Abstract
Nearly 7 00000 tons of dyes are produced annually throughout the world. Azo dyes are widely used in the textile and paper industries due to their low cost and ease of application. Their extensive use results in large volumes of wastewater being discharged into aquatic ecosystems. Large volume discharges constitute a health risk since many of these dyes, such as Congo Red, are elaborated with benzidine, a known carcinogenic compound. Information regarding dye toxicity in aquatic ecosystems is limited. Therefore, the aim of the present study was to evaluate the effect of Congo Red on survival and reproduction of Ceriodaphnia dubia. We determined the 48 h median lethal concentration (LC50) and evaluated the effects of sublethal concentrations in subchronic exposures by using as food either fresh algae or algae previously exposed to the dye. LC50 was 13.58 mg L−1. In subchronic assays, survival was reduced to 80 and 55 %, and fertility to 40 and 70 %, as compared to the control, in C. dubia fed with intoxicated cells or with the mix of intoxicated + fresh algae, respectively, so the quantity and type of food had a significant effect. We determined that Congo Red is highly toxic to C. dubia since it inhibits survival and fertility in concentrations exceeding 3 mg L−1. Our results show that this dye produces negative effects at very low concentrations. Furthermore, our findings warn of the risk associated with discharging dyes into aquatic environments. Lastly, the results emphasize the need to regulate the discharge of effluents containing azo dyes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution is a global issue of concern due to the supply crisis that afflicts several countries. In particular, the textile industry contributes significantly to the deterioration of water quality, since it discharges large volumes of wastewater effluents into aquatic systems. Thus, the textile industry is considered one of the most polluting industrial activities (Savin and Butnaru 2008). Wastewaters containing azo dyes significantly affect photosynthetic activity in aquatic ecosystems, since colorization greatly modifies light quality and decreases underwater light penetration (Hernández-Zamora et al. 2014), thereby reducing dissolved O2 concentrations (Ali 2010). Moreover, some colorants have been reported as toxic, mutagenic, and carcinogenic for aquatic biota (Bafana et al. 2008; Saratale et al. 2011).
Many of the azo dyes used in textile dyeing are manufactured with carcinogenic compounds, such as benzidine (Golka et al. 2004). Among these dyes is Congo Red (1-naphthalenesulfonic acid, 3,3′-(4,4′-biphenylenebis (azo)) bis (4-amino-) disodium salt), also known as Congo Red 4B, Cosmos Red, Cotton Red B, Cotton Red C, Direct Red 28, Direct Red R, Direct Red Y. It is a benzidine-based anionic diazo dye prepared by coupling tetrazotized benzidine with two molecules of napthionic acid (Purkait et al. 2007). Its molecular formula is C32H22N6O6S2Na2, and its molecular weight is 696.7 g mol−1. This dye is used to stain cotton, jute, leather, paper, silk, and wool. Despite evidence that during its degradation, metabolic conversion leads to generation of carcinogenic amines (Pielz 1999; Sponza and Isik 2005) and its ban by the European Union in 1999, it is widely used in the textile industry given its low cost.
Bafana et al. (2009) proved that Congo Red toxicity is primarily linked to its intermediary metabolites (benzidine and 4-aminobiphenyl), which affect DNA and induce apoptosis in HL-60 cells (human promyelocytic leukemia cell line).
Toxic pollutants have diverse effects at different levels within the structure of aquatic ecosystems. Negative effects can be seen in primary producers (cyanobacteria, algae, and plants) as well as consumers at all trophic levels (Sharma et al. 2007; Gómez et al. 2008). Some basic, acidic, and direct azo dyes are classified as toxic or highly toxic for fishes, crustaceans, algae, and bacteria, whereas reactive azo dyes are toxic only at concentrations exceeding 100 mg L−1 (Novotný et al. 2006; Øllgaard et al. 1998). The evaluation of the toxic effects of pollutants in freshwater ecosystems is conducted through the controlled exposure of selected test organisms. Selected organisms are preferably representative of large communities in aquatic ecosystems. Due to their ecological importance, cladocerans are frequently used as test organisms since they act as key components of the zooplankton community (Martínez-Jerónimo et al. 2000). Among cladocerans, Daphnia magna and Ceriodaphnia dubia are recognized as reference species for ecotoxicological studies (Blaise and Férard 2005). These organisms act as fundamental links between primary producers and secondary consumers. C. dubia is often used in ecotoxicity studies given its high sensitivity and short life cycle (Versteeg et al. 1997; Brander et al. 2012).
Research on the toxic effects on aquatic ecosystems generated by azo dyes-containing effluents is limited. Therefore, the aim of this study is to determine the acute and subchronic toxicity of Congo Red in C. dubia. Furthermore, we evaluated exposure to different sublethal dye concentrations, as well as to diverse food (microalgae) concentrations grown in a normal culture medium or a medium to which the dye had been added to determine whether ingestion of microalgae exposed to this compound affects the development of the studied cladoceran.
Materials and methods
Dye
We used the azo dye Congo Red of analytic grade (C32H22N6O6S2Na2, Sigma-Aldrich®, CAS number: 573-58-0, purity >97 %). Test solutions were prepared from a concentrated stock solution, and were sterilized by filtration using sterile nitrocellulose membranes (0.22 μm pore diameter).
Test organisms
The Ceriodaphnia dubia clonal strain used in this study was obtained from the cladoceran strain collection at the Laboratorio de Hidrobiología Experimental (LHE) of the Escuela Nacional de Ciencias Biológicas (ENCB) at the Instituto Politécnico Nacional (IPN). This strain has been successfully maintained in controlled cultures for over twenty years. An axenic strain of the green alga Chlorella vulgaris (LHE-Chl 01) was used as food in the cladoceran diet. The strain was obtained from the LHE-ENCB-IPN microalgae collection. C. vulgaris was cultivated in Bold’s basal mineral medium (250 mg L−1 NaNO3, 25 mg L−1 CaCl2•2H2O, 75 mg L−1 MgSO4•7H2O, 75 mg L−1 K2HPO4, 175 mg L−1 KH2PO4, 25 mg L−1 NaCl, 4.98 mg L−1 of FeSO4•7H2O, 0.001 mL L−1 H2SO4, 11.42 mg L−1 H3BO3, 50 mg L−1 EDTA, 31 mg L−1 KOH, 8.82 mg L−1 ZnSO4•7H2O, 1.44 mg L−1 MnCl2•4H2O, 0.71 mg L−1 MoO3, 1.57 mg L−1 CuSO4•5H2O, 0.49 mg L−1 Co(NO3)2•6H2O), under aseptic conditions at 24 ± 1oC, light intensity of 120 μmoles m−2 s−1, 12:12 h (light:dark) photoperiod, and bubbled air (200 mL min−1).
It is worth mentioning that C. dubia is commonly cultivated in semi-hard reconstituted water (EPA medium, USEPA 2002a). However, in previous studies we observed that in tests using EPA medium as dilution water, the dye precipitated during the first hours of exposure. We attempted to avoid precipitation by reducing water hardness, but precipitation continued even in soft water. Testing alternatives, we proved that Congo Red maintains solubility when it is diluted in dechlorinated tap water (pH: 8.0–8.2; hardness: 128 mg L−1 as CaCO3; conductivity: 412.0 μS; salinity: 0.2 psu). Using this medium, we guaranteed bioavailability of the compound to assess its toxic effects. Thus, C. dubia was also cultivated in dechlorinated tap water (pH: 8.0–8.2, hardness: 128 mg L−1 as CaCO3, conductivity: 412.0 μs, salinity: 0.2 psu), at 25 °C, with 16:8 h (light:dark) photoperiod. To avoid possible biases due to the change in culture medium, the cladoceran stock was stabilized for two generations. The test organisms (neonates of less than 24 h of age, USEPA 2002b) were obtained from cultures of known ages, fed with 1 × 106 cells mL−1 of C. vulgaris.
Exposure of C. vulgaris to congo red
To determine the toxic effects of Congo Red on the microalgae used as food, its median inhibitory concentration (IC50) was calculated. For this purpose, bioassays with axenic inocula of C. vulgaris were exposed to different concentrations of Congo Red (5, 10, 15, 20, and 25 mg L−1) in Bold’s basal medium (BBM). Incubation conditions for the tests were: temperature 24 ± 1 °C, light intensity of 120 μmoles m−2 s−1, 12:12 h (light:dark) photoperiod, and air flow of 200 mL min−1. The incubation period was 96 h and every 24 h samples were taken to evaluate cellular density in a Neubauer chamber.
Once IC50 was determined, C. vulgaris was cultured under the previously described conditions, in a medium containing the equivalent to the IC50 of Congo Red. After 96 h of exposure, the cells were centrifuged at 3 500 rpm for 10 minutes. Immediately after, the microalgae pellet was washed three times using BBM in order to eliminate the dye possibly adsorbed by the cell wall, following the same centrifugation-resuspension procedure. Lastly, the cells were resuspended in dechlorinated tap water and stored in darkness at 4 °C to be used as food for C. dubia during the subchronic toxicity tests.
Determination of the median lethal concentration (LC50) in C. dubia
The acute toxic effects of Congo Red dye were determined in exposure times of 48 h following the procedure suggested by USEPA (2002b). We used test volumes of 33 mL and dechlorinated tap water as dilution water. The 1, 2, 4, 8, and 16 mg L−1 concentrations were tested in triplicate. Neonates (age < 24 h) obtained from the third brood and thereafter were used. Bioassays were incubated at 24 ± 1 °C, using 16:8 h (light: dark) photoperiod with no food supply. We recorded the number of affected, immobilized, or dead (no heartbeat through observation at the stereomicroscope) organisms at 24 and 48 h. To calculate the average LC50, three separate bioassays were carried out.
Evaluation of the subchronic toxicity of congo red
The subchronic toxicity of Congo Red was determined following Method 1002.0 Daphnid, Ceriodaphnia dubia, survival and reproduction test established by USEPA (2002a). The bioassays were started from neonates, documenting reproduction and survival during a 10-day period. Two experimental series were conducted:
Evaluation of the effect of direct exposure to the dye
C. dubia strains were exposed to three concentrations of Congo Red equivalent to LC1, LC10, and LC50 (0.94, 3.11, and 13.58 mg L−1, respectively) using C. vulgaris not previously exposed to the dye as nourishment.
Evaluation of the effect of consuming microalgae grown in culture medium with dye
The effect of three feeding conditions was studied: a) healthy, fresh algae (control, CA), b) algae grown in a culture medium containing the equivalent to the IC50 of Congo Red (EA), and c) 75 % CA + 25 % EA. In this experiment, the dilution water did not contain the toxicant.
The effects of all three concentrations of C. vulgaris: 4 × 105, 8 × 105, and 1.2 × 106 cells mL−1 were studied in both experiments. Each experiment had 10 replicates, starting with neonates. Neonates were individually placed in recipients containing 25 mL of test solution. Tests were incubated in an environmental chamber at 24 ± 1 °C and 16:8 h (light: dark) photoperiod. Survivorship was daily recorded. Once reproduction began, the progeny was separated and counted. The test medium and the respective concentrations of microalgae were renewed daily.
Statistical analysis
The median inhibitory concentration (IC50) and the median lethal concentration (LC50) as well as the LC1 and LC10 values and the corresponding 95 % confidence intervals were calculated via the Probit method, using RA software (Risk Assessment. Hazard Assessment Tools, v. 1.0). Fertility results were analyzed through a two-way analysis of variance (two-way ANOVA) employing dye concentration and food concentration as variable factors. When significant differences were recorded (P < 0.05), Dunnett’s test was applied to find differences with the control, and multiple comparisons were made using the Tukey’s test. To compute comparisons, SigmaPlot version 11.0 was used. Survival curves were analyzed using the Kaplan-Meier test; pairwise comparisons were analyzed via the Gehan-Breslow-Wilcoxon Test, using Prisma GraphPad v. 6.0.
Results and discussion
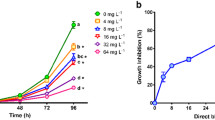
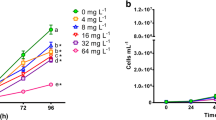
Congo Red significantly inhibited the population growth of the microalgae Chlorella vulgaris at all the tested concentrations, as observed in Fig. 1. ANOVA’s test evidenced significant differences (P < 0.05) for population density values among treatments, determined at 96 h. The Dunnett’s test demonstrated that cell densities at the five Congo Red concentrations were significantly lower than that observed in the control. These results are similar to those reported by other authors. Cheriaa et al. (2009) observed that growth of Chlorella was inhibited by exposing it to indigo dye at 10 mg L−1. Chia and Musa (2014) determined that the cell density of Scenedesmus quadricauda was the highest in the control, but decreased with increasing effluent concentration from the textile industry, containing indigo dye. The sensitivity of C. vulgaris to Congo Red in our study was evidenced by the determined IC50, the average value was 5.19 mg L−1 (95 % limits: 4.39–5.89 mg L−1), similar to that reported by Novotný et al. (2006) for the microalgae Selenastrum capricornutum (EC50 = 4.8 ± 1.0 mg L−1) after 96-h exposure to Congo Red.
The average LC50 value of Congo Red in C. dubia was 13.58 mg L−1 (95 % limits: 9.82–23.5 mg L−1). Additionally, the sublethal values were: LC1 0.94 mg L−1 (0.27–1.70 mg L−1), LC5 2.05 mg L−1 (0.92–3.10 mg L−1), LC10 3.11 mg L−1 (1.73–4.36 mg L−1), and LC15 4.13 mg L−1 (2.60–5.58 mg L−1). The recorded LC50 value agrees with findings reported by Øllgaard et al. (1998) and Novotný et al. (2006). The authors mention that some basic azo dyes and acidic, and direct dyes are classified as highly toxic or toxic for fishes, crustaceans, algae, and bacteria. The sublethal values here determined (LC1, LC5, and LC10) warn about the environmental risk posed by the presence of this compound, even at relatively low concentrations.
With respect to the effect of Congo Red on cladocerans, Wong et al. (2006) evaluated the acute toxicity of this dye in Moina macrocopa at 4 and 7 days, reporting LC50 values of 0.16 mg L−1 (95 % limits: 0.11–0.31 mg L−1) and 0.07 mg L−1 (0.03–0.09 mg L−1), respectively. However, these data are not comparable to ours because test conditions were rather different in terms of exposure time, test volume, and food supply during the assays. The lower LC50 values reported by Wong et al. (2006) can be explained by the longer exposure time, but species-specific sensitivity should also be considered; nevertheless, from that study it is clear that Congo Red was the most toxic azo dye from the three synthetic colorants evaluated by them.
In a study about Congo Red bioremoval using the microalgae Chlorella vulgaris, Hernández-Zamora et al. (2015) reported an EC50 of 3.32 mg L−1 (1.24–4.91 mg L−1) in C. dubia. This value is below the one found in this study. This difference could be attributed to the fact that Hernández-Zamora et al. (2015) used BBM as dilution water; this was the culture medium used herein for the microalgae, and was used for the toxic assays to avoid precipitation of the dye. It is possible that the complex mineral composition and the concentration of salts in this medium could have increased the Congo Red toxicity to C. dubia, because the salt content of BBM and the lower pH could cause stress and become additional lethality factors. Dilution water in the present study was dechlorinated tap water, with significantly lower salt content, as detailed in the methodology.
Studies testing other azo dyes’ effects on C. dubia indicate values of 25, 33, and 0.5 mg L−1, for Reactive Black 5, Acid Orange 7, and Vat Green 3, respectively (de Luna et al. 2014); the authors concluded that Vat Green 3 and Reactive Black 5 produced more toxic degradation products than the original dyes.
Regarding other cladocerans, Bae and Freeman (2007) studied the toxicity of different dyes and reported that the dye Direct Blue 218 was very toxic for daphnids, obtaining LC50 (48 h) values of 1–10 mg L−1. However, the acute toxicity of four other direct dyes was much lower, reporting LC50 > 100 mg L−1 values. These results suggest that copper molecules within the structure of Direct Blue 218 might increase toxicity. Conversely, Ferraz et al. (2010) reported higher toxicity values. The authors determined that the dyes Disperse Red 1 and Disperse Red 13 are highly toxic to Daphnia similis, with EC50 values of 127 and 18.7 μg L−1, respectively. Differences with the values reported here can be attributed not only to the type of dye, but also to species-specific responses, indicating that there are species of zooplankton that are more sensitive. These studies and our results elucidate the need to classify the toxicity of these dyes. Bhaskar et al. (2015) mentioned that, based on their EC50 values, emerging contaminants can be classified as harmful (EC50 as 10–100 mg L−1), toxic (EC50 as 1–10 mg L−1), or very toxic (EC50 < 1 mg L−1); according to this classification, some of the most common azo dyes could be categorized as toxic to very toxic.
When evaluating toxicity, it should be considered also that the acute or lethal toxic effects do not allow identification of other potential damages that could occur after exposure to sublethal concentrations (i.e., physiological, teratogenic, or carcinogenic effects). The toxic effects of azo dyes mainly depend on their chemical structure and/or their metabolites. Congo Red has the capability of producing benzidine, a carcinogenic aromatic amine formed through cleavage of one or more azo groups (Sponza and Isik 2005). The Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers reports that from approximately 4 000 dyes evaluated for their toxicity in mammals, more than 90 % have median lethal dose values (LD50) of less than 2 × 103 mg kg−1. The most toxic are basic and direct dis-azo dyes (Shore 1996; Robinson et al. 2001), such as Congo Red.
Fecundity registered in C. dubia exposed to 0.94 mg L−1 (LC1), 3.11 mg L−1 (LC10), and 13.58 mg L−1 (LC50) of Congo Red in combination with different concentrations of food (Chlorella vulgaris 4.0 × 105, 8.0 × 105, and 1.2 × 106 cells mL−1) for 10 days is shown in Fig. 2. Reproduction was only documented in organisms exposed to the lowest Congo Red concentration (0.94 mg L−1), although fecundity values were significantly lower than those shown by the control group. Females in the Congo Red concentrations of 3.11 mg L−1 (LC10) and 13.58 mg L−1 (LC50) presented 100 % mortality by day 6 and 3, respectively. Despite the fact that some organisms exposed to 3.11 mg L−1 lived up to 6 days, they did not reach sexual maturity. Infertility induced by the dye is evident. Therefore this finding represents an important contribution that enriches the body of knowledge regarding the chronic toxic effects of azo dyes on cladoceran species. Wong et al. (2006) reported similar effects in Moina macrocopa; they observed that the onset of reproduction and the number of neonates produced were reduced at azo dye concentrations as low as 0.01 mg L−1.
Fecundity of Ceriodaphnia dubia exposed during 10 days to three Congo Red dye concentrations: LC1 (0.94 mg L−1), LC10 (3.11 mg L−1), and LC50 (13.58 mg L−1), simultaneously fed with three food (C. vulgaris) concentrations. Average values and standard error bars are shown. Different letters above bars indicate significant differences (P < 0.05), after Tukey’s pairwise comparisons
With respect to food concentration, Kluttgen et al. (1996) observed that lower amounts, as opposed to higher concentrations of food, have a larger negative effect on fertility of Daphnia magna and Ceriodaphnia quadrangula exposed to 3–4 dichloroaniline. On the other hand, Kooijman and Metz (1984) mention that the toxicity of chemical compounds directly affects reproduction and does not depend on the concentration of food. This assessment coincides with our results. As observed in Fig. 2, there was no significant difference in the number of neonates in the different food concentrations exposed to 0.94 mg L−1. Notwithstanding, lower quantity of food in the control significantly reduced C. dubia fertility, which could be because lower food concentrations are associated to reduced energy available for reproduction (Rose et al. 2002).
As observed in Fig. 3, the survival of C. dubia exposed to different Congo Red quantities (0.94 mg Lmg L−1, 3.11 mg L−1, and 13.58 mg L−1) and to different concentrations of food dropped as the concentration of dye increased, independently of the quantity of food provided. However, in the organisms exposed to 0.94 mg L−1 (LC1), a higher concentration of microalgae-enabled higher survivorship.
Survivorship of Ceriodaphnia dubia exposed to three Congo Red dye concentrations: a LC1 (0.94 mg L−1), b LC10 (3.11 mg L−1), and c LC50 (13.58 mg L−1), and simultaneously fed with three food (C. vulgaris) concentrations. Asterisks indicate differences with respect to the control: ** P < 0.01 or *** P < 0.001. Kaplan-Meier test and pairwise comparisons (Gehan-Breslow-Wilcoxon Test)
The effects of food concentration as an influencing factor on the toxicity response in C. dubia have focused primarily on metals (Hauri and Horne 2004; Sofyan et al. 2007; Rodgher and Gaeta Espíndola 2008). However no clear trend has surfaced, since different concentrations of food produce either positive or negative effects on survival, reproduction, mortality, and fertility rates of this cladoceran. In our study, the survival of C. dubia exposed to 0.94 mg L−1 (LC1) and to the highest concentration of food (1.2 × 106 cells mL−1) was not different to the survival rates observed in the control group. Significantly lower survival values were recorded for organisms that fed on lower concentrations of food. This result indicates that a larger quantity of microalgae reduced mortality but fertility remained constant (as observed in Fig. 2).
Independently of the quantity of food provided, deaths were reported from days 2 and 3 onwards with the 3.11 mg L−1 concentration (Fig. 3b). As expected, with 13.58 mg L−1 (LC50) the longest life span was only 3 days (Fig. 3c). Only one report exists regarding the toxic effect of Congo Red on survival and reproduction of cladoceran species, specifically for Moina macrocopa (Wong et al. 2006). In that study the authors observed that fecundity was a more sensitive response than survival and, similarly to our results, reproduction was delayed and the total progeny was reduced, with no reproduction documented at Congo Red concentration as low as 1 mg L−1. When Wong et al. (2006) exposed M. macrocopa to different concentrations of Congo Red (0.01, 0.1, 1.0, 10, and 100 mg L−1), they observed that exposed animals divert energy to detoxification, thus reducing the amount of energy allocated to growth and reproduction. According to our results, no matter that food supply is not limiting, the toxic effects produced by the colorant override the capability of C. dubia to endure the intoxication, confirming that this dye is highly toxic for C. dubia in subchronic exposures.
The chronic toxic effects on C. dubia fertility caused by consuming food exposed to the dye (EA) or not-exposed food (CA) is graphed in Fig. 4. Consumption of algae that grew during 4 days in the Congo Red IC50 (Fig. 1) significantly reduced the cladoceran’s reproduction, regardless of the quantity of food provided. Independently of whether the microalgae were able to grow in presence of the dye, it is evident by their effect on C. dubia that they internally retained the dye and/or the dye’s metabolites. Reducing the diet to 25 % of intoxicated algae (CA + EA) allowed fertility rates to recover slightly, yet the different food concentrations did not produce significant differences (Fig. 4). Our results thus show that the consumption of microalgae grown in the presence of sub-inhibitory concentrations of Congo Red produces toxic effects on C. dubia, regardless if a portion of the diet had not been exposed to the dye.
Fecundity of Ceriodaphnia dubia exposed to fresh algae (CA, control), Congo Red exposed algae (EA), and a mixture of fresh and intoxicated algae (75 % CA + 25 % EA). Average values and standard error bars are shown. Different letters above bars indicate significant differences (P < 0.05, Tukey’s pairwise comparisons)
Antunes et al. (2004) studied the positive effect of microalgae concentration on the reduction of toxicity. The authors observed that a high density of Selenastrum capricornutum (currently Rhapidocelis subcapitata) (6 × 105 cells mL−1) reduces chronic toxicity of the lindane pesticide on D. magna. Hauri and Horne (2004) found that a high concentration of algae (according to that suggested as adequate by the USEPA 2002a, b) reduces the availability of copper in chronic toxicity tests in C. dubia. It is accepted generally that appropriate availability of food provides the nutrients and energy required for cladocerans reproduction and growth (Rodgher and Gaeta Espíndola 2008). An appropriate availability of algae also allows for specific detoxification or resistance mechanisms to be expressed more effectively. This finding is based on the principle that well-nourished organisms are more tolerant to environmental stress and in particular to the toxic effects of chemical contaminants. However, our results also show that food can be a pathway to intoxication. Independently of its concentration, the negative effect of Congo Red predominates over the positive effect shown by the presence of non-exposed microalgae, as was observed in the CA + EA mixture (Fig. 4).
Other toxic compounds, such as metals, can adhere to the cell walls of microalgae and in this way could be ingested by filter feeders. Rodgher and Gaeta Espíndola (2008) report that when C. dubia was exposed to cadmium in the presence of a high density of algae (1 × 106 cells mL−1), this cladoceran filtered less volume of medium and ingested higher amounts of algae with metal adhered to its cells. This caused a reduction in both reproduction and survival. Conversely at low food densities (1 × 104 cells mL−1), the cladoceran filtered more water containing metal and ingested fewer algal cells. Hence, the lower intake of food could mean less energy available for reproduction (Rose et al. 2002). Wong et al. (2006) observed that the azo dyes Congo Red, Procion Red, and Procion Yellow decrease the filtration rate of Moina macrocopa. They explained that a lower filtration rate means less food is ingested and, consequently, less energy is available for growth and reproduction. In our study, we did not obtain evidence on the change of filtration rate provoked by Congo Red exposure; yet, independently of food concentration (Figs. 2, 3, and 4), the toxic effect of the dye on reproduction rates was noteworthy and significant (P < 0.01).
Figure 5 depicts the survivorship curves of C. dubia during a 10-day period. Intake of intoxicated algae (EA) produced higher mortality rates. Mortality was highest in the sample with the lowest concentration of food (Fig. 5a). Comparatively, in the 75 % CA + 25 % EA mixture (Fig. 5b), the presence of fresh algae reduced the toxic effect of the dye present in the intoxicated food. Higher survival rates were recorded in the sample with the highest concentration of food.
Survivorship curves of Ceriodaphnia dubia fed with different concentrations of C. vulgaris grown in culture medium supplemented with Congo Red (EA) a, and with a mixture of 75 % fresh and 25 % intoxicated algae (CA + EA) b; control group was fed fresh algae with no exposure to the toxicant. Asterisks indicate differences with respect to the control: *P < 0.05, or **P < 0.01, Kaplan-Meier test and pairwise comparisons (Gehan-Breslow-Wilcoxon Test)
Congo Red can adhere to the cell walls of microalgae (Hernández-Zamora et al. 2015). Cell walls can be an entry route thus increasing the intoxication of cladocerans. In our case, this effect can be discarded since the washing method applied to C. vulgaris eliminated any trace of dye molecules on the cell wall. We can thus assume that the dye was found inside the cells and that feeding on them intoxicated organisms. Kilham et al. (1997) mention that food quality is less important than quantity for cladocerans fertility, growth, and survival. Quality is less important because compensatory mechanisms may occur through changes in filtration rates. However, this study shows that the intake of intoxicated microalgae negatively affected fertility and survival, regardless of whether or not part of the diet was constituted by nontoxic microalgae.
Lastly, Fliedner (1997) mentions that the digestive tracts of cladocerans are an important pathway toward chemical compound exposure. A toxic substance bound or incorporated in food particles can be easily filtrated and released into the digestive tract. Figure 6b clearly shows this phenomenon. The digestive tract of C. dubia presents red coloration, which contrasts with the green coloration resulting from consumption of nontoxic-containing microalgae seen in the control group (Fig. 6a). In this case red coloration is not given as a result of the filtration of microalgae previously exposed to Congo Red, but by consumption of fresh algae during subchronic exposure at 0.94 mg L−1 of the dye dissolved in the dilution water. This shows that even when food is not previously intoxicated, the dye possibly manages to enter through the flow of filtered water and/or through the adhesion of the dye to the cell wall of microalgae. The latter entry route represents an additional intoxication pathway as the test organisms come into contact with the toxicant dissolved in water. Similar results were reported by Yu et al. (2015) with Daphnia similis exposed to the dye Disperse Red 1.
The results obtained in this study prove that toxicity caused by Congo Red in daphnids is indicative of potential environmental and aquatic biota damage. Results suggest that it is necessary to regulate the discharge of effluents containing azo dyes, in particular Congo Red.
Conclusions
Ceriodaphnia dubia is an adequate test organism to evaluate the toxicity of azo dyes. Results show that Congo Red dye, even at very low concentration (0.94 mg L−1, equivalent to the LC1), has toxic effects on reproduction and survival rates of C. dubia.
The consumption of microalgae exposed to Congo Red also produces significant toxic effects on reproduction and survival of C. dubia. We determined that the quantity and quality of food were important aspects in the evaluation of the toxic effects produced by this dye.
It is necessary to review and evaluate the toxicity on organisms in aquatic ecosystems associated with discharges of the textile industry. Furthermore, we should study the effects on primary producers (which can retain dyes and make them available through feeding) and on zooplankton, which show toxic effects by direct exposure, as well as from consumption of intoxicated microalgae. The toxic effects on reproduction and survival were significant, even at concentrations that could be considered as acceptable for the protection of aquatic ecosystems.
References
Ali H (2010) Biodegradation of synthetic dyes—a review. Water Air Soil Pollut 213:251–273. doi:10.1007/s11270-010-0382-4
Antunes SC, Castro BB, Goncalves F (2004) Effect of food level on the acute and chronic responses of daphnids to lindane. Environ Pollut 127:367–375. doi:10.1016/j.envpol.2003.08.015
Bae JS, Freeman HS (2007) Aquatic toxicity evaluation of new direct dyes to the Daphnia magna. Dyes Pigments 73:81–85. doi:10.1016/j.dyepig.2005.10.015
Bafana A, Jain M, Agrawal G, Chakrabarti T (2009) Bacterial reduction in genotoxicity of Direct Red 28 dye. Chemosphere 74:1404–1406. doi:10.1016/j.chemosphere.2008.11.043
Bafana A, Krishnamurthi K, Devi S, Chakrabarti T (2008) Biological decolourization of C. I. Direct Black 38 by E. gallinarum. J Hazard Mater 157(1):187–193. doi:10.1016/j.jhazmat.2007.12.085
Bhaskar S, Kuldeep B, Faizal B (2015) Developments in Applied Phycology 7: Algae and environmental sustainability. Springer, New Delhi, India, p 194. doi:10.1007/978-81-322-2641-3, ISBN 978-81-322-2639-0
Blaise C, Férard JF (2005) Small-scale freshwater toxicity investigations. Vol. 1–Toxicity test methods. Springer, Berlin, Germany, p 422. ISBN 978-1-4020-3120-5
Brander SM, Mosser CM, Geist J, Hladik ML, Werner I (2012) Esfenvalerate toxicity to the cladoceran Ceriodaphnia dubia in the presence of green algae, Pseudokirchneriella subcapitata. Ecotoxicology 21:2409–2418. doi:10.1007/s10646-012-0996
Cheriaa J, Bettaieb F, Denden I, Bakhrouf A (2009) Characterization of new algae isolated from textile wastewater plant. J Food Agric Environ 7:700–704
Chia MA, Musa RI (2014) Effect of indigo dye effluent on the growth, biomass, production and phenotypic plasticity of Scenedesmus quadricauda (Chlorococcales). An Acad Bras Cienc 86(1):419–428. doi:10.1590/0001-3765201420130225
de Luna LA, da Silva TH, Nogueira RF, Kummrow F, Umbuzeiro GA (2014) Aquatic toxicity of dyes before and after photo-Fenton treatment. J Hazard Mater 276:332–338. doi:10.1016/j.jhazmat.2014.05.047
Ferraz ERA, Umbuzeiro GA, de-Alameida G, Caloto-Oliveira A, Chequer FMD, Zanoni MVB, Dorta DJ, Oliveira DP (2010) Differential toxicity of Disperse Red 1 and Disperse Red 13 in the Ames Test, HepG2 Cytotoxicity Assay, and Daphnia Acute Toxicity Test. Environ Toxicol 26(5):489–497. doi:10.1002/tox.20576
Fliedner A (1997) Ecotoxicity of poorly water-soluble substances. Chemosphere 35:295–305. doi:10.1016/S0045-6535(97)00156-2
Golka K, Kopps S, Myslak WZ (2004) Carcinogenicity of azo colorants: influence of solubility and bioavailability. Toxicol Lett 15:203–210. doi:10.1016/j.toxlet.2003.11.016
Gómez N, Sierra MV, Cortelizzi A, Capítulo AR (2008) Effects of discharges from textile industry on the biotic integrity of benthic assemblages. Ecotox Environ Safe 69:472–479. doi:10.1016/j.ecoenv.2007.03.007
Hauri JF, Horne HJ (2004) Reduction in labile copper in the 7-day Ceriodaphnia dubia toxicity test due to the interaction with zooplankton food. Chemosphere 56(7):717–723. doi:10.1016/j.chemosphere.2004.04.014
Hernández-Zamora M, Cristiani-Urbina E, Martínez-Jerónimo F, Perales-Vela HV, Ponce-Noyola T, Montes-Horcasitas M, Cañizares-Villanueva RO (2015) Bioremoval of the azo dye Congo Red by the microalga Chlorella vulgaris. Environ Sci Pollut Res Int. 14(22):10811–10823. doi:10.1007/s11356-015-4277-1
Hernández-Zamora M, Perales-Vela HV, Flores-Ortiz CM, Cañizares-Villanueva RO (2014) Physiological and biochemical responses of Chlorella vulgaris to Congo Red. Ecotox Environ Saf 108:72–77. doi:10.1016/j.ecoenv.2014.05.030
Kilham SS, Kreeger DA, Goulden CE, Lynn SG (1997) Effects of algal food quality on fecundity and population growth rates of Daphnia. Freshwater Biol 38:639–647. doi:10.1046/j.1365-2427.1997.00232.x
Kluttgen B, Kuntz N, Ratte HT (1996) Combined effects of 3,4-dichloroaniline and food concentration on life-table data of two related cladocerans, Daphnia magna and Ceriodaphnia quadrangula. Chemosphere 32:2015–2028. doi:10.1016/0045-6535(96)00081-1
Kooijman SAL, Metz JAJ (1984) On the dynamics of chemically stressed populations: The deduction of population consequences from effects on individuals. Ecotox Environ Saf 8:254–274
Martínez-Jerónimo F, Espinosa-Chávez F, Villaseñor-Córdova R (2000) Effect of culture volume and adult density on the neonate production of Daphnia magna, as test organisms for aquatic toxicity test. Environ Toxicol 15:155–159. doi:10.1002/1522-7278(2000)
Novotný C, Dias N, Kapanen A, Malachová K, Vándrovcová M, Itävaara M, Lima N (2006) Comparative use of bacterial, algal and protozoan tests to study toxicity of azo and anthraquinone dyes. Chemosphere 63:1436–1442. doi:10.1016/j.chemosphere.2005.10.002
Øllgaard H, Frost L, Galster J, Hansen O.C (1998) Survey of azo-colorants in Denmark: consumption, use, health and environmental aspects. Ministry of Environment and Energy, Denmark and Danish Environmental Protection Agency, No XX, Copenhagen, pp. 147–290
Purkait MK, Maiti A, DasGupta S, De S (2007) Removal of congo red using activated carbon and its regeneration. J Hazard Mater 145(1-2):287–295. doi:10.1016/j.jhazmat.2006.11.021
Pielz A (1999) The process of the reduction of azo dyes used in dyeing textiles on the basis of infrared spectroscopy analysis. J Mol Struct 511–512:337–344. doi:10.1016/S0022-2860(99)00176-3
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77(3):247–255. doi:10.1016/S0960-8524(00)00080-8
Rodgher S, Gaeta Espíndola LE (2008) Effects of interactions between algal densities and cadmium concentrations on Ceriodaphnia dubia fecundity and survival. Ecotox Environ Saf 71(3):106–114. doi:10.1016/j.ecoenv.2007.08.012
Rose RM, Warne MSTJ, Lim RP (2002) Food concentration affects the life history respond of Ceriodaphnia cf. dubia to chemicals with different mechanisms of action. Ecotox Environ Saf 51:106–114. doi:10.1006/eesa.2001.2137
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42:138–157. doi:10.1016/j.jtice.2010.06.006
Savin II, Butnaru R (2008) Wastewater characteristics in textile finishing mills. Environ Eng Manage J 7:859–864
Sharma KP, Sharma S, Sharma S, Singh PK, Kumar S, Grover R, Sharma PK (2007) A comparative study on characterization of textile wastewaters (untreated and treated) toxicity by chemical and biological test. Chemosphere 69:48–54. doi:10.1016/j.chemosphere.2007.04.086
Shore J (1996) Advances in direct dyes. Indian J Fibre Text 21:1–29
Sofyan A, Price DJ, Birge WJ (2007) Effects of aqueous, dietary and combined exposures of cadmium to Ceriodaphnia dubia. Sci Total Environ 385:108–116. doi:10.1016/j.scitotenv.2006.07.003
Sponza DT, Isik M (2005) Toxicity and intermediates of C.I. Direct Red 28 dye through sequential anaerobic/aerobic treatment. Process Biochem 40:2735–2744. doi:10.1016/j.procbio.2004.12.016
U.S. Environmental Protection Agency (2002a) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms. 4th edn, Office of Research and Development, Cincinnati, OH, EPA-821-R-02-013
U.S. Environmental Protection Agency (2002b) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organism. EPA-821-R-02-012. 5th edn, Office of Research and Development, Cincinnati, OH
Versteeg DJ, Stalmans M, Dyer SD, Janssen C (1997) Ceriodaphnia and Daphnia: A comparison of their sensitivity to xenobiotics and utility as a test species. Chemosphere 34:869–892. doi:10.1016/S0045-6535(97)00014-3
Wong K, Liu XJ, Lee AOK, Wong PK (2006) Effect of azo dyes on survivorship, oxygen consumption rate, and filtration rate of the freshwater cladoceran Moina macrocopa. Hum Ecol Risk Assess 12:89–300. doi:10.1080/10807030500531604
Yu TH, Dafre AL, de Aragao Umbuzeiro G, Franciscon E (2015) CYP-dependent induction of glutathione S-transferase in Daphnia similis exposed to a disperse azo dye. Ecotoxicology 24(1):232–237. doi:10.1007/s10646-014-1348-x
Acknowledgments
Miriam Hernández-Zamora received a graduate studies scholarship (204491) granted by the Consejo Nacional de Ciencia y Tecnología, and thanks to the Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional for the financial aid rendered and the Escuela Nacional de Ciencias Biológicas for providing the necessary facilities to conduct the present study. Fernando Martínez-Jerónimo acknowledges the Secretaria de Investigación y Posgrado I. P. N., and the Comisión de Operación y Fomento de Actividades Académicas del I. P. N. for the support provided. All the authors are also thankful to Mrs. Ingrid Mascher for copyediting this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests regarding the funding sources and findings of this research.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed according to the Ethics Committee of the Escuela Nacional de Ciencias Biológicas, I. P. N.
Rights and permissions
About this article
Cite this article
Hernández-Zamora, M., Martínez-Jerónimo, F., Cristiani-Urbina, E. et al. Congo red dye affects survival and reproduction in the cladoceran Ceriodaphnia dubia. Effects of direct and dietary exposure. Ecotoxicology 25, 1832–1840 (2016). https://doi.org/10.1007/s10646-016-1731-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-016-1731-x