Abstract
Purpose
Urinary metals can be used to identify metal exposure in humans from various sources in the environment. Decreased renal function and cardiovascular dysfunction may occur due to low levels of metal exposure in the general population. The purpose of this study is to assess the association between urinary arsenic and metals and a higher albumin to creatinine ratio (ACR) among adults in the general US population.
Methods
We conducted a cross sectional analyses using the 2015–2016 National Health and Nutrition Examination Survey (NHANES) dataset. Multiple linear logistic models were used to examine the association between 21 urinary arsenic and metal concentrations (arsenous acid, arsenic acid, arsenobetaine, arsenocholine, dimethylarsinic acid, monomethylarsonic acid, total arsenic, mercury, barium, cadmium, cobalt, cesium, molybdenum, manganese, lead, antinomy, tin, strontium, thallium, tungsten, uranium) and increased ACR (≥ 30 mg/g).
Results
The sample included 4122 adults, of whom approximately 9.4% of males and 10.7% females had increased ACRs. The exposure included urinary arsenic compounds (7) and urinary metal compounds (14) at or above the limit of detection. Urinary dimethylarsinic acid [OR 38.9, 95% CI 3.6–414.6], urinary monomethylarsonic acid [OR 18.6, 95% CI 1.1–308.2], urinary cadmium [OR 11.9, 95% CI 1.2–122.0], urinary cesium [OR 17.0, 95% CI 2.7–105.8], and urinary antimony [OR 10.7, 95% CI 2.2–51.3] were associated with an increased ACR. No other urinary metals were significantly associated with increased ACR.
Conclusion
Increased ACR was positively associated with urinary dimethylarsinic acid, monomethylarsonic acid, cadmium, cesium, and antimony.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metals are widely used and extensively disseminated in the environment. The toxicity of metals in human health is a clinical and public health concern. Toxic reactions can occur when excess amounts of metal are present in the human body resulting in pathology to multiple organs [1, 2]. Arsenic is found naturally in the soil and is considered toxic, the most common exposure occurs through food and water consumption [3]. Arsenic is a group 1 human carcinogen that has been associated with kidney, liver, skin, lung, and bladder cancers and is the second leading water borne cause of mortality [4]. Exposure to low levels of arsenic, cadmium, lead, and mercury may increase risks of cancer, kidney disease, cardiovascular and skeletal disease [1, 5,6,7,8,9]. Environmental exposures to low-level lead, mercury, and cadmium are widespread, particularly in industrialized countries. These metals can be present in cigarettes, gasoline, air, and foods, including seafood and foods grown in contaminated soil [10]. Arsenic exposure occurs in people that rely on well water and foods such as apple juice and rice. Workers are at risk with co-exposures of arsenic and wood dust, silica, and asbestos [11].
The kidney is a target organ for metals, which can accumulate in the nephron leading to changes in morphology and function, thought to be due to oxidative stress. The proximal tubule is known as a main site of metal accumulation and injury in addition to the distal nephron, glomeruli, and vessels [12]. The 2012 Kidney Disease Improving Global Outcome (KDIGO) guidelines define chronic kidney disease (CKD) as one or more markers of kidney damage or a decreased glomerular filtration rate (GFR) (< 60 ml/min/1.73 m2) for greater than 3 months. Markers of kidney damage include: albuminuria (albumin excretion rate (AER) ≥ 30 mg/24 h or ACR ≥ 30 mg/g), urine sediment abnormalities, electrolyte and other abnormalities due to tubular disorders, abnormalities detected by histology, structural abnormalities detected by imaging, and history of kidney transplantation [13]. The albumin to creatinine ratio (ACR) is beneficial in that it can be measured using a spot urine sample to detect abnormal albumin without waiting 24 h. Calculating the ratio improves sensitivity and is not affected by dilute or concentrated urine, correcting for variations in hydration [14, 15]. The National Health and Nutrition Examination Survey (NHANES) dataset provides ACR data on participants throughout the United States (US) using spot urine samples but does not track participants over the period of 3 months to establish a diagnosis of CKD [16]. GFR categorizes CKD into five stages, however, there can be error due to calibration of GFR measurements, selection of the GFR formula, and variations in serum creatinine [17].
The kidney is the major organ of excretion of metals, including arsenic. Several studies have investigated the adverse effects of arsenic toxicity on renal function. Since albuminuria is an early marker for glomerular damage and can lead to change in the GFR, several studies observed albuminuria as the outcome of interest for kidney function [18]. Increased albuminuria can identify the need for an immediate therapeutic intervention [19].
The cause of metal exposure in the general population and the association of nephrotoxicity is unknown [20]. Some studies found links between increased blood lead and cadmium, and increased urinary lead and chromium, with decreased GFR [21,22,23,24,25]. In addition, cesium is eliminated through the kidneys and studies have shown higher concentrations in the kidneys [26]. However, research is lacking studying the effect of cesium on kidney disease.
The prevalence of CKD is estimated to be 8–16% worldwide, with diabetes being the most common cause. The number of end stage renal disease (ESRD) cases has been increasing disproportionally in developing countries; increasing hypertension and diabetes will further enhance the prevalence of ESRD. CKD more commonly leads to death than ESRD, likely due to accelerated rates of atherosclerosis and heart failure. Therefore, those with CKD are in the highest risk group for cardiovascular disease. Furthermore, CKD imposes a substantial economic burden on patients. 2–3% of healthcare expenditures in developed countries go towards providing treatment for patients with ESRD. In 2010, costs for ESRD accounted for 6.3% of the Medicare budget. In 2007, US Medicare expenditures exceeded $60 billion for CKD [27].
The goal of the present study is to investigate the relationship between arsenic and metal exposure including: arsenous acid, arsenic acid, arsenobetaine, arsenocholine, dimethylarsinic acid (DMA), monomethylarsonic acid (MMA), total arsenic, mercury, barium, cadmium, cobalt, cesium, molybdenum, manganese, lead, antinomy, tin, strontium, thallium, tungsten, and uranium, as measured in urine, with the presence of albuminuria in US population using ACR.
Methods
Data sources
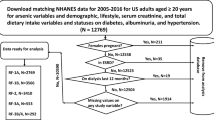
The data for this project was taken from the 2015–2016 NHANES data set, a long-standing study conducted by the National Center for Health Statistics (NCHS), a part of the Centers for Disease Control and Prevention (CDC). It combines interviews and physical examinations of children and adults throughout the US to determine the health and nutritional status of the US [28].
The ACR used to create the response variable came from the NHANES data file ALB_CR_I [29]. The NHANES data files for urinary arsenic and urinary metals, used as the variate data, were: UAS_I—Speciated urinary arsenic, UTAS_I—Total Urinary arsenic, UMS_I—Urinary metals, and UHG_I—Urinary mercury [16, 30,31,32]. The data for the covariates came from the following NHANES data files: ALQ_I—Alcohol consumption, BMX_I—Body mass index, DEMO_I—Demographic data, BPQ_I—Blood pressure questionnaire [33,34,35,36]. NHANES was approved by the Research Ethics Review Board of the National Center for Health Statistics. As this is a public-use dataset, this study was exempt from additional review by an institutional review board.
Data cleaning
The data cleaning consisted of four primary steps: (1) removing all missing responses and those for pregnant women, (2) categorizing the continuous covariate variables, (3) normalizing the concentration of the urinary chemical speicies by the creatinine concentration, and (4) creating the binary categorical variable for albuminuria. Missing responses in the demographic data, BMI, serum continine, and alcohol consumption were removed and the reduced data set was used for all subsequent modeling. If any one of these variables was missing, the entire response was eliminated. Missing responses for the urinary arsenic and urinary metals were removed from the complete data set just prior to creation of the logit regression model.
The continuous variables age, family income to poverty ratio (FIPR), and body mass index (BMI) were converted into categorical factors. Age was converted into a three-level categorical variable with the levels \(20\le age1\le 40\), \(41\le age2\le 65\) and \(65<age3\) [3]. FIPR was converted into a three-level categorical variable: \(0\le FIPR1\le 130\%\), \(130\%\le FIPR2\le 350\%\) and \(350\%<FIPR3\). BMI was converted into a four-level categorical variable with \(underweight: BMI<18.5\), \(normal weight: 18.5\le BMI<24.9\), \(overweight: 24.9\le BMI<30.0\), and \(obese: 30.0\le BMI\) per the CDC definitions [37].
Natural variation in urine dilution, due to variations in the urine flow rate (UFR), was addressed by taking the urinary arsenic and metals species and dividing them by the creatinine concentration [38]. No attempt was made to make the concentration units consistent, the values provided in the NHANES data were used directly. Once the concentration had been normalized, they were then log10 transformed to create a more nearly normal distribution of the concentrations.
CKD is defined as a urine ACR ≥ 30 mg/g over 3 months, defined in the most recent KDIGO 2012 guidelines [23]. Urinary albumin was measured in spot urine samples by solid-phase fluorescence immunoassay. The lower limit of detection for the assay was 0.3 μg/mL. Urinary creatinine was measured by the modified kinetic Jaffé method. The categorical binary variable for albuminuria was ACR ≥ 30 mg/g [39]. Only single albumin and creatinine levels were available in the NHANES dataset,therefore variation during the day and among different days was not assessed.
Dose–response relationships for the various urinary species and albuminuria were also investigated. For each normalized urinary species, a 5-level categorical variable was created. The first level included those below the lower limit of detection (LLoD). The remaining four levels were separated by the quantiles of the responses that were above the LLoD. One at a time these new categorical variables were used in multivariate linear logit regression models and the regression coefficients converted into odds ratios. The logit regression included the main effects of the same covariates as used above. For the concentration of arsenic and metal, the quantiles of urinary species normalized concentrations were calculated for the unweighted sample.
Statistical analyses
The statistical analysis was done in R version 3.6.3 using programs from the survey package to account for the complex design used in the construction of the NHANES survey [40,41,42,43]. The workhorse package used in this study was the survey package which was also used in illustrations of NHANES data analysis provided by the CDC [44]. Specifically, from the survey package the functions svyby, svymean, svyttest, svydesign, and svyglm were used to calculate the unweighted occurrences of responses, the weighted mean of the responses, the weighted pairwise t-tests of the responses, the survey design object, and the logit regression models, respectively. Additional R functions and packages used to simplify the programing included: the function nhanes_load_data in the package RNHANES was used to download the data files from the NHANES website and store them locally as comma separated values (*.csv) files [45, 46].
Using functions from the survey package, all analyses were weighted using the NHANES data on the survey design to account for the effects of design stratification and clustering. The descriptive statistics were calculated to determine the distribution of the participants across the covariates gender, ethnicity, highest level of education attained, marital status, age, FIPR, BMI, urinary cotinine concentration (a marker for smoking), and alcohol consumption. Multivariate logistic regression, with the covariates as cofactors, was used to compute the odds ratio (ORs) and their 95% confidence intervals (CI) for the association between albuminuria and the presence of urinary metals or arsenic species. Separate models were constructed for each metal or arsenic species.
Results
Preliminary data review: covariate factors
Given in Table 1 is a summary of the covariates used in this analysis. From the data presented in Table 1, the factors gender, serum cotinine, and alcohol consumption do not appear to significantly affect the percentage of respondents with albuminuria. The remaining factors do appear to affect the percentage of respondents with albuminuria in the following manners: ethnicity—non-Hispanic blacks have a higher likelihood of albuminuria than do the other ethnic groups, education—generally, the more education one has, the lower the likelihood of having albuminuria, marital status—those who are divorced, widowed, or separated have a higher likelihood of having albuminuria, age—the older one is the higher the likelihood of having albuminuria, FIPR—the higher one’s income ratio the lower the likelihood of having albuminuria, and BMI—the higher one’s BMI the higher the likelihood of having albuminuria. Table 2 revealed that age categories 40–64 years and 65 and over were significant at the α = 0.05 level for an increased likelihood of albuminuria. In addition, a BMI of > 30.0, or obese, was also significant for an increased likelihood of having albuminuria.
Five urinary metals were found to have a positive association with albuminuria at the α = 0.05 level as seen in Table 3. Among speciated arsenic, DMA (OR 38.9, 95% CI 3.6–414.6) and MMA (OR 18.6, 95% CI 1.1–308.2) had the highest odds ratios among the significant metals. Among the other metal compounds, cadmium (OR 11.9, 95% CI 1.2–122.0), cesium (OR 17.0, 95% CI 2.7–105.8), and antimony (OR 10.7, 95% CI 2.2–51.3) had a positive association with increased ACR, or albuminuria.
Concentration profiles in the form of quantiles for the various urinary arsenic and metal species are given in Table 4. There were detectable levels of arsenic in about 3% to 100% of the people in the sample depending on the specific arsenic species being considered. Detectable levels of the metal species were higher, ranging from about 30% to 100% of the individuals in the sample. For all species, the distribution of levels detected in the individuals was highly non-normal with a significant tail at higher concentrations.
The results of the dose–response study are shown in Table 5. The results indicate that of the arsenic species studied, only DMA showed a significant dose response trend in that respondents whose concentration fell in the 4th quartile were over 3 times as likely to have albuminuria. For cobalt, cesium, and tin, although there was a statistically significant difference between the below and above LLoD responses, the quartiles’ odds ratios were not significantly different from each other. For barium, those in the 2nd, 3rd, and 4th quartiles had odds ratios that were significantly different from the below LLoD respondents, but the odds ratios associated with these quartiles were not different from each other and were less than one. For manganese, only the 2nd quartile was significantly different from the other below LLoD respondents. The cesium results had all the odds ratios essentially equal to zero, which was a statistical artifact from the fact that only one respondent had urinary cesium levels below the lower limit of detection and therefore this result should not be taken as a valid response.
Discussion
This study linked multiple metal exposures measured in urine with kidney impairment defined by albuminuria using ACR. The effect of multiple metal exposures on kidney function and other organs is complex and difficult to assess [47].
End stage renal disease (ESRD) is one of the most dramatic health disparities in the US with rates in minorities ranging 1.5 to 4.0 times that of white counterparts. This can occur due to several mechanisms including clinical appropriateness and need, discrimination, biases and prejudice, stereotyping, and patient beliefs and preferences [48]. We determined a higher likelihood of albuminuria in non-Hispanic blacks. Crews et al. [49] found that race was not associated with CKD, but that African Americans had a much greater odds of advanced CKD defined as an eGFR < 30 mL/min/1.73 m2. Furthermore, a low social economic status (SES) was associated with a 59% greater odds of CKD. Low SES was associated with CKD in African Americans, but not in whites [49]. Our results supported a lower SES in relation to albuminuria in that a higher FIPR was associated with a lower likelihood for albuminuria.
Johnson et al. [50] found that among participants with CKD, a majority of participants were high school graduates, unemployed, female, unmarried, and/or earned less than or equal to $10,000 per year. This analysis found no difference among gender, but determined that those who were divorced, widowed, or separated had a higher likelihood of having albuminuria. In addition, Kramer et al. [51] calculated that the odds ratio of CKD in obese participants compared to those with an ideal BMI was 1.32. This study supported these findings in that a higher BMI resulted in a higher likelihood of having albuminuria. Nicotine exposure can cause exposures to lead, cadmium, arsenic and other nephrotoxic contaminants found in tobacco products. Cotinine is a biomarker for nicotine exposure. There was no significant association between albuminuria and cotinine.
Urinary arsenic
Arsenic is categorized into inorganic and organic forms; humans can be exposed to both types via food or drink, or in occupational settings. Arsenobetaine is an organic form of arsenic and is considered nontoxic or less toxic. The inorganic forms of arsenic acids are toxic and go through a complex biotransformation mechanism of detoxification and methylation and are converted to MMA and DMA before excretion through the kidney in the urine [52].
Weidemann et al. [52] determined that total arsenic and DMA were associated with a lower eGFR, but there was no association between total arsenic or DMA and ACR. Hsueh et al. [53] found an association between total arsenic and CKD determined by GFR < 60. In addition, participants with a high urinary total arsenic or participants with a low percentage of DMA had a positive association with CKD. Participants with CKD had significantly higher urinary total arsenic and MMA levels, but lower DMA percentage than controls [53]. Furthermore, Jin et al. [54] determined that DMA increased linearly with eGFR, suggesting that reduced renal function is associated with reduced urinary DMA. In this analysis, ACR was used as a marker for albuminuria, rather than GFR, both DMA and MMA were positively associated with an increased ACR.
The exact mechanism of arsenic species and toxicity on kidney function is not fully understood. Studies in dogs’ and humans’ proximal tubular cell (PTC) lines showed that the PTC can metabolize arsenic by converting arsenate to arsenite which can damage the mitochondria in cells, inducing cellular toxicity [55, 56]. Experiments using mice have confirmed that increased levels of arsenite methyltransferase in the kidney can lead to arsenic toxicity [57]. Mouse studies also showed that oxidative stress plays a major role in nephrotoxicity from arsenic exposure [58]. Acute tubular toxicity and tubulointerstitial nephritis were observed from acute arsenic toxicity in humans [59]. Animal and human experiments established association among superoxide production, peroxidation, and damage in DNA from arsenic exposure [60]. Animal and human studies also found links between arsenic exposure and kidney damage which can increase ACR. Our study also supported the role of arsenic species, such as MMA and DMA, increasing ACR, which could lead to the development of CKD.
Urinary metals
Limited studies have analyzed the effect of urinary cesium and kidney impairment. Jin et al. [54] determined that urinary cesium levels increased non-linearly with eGFR. In addition, urinary excretion rates of cesium significantly increased with increasing levels of eGFR, excretion rates were more sensitive in those with impaired renal function defined as GFR < 90 mL/min/1.73 m2) [54]. The accumulation of cesium has been noted to have higher concentrations in the kidneys, with 85% of cesium eliminated in the urine [26]. A positive association between increased ACR and urinary cesium levels was found in this study, suggesting a link between CKD and cesium exposure.
Studies have shown that compounds containing antimony can have toxic effects on the kidney following chronic use [61]. Limited research has been conducted on antimony exposure in humans with CKD. In mice studies, antimony treatment was significantly linked to kidney dysfunction in addition to biochemical alterations in the architecture of the kidney. Furthermore, serum urea and creatinine, a measure of the GFR levels, were significantly increased in mice treated with antimony, suggesting that antimony had adverse effects on the kidney [62]. A positive association was found between antimony and increased ACR, suggesting antimony may be linked to CKD.
Cadmium, lead, and mercury are heavy metals that are toxic to human health. High-level exposure to cadmium, lead, and mercury can cause damage to the renal system [10, 63,64,65]. Chronic exposure to cadmium, lead, and mercury can lead to deposition of these metals inside the body, the risk of adverse effects on the renal system with low-level exposure is unclear. A cross-sectional study conducted by Zhu et al. [66] with 2926 adults from the NHANES population showed that urinary and blood cadmium were significantly associated with increased ACR (≥ 30 mg/g), while urinary and blood lead and mercury were not associated with increased ACR. Furthermore, high level lead exposure is toxic to the renal system, low level exposure to lead and its adverse effects on the renal system are controversial [67]. Mujaj et al. [68] assessed for an association between ACR and eGFR and environmental lead exposure in 447 adult males. The study concluded that there was no significant association between lead exposure and renal impairment. A cross sectional study conducted by Grau-Perez et al. [69] in Spain with 1397 adults assessed for urinary albuminuria. The study concluded that urinary cadmium was associated with abnormal albuminuria. The findings from the above studies support our present findings with cadmium, lead, and mercury. Results were consistent with the findings that cadmium was associated with abnormal ACR or albuminuria. Lead and mercury were not associated with albuminuria.
Cadmium is one of the most toxic pollutants in the environment; studies have reported damage to numerous organs and systems in humans and animals, including the kidneys. In rats, studies have shown that cadmium exposure causes enhanced lipid peroxidation and oxidative damage to the cellular membranes [70]. Jacobo‐Estrada et al. [71] determined that cadmium exposure in rats significantly raised albumin and vascular endothelial growth factor (VEGF) in amniotic fluid, while decreasing creatinine. In addition, antimony has been linked to renal dysfunction in mice [62]. In a study evaluating metals in cats, copper, zinc, and manganese were highest in the liver followed by the renal cortex and renal medulla. The occurrence of CKD also altered the storage of elements, with higher cadmium in the liver and renal cortex in diseased cats [72].
Jayatilake et al. [73] found that uranium in sources of drinking water used by people with CKD were within normal limits. In addition, Kurttio et al. [74] found no evidence of renal damage in participants with uranium exposure in drinking water. No association was found among uranium in urine, water, hair, or toenails for 10 kidney toxicity indicators [74]. No association was found among kidney disease, measured by ACR, and urinary uranium in this study.
Strengths and limitations
This study used the concentration of 19 different metals in urine and assessed the impact of metals on abnormal ACR, which is a biomarker for albuminuria. This is the first study that measured 19 metal co-exposures and ACR in adults by using the most recent NHANES dataset to our knowledge. Cotinine, which is a biomarker for nicotine exposure, was adjusted for. Furthermore, the analysis was adjusted for gender, ethnicity, education, marital status, age, FIPR, BMI, and alcohol consumption. The effect of all the stated demographic variables’ exposure to metals and their association with ACR was analyzed. This study is one of the limited studies that analyzed nine demographic variables with multiple metal exposures and their association with ACR.
This study is a cross sectional design and therefore an inverse association cannot be ruled out. Albuminuria was measured by a single spot ACR in our study; there was no categorization between micro and macroalbuminuria and ACR was not measured over a period of 3 months to determine if the patient qualified for the diagnosis of CKD or not. The NHANES dataset only included the ACR measured at a single point in time, therefore there is no way to assess for interday or intraday variability of ACR and the results only apply to albuminuria rather than CKD. However, since albuminuria over 3 months can be classified as CKD, the results are suggestive of a possible link to CKD. Arsenocholine and arsenic acid were not included in our model. Once the data was cleaned, the remaining data was no longer distributed in such a way for a complete data analysis for these species. Therefore, the analysis began with 21 metals but only obtained results for 19. Also, the effects of interactions between the metals and CKD or interactions within the covariates were not examined. Possible associated conditions such as hypertension, diabetes, and cardiovascular disease were also not examined in this analysis.
Conclusion
ACR, a measure of albuminuria, was associated with urinary DMA, MMA, cadmium, cesium, and antimony. This is a possible link between DMA, MMA, cadmium, cesium, and antimony and CKD, suggesting that exposure to certain metals could put individuals at risk for CKD. Further research is needed to determine causation and understand the mechanism behind nephrotoxicity due to metal exposure.
Data availability
NHANES data is secondary data provided by the CDC to the public [75].
Code availability
R version 3.6.3, Upon request.
References
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182. https://doi.org/10.1093/bmb/ldg032
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Exp Suppl 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Rahman HH, Yusuf KK, Niemann D, Dipon SR (2020) Urinary speciated arsenic and depression among US adults. Environ Sci Pollut Res Int 27(18):23048–23053. https://doi.org/10.1007/s11356-020-08858-2
Chowdhury R, Ramond A, O’Keeffe LM et al (2018) Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 362:k3310. https://doi.org/10.1136/bmj.k3310
Nigra AE, Ruiz-Hernandez A, Redon J, Navas-Acien A, Tellez-Plaza M (2016) Environmental metals and cardiovascular disease in adults: a systematic review beyond lead and cadmium. Curr Environ Health Rep 3(4):416–433. https://doi.org/10.1007/s40572-016-0117-9
Satarug S, Moore MR (2004) Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect 112(10):1099–1103. https://doi.org/10.1289/ehp.6751
Tellez-Plaza M, Guallar E, Howard BV et al (2013) Cadmium exposure and incident cardiovascular disease. Epidemiology 24(3):421–429. https://doi.org/10.1097/EDE.0b013e31828b0631
Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, Guallar E (2012) Cadmium exposure and all-cause and cardiovascular mortality in the US general population. Environ Health Perspect 120(7):1017–1022. https://doi.org/10.1289/ehp.1104352
Wild P, Bourgkard E, Paris C (2009) Lung cancer and exposure to metals: the epidemiological evidence. Methods Mol Biol 472:139–167. https://doi.org/10.1007/978-1-60327-492-0_6
Soderland P, Lovekar S, Weiner DE, Brooks DR, Kaufman JS (2010) Chronic kidney disease associated with environmental toxins and exposures. Adv Chronic Kidney Dis 17(3):254–264. https://doi.org/10.1053/j.ackd.2010.03.011
Rahman HH, Niemann D, Singh D (2020) Arsenic exposure and association with hepatitis E IgG antibodies. J Occup Environ Med 8:111–122. https://doi.org/10.4236/odem.2020.83009
Reyes JL, Molina-Jijón E, Rodríguez-Muñoz R, Bautista-García P, Debray-García Y, Namorado M (2013) Tight junction proteins and oxidative stress in heavy metals-induced nephrotoxicity. Biomed Res Int. https://doi.org/10.1155/2013/730789
Stevens PE, Levin A (2013) Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158(11):825–830. https://doi.org/10.7326/0003-4819-158-11-201306040-00007
Basi S, Fesler P, Mimran A, Lewis JB (2008) Microalbuminuria in type 2 diabetes and hypertension: a marker, treatment target, or innocent bystander? Diabetes Care 31(Suppl 2):S194–S201. https://doi.org/10.2337/dc08-s249
Toto RD (2004) Microalbuminuria: definition, detection, and clinical significance. J Clin Hypertens (Greenwich) 6(11 Suppl 3):2–7. https://doi.org/10.1111/j.1524-6175.2004.4064.x
CDC (2018a) National Health and Nutrition Examination Survey, 2015–2016 Data Documentation, Codebook, and Frequencies, Speciated Arsenics - Urine (UAS_I). https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/UAS_I.htm
Glassock RJ, Warnock DG, Delanaye P (2017) The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol 13(2):104–114. https://doi.org/10.1038/nrneph.2016.163
Zheng L, Kuo CC, Fadrowski J, Agnew J, Weaver VM, Navas-Acien A (2014) Arsenic and chronic kidney disease: a systematic review. Curr Environ Health Rep 1(3):192–207. https://doi.org/10.1007/s40572-014-0024-x
Souweine JS, Corbel A, Rigothier C et al (2019) Interest of albuminuria in nephrology, diabetology and as a marker of cardiovascular risk. Intérêt de l’albuminurie en néphrologie, diabétologie et comme marqueur de risque cardiovasculaire. Ann Biol Clin (Paris) 77(1):26–35. https://doi.org/10.1684/abc.2018.1402
Lunyera J, Smith SR (2017) Heavy metal nephropathy: considerations for exposure analysis. Kidney Int 92(3):548–550. https://doi.org/10.1016/j.kint.2017.04.043
Fadrowski JJ, Navas-Acien A, Tellez-Plaza M, Guallar E, Weaver VM, Furth SL (2010) Blood lead level and kidney function in US adolescents: the third national health and nutrition examination survey. Arch Intern Med 170(1):75–82. https://doi.org/10.1001/archinternmed.2009.417
Ferraro PM, Costanzi S, Naticchia A, Sturniolo A, Gambaro G (2010) Low level exposure to cadmium increases the risk of chronic kidney disease: analysis of the NHANES 1999–2006. BMC Public Health 10:304. https://doi.org/10.1186/1471-2458-10-304
Kim NH, Hyun YY, Lee KB et al (2015) Environmental heavy metal exposure and chronic kidney disease in the general population. J Korean Med Sci 30(3):272–277. https://doi.org/10.3346/jkms.2015.30.3.272 ([published correction appears in J Korean Med Sci. 2015 Apr;30(4):507. Rhu, Seungho [corrected to Ryu, Seungho]])
Kim R, Rotnitsky A, Sparrow D, Weiss S, Wager C, Hu H (1996) A longitudinal study of low-level lead exposure and impairment of renal function. The Normative Aging Study. JAMA 275(15):1177–1181
Navas-Acien A, Tellez-Plaza M, Guallar E et al (2009) Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol 170(9):1156–1164. https://doi.org/10.1093/aje/kwp248
Melnikov P, Zanoni LZ (2010) Clinical effects of cesium intake. Biol Trace Elem Res 135(1–3):1–9. https://doi.org/10.1007/s12011-009-8486-7
Jha V, Garcia-Garcia G, Iseki K et al (2013) Chronic kidney disease: global dimension and perspectives. Lancet 382(9888):260–272. https://doi.org/10.1016/S0140-6736(13)60687-X (published correction appears in Lancet. 2013 Jul 20;382(9888):208)
CDC (2017a) About the National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm
CDC (2019b) National Health and Nutrition Examination Survey, 2015–2016 Data Documentation, Codebook, and Frequencies, Albumin & Creatinine - Urine (ALB_CR_I), https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/ALB_CR_I.htm
CDC (2018b) National Health and Nutrition Examination Survey, 2015–2016 Data Documentation, Codebook, and Frequencies, Arsenic - Total - Urine (UTAS_I). https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/UTAS_I.htm
CDC (2018c) National Health and Nutrition Examination Survey, 2015–2016 Data Documentation, Codebook, and Frequencies, Metals - Urine - Special Sample (UMS_I). https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/UMS_I.htm
CDC (2018d) National Health and Nutrition Examination Survey, 2015–2016 Data Documentation, Codebook, and Frequencies, Mercury - Urine (UHG_I). https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/UHG_I.htm
CDC (2017b) National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2015]
CDC (2017c) National Health and Nutrition Examination Survey, 2015–2016 Data Documentation, Codebook, and Frequencies, Body Measures (BMX_I). https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/BMX_I.htm
CDC (2017d) National Health and Nutrition Examination Survey, 2015–2016 Data Documentation, Codebook, and Frequencies, Demographic Variables and Sample Weights (DEMO_I). https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/DEMO_I.htm
CDC (2018e) National Health and Nutrition Examination Survey, 2015–2016 Data Documentation, Codebook, and Frequencies, Alcohol Use (ALQ_I). https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/ALQ_I.htm
CDC (2020a) Defining Adult Overweight and Obesity. https://www.cdc.gov/obesity/adult/defining.html
Jones MR, Tellez-Plaza M, Sharrett AR, Guallar E, Navas-Acien A (2011) Urine arsenic and hypertension in US adults: the 2003–2008 National Health and Nutrition Examination Survey. Epidemiology 22(2):153–161. https://doi.org/10.1097/EDE.0b013e318207fdf2
Buser MC, Ingber SZ, Raines N, Fowler DA, Scinicariello F (2016) Urinary and blood cadmium and lead and kidney function: NHANES 2007–2012. Int J Hyg Environ Health 219(3):261–267. https://doi.org/10.1016/j.ijheh.2016.01.005
Lumley T (2004) Analysis of complex survey samples. J Stat Softw 9(1):1–19. https://doi.org/10.18637/jss.v009.i08
Lumley T (2010) Complex surveys: a guide to analysis using R. Wiley
Lumley T (2020) Package ‘survey’: Analysis of Complex Survey Samples, version 4.0. https://cran.r-project.org/web/packages/survey/survey.pdf. Accessed 25 April 2021
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org. Accessed 25 April 2021
CDC (2019a) Module 3 examples - R code. https://wwwn.cdc.gov/nchs/data/tutorials/module3_examples_R.r
Susmann H (2016) Package ‘RNHANES’: Facilitates Analysis of CDC NHANES, version 1.1.0, https://cran.r-project.org/web/packages/RNHANES/RNHANES.pdf. Accessed 25 April 2021
Prabhakaran S (2016) Package ‘InformationValue’: Performance Analysis and Companion Functions for BinaryClassification Models, version 1.2.3, https://cran.r-project.org/web/packages/InformationValue/InformationValue.pdf. Accessed 25 April 2021
Jain RB (2019) Co-exposures to toxic metals cadmium, lead, and mercury and their impact on unhealthy kidney function. Environ Sci Pollut Res Int 26(29):30112–30118. https://doi.org/10.1007/s11356-019-06182-y
Norris K, Nissenson AR (2008) Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol 19(7):1261–1270. https://doi.org/10.1681/ASN.2008030276
Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR (2010) Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis 55(6):992–1000. https://doi.org/10.1053/j.ajkd.2009.12.032
Johnson AE, Boulware LE, Anderson CA et al (2014) Perceived barriers and facilitators of using dietary modification for CKD prevention among African Americans of low socioeconomic status: a qualitative study. BMC Nephrol 15:194. https://doi.org/10.1186/1471-2369-15-194
Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D (2005) Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis 46(4):587–594. https://doi.org/10.1053/j.ajkd.2005.06.007
Weidemann D, Kuo CC, Navas-Acien A, Abraham AG, Weaver V, Fadrowski J (2015) Association of arsenic with kidney function in adolescents and young adults: results from the National Health and Nutrition Examination Survey 2009–2012. Environ Res 140:317–324. https://doi.org/10.1016/j.envres.2015.03.030
Hsueh YM, Chung CJ, Shiue HS et al (2009) Urinary arsenic species and CKD in a Taiwanese population: a case-control study. Am J Kidney Dis 54(5):859–870. https://doi.org/10.1053/j.ajkd.2009.06.016
Jin R, Zhu X, Shrubsole MJ, Yu C, Xia Z, Dai Q (2018) Associations of renal function with urinary excretion of metals: evidence from NHANES 2003–2012. Environ Int 121(Pt 2):1355–1362. https://doi.org/10.1016/j.envint.2018.11.002
Ginsburg JM (1965) Renal mechanism for excretion and transformation of arsenic in the dog. Am J Physiol 208:832–840. https://doi.org/10.1152/ajplegacy.1965.208.5.832
Peraza MA, Carter DE, Gandolfi AJ (2003) Toxicity and metabolism of subcytotoxic inorganic arsenic in human renal proximal tubule epithelial cells (HK-2). Cell Biol Toxicol 19(4):253–264. https://doi.org/10.1023/b:cbto.0000003970.60896.49
Healy SM, Casarez EA, Ayala-Fierro F, Aposhian H (1998) Enzymatic methylation of arsenic compounds. V. Arsenite methyltransferase activity in tissues of mice. Toxicol Appl Pharmacol 148(1):65–70. https://doi.org/10.1006/taap.1997.8306
Sinha M, Manna P, Sil PC (2008) Arjunolic acid attenuates arsenic-induced nephrotoxicity. Pathophysiology 15(3):147–156. https://doi.org/10.1016/j.pathophys.2008.03.001
Prasad GV, Rossi NF (1995) Arsenic intoxication associated with tubulointerstitial nephritis. Am J Kidney Dis 26(2):373–376. https://doi.org/10.1016/0272-6386(95)90660-6
Sasaki A, Oshima Y, Fujimura A (2007) An approach to elucidate potential mechanism of renal toxicity of arsenic trioxide. Exp Hematol 35(2):252–262. https://doi.org/10.1016/j.exphem.2006.10.004
Winship KA (1987) Toxicity of antimony and its compounds. Adv Drug React Acute Poisoning Rev 6(2):67–90
Tanu T, Anjum A, Jahan M et al (2018) Antimony-induced neurobehavioral and biochemical perturbations in Mice. Biol Trace Elem Res 186(1):199–207. https://doi.org/10.1007/s12011-018-1290-5
Ekong EB, Jaar BG, Weaver VM (2006) Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int 70(12):2074–2084. https://doi.org/10.1038/sj.ki.5001809
Evans M, Elinder CG (2011) Chronic renal failure from lead: myth or evidence-based fact? Kidney Int 79(3):272–279. https://doi.org/10.1038/ki.2010.394
Johri N, Jacquillet G, Unwin R (2010) Heavy metal poisoning: the effects of cadmium on the kidney. Biometals 23(5):783–792. https://doi.org/10.1007/s10534-010-9328-y
Zhu XJ, Wang JJ, Mao JH, Shu Q, Du LZ (2019) Relationships of cadmium, lead, and mercury levels with albuminuria in US adults: results from the national health and nutrition examination survey database, 2009–2012. Am J Epidemiol 188(7):1281–1287. https://doi.org/10.1093/aje/kwz070
Rastogi SK (2008) Renal effects of environmental and occupational lead exposure. Indian J Occup Environ Med 12(3):103–106. https://doi.org/10.4103/0019-5278.44689
Mujaj B, Yang WY, Zhang ZY et al (2019) Renal function in relation to low-level environmental lead exposure. Nephrol Dial Transplant 34(6):941–946. https://doi.org/10.1093/ndt/gfy279
Grau-Perez M, Pichler G, Galan-Chilet I et al (2017) Urine cadmium levels and albuminuria in a general population from Spain: a gene-environment interaction analysis. Environ Int 106:27–36. https://doi.org/10.1016/j.envint.2017.05.008
Rogalska J, Pilat-Marcinkiewicz B, Brzóska MM (2011) Protective effect of zinc against cadmium hepatotoxicity depends on this bioelement intake and level of cadmium exposure: a study in a rat model. Chem Biol Interact 193(3):191–203. https://doi.org/10.1016/j.cbi.2011.05.008
Jacobo-Estrada T, Cardenas-Gonzalez M, Santoyo-Sánchez M et al (2016) Evaluation of kidney injury biomarkers in rat amniotic fluid after gestational exposure to cadmium. J Appl Toxicol 36(9):1183–1193. https://doi.org/10.1002/jat.3286
Paßlack N, Mainzer B, Lahrssen-Wiederholt M et al (2014) Liver and kidney concentrations of strontium, barium, cadmium, copper, zinc, manganese, chromium, antimony, selenium and lead in cats. BMC Vet Res 10:163. https://doi.org/10.1186/1746-6148-10-163 (Published 2014 Jul 17)
Jayatilake N, Mendis S, Maheepala P, Mehta FR, CKDu National Research Project Team (2013) Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol 14:180. https://doi.org/10.1186/1471-2369-14-180
Kurttio P, Harmoinen A, Saha H et al (2006) Kidney toxicity of ingested uranium from drinking water. Am J Kidney Dis 47(6):972–982. https://doi.org/10.1053/j.ajkd.2006.03.002
CDC (2020b) NHANES Questionnaires, Datasets, and Related Documentation. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2015
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HHR conceptualized the study and contributed to the introduction and discussion. SH. M-M conducted data analysis and contributed to the statistical methods and results section of the paper. DN contributed to the methods section and drafting of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study uses only secondary data analyses without any personal information identified using statistical data from the NHANES website, no further ethical approval for conducting the present study is required.
Consent to participate
This study uses only secondary data.
Consent for publication
Consent was given by all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahman, H.H., Niemann, D. & Munson-McGee, S.H. Association of albumin to creatinine ratio with urinary arsenic and metal exposure: evidence from NHANES 2015–2016. Int Urol Nephrol 54, 1343–1353 (2022). https://doi.org/10.1007/s11255-021-03018-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-03018-y