Abstract
Associations between environmental metals and chemicals and adverse human health effects have emerged recently, but the links among environmental metals and respiratory diseases are less studied. The aim of this study was to assess 14 urinary metals (cadmium, barium, cobalt, molybdenum, mercury, cesium, manganese, antimony, lead, tin, strontium, tungsten, thallium, and uranium), seven species of arsenic (arsenous acid, arsenic acid, arsenobetaine, arsenocholine, dimethylarsinic acid, monomethylarsonic acid, and total arsenic) and seven polycyclic aromatic hydrocarbon (PAH) (1-hydroxynaphthalene, 2-hydroxynaphthalene, 3-hydroxyfluorene, 2-hydroxyfluorene, 1-hydroxyphenanthrene, 1-hydroxypyrene, 2 & 3-hydroxyphenanthrene) compounds’ concentrations in urine and the correlation with chronic obstructive pulmonary disease (COPD) in the adult US population. A cross-sectional analysis using the 2013–2014 and 2015–2016 National Health and Nutrition Examination Survey (NHANES) dataset was conducted. Self-questionnaires related to COPD criteria were used to identify the COPD cases. The correlation between urinary metals and PAH compounds and COPD was calculated. The total study population analyzed included 2885 adults aged 20 years and older. Seven types of urinary PAHs including 1-hydroxynaphthalene [odds ratio (OR): 1.832, 95% confidence interval (CI): 1.210, 2.775], 2-hydroxynaphthalene [OR: 3.361, 95% CI: 1.519, 7.440], 3-hydroxyfluorene [OR: 2.641, 95% CI: 1.381, 5.053], 2-hydroxyfluorene [OR: 3.628, 95% CI: 1.754, 7.506], 1-hydroxyphenanthrene [OR: 2.864, 95% CI: 1.307, 6.277], 1-hydroxypyrene [OR: 4.949, 95% CI: 2.540, 9.643] and 2 & 3-hydroxyphenanthrene [OR: 3.487, 95% CI: 1.382, 8.795] were positively associated with COPD. Urinary cadmium [OR: 12.382, 95% CI: 4.459, 34.383] and tin [OR: 1.743, 95% CI: 1.189, 2.555] showed positive associations with increased odds of COPD. The other types of urinary metals were not associated with COPD. The study observed that urinary PAHs, cadmium, and tin are significantly associated with COPD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exposure to environmental chemicals and metals causing adverse health effects is a major concern leading to numerous conditions including hypertension, cardiovascular disease, sleep disorders, decrease in cognitive function, and allergies (Shiue 2013a, b, c, 2014a, b, 2015a, b, c, d, 2017; Rahman et al. 2020a, b, 2021a, b, c, d). Exposure to heavy metals has been associated with chronic diseases likely due to oxidative stress and inflammation in the lungs causing tissue destruction (Rokadia and Agarwal 2013). Among occupational respiratory diseases, there has been a shift from exposure to mineral dust such as coal and silica in the twentieth century to low-dose allergens and irritants. This has led to a change in respiratory diseases from pneumonoconiosis to occupational asthma. Approximately 15% of all chronic obstructive pulmonary disease (COPD) cases in western societies have been attributed to dust, fumes, vapor, or gas (De Matteis et al. 2017).
The incomplete combustion of coal, gas, and oil, which contain carbon, are sources of polycyclic aromatic hydrocarbons (PAHs). PAHs, composed of primarily hydrogen and carbon atoms, contain two or more aromatic rings and are categorized as environmentally harmful pollutants (Honda and Suzuki 2020; Kataria et al. 2015). Industrial activity and engine-driven vehicles on the roads are significant sources causing PAH emissions to contaminate the air. Exposure to polluted air containing PAHs can cause various diseases in humans (Błaszczyk et al. 2017; Liu et al. 2015). The major route of exposure to PAHs is via the respiratory system; therefore, the risk of developing diseases such as respiratory and cardiovascular diseases is common (Perez-Padilla et al. 2010; World Health Organization (WHO) 2018).
Cadmium is considered one of the toxic non-essential metals that can cause adverse effects due to exposure in humans and other living species. Cadmium is a naturally occurring pollutant in the environment resulting from industrial and agricultural procedures. Primary cadmium exposure routes include consumption of contaminated food and water, and inhalation from smoking. Studies show that exposure to cadmium has been linked with kidney, lung, breast, pancreas, prostate, and nasopharynx cancers (Genchi et al. 2020; McElroy et al. 2006; Elghany et al. 1990). Furthermore, tin is a naturally occurring metal. The primary exposure of tin comes from the burning of fossil fuels, waste incineration, and production of tin, organotins, iron, steel, and non-ferrous metals. Inorganic tin accumulates in the lungs because of a lack of absorption and insolubility, leading to the lungs being the target organ (Cima 2011). Tin has been linked to several adverse health effects including interstitial pneumonia, neurotoxicity, nausea, vomiting, diarrhea, abdominal pain, headaches, skin rashes, high blood pressure, palpitations, fatigue, shortness of breath, asthma, and insomnia (Homma et al. 2003; Nath 2008; Shiue 2014b, 2015d; Cima 2011).
COPD is a preventable disease characterized by persistent respiratory symptoms and airflow limitation due to airway and alveolar abnormalities. This is caused by both small airway disease and parenchymal destruction (emphysema). Chronic inflammation leads to narrowing of small airways and the destruction of lung parenchyma which decreases elastic recoil and causes the airways to not stay patent on exhalation. The terms emphysema and chronic bronchitis are no longer used in the definition of COPD; however, emphysema refers to the destruction of lung alveoli, and chronic bronchitis is the presence of cough or sputum for at least 3 consecutive months over the period of 2 years (Global Initiative for Chronic Obstructive Lung Disease 2020). Asthma, rhinitis, COPD, bronchiectasis, occupational lung disease, pulmonary hypertension, pulmonary interstitial diseases, and sleep disordered breathing are considered major chronic respiratory diseases (Bousquet and Kaltaev 2007). COPD is considered the most common respiratory disease. Approximately 10% of the population 40 years and older suffer from COPD worldwide (Bousquet et al. 2010). Globally, COPD is one of the leading causes of morbidity and mortality. Respiratory symptoms, persistent limitations in airflow, and frequent exacerbation symptoms are key features of COPD in addition to shortness of breath, wheezing, and cough (Lozano et al. 2012; Asia Pacific COPD Roundtable Group 2005; Donaldson et al. 2002; Seemungal et al. 1998). Intermittent exacerbations of COPD modify the disease trajectory leading to a decreased quality of life by reducing lung function and functional capacity (Donaldson et al. 2002; Seemungal et al. 1998).
Smoking is considered one of the primary risk factors for COPD; however, environmental factors and occupational exposure increase the risk of COPD (Eisner et al. 2010; Blanc and Torén 2007). Smoking cessation and supplemental oxygen are the only two available treatments for patients with COPD to improve mortality (Mannino et al. 2002; Halbert et al. 2003; Murray and Lopez 1997; Anthonisen et al. 1994). Currently, there are no studies showing that existing medications for COPD, including long-acting bronchodilators and inhaled corticosteroids, modify the long-term degradation of lung function (Burge et al. 2000; Anthonisen et al. 1994; Pauwels et al. 1999; Vestbo et al. 1999; Tashkin et al. 2008; Decramer et al. 2009; Celli et al. 2008; Global Initiative for Chronic Obstructive Lung Diseases 2020). Other contributing factors associated with COPD, such as environmental exposure and occupational exposure, should be reduced.
The purpose of this study is to analyze the effects of 14 forms of urinary metals including barium, cadmium, cobalt, cesium, molybdenum, manganese, lead, antimony, tin, strontium, thallium, tungsten, uranium, and mercury, seven forms of urinary speciated arsenic, and seven types of urinary PAHs, and the association with the risk of COPD in the adult United States (US) population.
Methods
Study population
Data for this study was taken from the National Health and Nutrition Examination Survey (NHANES), a long-standing study using physical exams and interviews to determine the health and nutritional status of adults and children in the USA. The survey sample is designed to represent the US population of all ages; with selected subgroups (e.g. people over 60 and certain race/ethnicities) oversampled to ensure reliable statistics. The survey examines a nationally representative sample of about 5000 people each year located in counties across the country, 15 of which are visited each year. Only adults aged ≥ 20 years were included in our analysis below (National Center for Health Statistics [NCHS] 2017a, b).
Metals, arsenic, and PAH assessment
As in Shiue (2015d), several urinary metals, arsenic, and PAHs were included in the same study in order to compare and contrast the effect of each potential exposure with depression. The metals, arsenic species, and PAHs were selected according to what was provided in the NHANES datasets. The 2013–2014 and 2015–2016 NHANES datasets: Polycyclic Aromatic Hydrocarbons (PAH) — Urine (PAH_I), Metals — Urine (UM_I), Mercury — Urine (UHG_I), Arsenic — Total — Urine (UTAS_I), and Speciated Arsenics — Urine (UAS_I) were used for data covering urinary barium, cadmium, cesium, cobalt, manganese, molybdenum, lead, antimony, strontium, thallium, tin, tungsten, uranium, mercury, total arsenic, arsenous acid, arsenic acid, arsenobetaine, arsenocholine, dimethylarsinic acid (DMA), monomethylarsonic acid (MMA), 1-hydroxynapthalene, 2-hydroxynapthalene, 3-hydroxyfluorene, 2-hydroxyfluorene, 1-hydroxyphenanthrene, 1-hydroxypyrene, 2-hydroxyphenanthrene & 3-hydroxyphenanthrene concentrations (CDC 2020b, 2018a, b, c, d). The urinary species were normalized with the creatinine concentration using the dataset Albumin & Creatinine — Urine (ALB_CR_J) (CDC 2017a). Details of the analytical methods and detection limits for the various compounds are provided in the CDC references. Metals, arsenic, and PAHs were all included in this analysis to evaluate the effect of each form while comparing the effects as in previous literature (Shiue 2015b, d; 2016).
COPD assessment
COPD was determined using variable MCQ160o in the 2013–2014 and 2015–2016 Medical Conditions (MCQ_I) datasets. Questions were asked via questionnaires using a Computer-Assisted Personal Interview (CAPI) system in the home. The questionnaire asked participants “Has a doctor or other health professional ever told you that you had COPD?” (CDC 2017b). This response was used to define which patients had COPD in our study.
Covariates
Datasets for covariates came from the NHANES data files ALQ_I — Alcohol consumption, BMX_I — Body mass index, and DEMO_I — Demographic data, and COT_I — Serum cotinine (CDC 2018e, 2017c, d, 2019). Among demographic categories, the following variables were included: gender (male, female), race/ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, non-Hispanic Asian, other race multi-racial), marital status (married, widowed, divorced, separated, never married, living with partner), highest educational level achieved (no high school, some high school, high school graduate, some college, college graduate), age (20–44 years, 45–60 years, 60 years and older), family income to poverty ratio (FIPR) (less than 1.5, 1.5–3.5, over 3.5), BMI was converted into a four-level categorical variable with underweight, BMI < 18.5; normal weight, BMI ≥ 18.5, < 24.9; overweight, BMI ≥ 24.9, < 30.0; and obese, BMI ≥ 30.0 per the CDC definitions, serum cotinine (below lower limit of detection, above lower limit of detection), alcoholic drink last 12 months (no, yes), and country of birth (USA, other) (CDC 2020a; Rahman et al. 2021a, e). NHANES was approved by the Research Ethics Review Board of the NCHS. This study was exempt from additional review by an institutional review board, as this is public-use data.
Statistical analysis
Data was first cleaned by removing missing responses, categorizing the continuous covariate variables, normalizing the concentrations of metals and PAHs with the creatinine concentration, and creating the binary categorical variable for COPD based on the binary NHANES variable M160o. The missing values for demographic categories, BMI, serum cotinine, alcohol use, and country of birth were removed, and the resulting data was used for all modeling. Missing responses for PAH and urinary metals were removed from the dataset before creation of the logit regression model. Values provided in NHANES data were used directly; the concentrations were normalized using the urinary creatinine concentration and then log10 transformed.
R version 3.6.3 was used for the statistical analysis with programs from the survey package and the functions svyby, svymean, svyttest, svydesign, and svyglm were used to calculate the unweighted occurrences of responses, the weighted mean of the responses, the weighted pairwise t-tests of the responses, the survey design object, and the logit regression models, respectively (R Core Team 2020; Lumley 2004, 2010, 2020). Other R functions and packages used to simplify the programing included: the function nhanes_load_data in the package RNHANES was used to download the data files from the NHANES and store as *.csv files (Susmann 2016).
The assessment of correlation between COPD and the environmental chemicals was conducted using a multi-factor, weighted logit regression model. Logit regression was selected as the appropriate statistical modeling tool since the response factor (absence/presence of COPD) was a binary, categorical variable. The model included one of the environmental chemicals and selected demographic and lifestyle covariates (see Table 2 for the covariates and the categories used in the analysis). This resulted in a regression model with 26 model parameters in which the absence/presence of COPD was modeled. The weighting factors for the model were taken from the NHANES dataset directly. The modeling results are given only for the environmental chemicals.
The COPD — covariate correlation summary given in Table 2 was done for the responses used in the COPD — PAH correlation summary considered later. Similar covariate correlation summaries were done (but not reported) for in the other environmental chemicals. There were slight differences in the numerical results but none that was significant.
Results
A total of N = 2885 participants were included in the study, 49.6% male and 50.4% female. About 4.0% of males and 2.9% of females were determined to have COPD in our sample (Table 1). Among race/ethnicity, non-Hispanic White had the highest percent with COPD at 6.2%; most participants with COPD were categorized as widowed, divorced, or separated, and were in the age group of 60 years and older. Higher percentages of participants with COPD had lower FIPR, and serum cotinine above the lower limit of detection and consumed alcohol. The factors determined significant for an increased odds of COPD were participants who were non-Hispanic White, ages 45–60 and 60 years and older, and a serum cotinine above the lower limit of detection. Factors that were significant for reducing the odds of COPD included living with a partner, and a FIPR of 1.5–3.5 and over 3.5 (Table 2).
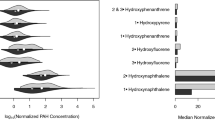
Figure 1 reveals a visibly higher concentration of both 2-hydroxynaphthalene and 1-hydroxynaphthalene in those with COPD compared to those who did not have COPD. Among arsenic species, Fig. 2 shows total arsenic, MMA, DMA, arsenobetaine, and arsenous acid which has concentrations that are lower in those with COPD. Among the metals, Fig. 3 shows strontium and cesium have higher concentrations in those with COPD, while molybdenum has a lower concentration in those with COPD.
Several significant associations were found among PAH and metals and COPD (Table 3). All forms of PAHs studied, including 1-hydroxynaphthalene, 2-hydroxynaphthalene, 3-hydroxyfluorene, 2-hydroxyfluorene, 1-hydroxyphenanthrene, 1-hydroxypyrene, and 2 & 3-hydroxyphenanthrene were found to have significant associations with COPD. Among metals, cadmium (OR = 12.382, 95% CI = 4.459, 34.383, p = 0.000) and tin (OR = 1.743, 95% CI = 1.189, 2.555, p = 0.008) had significant associations with COPD. No other metals had a significant association with arsenic (Tables 4–5).
Discussion
Worldwide, mortality from COPD ranked third, leading to COPD being considered a significant public health issue (Vestbo et al. 2013). Deaths due to COPD are ranked fourth in the USA but are expected to rise due to exposures from environmental and occupational pollutants (CDC 2017e; Ford et al. 2013a, b; World Health Organization (WHO) 2000, 2002). This study analyzed the association among COPD and 14 metals, seven arsenic species, and seven PAH compounds.
PAHs have commonly been studied among populations with COPD exposed to air pollution. Numerous studies have found a positive association between PAH exposure and asthma; however, other obstructive lung diseases have been less studied (Nwaozuzu et al. 2021). Shiue (2016) found a positive association using 2011–2012 NHANES data between 2-hydroxyfluorene and emphysema (OR: 1.60, 95% Cl: 1.26, 2.03) and chronic bronchitis (OR: 1.42, 95% CI: 1.04, 1.94), in 3-hydroxyfluorene with emphysema (OR: 1.42, 95% CI: 1.15, 1.77) and chronic bronchitis (OR: 1.40, 95% CI: 1.03, 1.91), and in 2-hydroxynaphthalene for chronic bronchitis (OR: 1.32, 95% CI: 1.02, 1.72). 9-hydroxyfluorene, 1-hydroxyphenanthrene, 2-hydroxyphenanthrene, 3-hydroxyphenanthrene, 1-hydroxypyrene, 1-hydroxynaphthalene, 4-hydroxyphenanthrene were not significant for emphysema or chronic bronchitis. In our study, we found 1-hydroxyphenanthrene, 1-hydroxypyrene, 2 & 3-hydroxyphenanthrene significant for COPD in addition to those that Shiue found significant associations for. Although similar questionnaires were used in our NHANES dataset, the 2011–2012 dataset did not include questionnaires on COPD but only for emphysema and chronic bronchitis. The 2015–2016 dataset used in our study specifically asked about COPD. Kilpatrick (2019) found a positive association between 1-napthol (OR: 1.150, 95% CI: 1.043, 1.268), 2-fluorene (OR: 1.257, 95% CI: 1.059, 1.492), and 2-phenanthrene (OR: 1.196, 95% CI: 1.025, 1.396) using NHANES 2007–2012 data.
Exposure to PAHs has been linked to DNA nucleotides, causing the production of DNA adducts that can cause mutations in DNA replication (Wogan et al. 2004; Sato and Aoki 2002; Pauk et al. 2013). In addition, PAHs can cause oxidative damage to DNA and participate in changes in oncogenes and tumor suppressor genes causing chromosomal aberrations (Farmer et al. 2003; Pauk et al. 2013). Those with large PAH exposures can be found to have anti-PAH antibodies. An increase in DNA adducts has been seen in patients with COPD who smoke and those with cancerous lung disease, suggesting that PAH exposure from smoking led to increased DNA changes (Pauk et al. 2013). Yang et al. (2017) determined that PAHs with high benzo(a)pyrene (BaP)-equivalent concentrations, rather than the major PAH components, have caused the increased risk of COPD. Therefore, determining the BaP toxic equivalency factors of PAH can determine risk for COPD (Yang et al. 2017).
Among metals, the study found a significant association between tin and cadmium and COPD. Feng et al. (2015) analyzed 23 urinary metals and their association with obstructive and restrictive lung disease measured with spirometry. Iron was found to have a dose–response association with a decreased risk of COPD, while lead had an increased risk of COPD (Feng et al. 2015). Mutti et al. (2006) studied the exhaled breath condensate of COPD patients and found that those with COPD had higher levels of lead, cadmium, and aluminum, and lower levels of iron and copper compared to the non-smoking controls (Mutti et al. 2006). Cadmium, a component of cigarette smoke, has been negatively correlated to forced expiratory volume in 1 s (FEV1) in current (− 2.06%, 95% CI: − 2.86, − 1.26 per 1 log increase in urinary cadmium) and former smokers (− 1.95%, 95% CI − 2.87, − 1.03), but not in never smokers. Higher levels of urinary cadmium were also associated with a significantly lower FEV1/FVC (forced vital capacity) ratio (Mannino et al. 2004). Among occupational studies, exposure to cadmium alloy has been linked to emphysema (Davison et al. 1988).
Tin has rarely been studied in the literature with its connection to COPD. Indium tin oxide (ITO) is a mixture of indium oxide and tin oxide and is commonly used in LCD displays (Liu et al. 2012). The increasing use has led to an increase in exposure of ITO resulting in interstitial pneumonia and pulmonary fibrosis (Liu et al. 2012; Homma et al. 2003; 2005). Liu et al. (2012) found significantly increased levels of SP-A and SP-D levels in ITO-manufacturing workers, which are markers of interstitial lung disease. In addition to interstitial lung diseases, Nakano et al. (2016) published a case report of an ITO worker who developed progressive lung destruction with accompanying severe centrilobular emphysema, with a FEV1/FVC of 76.5%. Nakano et al. (2016) suggests ITO exposure is a risk factor for emphysema. Among children with asthma, tin was not found to have a significant association with ln(FEV1), ln(FVC), or ln(FEV1/FVC) (Madrigal et al. 2021). However, other metals have been linked to COPD in the past. Feng et al. (2015) found significant dose–response associations between lead and iron and COPD. Among children, urinary manganese was inversely associated with FEV1/FVC and FEF25–75 forced expiratory flow between 25 and 75% of vital capacity) (Madrigal et al. 2018). Jones et al. (2007) conducted an occupational study investigating lung cancer among former employees at a tin smelter. Among cumulative exposures of arsenic, cadmium, lead, antimony, and polonium-210, significant associations were found between lung cancer mortality and exposures to arsenic, lead, and antimony. In our study, tin and cadmium were found to have a significant association with COPD; however, no other metals were significant.
Among arsenic’s effect on lung function, Wei et al. (2021) determined a positive linear dose–response association between urinary arsenic and increased annual declines in FEV1, predicted percent (pp)FEV1, FVC, and ppFVC in coke oven workers. No significant effects of urinary arsenic on annual changes in FEV1/FVC and FEF25–75 were found (Wei et al. 2021). Furthermore, in a meta-analysis of nine studies, Sanchez et al. (2018) found that arsenic exposure was associated with lower FEV1 and FVC but not with FEV1/FVC, suggesting that arsenic exposure leads to restrictive lung disease rather than obstructive lung diseases such as COPD. Our study also found no significant associations between any form of urinary speciated arsenic and COPD.
Among demographic factors, Parulekar et al. (2017) determined that COPD patients ≥ 65 years were more likely to require long-term oxygen therapy, had worse lung function measured by FEV1, and had worse exercise tolerance as measured by a 6-min walk distance. Older participants had higher percentages of emphysema (14% vs 8%, p < 0.001) and more gas trapping on CT (47% vs 36%, p < 0.001) compared to those < 65 years. Tilert et al. (2018) found a significant increase in the odds of participants with COPD > 60 years compared to 40–59 years (OR: 1.65, 95% CI: 1.07, 2.53). We found a significant increase in odds of COPD among ages 45–60 years and 60 years and older. Those with COPD also had a significantly higher percentage who were current (OR: 1.70, 95% CI: 1.17, 2.48) or former smokers (OR: 1.75, 95% CI: 1.23, 2.48) using NHANES data from 2007 to 2012. Our study using NHANES 2013–2016 data found increased odds when the cotinine level was above the LLoD, suggesting the participant was a smoker. When compared to non-Hispanic White, Tilert et al. (2018) determined that non-Hispanic Black and Hispanic had protective odds of COPD. Our data showed similar results in that non-Hispanic White had higher odds of COPD. Decreased odds for COPD were determined in those living with partner, and the participants with an FIPR of 1.5–3.5 or over 3.5. Eisner et al. (2011) found increased odds of COPD severity among those with low and medium incomes as compared to high.
Limitations
Data used in this study was obtained by NHANES and, therefore, we were limited by the method of COPD data collection. The dataset included COPD data by questionnaire from the participants but was not confirmed with pulmonary function testing with laboratory spirometry. In addition, the specific type of obstructive lung disease was not included, such as chronic bronchitis or emphysema. The question regarding the patients’ history of COPD was used and, therefore, we do not know the cause or severity of the participants’ COPD nor its timing relative to the urinalysis. Further clinical studies are needed to confirm the diagnosis of COPD among participants and understand the cause and management.
Conclusion
Our study found a positive association among urinary tin, cadmium, and seven forms of PAHs (1-hydroxynaphthalene, 2-hydroxynaphthalene, 3-hydroxyfluorene, 2-hydroxyfluorene, 1-hydroxyphenanthrene, 1-hydroxypyrene, 2 & 3-hydroxyphenanthrene) with COPD among US adults. No other metals or the arsenic compounds studied were found to be associated with COPD.
Availability of data and material
The datasets analyzed during the current study are available in the NHANES repository provided by the CDC to the public.
References
Anthonisen NR, Connett JE, Kiley JP et al (1994) Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study JAMA 272(19):1497–1505
Asia Pacific COPD Roundtable Group (2005) Global initiative for chronic obstructive lung disease strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: an Asia-Pacific perspective. Respirology 10(1):9–17. https://doi.org/10.1111/j.1440-1843.2005.00692.x
Blanc PD, Torén K (2007) Occupation in chronic obstructive pulmonary disease and chronic bronchitis: an update. Int J Tuberc Lung Dis 11(3):251–257
Błaszczyk E, Rogula-Kozłowska W, Klejnowski K, Fulara I, Mielżyńska-Švach D (2017) Polycyclic aromatic hydrocarbons bound to outdoor and indoor airborne particles (PM2.5) and their mutagenicity and carcinogenicity in Silesian kindergartens, Poland. Air Qual Atmos Health 10(3):389–400. https://doi.org/10.1007/s11869-016-0457-5
Bousquet J, Kaltaev N (2007) Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. World Health Organization. https://apps.who.int/iris/handle/10665/43776
Bousquet J, Kiley J, Bateman ED et al (2010) Prioritised research agenda for prevention and control of chronic respiratory diseases. Eur Respir J 36(5):995–1001. https://doi.org/10.1183/09031936.00012610
Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK (2000) Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 320(7245):1297–1303
CDC (2017a) Albumin & creatinine - urine (ALB_CR_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/ALB_CR_I.htm
CDC (2017b) Medical conditions (MCQ_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/MCQ_I.htm
CDC (2017c) Body measures (BMX_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/BMX_I.htm
CDC (2017d) Demographic variables and sample weights (DEMO_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/DEMO_I.htm
CDC (2017e) Demographic variables and sample weights (DEMO_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/DEMO_I.htm
CDC (2018a) Metals - urine (UM_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/UM_I.htm
CDC (2018b) Mercury - urine (UHG_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/UHG_I.htm
CDC (2018c) Arsenic - total - urine (UTAS_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/UTAS_I.htm
CDC (2018d) Speciated arsenics - urine (UAS_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/UAS_I.htm
CDC (2018e) Alcohol Use (ALQ_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/ALQ_I.htm
CDC (2019) Cotinine and hydroxycotinine - serum (COT_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/COT_I.htm
CDC (2020a) About adult BMI. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html#trends
CDC (2020b) Polycyclic aromatic hydrocarbons (PAH) - Urine (PAH_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/PAH_I.htm
Celli BR, Thomas NE, Anderson JA et al (2008) Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med 178(4):332–338
Cima F (2011) Tin: environmental pollution and health effects. Encyclopedia of Environmental Health 351–359. https://doi.org/10.1016/B978-0-444-52272-6.00645-0
Davison AG, Fayers PM, Taylor AJ et al (1988) Cadmium fume inhalation and emphysema. Lancet 1(8587):663–667. https://doi.org/10.1016/s0140-6736(88)91474-2
De Matteis S, Heederik D, Burdorf A et al (2017) Current and new challenges in occupational lung diseases. Eur Respir Rev 26(146):170080. https://doi.org/10.1183/16000617.0080-2017
Decramer M, Celli B, Kesten S et al (2009) Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet 374(9696):1171–1178. https://doi.org/10.1007/s11356-015-4944-2
Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA (2002) Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 57(10):847–852. https://doi.org/10.1136/thorax.57.10.847
Eisner MD, Anthonisen N, Coultas D et al (2010) An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182(5):693–718. https://doi.org/10.1164/rccm.200811-1757ST
Eisner MD, Blanc PD, Omachi TA et al (2011) Socioeconomic status, race and COPD health outcomes. J Epidemiol Community Health 65(1):26–34. https://doi.org/10.1136/jech.2009.089722
Elghany NA, Schumacher MC, Slattery ML, West DW, Lee JS (1990) Occupation, cadmium exposure, and prostate cancer. Epidemiology 1(2):107–115. https://doi.org/10.1097/00001648-199003000-00005
Farmer PP, Singh R, Kaur B, Sram RJ, Binkova B et al (2003) Molecular epidemiology studies of carcinogenic environmental pollutants. Effects of polycyclic aromatic hydrocarbons (PAHs) in environmental pollution on exogenous and oxidative DNA damage. Mutat Res 544:397–402
Feng W, Huang X, Zhang C et al (2015) The dose-response association of urinary metals with altered pulmonary function and risks of restrictive and obstructive lung diseases: a population-based study in China. BMJ Open 5(5):e007643. https://doi.org/10.1136/bmjopen-2015-007643
Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH (2013a) COPD surveillance–United States, 1999–2011. Chest 144(1):284–305. https://doi.org/10.1378/chest.13-0809
Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB (2013b) Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988–1994 to 2007–2010. Chest 143(5):1395–1406. https://doi.org/10.1378/chest.12-1135
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17(11):3782. https://doi.org/10.3390/ijerph17113782
Global Initiative for Chronic Obstructive Lung Disease (2020) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2021 Report. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf
Halbert RJ, Isonaka S, George D, Iqbal A (2003) Interpreting COPD prevalence estimates: what is the true burden of disease? Chest 123(5):1684–1692. https://doi.org/10.1378/chest.123.5.1684
Homma S, Miyamoto A, Sakamoto S, Kishi K, Motoi N, Yoshimura K (2005) Pulmonary fibrosis in an individual occupationally exposed to inhaled indium-tin oxide. Eur Respir J 25(1):200–204. https://doi.org/10.1183/09031936.04.10012704
Homma T, Ueno T, Sekizawa K, Tanaka A, Hirata M (2003) Interstitial pneumonia developed in a worker dealing with particles containing indium-tin oxide. J Occup Health 45(3):137–139. https://doi.org/10.1539/joh.45.137
Honda M, Suzuki N (2020) Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int J Environ Res Public Health 17(4):1363. https://doi.org/10.3390/ijerph17041363
Jones SR, Atkin P, Holroyd C et al (2007) Lung cancer mortality at a UK tin smelter. Occup Med (lond) 57(4):238–245. https://doi.org/10.1093/occmed/kql153
Kataria A, Trasande L, Trachtman H (2015) The effects of environmental chemicals on renal function. Nat Rev Nephrol 11(10):610–625. https://doi.org/10.1038/nrneph.2015.94
Kilpatrick DJ (2019) Investigating the relationship of COPD, lung cancer, and polycyclic aromatic hydrocarbons from ambient air pollution. Doctoral dissertation. https://scholarcommons.sc.edu/etd/5573
Liu HH, Chen CY, Chen GI, Lee LH, Chen HL (2012) Relationship between indium exposure and oxidative damage in workers in indium tin oxide production plants. Int Arch Occup Environ Health 85(4):447–453. https://doi.org/10.1007/s00420-011-0688-6
Liu J, Man R, Ma S, Li J, Wu Q, Peng J (2015) Atmospheric levels and health risk of polycyclic aromatic hydrocarbons (PAHs) bound to PM2.5 in Guangzhou, China. Mar Pollut Bull 100(1):134–143. https://doi.org/10.1016/j.marpolbul.2015.09.014
Lozano R, Naghavi M, Foreman K et al (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2095–2128. https://doi.org/10.1016/S0140-6736(12)61728-0
Lumley T (2004) Analysis of complex survey samples. J Stat Softw 9(8). https://doi.org/10.18637/jss.v009.i08
Lumley T (2020) Package ‘survey’: analysis of complex survey samples, version 4.0. https://cran.r-project.org/web/packages/survey/survey.pdf
Lumley TS (2010) Complex surveys: a guide to analysis using R. John Wiley & Sons, Hoboken
Madrigal JM, Persky V, Jackson BP et al (2021) Assessment of metal concentrations and associations with pulmonary function among children with asthma in Chicago, Illinois. Int J Environ Res Public Health 18(14):7279. https://doi.org/10.3390/ijerph18147279
Madrigal JM, Persky V, Pappalardo A, Argos M (2018) Association of heavy metals with measures of pulmonary function in children and youth: results from the National Health and Nutrition Examination Survey (NHANES). Environ Int 121(Pt 1):871–878. https://doi.org/10.1016/j.envint.2018.09.045
Mannino DM, Holguin F, Greves HM, Savage-Brown A, Stock AL, Jones RL (2004) Urinary cadmium levels predict lower lung function in current and former smokers: data from the Third National Health and Nutrition Examination Survey. Thorax 59(3):194–198. https://doi.org/10.1136/thorax.2003.012054
Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC (2002) Chronic obstructive pulmonary disease surveillance–United States, 1971–2000. MMWR Surveill Summ 51(6):1–16
McElroy JA, Shafer MM, Trentham-Dietz A, Hampton JM, Newcomb PA (2006) Cadmium exposure and breast cancer risk. J Natl Cancer Inst 98(12):869–873. https://doi.org/10.1093/jnci/djj233
Murray CJ, Lopez AD (1997) Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 349(9064):1498–1504. https://doi.org/10.1016/S0140-6736(96)07492-2
Mutti A, Corradi M, Goldoni M, Vettori MV, Bernard A, Apostoli P (2006) Exhaled metallic elements and serum pneumoproteins in asymptomatic smokers and patients with COPD or asthma. Chest 129(5):1288–1297. https://doi.org/10.1378/chest.129.5.1288
Nakano M, Tanaka A, Hirata M et al (2016) An advanced case of indium lung disease with progressive emphysema. J Occup Health 58(5):477–481. https://doi.org/10.1539/joh.16-0076-CS
Nath M (2008) Toxicity and the cardiovascular activity of organotin compounds: a review. Appl Organomet Chem 22(10):598–612. https://doi.org/10.1002/aoc.1436
National Center for Health Statistics [NCHS] (2017a) About the National Health and Nutrition Examination jSurvey. CDC. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm
National Center for Health Statistics [NCHS] (2017b). National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/data/factsheets/factsheet_nhanes.htm
Nwaozuzu CC, Partick-Iwuanyanwu KC, Abah SO (2021) Systematic review of exposure to polycyclic aromatic hydrocarbons and obstructive lung disease. J Health Pollut 11(31):210903. https://doi.org/10.5696/2156-9614-11.31.210903
Parulekar AD, Martinez C, Tsai CL et al (2017) Examining the effects of age on health outcomes of chronic obstructive pulmonary disease: results from the genetic epidemiology of chronic obstructive pulmonary disease study and evaluation of chronic obstructive pulmonary disease longitudinally to identify predictive surrogate endpoints cohorts. J Am Med Dir Assoc 18(12):1063–1068. https://doi.org/10.1016/j.jamda.2017.09.028
Pauk N, Klimesova S, Kara J, Topinka J, Labaj J (2013) The relevance of monitoring of antibodies against the polycyclic aromatic hydrocarbon (PAH) and PAH-DNA adducts in serum in relation to lung cancer and chronic obstructive pulmonary disease (COPD). Neoplasma 60(2):182–187. https://doi.org/10.4149/neo_2013_024
Pauwels RA, Lofdahl CG, Laitinen LA et al (1999) Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med 340(25):1948–53
Perez-Padilla R, Schilmann A, Riojas-Rodriguez H (2010) Respiratory health effects of indoor air pollution. Int J Tuberc Lung Dis 14(9):1079–1086
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna https://www.R-project.org/
Rahman HH, Niemann D, Munson-McGee SH (2021a) Association among urinary polycyclic aromatic hydrocarbons and depression: a cross-sectional study from NHANES 2015–2016. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-021-16692-3
Rahman HH, Niemann D, Munson-McGee SH (2021b) Environmental exposure to metals and the risk of high blood pressure: a cross-sectional study from NHANES 2015–2016. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-021-15726-0
Rahman HH, Niemann D, Bugajski A (2021c) Association of environmental toxic metals with high sensitivity C- reactive protein: a cross-sectional study. Occup Dis Environ Med 9(4):173–184. https://doi.org/10.4236/odem.2021.94013
Rahman HH, Niemann D, Munson-McGee SH (2021d) Association of albumin to creatinine ratio with urinary arsenic and metal exposure: evidence from NHANES 2015–2016. Int Urol Nephrol. https://doi.org/10.1007/s11255-021-03018-y
Rahman HH, Niemann D, Singh D (2020a) Arsenic exposure and association with hepatitis E IgG antibodies. Occup Dis Environ Med 8:111–122. https://doi.org/10.4236/odem.2020.83009
Rahman HH, Niemann D, Yusuf KK (2021e) Association of urinary arsenic and sleep disorder in the US population: NHANES 2015–2016. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-021-16085-6.10.1007/s11356-021-16085-6
Rahman HH, Yusuf KK, Niemann D, Dipon SR (2020b) Urinary speciated arsenic and depression among US adults. Environ Sci Pollut Res Int 27(18):23048–23053. https://doi.org/10.1007/s11356-020-08858-2
Rokadia HK, Agarwal S (2013) Serum heavy metals and obstructive lung disease: results from the National Health and Nutrition Examination Survey. Chest 143(2):388–397. https://doi.org/10.1378/chest.12-0595
Sanchez TR, Powers M, Perzanowski M, George CM, Graziano JH, Navas-Acien A (2018) A meta-analysis of arsenic exposure and lung function: is there evidence of restrictive or obstructive lung disease? Curr Environ Health Rep 5(2):244–254. https://doi.org/10.1007/s40572-018-0192-1
Sato H, Aoki Y (2002) Mutagenesis by environmental pollutants and bio-monitoring of environmental mutagens. Curr Drug Metab 3:311–319. https://doi.org/10.2174/1389200023337603
Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA (1998) Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157(5 Pt 1):1418–1422. https://doi.org/10.1164/ajrccm.157.5.9709032
Shiue I (2013a) Urine phthalate concentrations are higher in people with stroke: United States National Health and Nutrition Examination Surveys (NHANES), 2001–2004. Eur J Neurol 20:728–731
Shiue I (2013b) Association of urinary arsenic, heavy metal, and phthalate concentrations with food allergy in adults: National Health and Nutrition Examination Survey, 2005–2006. Ann Allergy Asthma
Shiue I (2013c) Urinary environmental chemical concentrations and vitamin D are associated with vision, hearing, and balance disorders in the elderly. Environ Int 53:41–46
Shiue I (2014a) Arsenic, heavy metals, phthalates, pesticides, hydrocarbons and polyfluorinated compounds but not parabens or phenols are associated with adult remembering condition: US NHANES, 2011–2012. Environ Sci Pollut Res Int 22:6381–6386
Shiue I (2014b) Higher urinary heavy metal, phthalate, and arsenic but not parabens concentrations in people with high blood pressure, U.S. NHANES, 2011–2012. Int J Environ Res Public Health 11:5989–5899
Shiue I (2015a) Are urinary polyaromatic hydrocarbons associated with adult hypertension, heart attack, and cancer? USA NHANES, 2011–2012. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-015-4922-8
Shiue I (2015b) Urinary heavy metals, phthalates, phenols, thiocyanate, parabens, pesticides, polyaromatic hydrocarbons but not arsenic or polyfluorinated compounds are associated with adult oral health: USA NHANES, 2011–2012. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-015-4561-0
Shiue I (2015c) Urinary parabens and polyaromatic hydrocarbons independent of health conditions are associated with adult emotional support needs: USA NHANES, 2005–2008. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-015-4749-3
Shiue I (2015d) Urinary heavy metals, phthalates and polyaromatic hydrocarbons independent of health events are associated with adult depression: USA NHANES, 2011–2012. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-015-4944-2
Shiue I (2016) Urinary polyaromatic hydrocarbons are associated with adult emphysema, chronic bronchitis, asthma, and infections: US NHANES, 2011–2012. Environ Sci Pollut Res Int 23(24):25494–25500. https://doi.org/10.1007/s11356-016-7867-7
Shiue I (2017) Urinary arsenic, pesticides, heavy metals, phthalates, polyaromatic hydrocarbons, and polyfluoroalkyl compounds are associated with sleep troubles in adults: USA NHANES, 2005–2006. Environ Sci Pollut Res Int 24(3):3108–3116. https://doi.org/10.1007/s11356-016-8054-6
Susmann H (2016) Package ‘RNHANES’: facilitates analysis of CDC NHANES, version 1.1.0. https://cran.r-project.org/web/packages/RNHANES/RNHANES.pdf
Tashkin DP, Celli B, Senn S et al (2008) A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 359(15):1543–1554
Tilert T, Paulose-Ram R, Howard D, Butler J, Lee S, Wang MQ (2018) Prevalence and factors associated with self-reported chronic obstructive pulmonary disease among adults aged 40–79: the National Health and Nutrition Examination Survey (NHANES) 2007–2012. EC Pulmonol Respir Med 7(9):650–662
Vestbo J, Hurd SS, Agustí AG et al (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187(4):347–365. https://doi.org/10.1164/rccm.201204-0596PP
Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K (1999) Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 353(9167):1819–1823
Wei W, Wu X, Bai Y et al (2021) Arsenic exposure and its joint effects with cigarette smoking and physical exercise on lung function impairment: evidence from an occupational cohort study. Environ Res 196:110419. https://doi.org/10.1016/j.envres.2020.110419
Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA (2004) Environmental and chemical carcinogenesis. Semin Cancer Biol 14:473–486. https://doi.org/10.1016/j.semcancer.2004.06.010
World Health Organization (WHO) (2000) Bronchial asthma. Fact sheet No. 206. http://www.who.int/inf-fs/en/fact206.html
World Health Organization (WHO) (2002) WHO strategy for prevention and control of chronic respiratory diseases. https://apps.who.int/iris/bitstream/handle/10665/67369/WHO_MNC_CRA_02.1.pdf?sequence=1&isAllowed=y
World Health Organization (WHO) (2018) Ambient (outdoor) air pollution. https://www.who.int/en/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health
Yang L, Wang WC, Lung SC et al (2017) Polycyclic aromatic hydrocarbons are associated with increased risk of chronic obstructive pulmonary disease during haze events in China. Sci Total Environ 574:1649–1658. https://doi.org/10.1016/j.scitotenv.2016.08.211
Author information
Authors and Affiliations
Contributions
Humairat H. Rahman conceptualized the study and contributed to the introduction and discussion. Stuart Munson-McGee conducted the data analysis and contributed to the drafting of the paper. Danielle Niemann contributed to the drafting of the paper. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable. This study uses only secondary data analyses without any personal information identified using statistical data from the NHANES website; no further ethical approval for conducting the present study is required.
Consent to participate
Consent was given by all the authors.
Consent for publication
Consent was given by all the authors.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Lotfi Aleya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahman, H.H., Niemann, D. & Munson-McGee, S.H. Association between environmental toxic metals, arsenic and polycyclic aromatic hydrocarbons and chronic obstructive pulmonary disease in the US adult population. Environ Sci Pollut Res 29, 54507–54517 (2022). https://doi.org/10.1007/s11356-022-19695-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19695-w