Abstract
The dyes Auramine and Auramine O are used in several industrial products, despite the scarce information regarding their ecotoxicity. The aim of the present study was to assess the acute and chronic toxicity of both dyes to aquatic organisms from different trophic levels (Raphidocelis subcapitata, Daphnia similis, Hydra attenuata, and Danio rerio) and calculate their predicted non-effect concentrations (PNEC). Auramine and Auramine O induced toxicity to all selected test organisms with L(E)C50 values ranging from 300 to 4800 ug/L. Both dyes induced inhibition in the growth rate of exposed algae, negatively affecting the reproduction of D. similis and induced deformities in H. attenuata (clubbed tentacles and shortened tentacles) and D. rerio (edemas, tail malformation and delay in yolk sac absorption). PNEC values of 0.92 μg/L and 4.0 μg/L were obtained for Auramine and Auramine O, respectively, based on results of the most sensitive test system (algae). Test results were analyzed using the Criteria of Reporting and Evaluating Ecotoxicity Data (CRED), confirming their reliability and relevance. Thus, PNEC values can be used in future risk assessments of those substances in freshwater systems.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyes are widely used in several industrial products, including textiles, paper, plastic, leather, food, cosmetics, and household products (Guaratini and Zanoni 2000; Bafana et al. 2011). In general, the conventional wastewater treatment processes are not efficient in their removal, and it is estimated that approximately 10–15% of dyes used in textile industries might be lost into the environment (Leite et al. 2016; USEPA 1990).
Several authors reported the occurrence of dyes in water and sediments from rivers located under the influence of textile industries discharges. In Brazil, dyes have been found in the aquatic environment with concentrations ranging from 0.0118 to 6.81 μg/L (Carneiro et al. 2010; de Aragão Umbuzeiro et al. 2005; Vacchi et al. 2016, 2017; Zocolo et al. 2015). In Canada, in a study conducted on the Yamaska River, dyes were detected in water samples, with concentrations ranging from 3 to 17 μg/L and in sediments in the range of 400–1500 (μg/Kg) (Maguire 1992). Rajaguru et al. (2002) registered a contamination by genotoxic compounds in groundwater, collected in an area under the influence of textile dyeing and bleaching industries in Tirupur, India. According to the results obtained by these authors, after chemical characterization, aromatic amines probably derived from discharges of textile effluents may be responsible for the DNA damaging activity of the water samples. Those studies suggest a high potential of contamination of textile dyes into the aquatic environment worldwide. Therefore, ecotoxicological studies are necessary to elucidate the possible adverse effects of this contamination on aquatic biota.
Several dyes have been tested and found to be toxic to aquatic organisms (Table 1). However, there are still few studies that evaluated the toxicity of the same dye with organisms representing three trophic levels or more (Vacchi et al. 2016; Martínez-jerónimo 2019; Hernández-Zamora and Martínez-Jerónimo 2019). Providing toxicity data for different organisms is necessary to derive reliable and relevant criteria for assessing the risk of dyes when it is desired to preserve aquatic life.
The dyes Auramine and Auramine O (also referred as Solvent Yellow 34 and Basic Yellow 2) are used for dyeing leather, jute, cotton, and paper (International Agency for Research on Cancer - IARC 2010, Gessner and Mayer 2000). The diphenylmethane dyes are usually grouped with the triarylmethane dyes (IARC 2010), which comprises synthetic colorants, widely used due to their versatility and bright colors (IARC 2010; Gessner and Mayer 2000). However, there are only few articles regarding the toxicity of triarylmethane’s dyes group to aquatic organisms (Table 1). According to the IARC, Auramine dyes are classified as Group 1 (carcinogenic to humans) with respect to production and as 2B (possibly carcinogenic to humans) with respect to their use. Despite that, these dyes are still being used, and were found even in food products in India and China (Tripathi et al. 2007; Lin 2007; Li et al. 2013; Tatebe et al. 2014). Information regarding auramine dyes toxicity in aquatic organisms is scarce. Moreover, it is expected to find auramine dyes in the aquatic environment because of their use in textile industries and high solubility when compared to other group of colorants, such as disperse dyes.
For the safety threshold, i.e., the predicted no-effect concentration (PNEC), the ideal is to have toxicity data on organisms representing three trophic levels (EUROPEAN COMMISSION 2011). PNEC values can be calculated based on the methodology adopted by the European Union, which uses toxicity data from a test substance and assessment factors, ranging from 10 to 1000, depending on the quality and quantity of available ecotoxicological data. Thus, the greater the set and/or quality of toxicity data, the greater the confidence to derive a criterion, and the lower the applied factor. PNEC values, derived from toxicological tests, can support an assessment of the toxicant's environmental risk and assist in regulations aiming at ensuring water quality for the protection of aquatic life (EUROPEAN COMMISSION 2011). Thus, PNEC derivation processes should be based on detailed, transparent, and unbiased assessments. Moreover, the aquatic ecotoxicity studies used for this purpose must be reliable and relevant (Moermond et al. 2016).
To calculate PNEC values for aquatic toxicity, algae, microcrustaceans, hydras, and fish can be used as test organisms. Algae are oxygen producers and staple food for primary consumer organisms. An imbalance at this basic trophic level could lead to imbalance throughout the trophic chain. Thus, adverse effects of both dyes on aquatic primary producers cannot be neglected.
Microcrustaceans are one of the most widely used organisms to evaluate the toxic potential of dyes in acute tests, and daphnids are a critical species in the aquatic food chain as an important dietary component of fish and invertebrate predators (Tatarazako and Oda 2007).
Hydras are well-established test organisms, although not often being used in toxicity assessment of aquatic pollutants. Since they represent secondary consumers in the trophic level (Suares-Ruppert 2005), the inclusion of this test system in ecotoxicological assessment of potential aquatic pollutants may be useful in the understanding of the toxic burden of determined compounds, such as dyes and other emerging contaminants, and the possible ecological effects that this toxicity may represent in an aquatic environment.
D. rerio is a vertebrate also inserted in the second level of the trophic chain and has increasingly being use in ecotoxicological assessments, especially with embryo exposure (Suares-Rocha et al. 2011).
Moreover, to evaluate the reliability and relevance of toxicological tests, a method called “Criteria for Reporting and Evaluating Ecotoxicity Data” (CRED) was developed (Moermond et al. 2016). In this method, key information need to be provided, e.g., CAS number, purity and source of the substances used for testing, and detailed information on the test organisms (scientific name, life stage, strain and source) as well as information on exposure conditions, including the real concentrations of the selected substances during the tests to assure a reliable PNEC derivation.
Thus, the aim of this study was to assess the ecotoxicity of two auramine dyes to aquatic organisms from three different trophic levels (Raphidocelis subcapitata, representing autotrophs, Dapnhia similis representing primary consumers, and Hydra attenuata and Danio rerio representing secondary consumers), providing highly reliable ecotoxicological data for PNEC derivation that can be used in future risk assessments.
Materials and methods
Chemicals and stock solutions
C.I. Auramine (CAS number 492-80-8, Color Index (C.I.) 41000B) and Auramine O (CAS number 2465-27-2, Color Index (C.I.) 41000) were purchased from Sigma-Aldrich, with 98 and 87% of purity, respectively. Stock solutions for Auramine O were prepared dissolving the dyes in the appropriate test medium for each organism. Because Auramine was less soluble than Auramine O, it was necessary to use dimethyl sulfoxide (DMSO) to prepare the stock solutions. However, the maximum final concentration of DMSO in the experiments was 0.01%. In all tests using DMSO, the solvent was added to the negative controls. This concentration was defined in previous tests in our laboratory as a maximum concentration causing no adverse effects in any of the selected organisms.
Chemical analysis
Chemical analysis was performed with aliquots of the test solutions to confirm the nominal concentration of dyes in ecotoxicity tests using high-performance liquid chromatography with diode array detection (HPLC-UV/DAD) (Shimadzu Corporation, Kyoto, Japan) using a Shim-pack G-ODS (4) 4-mm internal diameter guard column and a Capcell Pack C18 AG120 S-5 (Shiseido Co) 250 mm long 4.6 mm internal diameter separation column. The methanol and water acidified with formic acid (0.1%) (50/50) were used as the mobile phase, with isocratic elution at a flow rate of 0.8 mL/min and running time of 10 min. The quantitative determination was achieved at 437 nm for both dyes. The limit of detection (LOD) = 3.3 s/S and limit of quantification (LOQ) = 10 s/S for Auramine and Auramine O were determined using a calibration curve, with s = the estimate of the standard deviation of the blank samples (n = 10) and S = the slope of the analytical curve obtained from 15 to 200 μg/L. The lowest, middle, and highest concentrations were analyzed at the end of each test with selected aquatic organisms. In order to measure the exposure concentrations, extra replicates without the organisms were prepared. No extraction was required and the samples were diluted in the mobile phase of the analytical method (50/50 MeOH/H20) before being quantified by HPLC-UV/DAD (Azevedo et al. 2020). The analytical results are described in details in the Supplementary Material (SM1).
Ecotoxicity tests
All organisms used in this study were provided by the Laboratory of Ecotoxicology and Genotoxicity (LAEG), State University of Campinas (Limeira, Brazil), except the zebrafish embryos which were tested in cooperation with the Laboratory of Genetic Toxicology, University of Brasilia, (Brasilia, Brazil). The sensitivity of the test organisms was monitored by sensitivity tests and internal control charts, to warrant the use of only adequate cultures. Test concentrations were selected according to range finding tests, previously performed, in order to reach a range of concentrations inducing 0 to 100% effects in the exposed organisms.
Raw data from toxicity assays are presented in the Supplementary Material (SM2). Stock and exposure solutions for each dye tested were carefully prepared in adequate medium for each organism. No significant alterations in physical and chemical parameters (pH, conductivity, and dissolved oxygen) were observed after the addition of dyes to the media.
R. subcapitata
Algae R. subcapitata were maintained in a supplemented medium. Chronic toxicity tests using the freshwater algae were performed according to OECD guideline 201 (OECD 2011). Algae population was exposed to different dyes concentrations: 20, 60, 200, 600, and 2000 μg/L for Auramine and 40, 120, 400, 1200, and 4000 μg/L for Auramine O. Three replicates at each test concentration (treatments) were used. The inoculum was composed of algae harvested from a liquid stock algal culture, in an exponential growth phase of 3-day-old culture. The initial cell density was 10 000 ± 1000 cells/mL. The final volume of 50 mL, composed by algal inoculum and test dye in supplemented medium, was placed in an Erlenmeyer. The test was performed under static conditions for 72 h, at 24 ± 2 °C under continuous fluorescent light (4000 ± 400 lux), in a rotatory shaker with 150 revolutions per minute. To validate the test, the biomass in the control cultures should have increased exponentially by a factor of at least 16 within the end of the test.
Colored test chemicals can absorb photosynthetically active light and hence limit growth of algal cultures. Experiments were conducted with the highest concentration tested (4000 μg/L) to confirm that the toxic effects observed for the algae were not relate to inhibition of the photosynthesis caused by the color of the dye solutions. For that, solutions of highest concentrations of each dye were prepared and placed each in a beaker. Then, Erlenmeyers containing only algae in supplemented medium (10 mL) were placed into those beakers, in a way that the algae were totally covered by the dyes, but not in contact to them. Thus, the physical effect was evaluated without chemical interference. A control was performed at the same conditions, using only culture medium in the control beaker. Each independent test was performed in triplicate. At the end of 72 h, the growth inhibition of algae exposed to dye solution was compared to a control and the effective concentration inducing 50% growth inhibition (EC50) was calculated. Algae cell number was counted using a Neubauer chamber with optical microscope MB-E-200 (Nikon).
D. similis
D. similis culture was cultivated in synthetic medium (MS), with conductivity of 200 ± 20 mS/cm, hardness of 40–48 mg/L CaCO3, at 20 ± 2 °C and under a photoperiod of 16:8 h light:dark, according to ABNT NBR 12713 (ABNT 2016). The organisms were fed five times a week with the algae R. subcapitata. Neonates less than 24 h old, from a healthy culture, were used for the experiments.
Acute toxicity tests with D. similis were performed according to OECD guideline 202 (OECD 2004). Concentrations of Auramine (1000, 1500, 2000, 3000, and 4000 μg/L) and Auramine O (1000, 3000, 4500, 7000, and 10000 μg/L) were prepared in MS. Twenty neonates (< 24 h old) were placed in acrylic tubes with 10 mL of dyes solutions. Acute assays were performed in three replicates, at 21 ± 0.3 °C and photoperiod of 16:8 h light:dark. After 48 h, the number of immobilized daphnids was recorded, and the effective concentrations inducing 10 and 50% immobilization (EC10 and EC50) were recorded.
D. similis chronic toxicity tests were performed according to the OECD guideline 211 (OECD 2012) with adaptations described by Vacchi et al. (2016). This method measures the chronic toxicity using less than 24-h-old neonates exposed during 14 days to the chemical, with renewal of test medium every two days. Neonates < 24 h old were individually transferred to a 50-mL vessel containing 40 mL of treatments, in the following concentrations: 100, 200, 500, 1000, and 2000 μg/L for Auramine and 250, 500, 750, 1000, 1500, and 2000 μg/L of Auramine O. Ten control and treatment replicates were used for each tested concentration. The test organisms were fed daily with R. subcapitata at 20 ± 2 °C and under a photoperiod of 16:8 h light:dark. Chronic assays were performed in two replicates. After exposure period, total number of neonates per test concentrations was observed, and the effective concentrations inducing 10 and 50% reproduction inhibition (EC10 and EC50) were recorded.
H. attenuata
H. attenuata were cultivated in Hydra medium, pH 7.0 ± 0.1, maintained at 22 ± 2 °C, under a 16:8 h light:dark photoperiod, according to Trottier et al. (1997). The organisms were fed three times a week with newly hatched nauplii of Artemia salina. In Hydra sp., it is known that progressive morphological changes are indicative of increased toxicity. Here, the test was based on Trottier et al. (1997) that determines five stages of morphological changes: (A) hydras in their normal stage, with extended body and tentacles; (B) the appearance of bulbs at the tips of the tentacles, indicating the first sign of intoxication; (C) the second sign of intoxication, with the shortening of tentacles and body, (D) tulip stage, with tentacles and body dramatically reduced; and (E) the last phase of intoxication, leading to death by disintegration. Stages B and C are reversible and are considered sub-lethal effects, while stages D and E are irreversible and indicate lethal endpoints.
The organisms were exposed for 96 h to different concentrations of Auramine (500, 1000, 2000, 3500, and 7000 μg/L) and Auramine O (100, 500, 1000, 1500, 2000, and 3000 μg/L), dissolved in Hydra medium. The organisms were placed in 12-well microplates (three organism per well with 5 mL test solution), in triplicate (totalizing 9 organisms per concentrations and control). Three independent investigations were done.
The same temperature and photoperiod were used for cultivation and the tests. During exposure, animals were not fed. At the end of exposure, lethal (stages D and E) and sublethal effects (stages B and C) were observed under a stereomicroscope and LC/EC10 and LC/EC50 were calculated. Photographs of morphological changes of organisms were taken.
D. rerio embryo
D. rerio adults were maintained in aquariums with reverse osmosis and activated carbon filtered water, with a photoperiod cycle of 12:12 h (light:dark), temperature of 27 ± 1 °C, conductivity of 650 ± 100 μS/cm, pH of 7.0 ± 0.5, and dissolved oxygen ≥ 95% saturation. Fish embryo toxicity test was based on the OECD guideline 236 (OECD 2013). Zebrafish eggs were collected immediately after natural mating, rinsed in water, and checked for egg viability under a stereomicroscope (Stereoscopic Zoom Microscope—Stemi 2000, Zeiss, Germany). The unfertilized eggs and those showing cleavage irregularities or injuries were discarded. Experiments were initiated immediately after fertilization using 60 eggs per treatment, divided in 3 replicates, selected, and distributed in 24-well microplates in the climate chamber (SL-24 Solab Científica, Brazil). Embryos were exposed to different concentrations of Auramine (1000, 1900, 3700, 7100, and 13600 μg/L) and Auramine O (300, 600, 1000, 1900, 3700, 7100, and 13600 μg/L) for 96 h under static conditions and observed daily, under a stereomicroscope. Developmental parameters were evaluated in embryos over the test period, using a × 70 magnification for eggs and × 40 magnification for hatched embryos. Lethality and malformations were evaluated: lack of otolith formation, general delay in development, lack of eye and body pigmentation, lack or delay in somite formation, edemas, non-detachment of the tail-bud from the yolk sac, non-absorption of yolk sac, lack or delay in hatching, and mortality. All parameters were registered as presence and absence of effect. Three independents experiments were done.
Statistical analysis
The effective concentrations L(E)C10 and L(E)C50 with the corresponding 95% confidence intervals were calculated via the logistic model, using the package drc in R software (Christian Ritz 2005).
Evaluation of the quality of the ecotoxicity test
The CRED method (Moermond et al. 2016) was introduced to assess the reliability and relevance of the ecotoxicity data used in the derivation of PNEC, applying a set of 20 reliability and 13 relevance criteria. The CRED aimed at providing a complete report on the methodology performed during our tests, presenting unbiased and transparent results. Tables of the CRED are presented in the Supplementary Material (SM3 A and B).
PNEC derivation
PNEC values for protecting freshwater pelagic community from adverse effects of dyes Auramine and Auramine O were derived according the Technical Guidance for Deriving Environmental Quality Standards (TGD EQS) on the Water Framework Directive (2000/60/EC) (European Commission 2011) by using the deterministic approach, applying an adequate assessment factor (AF) to the lowest relevant EC10/NOEC value from the available dataset, to extrapolate to an environmentally protective concentration.
Results
Nominal and real concentrations
No alterations in concentrations of Auramine and Auramine O were observed during the test performed with algae, daphnia, and hydra (final concentrations remaining within 80–120% of nominal concentrations; see Supplementary Material, SM1). Therefore, it is possible to affirm that the test substances remained stable in the test solutions, during the exposure periods. Thus, the expression of results for all experiments was based on nominal values. Nominal concentrations were also considered for the experiments using D. rerio, because test conditions and duration were similar to those performed with H. attenuata. Therefore, no changes in concentrations were expected.
Ecotoxicity tests
For all experiments, the effects of dyes followed a dose response curve (Fig. 1), and L(E)C10 and L(E)C50 values with confidence intervals (95%) were calculated for both dyes (Table 2). Tables with toxicity tests data are presented in the Supplementary Material (SM2).
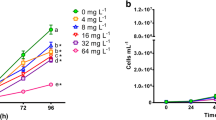
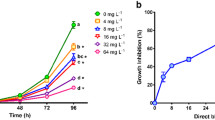
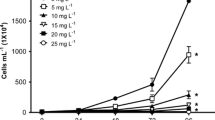
Dose-response curves for effects data obtained to aquatic organisms belonging to different trophic levels exposed to Auramine (AU) and Auramine O (AUO). Raphidocelis subcapitata, chronic test (4 days). Dapnhia similis, acute test (2 days), and chronic test (14 days). Hydra attenuata, acute test (4 days) with lethal and deformities (morphological changes). Danio rerio, acute test (4 days) with lethal and deformities (edema, tail malformation, and delay in yolk absorption)
R. subcapitata
All tests performed with algae were considered valid. The biomass in the control increased exponentially by a factor greater than 16 at the end of the test. The EC10 values for Auramine and Auramine O were 46 and 200 μg/L, and EC50 were 300 and 800 μg/L after 72 h of exposure, respectively (Table 2).
D. similis
In the acute immobilization test, no mortality was observed in the control group. Regarding the treatments, EC10 value of 1800 μg/L was registered for Auramine and of 1900 μg/L for Auramine O, while EC50 values of 2900 and 4300 μg/L were registered after 48 h of exposure, for each dye respectively. In the chronic test, after 14 days of exposure, similar results were obtained for both dyes, with EC10 value of 500 μg/L for Auramine and of 400 μg/L for Auramine O, while EC50 values of 900 and 800 μg/L were recorded at the end of the exposure period, respectively (Table 2).
H. attenuata
No mortality was observed in the control group, for the tests with H. attenuata.
The five stages of morphological alteration described in section 2.3.3 were observed for both dyes, in different concentrations (Fig. 2). In the assays with Auramine dye, in concentrations of 1000 μg/L, the first stage of morphological alteration (stage A) was observed in approximately 60% of organisms, while from concentrations of 2000 μg/L, all organisms already presented alterations in the formation of the tentacles (stages B and C). In the highest tested concentration (7000 μg/L), most of the organisms were in stages D or E, which are irreversible morphological effects.
Morphological stages in Hydra attenuata after 96 h exposure to increased concentrations of Auramine (AU) and Auramine O (AUO) illustrating stages from healthy to disintegrated polyp: a stage: (normal Hydra); b stage: minimal expression of toxicity, i.e., clubbed tentacles; c stage: shortened tentacles; d stage: tulip stage and e stage: disintegration. Magnification used for all each picture × 2.5
For Auramine O, the first signals of toxicity were observed in concentration of 500 μg/L, while in concentration of 1000 μg/L, all organisms presented some morphological alteration (stages B and C). In the highest tested concentration (3000 μg/L), all organisms were in stages D or E.
When considering lethality (stages D and E), LC10/50 values of 3200 and 4800 μg/L were recorded for Auramine and of 1000 and 1600 μg/L were recorded for Auramine O. Considering morphological changes (stages B and C), EC50 values of 1100 μg/L and EC10/50 of 500 and 600 μg/L were recorded for each dye, respectively (Table 2, Fig. 2).
D. rerio embryos
After 96 h exposure, no significant mortality was observed in the control group.
LC10/LC50 were calculated for lethality, with values of 1300 and 1900 μg/L for Auramine and of 2100 and 2400 μg/L for Auramine O.
Development deformities as edema and tail malformation were observed for both dyes at similar concentrations range, and delay in yolk absorption was observed more frequently in organisms exposed to Auramine. After 96 h, EC10/50 values of 900 and 1100 μg/L and of 900 and 1300 μg/L were obtained for each dye, respectively, considering all deformities. EC10/50 values were also calculated for each deformity (Table 2, Fig. 3).
PNEC calculation
PNEC calculations were performed by the deterministic method according to the guidelines of the European Commission (2011).
The ecotoxicity tests performed in this work were considered reliable and relevant for PNEC derivation (Supplementary Material, SM3) according to the CRED method. R. subpcapitata showed to be the most sensitive species to both dyes (Table 2); therefore, their EC10 values were selected for PNEC calculation. Because chronic data was available for two trophic levels, an AF of 50 was applied and PNECs of 0.92 μg/L and 4.6 μg/L were derived for Auramine and Auramine O, respectively.
Discussion
Solvent Yellow 34 Auramine and Auramine O dyes are toxic for the selected aquatic test organisms. The algae were the most sensitive organism for both Auramine and Auramine O dyes with IC10 of 46 and 200 μg/L, respectively. Auramine is up to 4 times more toxic to this test system when compared to Auramine O. In general, more water-soluble compounds tend to induce less toxicity, because they are less absorbed by cell membranes, while less soluble compounds tend to be more absorbed by organisms, and consequently are more likely to induce toxicity (Klassen et al. 2013). The higher maximum solubilities of Auramine (53.5 mg/L) in relation to Auramine O (10000 mg/L) may explain the higher toxicity observed for Auramine.
EC50 values of 300 and 800 μg/L were determined for Auramine and Auramine O, respectively, for algae. In general, algae has been shown to be very sensitive to dyes, since most of the dyes tested for these organisms are considered very toxic or toxic according to some authors (Croce et al. 2017b; Hernández-Zamora and Martínez-Jerónimo 2019; Novotný et al. 2006). Novotný et al. (2006) found values similar to those registered in this study, for the algae exposed for 96 h to Disperse Blue 3 (EC50 of 500 μg/L). Hernández-Zamora and Martínez-Jerónimo (2019) evaluated the toxicity of Congo Red dye for algae, microcrustaceans, and fish, and found that algae was the most sensitive organism for this compound, with EC50 of 3110 μg/L. Other authors registered EC50 values in the same order of magnitude, for algae exposed to several dyes, for instance: Novotný et al. (2006) reported EC50 of 7800 μg/L for Reactive Orange 16 after 96 h of exposure and Luna et al. (2014) EC50 of 5600 μg/L to Vat Green 3 in a 72-h test. Croce et al. (2017) investigated the toxicity of 42 dyes belonging to different chemical classes, and found that 30 of them had EC50 values lower than 100 000 μg/L, while 12 presented EC50 values ranging from 102,400 to 152,800 μg/L, after 72 h of exposure. Vacchi et al. (2016) recorded EC50 values of 102,000 μg/L for algae exposed to Disperse Red 1. According to those data, it is possible to conclude that Auramine and Auramine O presented similar or higher toxicity to these aquatic organisms, when compared to several other dyes.
Some studies have indicated that the algae sensitivity to dyes is related to the ability of these substances to inhibit or block light, making it less accessible to aquatic organisms. In experiments using algae as test system, high concentrations of dyes may result in inhibitory effects that can be comparable to the effects of the internal toxicity induced by those dyes (Øllgaard et al. 1998). In addition, in some cases, the inhibition of light can represent up to 50% of the observed grown inhibition (ETAD 1994 in Croce et al. 2017). To verify the possible effects related to inhibition of the photosynthesis caused by light-blocking, due to the color of the dye solutions, we performed preliminary tests with the highest concentration of each tested dye. Apparently, the observed toxicity was related to dyes themselves, and not to the light-blocking, because no statistically significant difference in algal growth was observed when compared to the control experiments (see Supplementary 3, Fig. S1). However, testing with more replicates and longer exposure periods would be required to confirm this hypothesis.
For D. similis, it was observed a small difference between the results of the acute tests, with a higher toxicity for Auramine (Table 2). D. similis and D. magna are the most used cladocerans to assess the toxicity of dyes. Around 50 dyes have already been tested for these species and the levels of the acute toxicity varied significantly in the available literature (Table 2).
Auramine and Auramine O showed similar results for the chronic tests with D. similis, (inhibition of reproduction) (Table 1). It means 6- to 10-fold increase in toxicity from the acute test to the chronic test. Vacchi et al. (2016) observed an almost 50-fold increase in toxicity from the acute test (EC50 = 0.13 μg/L) to the chronic test (NOEC = 0.003 μg/L) with D. similis exposed to Disperse Red 1. These results confirmed the relevance of sublethal effects evaluation in toxicity assessments. However, there is still a lack of information in the literature related to chronic toxicity of dyes to microcrustaceans, such as daphnids.
Both dyes induced lethal (stages D and E) and sublethal (stages B and C) effects in H. attenuata (Fig. 2). For lethality and morphological changes, the toxicity of Auramine O was approximately 2 times higher than the toxicity recorded for Auramine. (Table 2). H. attenuata was the only organism that showed greater sensitivity to Auramine O. Hydra has shown high sensitivity to chlorinated compounds (Vacchi et al. 2013). Maybe the fact that Auramine O is chlorinated salt (C17H21N3.HCl) could explain its higher toxicity in relation to Auramine.
The toxicity of dyes for H. attenuata is not well known, only Disperse Red 1 was investigated using this test organism. Vacchi et al. (2016) recorded an LC50 of 48000 μg/L (lethal effects) and a NOEC of 1000 μg/L (sublethal effects). Jong et al. (2016) also observed sublethal effects for H. attenuata even with lower concentrations (< 100 μg/L). Therefore, it is possible to conclude that dyes may induce toxic lethal and sublethal effects in H. attenuata.
Fish embryos are often used to assess dye toxicity and, in general, effects on organism survival are observed in the mg/L concentration range, in line with the results obtained to both Auramines in the present study (Table 1). Auramine and Auramine O caused similar sublethal and teratogenic effects in fish embryos, including edemas, tail malformation, and delay in yolk sac absorption (Fig. 3). Such effects occurred already in the first day of exposure, remaining until the end of the test. In the first 24 h, those effects were observed only in the highest concentrations. However, with the increasing of exposure period, they were recorded even at the lowest concentrations. No studies were found in the literature with Auramine and fish but Dach et al. (2019) studied the toxicity Auramine O on zebrafish development. They observed developmental effects such as alteration in craniofacial and body axis morphology and the presence of yolk-sac and pericardial edema after at 3.04 mg/L of Auramine O. Furthermore, teratological effects to developing Microhyla ornata embryos, such as malformation tail and eyes, were observed by Ghate and Mulherkar (1978) after the exposure of these organisms to effluents from a textile industry that used several dyes, including Auramine. To the best of our knowledge, no mechanistic explanation can be found in the current literature to explain the malformations induced by auramine in fish embryos. Auramine O caused DNA damage (comet assay) in human hepatocytes (Martelli et al. 1998). Auramine O was also positive in the Salmonella/microsome mutagenicity test (TA98, TA1535, TA1538, and YG1024 strains), in the presence of metabolic activation (IARC 2010).
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS 2009) classify the chemical compounds by criteria as very toxic (LC50 < 1000 μg/L), toxic (LC50 1000–10000 μg/L), or harmful (LC50 10000–100000 μg/L). According to this classification and based on the organisms and endpoints tested, the two dyes evaluated in this study can be considered toxic or very toxic to these organisms.
PNEC values of 0.92 μg/L for Auramine and 4.6 μg/L for Auramine O were derived based on the most sensitive organism in the chronic tests (algae R. subcapitata) divided by an assessment factor of 50. A PNEC of 2.3 μg/L was derived by the Canadian Government representing all azo dyes using the deterministic approach (Environment Canada 2016). Vacchi et al. (2016) determined a PNEC for the dye Disperse Red 1 of 0.06 μg/L and 1.8. The PNECs derived in this study for Auramine and Auramine O can be considered environmentally relevant because they are in the same order of magnitude of the concentration of other dyes found in surface waters (Carneiro et al. 2010; Zocolo et al. 2015; Vacchi et al. 2017).
Conclusions
We concluded that both synthetic dyes, Auramine and Auramine O, are toxic to aquatic organisms from different trophic levels, which could lead to an imbalance in an ecosystem contaminated with these substances. All 50% effective concentrations values were below 5000 μg/L. Concentrations ranging from 600 to 1300 μg/L negatively affected daphnids’ reproduction and induced deformities in hydra and fish embryos, representing a risk to their populations when exposed to those concentrations in the field.
From all tested organisms, algae were the most sensitive, and 50% inhibition growth was observed in concentrations equal or lower than 800 μg/L. Because they are in the first trophic level, an imbalance in algae population in the field could lead to an imbalance throughout the trophic chain. The toxicity observed to algae does not seem to be related to the blockage of light caused by the dye solution color.
PNEC values based on algae chronic effects were derived (0.92 μg/L for Auramine and 4.6 μg/L for Auramine O), CRED showed the reliable values and, may be useful in future risk assessments related to the presence of these dyes in aquatic environments.
References
Abe FR, Mendonça JN, Moraes LAB, Oliveira GAR, Gravato C, Soares AMVM, Oliveira DP (2017) Toxicological and behavioral responses as a tool to assess the effects of natural and synthetic dyes on zebrafish early life. Chemosphere 178:282–290. https://doi.org/10.1016/j.chemosphere.2017.03.030
ABNT (2016) Aquatic Ecotoxicology - Acute Toxicity - Bioassay Methodology with Daphnia Spp (Crustacea, Cladocera). NBR 12713. Brazilian Association of Technique Standards
de Aragão Umbuzeiro G, Freeman HS, Warren SH et al (2005) The contribution of azo dyes to the mutagenic activity of the Cristais River. Chemosphere 60:55–64. https://doi.org/10.1016/j.chemosphere.2004.11.100
Azevedo CCJ, Kasahara MK, Montagner CC (2020) Development and validation of method to quantify auramine dyes in toxicity tests and samples environmental using HPLC/DAD. Submitted as Quim Nova.
Bae JS, Freeman HS (2007) Aquatic toxicity evaluation of new direct dyes to the Daphnia magna. Dyes Pigments 73:81–85
Bafana A, Devi S, Chakrabarti T (2011) Azo dyes: past, present and the future. Environ Rev 370:350–370. https://doi.org/10.1139/A11-018
Carneiro PA, Oliveira DP, Umbuzeiro GA, Zanoni MVB (2010) Mutagenic activity removal of selected disperse dye by photoeletrocatalytic treatment. J Appl Electrochem 40:485–492. https://doi.org/10.1007/s10800-009-0018-9
Christian Ritz JCS (2005) Bioassay Analysis Using R
Croce R, Cinà F, Lombardo A, Crispeyn G, Cappelli CI, Vian M, Maiorana S, Benfenati E, Baderna D (2017) Aquatic toxicity of several textile assays with Daphnia magna and Raphidocelis subcapitata. Ecotoxicol Environ Saf 144:79–87
Dach K, Yaghoobi B, Schmuck MR et al (2019) Teratological and behavioral screening of the National Toxicology Program 91-Compound Library in Zebrafish ( Danio rerio ). 167:77–91. https://doi.org/10.1093/toxsci/kfy266
Environment Canada (2016) Environment Canada Certain solvent dyes of the Aromatic Azo and Benzidine-based substance grouping - Canada. https://www.canada.ca/en/healthcanada/services/chemical-substances/fact-sheets/chemicals-glance/certain-solvent-dyes-aromatic-benzidine-based-substance-grouping.html. Accessed 13 Jun 2019
European Commission (2011) Common implementation strategy for the Water Framework Directive (2000/60/EC). Guidance Document No. 27. Technical guidance for deriving Environmental Quality Standards. European Union. 204 pp.
Ferraz ERA, Umbuzeiro GA, de Almeida G et al (2011) Differential toxicity of Disperse Red 1 and Disperse Red 13 in the Ames test, HepG2 cytotoxicity assay, and Daphnia acute toxicity test. Environ Toxicol 26:489–497. https://doi.org/10.1002/tox.20576
Gessner T, Mayer U (2000) Triarylmethane and Diarylmethane Dyes. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, In
Ghate HV, Dodakundi GB (1978) Effect of dye factory effluent on the developing embryos of microphyla O. Indian J Environ Health 20:359–365
GHS (2009) Globally Harmonized System of Classification and Labelling of Chemicals (GHS). Globally Harmonized System, GHS, New York and Geneva, United Nations. ST/SG/AC.10/30/Rev.3.
Guaratini CCI, Zanoni MVB (2000) Corantes têxteis. Quim Nova 23:71–78
Hernández-Zamora M, Martínez-Jerónimo F (2019) Congo red dye diversely affects organisms of different trophic levels: a comparative study with microalgae, cladocerans, and zebrafish embryos. Environ Sci Pollut Res 26:11743–11755. https://doi.org/10.1007/s11356-019-04589-1
IARC (2010) IARC Monographs on the evaluation of carcinogenic risks to humans VOLUME 99 Some Aromatic Amines, Organic Dyes, and Related Exposures
Jiang L-L, Li K, Yan D-L, Yang M-F, Ma L, Xie L-Z (2020) Toxicity assessment of 4 azo dyes in zebrafish embryos. Int J Toxicol 39(2):115–123
de Jong L, Pech N, de Aragão Umbuzeiro G, Moreau X (2016) Multi-scale biomarker evaluation of the toxicity of a commercial azo dye (Disperse Red 1) in an animal model, the freshwater cnidarian Hydra attenuata. Water Res 96:62–73. https://doi.org/10.1016/j.watres.2016.03.043
Joshi V, Pancharatna K (2018) Food colorant Sunset Yellow (E110) intervenes developmental profile of zebrafish. J Appl Toxicol 39(4):571–581
Jung H, Seok S-H, Han J-H, Abdelkader TS, Kim T-H, Chang S-N, Ko A-S, Choi S-K, Lee C-R, Seo J-E, Byun S-H, Kim J-A, Park J-H (2012) Effect of fluorescent whitening agent on the transcription of cell damage-related genes in zebrafish embryos. J Appl Toxicol 32(9):654–661
Jungtanasombut W, Preeprem P (2014) Effects of reactive red 239 on developing zebra fish (Danio rerio) embryos. Kasetsart J (Nat Sci) 48:619–628
Klassen CD, Casarett LJ, Doull J (2013) Casarett and doull’s toxicology: the basic science of poisons. New York.
Leite S, Souza B De, Arag G De, Pupo RF (2016) Chemosphere monitoring ecotoxicity of disperse red 1 dye during photo-Fenton degradation. 148:. https://doi.org/10.1016/j.chemosphere.2016.01.053
Li J, Ding X-M, Liu D-D, Guo F, Chen Y, Zhang YB, Liu HM (2013) Simultaneous determination of eight illegal dyes in chili products by liquid chromatography–tandem mass spectrometry. J Chromatogr B 942–943:46–52. https://doi.org/10.1016/J.JCHROMB.2013.10.010
Lin Q (2007) Simultaneous determination of chrysoidine and auramine O in bean products by HPLC. Se pu = Chinese J Chromatogr 25:776–777
Liu H, Yu H, Giesy JP, Sun Y, Wang X (2007) Toxicity of HC Orange No. 1 to Daphnia magna, Zebrafish (Brachydanio rerio) embryos, and goldfish (Carassius auratus). Chemosphere 66(11):2159–2165
Luna LAV, da Silva THG, Nogueira RFP, Kummrow F, Umbuzeiro GA (2014) Aquatic toxicity of dyes before and after photo-Fenton treatment. J Hazard Mater 276:332–338. https://doi.org/10.1016/j.jhazmat.2014.05.047
Maguire RJ (1992) Occurrence and persistence of dyes in a canadian river. 25:265–270
Manimaran D, Sulthana A s, Elangovan N (2018) Reactive black 5 induced developmental defects via potentiating apoptotic cell death in Zebrafish (Danio rerio) embryos. Pharm Pharmacol Int J 6(6)
Martelli A, Brambilla Campart G, Canonero R, Carrozzino R, Mattioli F, Robbiano L, Cavanna M (1998) Evaluation of auramine genotoxicity in primary rat and human hepatocytes and in the intact rat. Mutat Res Toxicol Environ Mutagen 414:37–47. https://doi.org/10.1016/S1383-5718(98)00037-0
Martínez-jerónimo MHF (2019) Exposure to the azo dye Direct blue 15 produces toxic effects on microalgae, cladocerans, and zebra fish embryos. Ecotoxicology 28:890–902. https://doi.org/10.1007/s10646-019-02087-1
Moermond CTA, Kase R, Korkaric M, Ågerstrand M (2016) CRED: criteria for reporting and evaluating ecotoxicity data. Environ Toxicol Chem 35:1297–1309. https://doi.org/10.1002/etc.3259
Novotný Č, Dias N, Kapanen A, Malachová K, Vándrovcová M, Itävaara M, Lima N (2006) Comparative use of bacterial, algal and protozoan tests to study toxicity of azo- and anthraquinone dyes. Chemosphere 63:1436–1442. https://doi.org/10.1016/J.CHEMOSPHERE.2005.10.002
OECD (2004) Test No. 202: Daphnia sp. acute immobilisation test. OECD Guidel Test Chem. https://doi.org/10.1787/9789264069947-en
OECD (2011) Test No. 201: alga, growth inhibition test. OECD Guidel Test Chem. https://doi.org/10.1787/9789264069923-en
OECD (2012) Test No. 211: Daphnia magna reproduction test. OECD Guidel Test Chem. https://doi.org/10.1787/9789264185203-en
OECD (2013) Test No. 236: Fish embryo acute toxicity (FET) Test. OECD Guidel Test Chem. https://doi.org/10.1787/9789264203709-en
Øllgaard H, Frost L, Galster J, Hansen OC (1998) Survey of azo-colorants in Denmark: consumption, use, health and envi-ronmental aspects
Ozmen N, Erdemoglu S, Gungordu A, Asilturk M, Turhan DO, Akgeyik E, Harper SL, Ozmen M (2018) Photocatalytic degradation of azo dye using core@shell nano-TiO2 particles to reduce toxicity. Environ Sci Pollut Res 25(29):29493–29504
Parrott JL, Bartlett AJ, Balakrishnan VK (2016) Chronic toxicity of azo and anthracenedione dyes to embryo-larval fathead minnow. Environ Pollut 210:40–47
Rajaguru P, Vidya L, Baskarasethupathi B, Kumar PA, Palanivel M, Kalaiselvi K (2002) Genotoxicity evaluation of polluted ground water in human peripheral blood lymphocytes using the comet assay. Mutat Res 517:29–37
Rocha PS, Bernecker C, Strecker R, Mariani CF, Pompêo MLM, Storch V, Hollert H, Braunbeck T (2011) Sediment-contact fish embryo toxicity assay with Danio rerio to assess particle-bound pollutants in the Tietê River Basin (São Paulo, Brazil). Ecotoxicol Environ Saf 74:1951–1959. https://doi.org/10.1016/j.ecoenv.2011.07.009
Rocha OP, Cesila CA, Christovam EM, Barros SBM, Zanoni MVB, de Oliveira DP (2017) Ecotoxicological risk assessment of the “Acid Black 210” dye. Toxicology 376:113–119. https://doi.org/10.1016/j.tox.2016.04.002
Shen B, Liu H-C, Ou W-B, Eilers G, Zhou SM, Meng FG, Li CQ, Li YQ (2015) Toxicity induced by Basic Violet 14, Direct Red 28 and Acid Red 26 in zebrafish larvae. J Appl Toxicol n/a-n/a 35:1473–1480. https://doi.org/10.1002/jat.3134
Suares-Ruppert EE Zoologia dos Invertebrados - 7aed. 2005.
Tatarazako N, Oda S (2007) The water flea Daphnia magna (Crustacea, Cladocera) as a test species for screening and evaluation of chemicals with endocrine disrupting effects on crustaceans. Ecotoxicology 16:197–203. https://doi.org/10.1007/s10646-006-0120-2
Tatebe C, Zhong X, Ohtsuki T, Kubota H, Sato K, Akiyama H (2014) A simple and rapid chromatographic method to determine unauthorized basic colorants (rhodamine B, auramine O, and pararosaniline) in processed foods. Food Sci Nutr 2:547–556. https://doi.org/10.1002/fsn3.127
Tippabathani J, Nellore J, Kathirkannan P, Nachiyar CV (2020) Developmental effects of three textile chemicals on locomotor activity, antioxidant markers and acetylcholine esterase activity in zebrafish
Tripathi M, Khanna SK, Das M (2007) Surveillance on use of synthetic colours in eatables vis a vis Prevention of Food Adulteration Act of India. Food Control 18:211–219. https://doi.org/10.1016/J.FOODCONT.2005.09.016
Trottier S, Blaise C, Kusui T, Johnson EM (1997) Acute toxicity assessment of aqueous samples using a microplate-basedHydra attenuata assay. Environ Toxicol Water Qual 12:265–271. https://doi.org/10.1002/(SICI)1098-2256(1997)12:3<265::AID-TOX10>3.0.CO;2-9
USEPA (1990) Aerobic and anaerobic treatment of C.I. Disperse Blue 79. 6 pp
Vacchi FI, Albuquerque AF, Vendemiatti JA, Morales DA, Ormond AB, Freeman HS, Zocolo GJ, Zanoni MVB, Umbuzeiro G (2013) Chlorine disinfection of dye wastewater: implications for a commercial azo dye mixture. Sci Total Environ 442:302–309. https://doi.org/10.1016/j.scitotenv.2012.10.019
Vacchi FI, Von der Ohe PC, de Albuquerque AF et al (2016) Occurrence and risk assessment of an azo dye—the case of Disperse Red 1. Chemosphere 156:95–100. https://doi.org/10.1016/j.chemosphere.2016.04.121
Vacchi FI, de Vendemiatti JA, S, da Silva BF et al (2017) Quantifying the contribution of dyes to the mutagenicity of waters under the influence of textile activities. Sci Total Environ 601–602:230–236. https://doi.org/10.1016/j.scitotenv.2017.05.103
Verma Y (2008) Acute toxicity assessment of textile dyes and textile and dye industrial effluents using Daphnia magna bioassay. Toxicol Ind Health 24:491–500. https://doi.org/10.1177/0748233708095769
White CR, Davies SJ, Henry TB (2012) Malachite green toxicity and effects on reproductive success in zebrafish. Zebrafish 9(3):135–139
Wong CK, Liu XJ, Lee AOK, Wong PK (2007) Effect of Azo Dyes on survivorship, oxygen consumption rate, and filtration rate of the freshwater Cladoceran Moina macrocopa. Hum Ecol Risk Assess An Int J 12:289–300. https://doi.org/10.1080/10807030500531604
Zocolo GJ, Pilon dos Santos G, Vendemiatti J, Vacchi FI, Umbuzeiro GA, Zanoni MVB (2015) Using SPE-LC-ESI-MS/MS analysis to assess disperse dyes in environmental water samples. J Chromatogr Sci 53:1–8. https://doi.org/10.1093/chromsci/bmu221
Funding
This study was supported by the Brazilian Ministry of Education, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES) (Finance Code 001) through a personal grant provided to CCJA, and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) RO (grant no. 2018/03108-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The experiments are in accordance with the current laws of the country in which they were performed. The study was approved by the Ethics Committee at the University of Brasilia (reference no 100226/2014).
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Assessment of aquatic toxicity of auramine dyes using organisms from different trophic levels, Raphidocelis subcapitata (autotrophs), Dapnhia similis (primary consumers), and Hydra attenuata and Danio rerio (secondary consumers).
• Lethality for D. similis, H. attenuata, and D. rerio in concentrations below of 4800 μg/L.
• Induced inhibition in the growth rate of exposed algae, negatively affecting the reproduction of D. similis and induced deformities in H. attenuata (clubbed tentacles and shortened tentacles) and D. rerio (edemas, tail malformation and delay in yolk sac absorption) in sublethal concentrations.
• PNEC values of 0.92 μg/L (Auramine) and 4.6 μg/L (Auramine O) were derived.
Electronic supplementary material
ESM 1
(DOCX 102 kb)
ESM 2
(DOCX 38087 kb)
Supplemental material SM3A
CRED evaluation Auramine (XLSX 20 kb)
Supplemental material SM3B
CRED evaluation Auramine O (XLSX 21 kb)
Rights and permissions
About this article
Cite this article
de Jesus Azevedo, C.C., de Oliveira, R., Suares-Rocha, P. et al. Auramine dyes induce toxic effects to aquatic organisms from different trophic levels: an application of predicted non-effect concentration (PNEC). Environ Sci Pollut Res 28, 1866–1877 (2021). https://doi.org/10.1007/s11356-020-10462-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10462-3