berritta D, Trovato A, Ontario M, Bianchini R, Calabrese E (2016) Hormesis, cellular stress response, and redox homeostasis in autism spectrum disorders. J Neurosci Res 94:1488–1498
Crăciun EC, Bjørklund G, Tinkov AA, Urbina MA, Skalny AV, Rad F, Dronca E (2016) Evaluation of whole blood zinc and copper levels in children with autism spectrum disorder. Metab Brain Dis 31:887–890
Dickerson AS, Rahbar MH, Bakian AV, Bilder DA, Harrington RA, Pettygrove S, Kirby RS, Durkin MS, Han I, Moyé LA (2016) Autism spectrum disorder prevalence and associations with air concentrations of lead, mercury, and arsenic. Environ Monitoring assessment 188:1–15
El-Ansary A, Bjørklund G, Tinkov AA, Skalny AV, Al Dera H (2017) Relationship between selenium, lead, and mercury in red blood cells of Saudi autistic children. Metab Brain Dis 32:1073–1080
Eqani SAMAS, Khuram F, Alamdar A, Tahir A, Shah STA, Nasir A, Javed S, Bibi N, Hussain A, Rasheed H (2020) Environmental exposure pathway analysis of trace elements and autism risk in Pakistani children population. Sci Total Environ 712:136471
Fiłon J, Ustymowicz-Farbiszewska J, Krajewska-Kułak E (2020) Analysis of lead, arsenic and calcium content in the hair of children with autism spectrum disorder. BMC Public Health 20:1–8
Fuentes-Albero M, Puig-Alcaraz C, Cauli O (2015) Lead excretion in Spanish children with autism spectrum disorder. Brain Sci 5:58–68
Imbriani G, Panico A, Grassi T, Idolo A, Serio F, Bagordo F, De Filippis G, De Giorgi D, Antonucci G, Piscitelli P (2021) Early-life exposure to environmental air pollution and autism spectrum disorder: a review of available evidence. Int J Environ Res Public Health 18:1204
Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QM (2015) Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 16:29592–29630
Kloke JD, McKean JW (2012) Rfit: rank-based estimation for linear models. R J 4:57
Li S-o, Wang J-l, Bjørklund G, Zhao W-n, Yin C-h (2014) Serum copper and zinc levels in individuals with autism spectrum disorders. Neuroreport 25:1216–1220
Meguid NA, Anwar M, Bjørklund G, Hashish A, Chirumbolo S, Hemimi M, Sultan E (2017) Dietary adequacy of Egyptian children with autism spectrum disorder compared to healthy developing children. Metab Brain Disease 32:607–615
Mohammadi MR, Ahmadi N, Khaleghi A, Zarafshan H, Mostafavi S-A, Kamali K, Rahgozar M, Ahmadi A, Hooshyari Z, Alavi SS (2019) Prevalence of autism and its comorbidities and the relationship with maternal psychopathology: a national population-based study. Arch Iranian Med 22:546–553
Mostafa GA, Bjørklund G, Urbina MA, Al-Ayadhi LY (2016) The positive association between elevated blood lead levels and brain-specific autoantibodies in autistic children from low lead-polluted areas. Metab Brain Disease 31:1047–1054
Nevison CD (2014) A comparison of temporal trends in United States autism prevalence to trends in suspected environmental factors. Environ Health 13:1–16
Obrenovich ME, Shamberger RJ, Lonsdale D (2011) Altered heavy metals and transketolase found in autistic spectrum disorder. Biol Trace Element Res 144:475–486
Ousley O, Cermak T (2014) Autism spectrum disorder: defining dimensions and subgroups. Current Dev Disorders Reports 1:20–28
Priya MDL, Geetha A (2011) Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol Trace Element Res 142:148–158
Rahbar MH, Samms-Vaughan M, Lee M, Zhang J, Hessabi M, Bressler J, Bach MA, Grove ML, Shakespeare-Pellington S, Beecher C (2020) Interaction between a mixture of heavy metals (lead, mercury, arsenic, cadmium, manganese, aluminum) and GSTP1, GSTT1, and GSTM1 in relation to autism spectrum disorder. Res Autism Spectrum Disorders 79:101681
Roberts AL, Lyall K, Hart JE, Laden F, Just AC, Bobb JF, Koenen KC, Ascherio A, Weisskopf MG (2013) Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect 121:978–984
Saghazadeh A, Rezaei N (2017) Systematic review and meta-analysis links autism and toxic metals and highlights the impact of country development status: higher blood and erythrocyte levels for mercury and lead, and higher hair antimony, cadmium, lead, and mercury. Prog Neuro-Psychopharmacol Biol Psychiat 79:340–368
Santos JX, Rasga C, Vicente AM (2021) Exposure to xenobiotics and gene-environment interactions in autism spectrum disorder: a systematic review. . In: Michael F (Editor), Autism spectrum disorder: profile, heterogeneity, neurobiology and intervention. doi: https://doi.org/10.5772/intechopen.95758. BoD – Books on Demand, pp. 133
Skalny AV, Simashkova NV, Klyushnik TP, Grabeklis AR, Radysh IV, Skalnaya MG, Tinkov AA (2017) Analysis of hair trace elements in children with autism spectrum disorders and communication disorders. Biol Trace Element Res 177:215–223
Sulaiman R, Wang M, Ren X (2020) Exposure to aluminum, cadmium, and mercury and autism spectrum disorder in children: a systematic review and meta-analysis. Chem Res Toxicol 33:2699–2718
Vakilizadeh N, Abedi A, Mohseni Ezhiyeh A (2017) Investigating validity and reliability of early screening for autistic traits-Persian version (ESAT-PV) in toddlers. Arch Rehabilit 18:182–193
Weitlauf AS, McPheeters ML, Peters B, Sathe N, Travis R, Aiello R, Williamson E, Veenstra-VanderWeele J, Krishnaswami S, Jerome R (2014) Therapies for children with autism spectrum disorder: behavioral interventions update. AHRQ Comparative Effectiveness Reviews. No. 137. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Aug. Report No: 14-EHC036-EF
Yao Y, Walsh WJ, McGinnis WR, Praticò D (2006) Altered vascular phenotype in autism: correlation with oxidative stress. Arch Neurol 63:1161–1164
Yasuda H, Yasuda Y, Tsutsui T (2013) Estimation of autistic children by metallomics analysis. Scientific Reports 3:1–8
Yorbik Ö, Kurt İ, Haşimi A, Öztürk Ö (2010) Chromium, cadmium, and lead levels in urine of children with autism and typically developing controls. Biol Trace Element Res 135:10–15
Yorbik O, Sayal A, Akay C et al (2002) Investigation of antioxidant enzymes in children with autistic disorder. Prostaglandins Leukot Essent Fatty Acids 67(5):341–343
Acknowledgements
We would like to acknowledge the assistance of the Medical Toxicology and Drug Abuse Research Center (MTDRC) and also Clinical Research Development Unit of Imam Reza Hospital at the Birjand University of Medical Sciences. Special thanks to Dr. Lukasz J. Binkowski and Dr. Martyna Błaszczyk for their assistance in improving the manuscript. In addition, our sincere appreciation goes to all patients who participated in this study.
Availability of data and materials
The datasets used and/or analyzed during the current research are available from the corresponding author on request.
Funding
This study was supported by the Birjand University of Medical Sciences (Grant number: 1398.4868).
Author information
Authors and Affiliations
Contributions
MR and BM were the overall coordinators. MR, AR, AE, SN, NA, and BM contributed to the design of the study, interpretation of the results, and drafting of the manuscript. MR and AR conducted the data collection. NA did data analysis. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the research and ethics committee of Birjand University of Medical Sciences (no. IR.BUMS.REC.1397.204).
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rezaei, M., Rezaei, A., Esmaeili, A. et al. A case–control study on the relationship between urine trace element levels and autism spectrum disorder among Iranian children. Environ Sci Pollut Res 29, 57287–57295 (2022). https://doi.org/10.1007/s11356-022-19933-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19933-1