Abstract
Purpose
Metal and chemical exposure can cause acute and chronic respiratory diseases in humans. The purpose of this analysis was to analyze 14 types of urinary metals including mercury, uranium, tin, lead, antimony, barium, cadmium, cobalt, cesium, molybdenum, manganese, strontium, thallium, tungsten, six types of speciated arsenic, total arsenic and seven forms of polycyclic aromatic hydrocarbons (PAHs), and the link with self-reported emphysema in the US adult population.
Methods
A cross-sectional analysis using the 2011–2012, 2013–2014 and 2015–2016 National Health and Nutrition Examination Survey datasets was conducted. A specialized weighted complex survey design analysis package was used in analyzing the data. Multivariate logistic regression models were used to assess the association between urinary metals, arsenic, and PAHs and self-reported emphysema among all participants and among non-smokers only. Models were adjusted for lifestyle and demographic factors.
Results
A total of 4,181 adults were analyzed. 1-Hydroxynaphthalene, 2-hydroxynaphthalene, 3-hydroxyfluorene, 2-hydroxyfluorene, 1-hydroxypyrene, and 2 & 3-hydroxyphenanthrene were positively associated with self-reported emphysema. Positive associations were also observed in cadmium and cesium with self-reported emphysema. Among non-smokers, quantiles among 2-hydroxynaphthalene, arsenocholine, total urinary arsenic, cesium, and tin were associated with increased odds of self-reported emphysema. Quantiles among 1-hydroxyphenanthrene, cadmium, manganese, lead, antimony, thallium, and tungsten were associated with an inverse relationship with self-reported emphysema in non-smokers.
Conclusion
The study determined that six types of urinary PAHs, cadmium, and cesium are positively associated with self-reported emphysema. Certain quantiles of 2-hydroxynaphthalene, arsenocholine, total urinary arsenic, cesium, and tin are positively associated with self-reported emphysema among non-smokers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Emphysema is an airflow limitation due to parenchymal destruction in which there is destruction and enlargement of the gas-exchanging surfaces of alveoli distal to the terminal bronchiole [1]. Emphysema is a subtype of chronic obstructive pulmonary disease (COPD), which is characterized by persistent respiratory symptoms due to abnormalities in the airway and alveoli often due to exposure to noxious particles or gases [2,3,4,5]. The most common cause of emphysema is cigarette smoking.
Polycyclic aromatic hydrocarbons (PAHs) are chemicals that contain fused aromatic rings and are organic in nature, containing hydrogen and carbon molecules [6]. The principal source of PAHs is the partial combustion of organic materials, in addition to exhaust from vehicles, smoking tobacco, agricultural burning, occupational sources, smoked and grilled food, and coal tar in the United States (US) [7]. Correlational studies in humans have found associations with PAH exposure and multiple chronic diseases in humans including emphysema, impaired respiratory function, lung cancer, and ischemic heart disease [8,9,10,11,12].
Exposure to cadmium can occur through food and through accumulation in tobacco plants, resulting in tobacco smoking being an important route of exposure in the human population. Cadmium accumulates inside the lungs, which chronically can lead to smoking-related lung diseases including emphysema and chronic bronchitis [13]. Exposure to cadmium in occupational settings has been associated with impaired lung function and emphysema [14, 15]. Furthermore, arsenic is a naturally occurring element and metalloid found in earth’s crust. It can contaminate groundwater sources, creating a public health threat [16]. Low–moderate arsenic exposure has been linked with lower lung function and emphysema [17]. Cesium exposure has been attributed to drinking water and prior nuclear accidents and has been associated with several health conditions [18,19,20,21]. There is minimal literature regarding cesium exposure, particularly in relation to its effect on lung function [19, 22].
The purpose of this study is to analyze the association between urinary PAHs, arsenic (speciated and total), and metal exposure and self-reported emphysema in the US adult population among all participants and non-smokers. The study uses the National Health and Nutrition Examination Survey (NHANES) dataset including six forms of urinary PAHs, seven forms of urinary speciated arsenic, and 14 urinary metals to determine the association with self-reported emphysema.
Methods
The 2011–2012, 2013–2014, and 2015–2016 NHANES datasets were used. NHANES is a national study evaluating the health and nutritional status of children and adults in the US. Demographic, laboratory, examination, and questionnaire data were collected as part of the datasets [23].
For emphysema data, the variable “MCQ160G” in medical conditions (MCQ_G), (MCQ_H), and (MCQ_I) datasets was used [24,25,26]. Patients were asked on a questionnaire, “Ever told you had emphysema?” [31]. For urinary metals data, the metals—urine (UHM_G), (UM_H), and (UM_I) datasets including urinary barium, cadmium, cesium, cobalt, manganese, molybdenum, lead, antimony, strontium, thallium, tin, tungsten, and uranium were used [27,28,29] in addition to mercury data: inorganic, urine (UHG_G), mercury—urine (UHG_H), and (UHG_I) datasets [30,31,32]. Both urinary and blood cadmium are used as biomarkers to assess exposure and body burden of cadmium. Urinary cadmium is considered to reflect the kidney burden of cadmium, while blood cadmium is often considered the best to reveal recent cadmium exposure [33,34,35,36]. Urinary total and speciated arsenic datasets including total arsenic, arsenous acid, arsenic acid, arsenobetaine, arsenocholine, dimethylarsinic acid (DMA), and monomethylarsonic acid (MMA) including arsenics—total and speciated—urine (UAS_G), arsenic—total—urine (UTAS_H), arsenics—speciated—urine (UAS_H), arsenic—total—urine (UTAS_I) and speciated arsenics—urine (UAS_I) were used [37,38,39,40,41]. For PAH data including 1-hydroxynapthalene, 2-hydroxynapthalene, 3-hydroxyfluorene, 2-hydroxyfluorene, 1-hydroxyphenanthrene, 1-hydroxypyrene, 2-hydroxyphenanthrene & 3-hydroxyphenanthrene, Polyaromatic Hydrocarbons (PAHs)—Urine (PAH_G), (PAH_H), and (PAH_G) datasets were used [42,43,44]. The NHANES datasets included utilized urinary samples, which were consistent throughout all PAHs, arsenic, and metals. Therefore, to accurately compare the association with emphysema, we used urinary rather than blood samples [45, 46]. Adults ≥ 20 years were included in the study.
The following demographics and data files were used as covariates: gender (male, female), race/ethnicity (Mexican American, other Hispanic, Non-Hispanic White, Non-Hispanic Black, Non-Hispanic Asian, other race multi-racial), marital status (married, widowed, divorced, separated, never married, living with partner), highest level of education achieved (no high school, some high school, high school graduate, some college, college graduate), age (20–44, 45–59, 60 and older), family income to poverty ratio (FIPR) (< 1.5, 1.5–3.5, > 3.5), BMI (normal weight, underweight, overweight, obese) (underweight: BMI < 18.5, normal weight: 18.5 ≤ BMI ≤ 24.9, overweight: 25.0 ≤ BMI ≤ 29.9, and obese: 30.0 ≤ BMI), serum cotinine (below lower limit of detection (LLoD), above LLoD), and alcoholic drink in last 12 months (no, yes), and country of birth (USA, other) [2, 21, 47,48,49,50,51,52,53,54,55]. Smoking was controlled for through the variable cotinine. Cotinine is used as a determinant of smoking status as it is the main metabolite of nicotine biotransformation [56, 57].
For the non-smoker analysis, the data were censored by eliminating all participants who had smoked more than 100 cigarettes in their lifetime using the NHANES variable "SMQ020—smoked at least 100 cigarettes in life" in the datasets smoking—cigarette use (SMQ_G), (SMQ_H), and (SMQ_I) [64,65,66]. Once the data had been reduced to include only non-smokers, the urinary concentrations of PAHs, arsenic, and metal species were normalized by the urinary creatine concentration. The data were then categorized into quantiles by each urinary species. The first quantile included those with concentrations of the urinary species that were below the LLOD. Each quantile typically had between 630 and 660 data points depending on which species was being modeled. Demographic data for non-smokers was analyzed but not presented.
R version 3.6.3 was used for the statistical analysis. Data was cleaned and missing responses removed. Concentrations of PAHS, arsenic, and metals were normalized with creatinine concentrations [58] and a binary categorical variable was created for self-reported emphysema. Data was used as directly reported by NHANES, concentrations were then normalized and log10 transformed. Programs from the survey package and svyby, svymean, svyttest, svydesign, and svyglm functions were used for data analysis and to calculate pairwise t-tests and logit regression models [59,60,61,62]. The function nhanes_load_data in the package RNHANES was used in downloading and processing data [63].
Results
Among three NHANES datasets, 2,026 participants were included in reduced sample. Several significant demographic findings were determined among those who identified as being diagnosed with emphysema in the past (Tables 1 and 2). Marital status of separated, ages 45–59 and 60 years and older, and serum cotinine above the LLOD were seen to have an increased odds of self-reported emphysema, as seen in Table 2. Race of Non-Hispanic Black, some college, FIPR over 3.5, underweight BMI, and country of birth outside USA were found to have an inverse relationship with self-reported emphysema.
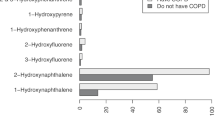
Figures 1, 2, and 3 display the log10 and median normalized concentration distributions of urinary metals, arsenic, and PAHs in the sample population. For all the species studied, the concentration distributions were non-normal even in the transformed space with high concentration outliers. For some of the species, e.g., 2-hydroxyfluorene, the distributions were bimodal.
All PAHs analyzed (Table 3), 1-hydroxynaphthalene, 2-hydroxynaphthalene, 3-hydroxyfluorene, 2-hydroxyfluorene, 1-hydroxypyrene, and 2 & 3-hydroxyphenanthrene, with the exception of 1-hydroxyphenanthrene, were found to have an increased odds of self-reported emphysema. Therefore, there was an increased odds of self-reported emphysema given the exposure to 1-hydroxynaphthalene, 2-hydroxynaphthalene, 3-hydroxyfluorene, 2-hydroxyfluorene, 1-hydroxypyrene, and 2 & 3-hydroxyphenanthrene, compared to the odds of self-reported emphysema occurring in the absence of these PAH exposures. Among urinary arsenic species (Table 4), urinary arsenobetaine and total urinary arsenic had significant inverse associations with self-reported emphysema. Urinary arsenobetaine and total urinary arsenic exposure revealed reduced odds of self-reported emphysema compared with no exposure to these species. Among urinary metals (Table 5), cadmium and cesium were both found to have an increased odds of self-reported emphysema. There was increased odds of self-reported emphysema with exposure to cadmium and cesium compared to the odds of self-reported emphysema with no exposure. No other urinary arsenic or metal species were found to have significant associations with self-reported emphysema.
Of the PAH compounds studied in non-smokers (Table 6), only the 2nd and 4th quartiles of 2-hydroxynaphthalene had odds ratios that were statistically significant (OR 20.73, 95% CI 1.59–269.73 and OR 42.42, 95% CI 1.25–1435.96 respectively). On the other hand, only the 3rd quartile of 1-hydroxyphenanthrene had odds ratios that were statistically less than one.
Of the urinary arsenic compounds studied (Table 7), both arsenocholine and total urinary arsenic had odds ratios that were significantly different than one. In both cases, the odds ratios were very large. This is most likely due to the few respondents with self-reported emphysema and a low measured concentration of the arsenic species, i.e., the data for the 1st quartile (see Fig. 2) were entirely due to those who did not report having emphysema.
Of the 14 urinary metal compounds studied (Table 8), only cesium (all three quartiles) and the 3rd and 4th quartiles of tin had odds ratios that were statistically significantly greater than one. Conversely, cadmium, manganese, antimony, thallium, and tungsten all had odds ratios for at least one quartile that was significantly smaller than one.
Discussion
This study found an association among PAHs and self-reported emphysema, with six of seven PAHs studied having a significant positive association (P < 0.05) including 1-hydroxynaphthalene, 2-hydroxynaphthalene, 3-hydroxyfluorene, 2-hydroxyfluorene, 1-hydroxypyrene, and 2 & 3-hydroxyphenanthrene. Shiue [8] analyzed the association among PAHs and emphysema using NHANES 2011–2012 data. Significant positive associations between emphysema and 2-hydroxyfluorene and 3-hydroxyfluorene were found; concordant findings were found among 2-hydroxyfluorene, 3-hydroxyfluorene, and 1-hydroxyphenanthrene. No association was found among the other forms of PAHs. Among those with self-reported emphysema who were non-smokers, there was increased odds among some quartiles with 2-hydroxynaphthalene, similar to the original group, and a protective factor for 1-hydroxyphenanthrene. The NHANES datasets, used in this study, did not include 9-hydroxyfluorene and 4-hydroxyphenanthrene which were included in the dataset in Shiue’s study [8]. This study did, however, find a positive association among 1-hydroxynaphthalene, 2-hydroxynaphthalene, 1-hydroxypyrene, and 2 & 3-hydroxyphenanthrene which were not significant in Shiue [8].
In mice studies, co-exposure to tobacco smoke and arsenite significantly induced emphysema-like lesions, characterized by enlarged alveolar spaces and destruction of alveolar structure [67]. Arsenic exposure in humans has been correlated with increased incidence of chronic bronchitis, chronic cough, shortness of breath and obstructive lung diseases [68,69,70,71]. Arsenobetaine and arsenocholine, organic forms of speciated arsenic, are the major forms of arsenic in most fish, which is non-toxic and not metabolized. The findings in this study are likely attributed to consumption of seafood rather than inorganic forms of arsenic, which are the main forms in drinking water [72]. In contrast, arsenocholine and total urinary arsenic had significant increased odds for emphysema in the non-smoker group. A previous study [73] utilizing 2003–2006 NHANES data found no association in low or high quintiles between organic arsenic and emphysema.
Cadmium has been linked to emphysema most commonly due to smoking [74]. Mannino et al. [75] determined a significant association between reduced forced expiratory volumes in 1 s (FEV1) and cadmium in current and former smokers, but not in never smokers. However, that study only evaluated COPD, rather than emphysema specifically. This study found a positive association among urinary cadmium and self-reported emphysema using NHANES 2011–2016 data. It is suggested that prolonged exposure to cadmium contributes to airway inflammation likely due to the induction of oxidative stress, cadherin activity, and impaired DNA repair and apoptosis [76,77,78,79,80,81,82]. In contrast to the overall group, the non-smoker group was found to have an inverse association with emphysema among multiple quartiles. This is likely due to alterative sources of cadmium as compared to smokers exposed to cadmium in cigarettes.
The association among cesium and emphysema has been relatively unreported in literature [19]. Almulla et al. [83] determined an association among cesium levels and immune biomarkers in immune inflammatory pathways. In studies from the 1986 Chernobyl disaster, radioactive Cesium 137 was linked to pediatric obstructive and restrictive lung function. This study found a statistically significant association with cesium among all participants (OR 9.045, 95% CI 2.083–39.283) and in the 2nd, 3rd, and 4th quantiles among non-smokers.
Specific to the non-smoker group, some quantiles of tin were associated with increased odds of self-reported emphysema, while some quantiles of manganese, lead, antimony, thallium, and tungsten were associated with an inverse relationship with self-reported emphysema. Heavy metals have commonly been linked to lung diseases such as lung cancer through smoking. The protective factor seen with several metals in non-smokers suggests that the smoking itself, rather than the metal may be causing the emphysema in these patients [84].
Among demographic data, participants with self-reported emphysema were more likely to be separated, ages 45–60 or 60 years and older, and have a serum cotinine above the LLOD. Buendia-Roldan et al. [85] determined that those with subclinical pulmonary emphysema were older, smoker males, and had a low BMI. Those with emphysema also had a higher mean age. There was no association with male gender, and underweight BMI (< 18.5) was determined to be a protective factor. Buendia-Roldan et al. [85] found that the average BMI of the emphysema group was 24, which is considered normal, and 27 in the control group, which we classified as overweight. Among protective factors, Non-Hispanic Black, some college, FIPR > 3.5, and birth outside the US were protective factors. Non-Hispanic Black was also found to be a protective factor for self-reported emphysema. African Americans had a higher odds (P ≤ 0.0001) of not having a prior COPD diagnosis despite having airflow obstruction consistent with COPD compared to Non-Hispanic White participants. This suggests that African Americans may be at higher risk to getting forms COPD, such as emphysema, at a younger age and with fewer pack years, and are more likely to be undiagnosed [86].
Limitations
This study was conducted using three NHANES datasets. The data collection was conducted by the Centers for Disease Control and Prevention (CDC) and the methods and datapoints could not be changed. Therefore, the diagnosis of emphysema was self-reported, through a questionnaire asking participants if they had been diagnosed with emphysema, rather than through spirometry or more definitive diagnostic modalities. In addition, urinary PAH, arsenic, and metal concentrations were used to assess the association with self-reported emphysema as this was included in the NHANES datasets, rather than blood samples. No data on individual participants’ exposures to PAHs or urinary metals was included in the NHANES dataset. Therefore, this study was unable to link potential sources of exposure that could have resulted in increased concentrations of certain PAHs or metals. Furthermore, this is a cross-sectional study, and causality cannot be determined.
Conclusion
Six forms of urinary PAHs including 1-hydroxynaphthalene, 2-hydroxynaphthalene, 3-hydroxyfluorene, 2-hydroxyfluorene, 1-hydroxypyrene, and 2 & 3-hydroxyphenanthrene and two forms of urinary metals, cadmium and cesium, were linked to an increased odds of self-reported emphysema in the US adult population. Urinary arsenobetaine and total urinary arsenic were found to be inversely associated with self-reported emphysema. Among non-smokers, quantiles of 2-hydroxynaphthalene, arsenocholine, total urinary arsenic, cesium, and tin were associated with increased odds of self-reported emphysema. Quantiles of 1-hydroxyphenanthrene, cadmium, manganese, lead, antimony, thallium, and tungsten were associated with protective factors for self-reported emphysema in non-smokers. Further studies are needed to determine the causation for this link between urinary PAHs, arsenic, and metals and their contribution to lung dysfunction in emphysema.
Data Availability
The datasets analyzed during the current study are available in the NHANES repository provided by the CDC to the public.
References
Global Initiative for Chronic Obstructive Lung Disease (2020) Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2021 report. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf. Accessed 25 Nov 2021
Shapiro SD (2000) Animal models for chronic obstructive pulmonary diseases. Am J Respir Cell Mol Biol 22:4–7
GOLD (2018) Global strategy for the diagnosis, management and prevention of COPD, global initiative for chronic obstructive lung disease (GOLD) 2018. https://goldcopd.org/. Accessed 25 Nov 2021
Vogelmeier CF, Criner GJ, Martinez FJ et al (2017) Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med 195(5):557–582
Mirza S, Clay RD, Koslow MA, Scanlon PD (2018) COPD guidelines: a review of the 2018 GOLD report. Mayo Clin Proc 93(10):1488–1502. https://doi.org/10.1016/j.mayocp.2018.05.026
Kumar V, Kothiyal NC, Saruchi VP, Sharma R (2016) Sources, distribution, and health effect of carcinogenic polycyclic aromatic hydrocarbons (PAHs) —current knowledge and future directions. J Chin Adv Mater Soc 4(4):302–321. https://doi.org/10.1080/22243682.2016.1230475
Boström CE, Gerde P, Hanberg A et al (2002) Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect 110(Suppl 3):451–488. https://doi.org/10.1289/ehp.110-1241197
Shiue I (2016) Urinary polyaromatic hydrocarbons are associated with adult emphysema, chronic bronchitis, asthma, and infections: US NHANES, 2011–2012. Environ Sci Pollut Res Int 23(24):25494–25500. https://doi.org/10.1007/s11356-016-7867-7
Burstyn I, Kromhout H, Partanen T et al (2005) Polycyclic aromatic hydrocarbons and fatal ischemic heart disease. Epidemiology 16(6):744–750. https://doi.org/10.1097/01.ede.0000181310.65043.2f
Burstyn I, Boffetta P, Heederik D et al (2003) Mortality from obstructive lung diseases and exposure to polycyclic aromatic hydrocarbons among asphalt workers. Am J Epidemiol 158(5):468–478. https://doi.org/10.1093/aje/kwg180ae
Gammon MD, Sagiv SK, Eng SM et al (2004) Polycyclic aromatic hydrocarbon-DNA adducts and breast cancer: a pooled analysis. Arch Environ Health 59(12):640–649. https://doi.org/10.1080/00039890409602948
Zhang Y, Tao S, Shen H, Ma J (2009) Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proc Natl Acad Sci USA 106(50):21063–21067. https://doi.org/10.1073/pnas.0905756106
Ganguly K, Levänen B, Palmberg L, Åkesson A, Lindén A (2018) Cadmium in tobacco smokers: a neglected link to lung disease? Eur Respir Rev 27(147):170122. https://doi.org/10.1183/16000617.0122-2017
Davison AG, Fayers PM, Taylor AJ et al (1988) Cadmium fume inhalation and emphysema. Lancet 1(8587):663–667. https://doi.org/10.1016/s0140-6736(88)91474-2
Friberg L (1950) Health hazards in the manufacture of alkaline accumulators with special reference to chronic cadmium poisoning; a clinical and experimental study. Acta Med Scand Suppl 240:1–124
Chung JY, Yu SD, Hong YS (2014) Environmental source of arsenic exposure. J Prev Med Public Health 47(5):253–257. https://doi.org/10.3961/jpmph.14.036
Powers M, Sanchez TR, Grau-Perez M et al (2019) Low-moderate arsenic exposure and respiratory in American Indian communities in the strong heart study. Environ Health 18(1):104. https://doi.org/10.1186/s12940-019-0539-6
Concha G, Broberg K, Grandér M, Cardozo A, Palm B, Vahter M (2010) High-level exposure to lithium, boron, cesium, and arsenic via drinking water in the Andes of northern Argentina. Environ Sci Technol 44(17):6875–6880. https://doi.org/10.1021/es1010384
Svendsen ER, Kolpakov IE, Karmaus WJ et al (2015) Reduced lung function in children associated with cesium 137 body burden. Ann Am Thorac Soc 12(7):1050–1057. https://doi.org/10.1513/AnnalsATS.201409-432OC
Rahman HH, Niemann D, Munson-McGee SH (2022) Environmental exposure to metals and the risk of high blood pressure: a cross-sectional study from NHANES 2015–2016. Environ Sci Pollut Res Int 29(1):531–542. https://doi.org/10.1007/s11356-021-15726-0
Rahman HH, Niemann D, Munson-McGee SH (2021) Association of albumin to creatinine ratio with urinary arsenic and metal exposure: evidence from NHANES 2015–2016 [published online ahed of print, 2021 Oct 13]. Int Urol Nephrol. https://doi.org/10.1007/s11255-021-03018-y
Melnikov P, Zanoni LZ (2010) Clinical effects of cesium intake. Biol Trace Elem Res 135(1–3):1–9. https://doi.org/10.1007/s12011-009-8486-7
National Center for Health Statistics (NCHS) (2017) About the national health and nutrition examination jsurvey. CDC. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed 25 Nov 2021
CDC (2013) Medical conditions (MCQ_G). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/MCQ_G.htm. Accessed 25 Nov 2021
CDC (2015) Medical conditions (MCQ_H). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/MCQ_H.htm. Accessed 25 Nov 2021
CDC (2017) Medical conditions (MCQ_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/MCQ_I.htm. Accessed 25 Nov 2021
CDC (2013) Metals—urine (UHM_G). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/UHM_G.htm. Accessed 25 Nov 2021
CDC (2016) Metals—urine (UM_H). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/UM_H.htm. Accessed 25 Nov 2021
CDC (2018) Metals—Urine (UM_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/UM_I.htm. Accessed 25 Nov 2021
CDC (2013) Mercury—inorganic, Urine (UHG_G). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/UHG_G.htm. Accessed 25 Nov 2021
CDC (2016) Mercury—urine (UHG_H). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/UHG_H.htm. Accessed 25 Nov 2021
CDC (2018) Mercury—urine (UHG_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/UHG_I.htm. Accessed 25 Nov 2021
Akerstrom M, Barregard L, Lundh T, Sallsten G (2013) The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol Appl Pharmacol 268(3):286–293. https://doi.org/10.1016/j.taap.2013.02.009
Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M (1998) Health effects of cadmium exposure—a review of the literature and a risk estimate. Scand J Work Environ Health 24(Suppl 1):1–51
European Food Safety Authority [EFSA] (2009) Cadmium in food1Scientific opinion of the panel on contaminants in the food chain. EFSA J 980:1–139. https://doi.org/10.2903/j.efsa.2009.980
Nordberg G, Fowler BA, Nordberg M (2014) Handbook on the toxicology of metals. Academic Press, Cambridge
CDC (2013) Arsenics—total & speciated—urine (UAS_G). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/UAS_G.htm. Accessed 25 Nov 2021
CDC (2016) Arsenic—total—urine (UTAS_H). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/UTAS_H.htm. Accessed 25 Nov 2021
CDC (2016) Arsenics—speciated—urine (UAS_H). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/UAS_H.htm. Accessed 25 Nov 2021
CDC (2018) Arsenic—total—urine (UTAS_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/UTAS_I.htm. Accessed 25 Nov 2021
CDC (2018) Speciated Arsenics - Urine (UAS_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/UAS_I.htm. Accessed 25 Nov 2021
CDC (2014) Polyaromatic hydrocarbons (PAHs)—urine (PAH_G). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/PAH_G.htm. Accessed 25 Nov 2021
CDC (2016) Polycyclic aromatic hydrocarbons (PAH)—urine (PAH_H). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/PAH_H.htm. Accessed 25 Nov 2021
CDC (2020) Polycyclic aromatic hydrocarbons (PAH)—urine (PAH_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/PAH_I.htm. Accessed 25 Nov 2021
Shiue I (2015) Urinary heavy metals, phthalates and polyaromatic hydrocarbons independent of health events are associated with adult depression: USA NHANES, 2011–2012. Environ Sci Pollut Res Int 22(21):17095–17103. https://doi.org/10.1007/s11356-015-4944-2
Rahman HH, Niemann D, Munson-McGee SH (2022) Association among urinary polycyclic aromatic hydrocarbons and depression: a cross-sectional study from NHANES 2015–2016. Environ Sci Pollut Res Int 29(9):13089–13097. https://doi.org/10.1007/s11356-021-16692-3
CDC (2017) Body measures (BMX_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/BMX_I.htm. Accessed 25 Nov 2021
CDC (2017) Demographic Variables and Sample Weights (DEMO_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/DEMO_I.htm. Accessed 25 November 2021
CDC (2018) Alcohol use (ALQ_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/ALQ_I.htm. Accessed 25 Nov 2021
CDC (2019) Cotinine and hydroxycotinine—serum (COT_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/COT_I.htm. Accessed 25 Nov 2021
CDC (2020) About adult BMI. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html#trends. Accessed 25 Nov 2021
Rahman HH, Niemann D, Singh D (2020) Arsenic exposure and association with hepatitis E IgG antibodies. Occup Dis Environ Med 8:111–122. https://doi.org/10.4236/odem.2020.83009
Rahman HH, Yusuf KK, Niemann D, Dipon SR (2020) Urinary speciated arsenic and depression among US adults. Environ Sci Pollut Res Int 27(18):23048–23053. https://doi.org/10.1007/s11356-020-08858-2
Rahman HH, Niemann D, Yusuf KK (2022) Association of urinary arsenic and sleep disorder in the US population: NHANES 2015–2016. Environ Sci Pollut Res Int 29(4):5496–5504. https://doi.org/10.1007/s11356-021-16085-6
Rahman HH, Niemann D, Munson-McGee SH (2021) Association of environmental toxic metals with high sensitivity C-reactive protein: a cross-sectional study. Occup Dis Environ Med 9(4):173–184. https://doi.org/10.4236/odem.2021.94013
Tutka P, Mosiewicz J, Wielosz M (2005) Pharmacokinetics and metabolism of nicotine. Pharmacol Rep 57(2):143–153
Fernandes AGO, Santos LN, Pinheiro GP et al (2020) Urinary cotinine as a biomarker of cigarette smoke exposure: a method to differentiate among active, second-hand, and non-smoker circumstances. Open Biomark J 10:60–68. https://doi.org/10.2174/1875318302010010060
CDC (2017) Albumin & creatinine—urine (ALB_CR_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/ALB_CR_I.htm. Accessed 25 Nov 2021
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Lumley T (2004) Analysis of complex survey samples. J Stat Soft. https://doi.org/10.18637/jss.v009.i08
Lumley TS (2010) Complex surveys: a guide to analysis using R. Wiley, Hoboken
Lumley T (2020) Package ‘survey’: analysis of complex survey samples, version 4.0. https://cran.r-project.org/web/packages/survey/survey.pdf. Accessed 25 Nov 2021
Susmann H (2016) Package ‘RNHANES’: “facilitates analysis of CDC NHANES,” version 1.1.0. Accessed from https://cran.r-project.org/web/packages/RNHANES/RNHANES.pdf
CDC (2015) Smoking—cigarette use (SMQ_G). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/SMQ_G.htm. Accessed 5 Feb 2022
CDC (2016) Smoking—cigarette use (SMQ_H). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/SMQ_H.htm. Accessed 5 Feb 2022
CDC (2017) Smoking—cigarette use (SMQ_I). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/SMQ_I.htm. Accessed 5 Feb 2022
Wang CK, Lee HL, Chang H, Tsai MH, Kuo YC, Lin P (2012) Enhancement between environmental tobacco smoke and arsenic on emphysema-like lesions in mice. J Hazard Mater 221–222:256–263. https://doi.org/10.1016/j.jhazmat.2012.04.042
Mazumder DN, Haque R, Ghosh N et al (2000) Arsenic in drinking water and the prevalence of respiratory effects in West Bengal. India Int J Epidemiol 29(6):1047–1052. https://doi.org/10.1093/ije/29.6.1047
von Ehrenstein OS, Mazumder DN, Yuan Y et al (2005) Decrements in lung function related to arsenic in drinking water in West Bengal. India Am J Epidemiol 162(6):533–541. https://doi.org/10.1093/aje/kwi236
Parvez F, Chen Y, Brandt-Rauf PW et al (2008) Nonmalignant respiratory effects of chronic arsenic exposure from drinking water among never-smokers in Bangladesh. Environ Health Perspect 116(2):190–195. https://doi.org/10.1289/ehp.9507
Guha Mazumder DN (2007) Arsenic and non-malignant lung disease. J Environ Sci Health A 42(12):1859–1867. https://doi.org/10.1080/10934520701566926
Taylor V, Goodale B, Raab A et al (2017) Human exposure to organic arsenic species from seafood. Sci Total Environ 580:266–282. https://doi.org/10.1016/j.scitotenv.2016.12.113
Amster ED, Cho JI, Christiani D (2011) Urine arsenic concentration and obstructive pulmonary disease in the U.S. population. J Toxicol Environ Health A 74(11):716–727. https://doi.org/10.1080/15287394.2011.556060
Torén K, Olin AC, Johnsson Å et al (2019) The association between cadmium exposure and chronic airflow limitation and emphysema: the Swedish CArdioPulmonary BioImage Study (SCAPIS pilot). Eur Respir J. https://doi.org/10.1183/13993003.00960-2019
Mannino DM, Holguin F, Greves HM, Savage-Brown A, Stock AL, Jones RL (2004) Urinary cadmium levels predict lower lung function in current and former smokers: data from the third national health and nutrition examination survey. Thorax 59(3):194–198. https://doi.org/10.1136/thorax.2003.012054
Rokadia HK, Agarwal S (2013) Serum heavy metals and obstructive lung disease: results from the national health and nutrition examination survey. Chest 143(2):388–397. https://doi.org/10.1378/chest.12-0595
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160(1):1–40
Forti E, Bulgheroni A, Cetin Y et al (2010) Characterisation of cadmium chloride induced molecular and functional alterations in airway epithelial cells. Cell Physiol Biochem 25(1):159–168. https://doi.org/10.1159/000272060
Gong Q, Hart BA (1997) Effect of thiols on cadmium-induced expression of metallothionein and other oxidant stress genes in rat lung epithelial cells. Toxicology 119(3):179–191. https://doi.org/10.1016/s0300-483x(96)03608-
Koizumi S, Gong P, Suzuki K, Murata M (2007) Cadmium-responsive element of the human heme oxygenase-1 gene mediates heat shock factor 1-dependent transcriptional activation. J Biol Chem 282(12):8715–8723. https://doi.org/10.1074/jbc.M609427200
Croute F, Beau B, Arrabit C et al (2000) Pattern of stress protein expression in human lung cell-line A549 after short- or long-term exposure to cadmium. Environ Health Perspect 108(1):55–60. https://doi.org/10.1289/ehp.0010855
Souza V, Carmen ME, Gómez-Quiroz L et al (2004) Acute cadmium exposure enhances AP-1 DNA binding and induces cytokines expression and heat shock protein 70 in HepG2 cells. Toxicology 197(3):213–228. https://doi.org/10.1016/j.tox.2004.01.006
Almulla AF, Moustafa SR, Al-Dujaili AH, Al-Hakeim HK, Maes M (2021) Lowered serum cesium levels in schizophrenia: association with immune-inflammatory biomarkers and cognitive impairments. Braz J Psychiatry 43(2):131–137. https://doi.org/10.1590/1516-4446-2020-0908
Vanidassane I, Malik P, Gupta P et al (2021) P54.04 A study to determine the association of trace eelments and heavy metals with lung cancer and their correlation with smoking. J Thorac Oncol 16(3):S531. https://doi.org/10.1016/j.jtho.2021.01.940
Buendia-Roldan I, Palma-Lopez A, Chan-Padilla D et al (2020) Risk factors associated with the detection of pulmonary emphysema in older asymptomatic respiratory subjects. BMC Pulm Med 20(1):164. https://doi.org/10.1186/s12890-020-01204-9
Mamary AJ, Stewart JI, Kinney GL et al (2018) Race and gender disparities are evident in COPD underdiagnoses across all severities of measured airflow obstruction. Chronic Obstr Pulm Dis 5(3):177–184. https://doi.org/10.15326/jcopdf.5.3.2017.0145
Acknowledgements
None.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
HHR conceptualized the study and contributed to the introduction, discussion and drafting of the paper. SMM conducted the data analysis, methods and contributed to the drafting of the paper. DN contributed to the introduction, discussion and drafting of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Not applicable. This study uses only secondary data analyses without any personal information identified using statistical data from the NHANES website, no further ethical approval for conducting the present study is required.
Consent to Participate
Consent was given by all the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahman, H.H., Niemann, D. & Munson-McGee, S.H. Urinary Metals, Arsenic, and Polycyclic Aromatic Hydrocarbon Exposure and Risk of Self-reported Emphysema in the US Adult Population. Lung 200, 237–249 (2022). https://doi.org/10.1007/s00408-022-00518-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-022-00518-1