Abstract
Aquatic pollution caused by dyes has increased together with the growth of activities using colorants such as the textile, leather, food, and agrochemicals industries. Because most popular azo dyes are synthesized from benzidine, a carcinogenic compound, a threat to aquatic biota could be expected. The use of single species for toxicity assessment provides limited data, so a battery of test organisms, including representatives of different trophic levels such as algae, zooplankters, and fish, could undoubtedly provide more information. Therefore, our study was aimed at evaluating the toxic effect of the azo dye Direct blue 15 (DB15) on a battery of bioassays using a primary producer (Pseudokirchneriella subcapitata), a primary consumer (Ceriodaphnia dubia), and a secondary consumer (Danio rerio). P. subcapitata was more sensitive to DB15 (IC50 = 15.99 mg L−1) than C. dubia (LC50: 450 mg L−1). In the algae exposed to DB15, chlorophyll-a and -b were significantly increased, and carotenoids were reduced. The concentrations of protein, carbohydrates, and lipids per cell in P. subcapitata exposed to all DB15 concentrations were significantly higher than that measured in control. At 25 mg L−1 of DB15, survival, total progeny, and the number of released clutches were significantly decreased, and the start of reproduction was delayed in C. dubia. DB15 did not induce lethal or sublethal effects in D. rerio embryos at any of the tested concentrations from 24 to 72 h post-fertilization (hpf), but from 96 to 144 hpf, the larvae exposed to 100 and 500 mg L−1 developed yolk sac edema, curved tail, and skeletal deformations. After 144 hpf, DB15 produced a significant increase in embryos without a heartbeat, as the concentration of dye raised. The textile-used, azo dye DB15, caused toxic effects of different magnitude on microalgae, cladocerans, and zebrafish embryos; for this reason, the discharge of this colorant into waterbodies should be regulated to prevent environmental impacts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The dyeing process of fabrics produces approximately 90% of the total textile industry wastewaters containing significant concentrations of dyes (Zaharia and Suteau 2013). The main synthetic colorants are azo dyes (Balapure et al. 2015), being the most important producers USA, China, India, and Middle East countries (Zaharia and Suteu 2012).
Effluents containing dyes reduce light penetration in waterbodies and affect the photosynthesis of phytoplankton (Hernández-Zamora et al. 2014). Azo dyes (such as Direct Blue 15, DB15), are made from benzidine, a carcinogenic compound (Golka et al. 2004). DB15 has a strong affinity to cellulose fibers and is, thus, employed in textile industries (Rehman et al. 2017). According to European regulations (SCCNFP 2002), DB15, when degraded, produces carcinogenic amines. Its use has been banned in India since 1997 (Ministry of Environment and Forest 1997); however, it is manufactured in India for exportation and consumption in other countries.
The environmental impact caused by the discharge of textile dyes effluents has been scarcely studied. Even at low concentration (1 mg L−1), dyes could be highly perceptible in waterbodies, causing not only aesthetic pollution but disturbing aquatic ecosystems and water resources (Vimonses et al. 2009). In recent years, information about concentrations of azo dyes in the aquatic environment has been published. In the rivers Piracicaba and Quilombo (Brazil), Vacchi et al. (2016) reported that Disperse Red 1 was detected in a range of 50 to 500 ng L−1. Zocolo et al. (2015) estimated that the concentrations of the dyes Disperse 1, Disperse blue 373, and Disperse violet 93 ranged from 84.4 to 3453.3 ng L−1 in samples from the Piracicaba and Cristais Rivers, in the state of Sao Paulo, Brazil. In North America, Environment Canada and Health Canada (2009, 2013a, 2013b) estimated concentrations of 3.7, 0.40–10.8, and 0.12–102.7 µg L−1 for disazo dyes, azo solvents, and azo disperse dyes, respectively in Canadian waters. Disperse blue 79, Disperse blue 26, and Disperse red 60 were detected in samples of water, suspended solids, and sediments in a river in Canada (Maguire and Tkacz 1991).
Not enough data is available for Mexico; however, Villegas-Navarro et al. (1999) reported that in the State of Puebla, Mexico, were 353 textile industries that use great quantities of water. From this number, 53 facilities are for dyeing of textiles, and they discharge polluted colored effluents to water bodies.
The toxic impact of dyes has been demonstrated on aquatic and terrestrial organisms and in humans (Umbuzeiro et al. 2005; Puvaneswari et al. 2006; Copaciu et al. 2013). Toxicity of some azo dyes has been studied in aquatic organisms including algae, crustaceans, and fish (Bafana et al. 2011; Hernández-Zamora et al. 2016); nevertheless, some of these dyes have been studied only with a reduced number of test organisms.
Due to their importance as primary producers and their sensitivity to chemical stressors, microalgae have been used to assess environmental perturbations and as indicators of water quality (Mohamed 1994). Microalgae have been used to evaluate the toxicity of chemical pollutants as metals, herbicides, insecticides, and, recently, dyes (Levy et al. 2007; Wen et al. 2011; Jena et al. 2012; Hernández-Zamora et al. 2016).
Cladocerans are one of the main components of the zooplankton community and are important inhabitants of most water bodies (Wong et al. 2006). Daphnia magna and Ceriodaphnia dubia are reference test organisms for ecotoxicological studies (Blaise and Férard 2005). Although information about the effects of dyes to aquatic invertebrates is scarce, some studies have determined the toxicity of azo dyes to cladocerans (Villegas-Navarro et al. 1999; Bae and Freeman 2007; Ferraz et al. 2011; Malik et al. 2018; Oliveira et al. 2018).
Zebrafish embryos have been widely used as a model for toxicity studies of chemicals due to their rapid development and easy identification of different endpoints, easy maintenance in the laboratory and large fecundity; in addition, the high transparency of the chorion allow elucidating toxic effects on embryogenesis and larval development (Kimmel et al. 1995; Hill et al. 2005; Scholz et al. 2008; Lammer et al. 2009). Recently, zebrafish have been used to determine the toxicity of some dyes (Parrott et al. 2016; Rodrigues de Oliveira et al. 2016; Abe et al. 2017; Meireles et al. 2018).
The environmental impact caused by the discharge of textile dyes into water bodies and the toxicity to aquatic biota have not been fully documented. Regarding the toxic effects of azo dyes, most of the few available studies have been done with single-species tests, thus, no complete scenario can be elucidated to establish the expected, variable damages, when diverse organisms are exposed to these potential pollutants. With this aim, we studied the toxic effect of the azo dye Direct blue 15 on a battery of bioassays, including microalgae (Pseudokirchneriella subcapitata), a cladoceran (Ceriodaphnia dubia), and a fish fed on zooplankton (Danio rerio).
Materials and methods
Direct blue 15
This anionic diazo direct dye was obtained from Sigma-Aldrich® (C34H24N6O6S4Na4; molecular weight: 992.80 g mol−1; CAS No: 2429-74-5). For all experiments, the dye was prepared as a stock solution at 1000 mg L−1. This compound is a deep purple to dark blue microcrystalline powder that decomposes when exposed to air. It is highly soluble in water (60 g L−1 at 85 °C), and insoluble in most organic solvents. It is also called Direct sky blue A and Direct sky blue 5B (Rehman et al. 2017).
Test organisms
The test organisms (Pseudokirchneriella subcapitata, Ceriodaphnia dubia, and Danio rerio embryos) were obtained from the collections of the Laboratorio de Hidrobiología Experimental, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, México.
The microalga was grown in Bold’s basal mineral medium (Stein 1973) using flat-sided glass bottles with a total capacity of 0.5 L and a test volume of 0.25 L. Incubation conditions were: temperature: 24 ± 1 °C, light intensity: 120 μmol m−2 s−1, constant illumination, and continuous bubbling with air flow of 200 mL min−1.
Ceriodaphnia dubia was cultured in ISO medium (2 mM CaCl2·2H2O, 0.5 mM MgSO4·7H2O, 0.75 mM NaHCO3, 0.07 mM KCl) (ISO 1996) and deionized water 1:5. Cultures were maintained at 25 ± 1 °C, and 16:8 h (light: darkness photoperiod). The cladocerans were fed with the microalga P. subcapitata at a concentration of 1 × 106 cells mL−1. For neonate production, 24 h before the experiments, adult parthenogenetic females were separated and neonates produced from these adults were used in the study (USEPA 2002).
Adult zebrafish males and females have been successfully cultured under controlled conditions for over eight years. Fish are maintained in 40-L glass aquaria at 26 ± 1 °C and photoperiod 16:8 h (light: darkness). Fish are fed with commercial micropellets (Azoo® Plus Ultra Fresh Tropical Bits) 3-times daily at regular intervals, complemented sporadically with cultured live food (small daphnids). One week before spawning, reproducers were fed daily with daphnids to promote optimal mating.
Direct blue 15 toxicity to microalga P. subcapitata
Tha algal growth inhibition test was performed according to the OECD protocol 201 (OECD 2011) to determine the toxic effects of DB15. For the bioassays, vials with inocula of P. subcapitata (1 × 104 cells mL−1) were exposed to various concentrations of DB15 (4, 8, 16, 32, and 64 mg L−1) in OECD medium (pH 8.1). Incubation conditions for the tests were 24 ± 1 °C, and continuous fluorescent light (120 μmoles m−2 s−1). Algal growth was determined in the samples with a Neubauer hemocytometer every 24 h for four days. The median inhibitory concentration (IC50) was obtained from three independent assays, with three replicates for each bioassay.
Sub-inhibitory toxicity test in P. subcapitata
Experiments were performed in flat-sided glass bottles with a total capacity of 0.5 L and a test volume of 0.25 L of OECD medium, inocula of P. subcapitata (1 × 104 cells mL−1) and the concentrations of 1.63, 3.57, 6.29, 10.19, and 15.99 mg L−1 of DB15 equivalent to the IC10, IC20, IC30, IC40, and IC50, respectively. Test organisms were incubated at 24 ± 1 °C, continuous illumination of 120 μmol m−2 s−1, and constant bubbling with an air flow of 200 mL min−1. The cell density was determined daily with a Neubauer chamber. After 96 h of exposure, the microalgae pellet was washed three times using OECD medium to eliminate all the dye remnants and the dye possibly adhered to the cell wall, following the centrifugation at 3500 rpm for 10 min. Finally, the cells were resuspended in OECD medium and stored in darkness at 4 °C to determine photosynthetic pigments and macromolecules.
Photosynthetic pigments
Photosynthetic pigments of the cells contained in 500 μL sample were extracted with 600 μL dimethyl sulfoxide. Absorbance was read at 665, 649, and 470 nm. The concentration of Chl-a, Chl-b, and total carotenoids was determined according to the equations of Wellburn (1994):
Protein content in P. subcapitata
Proteins were quantified according to Lowry et al. (1951) using a calibration curve of albumin in a standard solution; 500 μL of P. subcapitata cells was extracted with 300 μL of a 1 N solution of NaOH at 90 °C for 15 min to extract soluble protein. After this, the sample was centrifuged at 14,000 rpm for 5 min, and 100 μL of supernatant was used to determine the amount of protein. Absorbance of samples and standard solution was read at 590 nm.
Carbohydrates content in P. subcapitata
Cells contained in 500 μL were pretreated with 200 μL of 2 N HCl at 90 °C for 1 h. Total carbohydrates were determined according to Dubois et al. (1951). Carbohydrates quantitation was based on calibration curves using glucose as standard. Absorbance of samples and standard solution was read at 490 nm.
Lipids
Lipids of algal cells (500 μL) were extracted with a solution of chloroform: methanol (2:1) and, subsequently, evaporated at 80 °C. Lipids were determined according to Zöllner and Kirsch (1962). Cholesterol was used as standard solution. Absorbance of samples and standard solution was read at 525 nm.
Direct blue 15 toxicity to Ceriodaphnia dubia: acute and chronic toxic effects
The acute toxic effects of DB15 dye on C. dubia were determined according to USEPA (2002) guidelines. For the bioassays, we used different concentrations of DB15 (25, 50, 100, 200, 300, and 400 mg L−1) diluted in ISO medium and deionized water (1:5); test volume was 30 mL in each of three replicates per test. Thereafter, 10 neonates were transferred to each test vessel and incubated in an environmental chamber at 24 ± 1 °C, with a photoperiod of 16:8 h (light: darkness). No food was supplied during the assays. The number of affected, immobilized, and dead (no heartbeat, through observation with the stereomicroscope) organisms were recorded at 24 and 48 h. Three independent bioassays were carried out to calculate the average LC50.
The chronic toxicity of DB15 was determined following Method 1002.0 Daphnid, Ceriodaphnia dubia, survival, and reproduction test (USEPA 2002). The bioassays were started with neonates, and each experiment had 10 replicates. Neonates were individually placed in recipients containing 25 mL of test solution (ISO medium and deionized water 1:5) and different concentration of DB15 (5, 10, 15, 20, and 25 mg L−1). C. dubia organisms were fed daily the microalga P. subcapitata (1 × 106 cells mL−1). The test solutions and food were renewed daily.
Bioassays were incubated in an environmental chamber at 24 ± 1 °C and 16:8 h (light: darkness) photoperiod. Survival, accumulated progeny, age at first reproduction, and the number of clutches were daily recorded for seven days.
Direct blue 15 toxicity to zebrafish embryos: lethal and sublethal endpoints
To obtain fertilized zebrafish eggs, we followed the protocol proposed by the OECD Guideline 236 (OECD 2013). Briefly, the day before the test, adult, mature zebrafish males and females (ratio 2:1) were placed in spawning tanks in a room with controlled temperature and photoperiod (16:8, light: darkness). After the light turned on the next morning, spawn was promoted, and fertilized eggs were obtained by natural mating. After spawning, the eggs were collected by hand, rinsed with ISO medium, and checked under a stereomicroscope; unfertilized eggs were discarded, and live embryos were used for the toxicity tests.
We followed the procedure described in the OECD guideline 236 (OECD 2013) and by Busquet et al. (2014). Two sets of DB15 concentrations were tested: (1) 6.25, 12.5, 25, 50, 100 mg L−1 and (2) 100, 200, 300, 400, and 500 mg L−1. 3, 4-Dichloroaniline (4 mg L−1) was used as positive control, and ISO medium: deionized water (1:5) was the negative control and dilution water.
Viable eggs were individually placed into 24-well plates, each well containing 2 mL of test solution. For all experiments, 20 eggs for each concentration of DB15 (one plate for each concentration) and four eggs as the internal negative control (ISO medium: deionized water 1:5) were used, as well as one plate with 20 eggs as the positive control and 24 eggs as negative external control. The samples were incubated at 26 ± 1 °C for 144 h with a photoperiod of 16:8 h (light: darkness) in an environmental chamber. Test solutions were fully renewed at 24, 72, and 120 h, as indicated in the guideline.
Toxic effects of DB15 on early life stages (ELS) of zebrafish were determined according to the criteria described in the OECD guideline 236 through observations under a stereomicroscope at 24, 48, 72, and 96 h, but extended observations were performed at 120 and 144 h post fertilization (hpf). Lethal endpoints assessed were: coagulation, no tail detachment, no somite formation, and no heartbeat (this last one measured as presence and absence after 48 h). Larval survival and malformations were documented and photographed every day after hatching. Sublethal endpoints used to assess the effects of DB15 on zebrafish development included the presence of yolk sac edema, pericardial sac edema, skeletal deformities (curved tail) and delayed pulse. These endpoints were qualitatively evaluated as presence or absence; the affected embryos and larvae were also counted.
Statistical analysis
The median inhibitory concentration (IC50) and the median lethal concentration (LC50) with corresponding 95% confidence intervals were calculated via the Probit method, using RA software (Risk Assessment. Hazard Assessment Tools, v. 1.0).
In P. subcapitata and C. dubia, an analysis of variance (ANOVA) was performed using Dunnett’s comparison test to identify significant differences between the concentrations of DB15 and the control. Tukey’s pairwise multiple comparisons test was carried out to identify significant differences among all the treatments, including the control. Kruskal–Wallis test was used to analyze the toxicity of Direct blue 15 on zebrafish, followed by Dunnett’s and Tukey’s pairwise tests. All statistical analyses were completed using Statistica ver. 10.0 and SigmaPlot ver. 12.0.
Results
Direct blue 15 effects on the growth of P. subcapitata
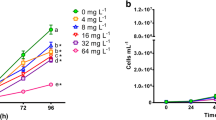
The 96-h growth inhibition trend lines of algae exposed to DB15 are shown in Fig. 1a, b. P. subcapitata growth was affected by exposure to the dye at all tested concentrations. ANOVA test demonstrated significant differences (P< 0.05) for population density values among treatments, determined at 96 h. Cell density of microalgae in the control group was significantly higher than at all the tested DB15 concentrations (Dunnett’s test, Fig. 1a).
Population growth (a) and growth inhibition (b) of P. subcapitata exposed during 96 h to different concentrations of Direct Blue 15. Mean values ± standard error. * Indicates significant differences (P < 0.05) with respect to the control (Dunnett’s test). Different letters indicate significant differences (P < 0.05) after Tukey’s pairwise comparisons
The 96-h growth inhibition at the concentrations of 4, 8, 16, 32, and 64 mg L−1 was 29%, 41%, 48%, 68%, and 80%, respectively, compared to the control (Fig. 1b). The determined median inhibition concentration (IC50) for DB15 was 15.99 mg L−1 (95% CI limits: 12.94–19.75 mg L−1).
Direct blue 15 effects on the pigments and macromolecules content of P. subcapitata
Figure 2a shows the concentration of photosynthetic pigments quantified at the end of the test (96 h). Chlorophyll a in cells exposed to 3.57, 10.19, and 15.99 mg L−1 DB15 was significantly higher than in the control (Dunnett’s test, P< 0.05). Chlorophyll b content was significantly lower in the control that at all the dye concentrations, but carotenoids content displayed an inverse situation. Tukey’s differences among treatments and the control are shown in Fig. 2a.
Biomarkers in cells of P. subcapitata exposed to sub-inhibitory concentrations of Direct Blue 15 during 96 h: (a) concentration of photosynthetic pigments, and (b) concentration of macromolecules. Mean values ± standard error bars. *Indicates significant differences (P < 0.05), comparing with the control (Dunnett’s test). Different letters above the bars indicate significant differences (P< 0.05) for each set of pigments or macromolecules (proteins, carbohydrates, and lipids) data, after Tukey’s pairwise comparisons
Figure 2b shows significant differences in the concentration of macromolecules (protein, carbohydrates, and lipids) at the end of the sub-inhibitory assay. The concentration of protein per cell in P. subcapitata exposed to any of the dye concentrations was significantly higher than that measured in the control (Dunnet’s test, P< 0.05), and was significantly different among them (Tukey’s test, P< 0.05). The highest protein concentration was observed in cells exposed to 6.29 mg L−1 of dye.
The carbohydrate concentrations in cells exposed to any of the DB15 concentrations were significantly higher, according to the Dunnett’s test (P < 0.05), than that measured in the control. Figure 2b shows that, among treatments (1.63, 13.57, 10.19, and 15.99 mg L−1), there were no significant differences according to Tukey’s test (P < 0.05). The highest carbohydrate content was measured in cells exposed to 6.29 mg L−1 of dye.
DB15 exposure significantly augmented lipids concentration in cells of microalgae respect to the control (Dunnett’s test). The concentration of lipids per cell was not significantly different among treatments, except for 6.29 mg L−1 according to Tukey’s test (P < 0.05), as shown in Fig. 2b.
Effect of Direct blue 15 on cladocerans
Acute toxicity test results demonstrated that the median lethal concentration (LC50) for C. dubia was 450 mg L−1 of DB15 (95% limits: 422.65–476.34 mg L−1). Although the LC50 is not an environmentally relevant concentration for cladocerans, we observed that the digestive tract of test organisms was remarkably blue colored, hence toxic effects could be expected at chronic exposures.
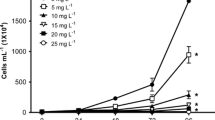
Figure 3a–d show the effect of chronic DB15 exposures on survival, accumulated progeny, age at first reproduction, and the number of clutches of C. dubia. The percentage of survival was 100% after seven days of exposure at all concentrations of DB15, except for 25 mg L−1 (Fig. 3a). Accumulated progeny decreased significantly (Fig. 3b, P< 0.05) in organisms exposed to 20 and 25 mg L−1 respect to the control (according to Dunnett’s test). As observed in Fig. 3c, the age at first reproduction was significantly higher (P< 0.05) at the 25 mg L−1 concentration respect to the control and the other dye concentrations. The two highest concentrations of DB15 (20 and 25 mg L−1) caused the reduction of clutches in 37 and 70%, respectively, compared to the control (Dunnett’s test, Fig. 3d).
Effect of Direct Blue 15 on Ceriodaphnia dubia (a) survival, (b) accumulated progeny, (c) age at first reproduction and (d) number of clutches. Mean values ± standard error. *Indicates significant difference (P < 0.05) compared with the control (Dunnett’s test). Different letters above the bars indicate significant differences (P < 0.05), after Tukey’s pairwise comparisons
Effect of Direct blue 15 on zebrafish embryos
Fish embryos were exposed to two different DB15 concentration ranges (0–100 and 0–500 mg L−1) to determine their toxic effects. In the first assay, hatching started at 72 h in the controls and at all DB15 concentrations (Fig. 4a), and 100% hatched larvae were observed at 96 h. However, in the experiment where the range of 0–500 mg L−1 was tested, hatching was significantly reduced in all the DB15 concentrations, except in 100 mg L−1; at 144 h hatching percentage was 35%, 35%, 10% and 5%, respectively in the concentrations of 200, 300, 400 and 500 mg L−1 (Dunnett’s test) (Fig. 4b, P < 0.05).
Figure 5 shows the development of the zebrafish embryos exposed to 6.25, 100, and 500 mg L−1 DB15. Exposure to the dye from 24 to 72 hpf did not induce lethal effects (coagulation, no tail detachment, no heartbeat, or no somite formation) or sublethal effects (skeletal deformations and yolk sac edema) at all tested concentrations (Fig. 5a–d). However, from 96 to 144 hpf, the organisms exposed to 100 and 500 mg L−1 showed yolk sac edema, curved and deformed tail (Fig. 5c, d).
Toxic effects of Direct blue 15 on the development of zebrafish embryos. (a) Normal embryo and larva at 144 hpf, (b) Normal embryo in 6.25 mg L−1 of dye at 144 hpf, (c) Larvae of 96–144 hpf with pericardial sac edema and yolk sac edema (arrows) exposed to 100 mg L−1 of dye, (d) Larvae of 120–144 hpf with tail deformation (arrows), observed at 500 mg L−1 of DB15
The percentage of lethal (no heartbeat) and sublethal effects (delayed pulse, curved tail, and yolk sac edema) observed at 144 hpf in early life stages of zebrafish are shown in Fig. 6. Exposure to DB15 produced a significant increase in no heartbeat frequency, as the dye concentration increased (P< 0.05), however, yolk sac edema only increased significantly in the organisms exposed to 100, 200, and 300 mg L−1, according to Tukey’s test. The percentage of larvae with delayed pulse and curved tail did not show significant differences at DB15 concentrations from 100 to 500 mg L−1 (P< 0.05).
Effects on zebrafish embryos after 144 hpf exposure to different concentrations of Direct Blue 15. The effects included no heartbeat (NH), delayed pulse (DP), curved tail (CT), and yolk sac edema (YSE). Mean values ± standard error bars (n = 20). Different letters above the bars indicate significant differences (P< 0.05) for each endpoint after Kruskal-Wallis’s test followed by Tukey’s pairwise comparisons
Discussion
The green alga P. subcapitata is ubiquitous in freshwater ecosystems and is frequently used as test organism due to its high sensitivity to toxicants (Greene and Baughman 1996; Croce et al. 2017). Results demonstrated that the growth of the microalga P. subcapitata exposed to DB15 was inhibited as the dye concentration increased (Fig. 1a, b). Similar results were reported by Lim et al. (2010) when the microalga Chlorella vulgaris was grown on textile wastewaters containing azo dyes. Chia and Musa (2014) showed that effluents containing indigo dye were toxic to Scenedesmus quadricauda, having negative effects on growth, biomass production, and the number of cells per coenobium. Moreover, Greene and Baughman (1996) demonstrated that six dyes inhibited population growth in more than 20% in Selenastrum capricornutum; however, seven basic textile dyes stimulated growth by >20% compared to control cultures. These opposite results can be related to the exposure conditions and the species-specific effects; this should be taken with care to discard toxic effects, because many others results, including ours, have demonstrated inhibitory effects in the population growth of microalgae.
Øllgaard et al. (1998) mentioned that when the dye is present at high concentrations, the resulting growth inhibition in microalgae is produced by toxicity inside the cell. Roy et al. (2010) showed that textile dyes produced growth inhibition in freshwater microalgae because of the reduction in cell division and photosynthetic activity. Hernández-Zamora et al. (2014) reported that the alteration of photosynthetic activity at the electron donor stage of photosystem II was one of the possible causes of the growth inhibition in the microalga C. vulgaris exposed to Conge Red dye.
The sensitivity of P. subcapitata to DB15 was demonstrated as the mean inhibitory concentration (IC50 = 15.99 mg L−1, 95% confidence limits: 12.94–19.75 mg L−1). Greene and Baughman (1996) reported that P. subcapitata had EC50 values of 0.025 and 0.247 mg L−1 for dyes 28 and 41, respectively. The 96-h EC50 values of Reactive Orange 16, Congo Red, Remazol Brillant Blue R, and Disperse Blue 3 reported for P. subcapitata are 7.8, 4.8, 81.1, and 0.5 mg L−1, respectively (Novotný et al. 2006); some of these values are lower than the one determined in the present study for DB15, but differences could be related to the conditions used for their determination.
Moreover, Tigini et al. (2011) demonstrated that P. subcapitata was sensitive to dyes such as Acid Bath for wool (EC50 18.8%), Acid Bath for leather (EC50 17.7%), Reactive Bath for cotton (EC50 2.2%), and Direct Bath for cotton (EC50 31.7%). Vacchi et al. (2016) and de Luna et al. (2014) reported comparatively higher values of EC50 for P. subcapitata exposed to Disperse Red and Acid Orange 7 (102 and >100 mg L−1, respectively). Similarly, another study with P. subcapitata demonstrated that Direct Blue 71 and Direct Blue 293 produced EC50 values of 62.260 and 137.975 mg L−1, respectively (Croce et al. 2017). However, other studies showed algae susceptibility below 100 mg L−1 for different azo dyes, in the following order: mordant > basic/acid/disperse > direct > hydrolyzed > reactive dyes (ETAD 1992; Øllgaard et al. 1998).
Based on the presented information, we can infer that the sensitivity of P. subcapitata to dyes could be related mainly to the chemical structure of colorants, the exposure time, and the exposure conditions, but invariably toxic effects on the growth of microalgae have been documented. Most of the textile dyes are not available as pure reagents; DB15 contains other components, such as organic compounds related to the synthesis of the dye, and inorganic salts (sodium chloride, sodium sulfate); these impurities are present in the product sold as a chemical compound (from Sigma-Aldrich and other suppliers), and in the commercial form (Shan and Harbin 1988). The dye and all these accompanying impurities are used as a whole during the dyeing process and could contribute to the toxic effects produced, but also could be related with the variable effects reported in the growth of P. subcapitata.
The photosynthetic pigments content has been used as a sensitive parameter for microalgae under environmental stress conditions caused by heavy metals and dyes (Hernández-Zamora et al. 2014; Martínez-Ruiz and Martínez-Jerónimo 2015). Toxic compounds could affect the synthesis of chlorophyll-a, -b and carotenoids in algae. DB15 exposure stimulated the content of chlorophylls in P. subcapitata; however, the carotenoids content was diminished, as observed in Fig. 2a. Similar effects were observed by Hernández-Zamora and Martínez-Jerónimo (2019) when P. subcapitata was exposed to Congo Red dye. The increase in photosynthetic pigments could be a response to compensate the chemical stress produced by the dye and to reduce the toxic effect; this reaction can also be related to adaptations to perform the photosynthesis in the colored culture media, after the addition of the dye.
Mahalakshmi et al. (2015) reported that when Haematococcus sp. was exposed to different concentrations of Congo Red, the content of chlorophyll-a showed the highest accumulation on the 5th day at 12 mg L−1. In contrast, Gita et al. (2019) observed that the textile dyes Optilan yellow, Drimarene blue and Lanasyn brown, inhibited chlorophyll and carotenoids content in Chlorella vulgaris; in this case, the observed effects could be related to a toxic response. Nevertheless, in Chlamydomonas reinhardtti exposed to 10 and 20 mg L−1 of Malachite green and Crystal violet, Yadav et al. (2014) reported that the chlorophyll content was not significantly modified; this result could indicates that the concentration of these dyes was low enough to produce changes in this endpoint.
The concentration of proteins, carbohydrates, and lipids per cell increased significantly in P. subcapitata under DB15 stress (Fig. 2b). The increase of proteins in P. subcapitata is an effect similar to that reported by Einicker-Lamas et al. (2002) with Euglena gracilis and by Rocha et al. (2016) with Selenastrum gracile; these authors suggested that the inability of cells to divide could be responsible for the increase in this biomolecule production. However, Gita et al. (2019) reported that comparatively higher concentrations of Optilan yellow, Drimarene blue, and Lanasyn brown reduce the content of protein in Chlorella vulgaris.
Algae use light and nutrients to produce proteins, carbohydrates, and lipids during photosynthesis; however, under unfavorable conditions, microalgal growth is arrested, and the energy in excess could be stored as carbohydrates and lipids (George et al. 2014). D’Alessandro and Antoniosi Filho (2016) reported that lipid metabolism could be modified in response to several factors that induced lipid accumulation, as a strategy to assure their growth and reproduction via cell division; this effect was observed in P. subcapitata exposed to DB15 as a reduction in the population growth (Fig. 1b), and increase in lipid content (Fig. 2b).
Seo et al. (2015) reported in C. vulgaris exposed to rhodamine 101 (red solution) and 9,10-diphenyl anthracene (blue solution) increases in lipid content of 1.4 and 1.6 times, respectively, compared with the control. Ruyters (1984) reported that blue light stimulates nitrogen metabolism and the activation of enzymes related to lipid synthesis. We can assume that the increase in lipid content we observed in P. subcapitata is produced by the color of the dye solution, in addition to the toxicity of DB15.
Hernández-Zamora and Martínez-Jerónimo (2019) reported that when P. subcapitata was exposed to Congo red, the proteins, carbohydrates and lipids content increased in almost all the tested concentrations, similar to the effects we observed in the present study. These changes, as a toxic response to DB15, could affect the nutrimental quality of microalgae, with consequences to filter-feeder consumers in natural conditions, but this issue must be further studied.
Direct Blue 15 did not induce significant acute toxic effects on C. dubia after 48 h (LC50 450 mg L−1). Similarly, Rocha et al. (2017) demonstrated that Acid Black 210 dye does not cause acute toxicity in the cladoceran D. similis (EC50 was 2993.73 mg L−1). Croce et al. (2017) reported that the LC50 for D. magna exposed to Direct Blue 293, Direct Blue 71, and Direct Red 227 was higher than 100 mg L−1. Dyes with a 48-h LC50 higher than 100 mg L−1 are considered as non-toxic, whereas those with 48-h LC50 values lower than 100 mg L−1 are classified as toxic and of toxicological concern for aquatic biota (Zucker 1985; OECD 2004). According to this classification, DB15 should be classified as non-toxic, but this classification is valid only for acute, lethal effects because chronic exposure to sublethal concentrations could produce toxic effects that can be a risk for aquatic organisms.
Survival, accumulated progeny, age at first reproduction, and the number of clutches were examined during chronic exposure of C. dubia to DB15 (Fig. 3a–d). Survival of C. dubia was 100% after seven days at almost all tested concentrations (Fig. 3a). For other cladocerans, Wong et al. (2006) observed that the survival of Moina macrocopa exposed to different concentrations of Procion Red, Procion Yellow, and Congo Red decreased with increasing dye concentration.
Walthall and Stark (1999) reported that Daphnia pulex exposed to up to 250 mg L−1 of fluorescein did not show a significant reduction in survivorship after ten days; however, a decrease of 80% was documented at 350 mg L−1. In our study, DB15 tested concentrations were lower than the used by Walthall and Stark (1999), and no significant effects were observed in this endpoint.
Regarding reproductive effects, our results demonstrated that the accumulated progeny was significantly reduced in C. dubia exposed to 20 and 25 mg L−1 DB15 (Fig. 3b). Similarly, Wong et al. (2006) reported that the total number of neonates produced by M. macrocopa after seven days exposure to Procion Red, Procion Yellow, and Congo Red was reduced in 49.8, 44.5 and 69% respectively, compared with the control. In D. pulex exposed to fluorescein Walthall and Stark (1999) observed that reproduction in control was significantly higher (P < 0.05) than that observed in all the tested concentrations (200, 250, 350, 450, and 600 mg L−1).
Reduction in cladoceran fecundity is the consequence of intoxication produced by dye exposure. Schroder et al. (1991) reported that municipal effluents, containing toxic wastes, were also extremely toxic to C. dubia reducing significantly survival and reproduction even at low concentrations (6.25% and 10%). Reproduction in cladocerans exposed to chemical stressors is negatively affected because energy is invested in the detoxification process, thus reducing the amount of energy allocated to growth and reproduction, which could explain our present results.
The first reproduction of control C. dubia was recorded at 4-d age; however, cladocerans exposed to 25 mg L−1 of DB15 delayed reproduction until the sixth day (Fig. 3c). Wong et al. (2006) reported that reproduction in M. macrocopa was delayed by 1–1.5 days in organisms exposed to diazo dyes; delay in reproduction began at 0.01 mg L−1 for all three dyes, and cladocerans exposed to Procion Red at 100 mg L−1, and Congo Red at 1, 10, and 100 mg L−1 did not reproduce.
In our study, the number of clutches per female was significantly decreased in C. dubia by high DB15 concentrations (Fig. 3d). In M. macrocopa, Wong et al. (2006) reported that the onset of reproduction was delayed and the number of produced neonates was reduced at azo dye concentrations as low as 0.01 mg L−1. With other toxicants, similar results were reported by Souza et al. (2014) for the cladoceran Simocephalus serrulatus fed with cadmium-contaminated algae; they found significantly reduced clutches per female. Negative changes in accumulated progeny, age at first reproduction, and the number of released clutches by C. dubia exposed to DB15 can be regarded as a physiologically impaired response to DB15 stress.
The hatching rate of zebrafish embryos did not decrease at 100 mg L−1 of DB15 (Fig. 4a); however, from 200 to 500 mg L−1 hatching was diminished as the concentration of dye increased (Fig. 4b). Similarly, Manjunatha et al. (2014) reported that hatching of zebrafish embryos was affected by henna dye proportionally to its concentration. Parrott et al. (2016) reported that Acid Blue 80 and Acid Blue 129 caused no effects on larval fish at the highest measured concentrations they tested (7700 and 6700 μg L−1, respectively); however, Disperse Yellow 7 and Sudan Red G decreased survival of larval fish at 25.4 and 16.7 μg L−1, respectively. One probable explanation for the delayed hatching in our results could be due to the inability of the embryos to break the chorion, which was observed in some eggs. Another reason could be the inhibition of enzymes involved in hatching provoked by the dye (Du et al. 2012; Manjunatha et al. 2014).
After 72 hpf, DB15 did not induce lethal or sublethal effects in the range of 6.25 to 500 mg L−1. At 96 hpf abnormalities originated since the early exposure stages were now visible in the embryos, such as yolk sac edema, curved tail, and tail deformities (Fig. 5c, d). Joshi and Katti (2018) observed that 50% of hatched larvae exhibited tail distortion, yolk sac edema, and cardiac edema with 20 to 30 mM tartrazine (a synthetic colorant used in the food industry). Abe et al. (2017) reported that the synthetic dye Basic Red 51 and the natural dye, erythrostominone, induced in zebrafish embryos a large yolk sac that is related to deformities or delayed yolk resorption. Yolk sac malformation might be associated also with the hypoactivity of zebrafish larvae. Yolk sac plays a fundamental role in growth, associated with secretion of growth factors, cytokines, and proteins (Herbomel et al. 1999). Moreover, pericardial edema in zebrafish embryos has been associated with an abnormal intracellular Ca2+ signaling pattern (Tsuruwaka et al. 2015).
Lethal and sublethal effects such as no heartbeat, delayed pulse, curved tail, and yolk sac edema were observed with different DB15 concentrations in zebrafish larvae at 144 hpf (Fig. 6). Joshi and Katti (2018) observed that from 40 to 50 mM tartrazine, the larvae exhibited a reduction in heartbeat frequency followed by mortality within 96 to 144 hpf. Rodrigues de Oliveira et al. (2016) reported that Direct Black 38 and Vat Green 3 induced curved tail and yolk sac edema in zebrafish embryos at 25 and 100 mg L−1, respectively. Jang et al. (2009) observed blood circulation defects, including slow heartbeat, heart edema, blood accumulation near the heart region, and a big yolk in D. rerio embryos exposed to malachite green (150 mg L−1) at 72 hpf. According to our results, we could suppose that DB15 dye crossed the chorion barrier and for this reason, zebrafish had sublethal effects in embryos, evidenced after larvae hatched (Fig. 5c, d).
In contrast to our results, Abe et al. (2017) demonstrated that zebrafish embryos exposed to Basic Red 51 and erythrostominone did not present lethal or sublethal effects such as coagulation, not detached tail, malformation of somites, changes in the development of eyes, alterations in the pigmentation patterns, malformation of head, tail and otoliths, scoliosis, and retarded growth. These variable reports indicate that different synthetic dyes provoke diverse acute and chronic effects in early life stages of fish; these effects could be related with the chemical structure and purity of dyes, but differences in test conditions must be considered also. It is important to mention that some azo dyes are toxic, and some of the observed sublethal effects are difficult to quantify and hinder inferring their consequences in the future development of fish.
Conclusions
The magnitude of toxic effects in organisms exposed to chemical stressors is species-specific. In our study, the green microalga P. subcapitata was the most sensitive organism to Direct blue 15, because this azo dye inhibited population growth, altered the photosynthetic pigments concentration, and modified the content of proteins, lipids, and carbohydrates. Toxic damages to primary photosynthetic producers are relevant because a general disruption in aquatic food webs could be expected when compounds like this azo dye are discharged in waterbodies.
Despite the acute effects of DB15 on the planktonic crustacean Ceriodaphnia dubia were comparatively slight, this cladoceran was significantly sensitive in chronic exposure, displaying affectations in survival, fecundity, the beginning of the reproductive process, and a reduction in the number of released clutches.
Direct blue 15 can be an environmental toxic compound with the potential to provoke lethal and sublethal effects in early life stages of the Danio rerio fish.
The results obtained in these experiments confirmed that a battery of toxicity tests undoubtedly provide more information on the effects of emergent pollutants (like the azo dyes), than the use of single-species toxicity tests. P. subcapitata, C. dubia, and D. rerio can be used as test organisms to establish safe levels of dyes in the aquatic environment.
References
Abe FR, Mendonca JN, Moraes LAB, de Oliveira GAR, Gravato C, Soares AMVM, de Oliveira DP (2017) Toxicological and behavioral responses as a tool to assess the effects of natural synthetic dyes on zebrafish early life. Chemosphere 178:282–290. https://doi.org/10.1016/j.chemosphere.2017.03.030
Bae JS, Freeman HS (2007) Aquatic toxicity evaluation of new direct dyes to the Daphnia magna. Dyes Pigments 73:81–85. https://doi.org/10.1016/j.dyepig.2005.10.015
Bafana A, Sivanesan SD, Tapan C (2011) Azo dyes: past, present and the future. Environ Rev 19:350–371. https://doi.org/10.1139/a11-018
Balapure K, Bhatt N, Madamwar D (2015) Mineralization of reactive azo dyes present in simulated textile waste water using down flow microaerophilic fixed film bioreactor. Bioresour Technol 175:1–7. https://doi.org/10.1016/j.biortech.2014.10.040
Blaise C, Férard JF (2005) Small-scale freshwater toxicity investigations. vol. 1–Toxicity test methods. Springer, Berlin, Germany, p 422
Busquet F, Strecker R, Rawlings JM, Belanger SE, Braunbeck T, Carr GJ, Cenijn P, Fochtman P, Gourmelon A, Hübler N, Kleensang A, Knöbel M, Kussatz C, Legler J, Lillicrap A, Martínez-Jerónimo F, Polleichtner C, Rzodeczko H, Salinas E, Schneider KE, Scholz S, van den Brandhof EJ, van der Ven LT, Walter-Rohde S, Weigt S, Witters H, Halder M (2014) OECD validation study to assess intra- and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul Toxicol Pharmacol 69(3):496–511. https://doi.org/10.1016/j.yrtph.2014.05.018
Chia MA, Musa RI (2014) Effect of indigo dye effluent on the growth, biomass, production and phenotypic plasticity of Scenedesmus quadricauda (Chlorococcales). Acad Bras Cienc 86(1):419–428. https://doi.org/10.1590/0001-3765201420130225
Copaciu F, Opris O, Coman V, Ristoiu D, Niinemets Ü, Copolovici L (2013) Diffuse water pollution by anthraquinone and azo dyes in environment importantly alters foliage volatiles, carotenoids and physiology in wheat (Triticum aestivum). Water Air Soil Pollut 224:1478. https://doi.org/10.1007/s11270-013-1478-4
Croce R, Ciná F, Lombardo A, Crispeyn G, Cappelli CI, Vian M, Maiorana S, Benfenati E, Baderna D (2017) Aquatic toxicity of several textile dye formulations: acute and chronic assays with Daphnia magna and Raphidocelis subcapitata. Ecotoxicol Environ Saf 144:79–87. https://doi.org/10.1016/j.ecoenv.2017.05.046
D’Alessandro EB, Antoniosi Filho NR (2016) Concepts and studies on lipid and pigments of microalgae: a review. Renew Sust Energ Rev 58:832–841
de Luna LA, Silva TH, Nogueira RF, Kummrow F, Umbuzeiro GA (2014) Aquatic toxicity of dyes before and after photo-Fenton treatment. J Hazard Mater 276:332–338. https://doi.org/10.1016/j.jhazmat.2014.05.047
Du M, Zhang D, Yan C, Zhang X (2012) Developmental toxicity evaluation of three hexabromocyclododecane diastereoisomers on zebrafish embryos. Aquat Toxicol 112–113:1–10
Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F (1951) A colorimetric method for the determination of sugars. Nature 168(4265):167. https://doi.org/10.1038/168167a0
Einicker-Lamas M, Mezian GA, Fernandes TB, Silva FLS, Guerra F, Miranda K, Attias M, Oliveira MM (2002) Euglena gracilis as a model for the study of Cu2+ and Zn2+ toxicity and accumulation in eukaryotic cells. Environ Pollut 120:779–786. https://doi.org/10.1016/S0269-7491(02)00170-7
Environment Canada and Health Canada (2009) Screening assessment for the challenge: five disazo dyes
Environment Canada and Health Canada (2013a) Draft screening assessment-aromatic azo and benzidine-based substance grouping-certain azo disperse dyes
Environment Canada and Health Canada (2013b) Draft screening assessment-aromatic azo and benzidine-based substance grouping- certain azo solvent dyes
Environmental Hazard and Risk Assessment of Organic Colorants (ETAD) (1992) Including life-cycle analysis. Materials for ETAD seminar, May 22, Denmark
Ferraz ER, Umbuzeiro GA, de-Almeida G, Caloto-Oliveira A, Chequer FM, Zanoni MV, Oliveira DP (2011) Differential toxicity of Disperse Red 1 and Disperse Red 13 in the Ames test, HepG2 cytotoxicity assay, and Daphnia acute toxicity test. Environ Toxicol 26(5):489–497. https://doi.org/10.1002/tox.20576.
George B, Pancha I, Desai C, Choskshi K, Paliwal C, Ghosh T, Mishra S (2014) Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus –a potential strain for bio-fuel production. Bioresour Technol 171:367–374. https://doi.org/10.1016/j.biortech.2014.08.086
Gita S, Shukla SP, Saharan N, Prakash C, Deshmukhe G (2019) Toxic Effects of selected textile dyes on elemental composition, photosynthetic pigments, protein content and growth of a freshwater chlorophycean alga Chlorella vulgaris. Bull Environ Contam Toxicol 102:795–801. https://doi.org/10.1007/s00128-019-02599-w
Golka K, Kopps S, Myslak WZ (2004) Carcinogenicity of azo colorants: influence of solubility and bioavailability. Toxicol Lett 151(1):203–210. https://doi.org/10.1016/j.toxlet.2003.11.016
Greene JC, Baughman GL (1996) Effects of 46 dyes on population growth of freshwater green alga Selenastrum capricornutum. Text Chem Color 28(4):23–30
Herbomel P, Thisse B, Thisse C (1999) Ontogeny and behavior of early macrophages in the zebrafish embryo. Development 126:3735–3745
Hernández-Zamora M, Perales-Vela HV, Flores-Ortiz CM, Cañizares- Villanueva RO (2014) Physiological and biochemical responses of Chlorella vulgaris to Congo Red. Ecotoxicol Environ Saf 108:72–77. https://doi.org/10.1016/j.ecoenv.2014.05.030
Hernández-Zamora M, Martínez-Jerónimo F, Cristiani-Urbina E, Cañizares-Villanueva RO (2016) Congo red dye affects survival and reproduction in the cladoceran Ceriodaphnia dubia. Effects of direct and dietary exposure. Ecotoxicology 25(10):1832–1840. https://doi.org/10.1007/s10646-016-1731-x
Hernández-Zamora M, Martínez-Jerónimo F (2019) Congo red diversely affects organisms of different trophic levels: a comparative study with microalgae, cladocerans and zebrafish embryos. Environ Sci Pollut R 26:11743–11755. https://doi.org/10.1007/s11356-019-04589-1
Hill AJ, Teraoka H, Heideman W, Peterson RE (2005) Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 86(1):6–19. https://doi.org/10.1093/toxsci/kfi110
ISO. International Standard Organization (1996) Water quality – Determination of the inhibition of the mobility of Daphnia magna Straus (Cladocera, Crustacea) – Acute toxicity test. ISO 6341, 3rd Ed
Jang GH, Park IS, Lee SH, Huh TL, Lee YM (2009) Malachite green induces cardiovascular defects in developing zebrafish (Danio rerio) embryos by blocking VEGFR-2 signaling. Biochem Biophys Res Commun 382(3):486–491. https://doi.org/10.1016/j.bbrc.2009.01.118
Jena S, Acharya S, Mohapatra PK (2012) Variation in effects of four OP insecticides on photosynthetic pigment fluorescence of Chlorella Vulgaris Beij. Ecotox Environ Saf 80:111–117. https://doi.org/10.1016/j.ecoenv.2012.02.016
Joshi V, Katti P (2018) Developmental toxicity assay for food additive tartrazine using Zebrafish (Danio rerio) embryo cultures. Int J Toxicol 37(1):38–44. https://doi.org/10.1177/1091581817735227
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310. https://doi.org/10.1002/aja.1002030302
Lammer E, Carr GJ, Wendler K, Rawlings JM, Belanger SE, Braunbeck T (2009) Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test. Comp Biochem Physiol C 149:196–209. https://doi.org/10.1016/j.cbpc.2008.11.006
Levy JL, Stauber JL, Jolley DF (2007) Sensitivity of marine microalgae to copper: the effect of biotic factors on copper adsorption and toxicity. Sci Total Environ 387(1–3):141–154. https://doi.org/10.1016/j.scitotenv.2007.07.016
Lim SL, Chu WL, Phang SM (2010) Use of Chlorella vulgaris for bioremediation of Textile Wastewater. Bioresour Technol 101(19):7314–7322. https://doi.org/10.1016/j.biortech.2010.04.092
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maguire RJ, Tkacz RJ (1991) Occurrence of dyes in the Yamaska River, Quebec. Water Pollut Res J Can New Ser 26(2):145–161
Mahalakshmi S, Lakshmi D, Menaga U (2015) Biodegradation of different concentration of dye (Congo red dye) by using green and blue green algae. Int J Environ Res 9(2):735–744
Malik, NH, Zain, H, Ali, N (2018) Organismic-level acute toxicology profiling of reactive azo dyes. Environ. Monit. Asess. 190–612. https://doi.org/10.1007/s10661-018-6986-7
Manjunatha B, Wei-bing P, Ke-chun L, Marigoudar AR, Xi-Qiang C, Xi-min W, Xue W (2014) The effects of henna (hair dye) on the embryonic development of zebrafish (Danio rerio). Environ Sci Pollut Res 21:10361–10367. https://doi.org/10.1007/s11356-014-2968-7
Martínez-Ruiz EB, Martínez-Jerónimo F (2015) Nickel has biochemical, physiological, and structural effects on the Green microalga Ankistrodesmus falcatus: an integrative study. Aquat Toxicol 169:27–36
Meireles G, Daam MA, Sanches ALM, Zanoni MVB, Soares AMVM, Gravato C, Oliveira DP (2018) Red disperse dyes (DR 60, DR 73 and DR 78) at environmentally realistic concentrations impact biochemical profile of early life stages of zebrafish (Danio rerio) Chem Biol Interact 292:94–100. https://doi.org/10.1016/j.cbi.2018.07.007
Ministry of Environment and Forest (1997) Government of India, Gazette on 26th March
Mohamed NA (1994) Application of algal ponds for wastewater treatment and algal production. M.Sc. Thesis, Faculty of Science, Cairo University, Bani-Sweef Branch
Novotný N, Dias A, Kapanen K, Malachová M, Vándrovcová M, Itävaara N, Lima N (2006) Comparative use of bacterial, algal and protozoan tests to study toxicity of azo and anthraquinone dyes. Chemosphere 63:1436–1442. https://doi.org/10.1016/j.chemosphere.2005.10.002
Oliveira GAR, Leme DM, de LaPuente J, Brito LB, Porredón C, Rodrigues LB, Brull N, Serret JT, Borrás M, Disner GR, Cestari MM, Oliveira DP (2018) A test battery for assessing the ecotoxic effects of textile dyes. Chem Biol Interact 291:171–179. https://doi.org/10.1016/j.cbi.2018.06.026
Organisation for Economic Co-operation and Development OECD (2004) Test No. 202: Daphnia sp. acute immobilisation test. OECD guidelines for the testing of chemicals (Section 2). OECD Publishing, Paris
Organisation for Economic Co-operation and Development OECD (2011) Test No. 201: freshwater alga and cyanobacteria, growth inhibition test. OECD guidelines for the testing of chemicals, Section 2. OECD Publishing, Paris
Organisation for Economic Co-operation and Development OECD (2013) Test no. 236: fish embryo acute toxicity (FET) test. OECD guidelines for the testing of chemicals. Paris, France
Øllgaard, H, Frost, L, Galster, J, Hansen, OC (1998) Survey of azo-colorants in Denmark: consumption, use, health and environmental aspects. Danish Environmental Protection Agency, Denmark
Parrott JL, Bartlett AJ, Balakrishnan VK (2016) Chronic toxicity of azo and anthracenedione dyes to embryo-larval fathead minnow. Environ Pollut 210:40–47
Puvaneswari N, Muthukrishnan J, Gunasekaran P (2006) Toxicity assessment and microbial degradation of azo dyes. Indian J Exp Biol 44(8):618–626
Rehman R, Mahmud T, Ejaz R, Rauf A, Mitu L (2017) Sorptive removal of Direct blue-15 dye from water using Camellia sinensis and Carica papaya leaves. Bulg Chem Comm 49(1):20–25
Ruyters G (1984) Effects of blue light on enzymes. Blue light effects in biological systems. Proc Life Sci 283–301
Rocha OP, Cesila CA, Christovam EM, Barros SB, Zanoni MV, de Oliveira DP (2017) Ecotoxicological risk assessment of the “Acid Black 210” dye. Toxicology 376:113–119. https://doi.org/10.1016/j.tox.2016.04.002
Rocha GS, Parrish CC, Lombardi AT, da GG, Melão M (2016) Copper affects biochemical and physiological responses of Selenastrum gracile (Reinsch). Ecotoxicology 25(8):1468–1477. https://doi.org/10.1007/s10646-016-1698-7
Rodrigues de Oliveira GA, de Lapuente J, Teixidó E, Porredón C, Borràs M, de Oliveira DP (2016) Textile dyes induce toxicity on zebrafish early life stages. Environ Toxicol Chem 35(2):429–434. https://doi.org/10.1002/etc.3202
Roy R, Fakhruddin ANM, Khatun R, Islam MS, Ahsan MA, Neger AJMT (2010) Characterization of textile industrial effluents and its effects on aquatic macrophytes and algae. Bangladesh J Sci Ind Res 45(1):79–84. https://doi.org/10.3329/bjsir.v45i1.5187
SCCNFP (2002) Opinion concerning the safety review of the use of certain azo-dyes in cosmetic products according to the cosmetic directive 76/768/EFC. Opinion expressed at the 19th plenary meeting
Scholz S, Fischer S, Gündel U, Küster E, Luckenbach T, Voelker D(2008) The zebrafish embryo model in environmental risk assessment—applications beyond acute toxicity testing Environ Sci Pollut Res Int 15:394–404. https://doi.org/10.1007/s11356-008-0018-z
Schroder GD, Rosslewandowski S, Davis EM (1991) Evaluation of the toxic effects of selected municipal wastewater effluents on aquatic invertebrates. Environ Technol 12:757–768. https://doi.org/10.1080/09593339109385067
Shan A, Harbin D (1988) Analyses of two azo dyes by high-performance liquid chromatography. J Chromatogr Sci 26:439–442
Seo YH, Lee Y, Jeon DY, Han JI (2015) Enhancing the light utilization efficiency of microalgae using organic dyes. Bioresour Technol 181:355–359. https://doi.org/10.1016/j.biortech.2015.01.031
Souza JP, Melo DC, Lombardi AT, Melão MG (2014) Effects of diet-borne cadmium on life history and secondary production of a tropical freshwater cladoceran. Ecotoxicology 23(9):1764–1773. https://doi.org/10.1007/s10646-014-1341-4
Stein JR (1973) Handbook of phycological methods. Culture methods and growth measurements. Cambridge University Press, London, p 7–24
Tigini V, Giansanti P, Mangiavillano A, Pannocchia A, Varese GC (2011) Evaluation of toxicity,genotoxicity and environmental risk of simulated textile and tannery wastewaters with a battery of biotests. Ecotoxicol Environ Saf 74(4):866–873. https://doi.org/10.1016/j.ecoenv.2010.12.001
Tsuruwaka Y, Konishi M, Shimada E (2015) Loss of wwox expression in zebrafish embryos causes edema and alters Ca2+ dynamics. PeerJ 3:1–12. https://doi.org/10.7717/peerj.727
U.S. Environmental Protection Agency (2002) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. EPA-821-R-02-012. 5th edn, Office of Research and Development, Cincinnati, OH
Umbuzeiro G, de A, Freeman HS, Warren SH, De Oliveira DP, Terao Y, Watanabe T, Claxton LD (2005) The contribution of azo dyes to the mutagenic activity of the Cristais River. Chemosphere 60:55–64. https://doi.org/10.1016/j.chemosphere.2004.11.100
Vacchi FI, Von der Ohe PC, Albuquerque AF, Vendemiatti JA, Azevedo CC, Honório JG, Silva BF, Zanoni MV, Henry TB, Nogueira AJ, Umbuzeiro GdeA (2016) Occurrence and risk assessment of an azo dye—the case of Disperse Red 1. Chemosphere 156:95–100. https://doi.org/10.1016/j.chemosphere.2016.04.121
Villegas-Navarro A, Romero-González MC, Rosas-López E, Domínguez-Aguilar R, Sachetin Marcal W (1999) Evaluation of Daphnia magna as an indicator of toxicity and treatment efficacy of textile wastewaters. Environ Int 25(5):619–624. https://doi.org/10.1016/S0160-4120(99)00034-3
Vimonses V, Lei S, Jin B, Chow CWK, Saint C (2009) Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem Eng J 148:354–364. https://doi.org/10.1016/j.cej.2008.09.009
Walthall WK, Stark JD (1999) The acute and chronic toxicity of two xanthene dyes, fluorescein sodium salt and phloxine B, to Daphnia pulex. Environ Pollut 104:207–215. https://doi.org/10.1016/S0269-7491(98)00189-4
Wellburn AR (1994) The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Wen YZ, Chen H, Shen CS, Zhao MR, Liu WP (2011) Enantio selectivity tuning of chiral herbicide dichlorprop by copper: roles of reactive oxygen species. Environ Sci Technol 45:4778–4784. https://doi.org/10.1021/es2003793
Wong K, Liu XJ, Lee AOK, Wong PK (2006) Effect of azo dyes on survivorship, oxygen consumption rate, and filtration rate of the freshwater cladoceran Moina macrocopa. Hum Ecol Risk Assess 12:89–300. https://doi.org/10.1080/10807030500531604
Yadav S, Saravanan PS, Renganathan S (2014) Accumulation of malachite green and cristal violet dye from synthetic effluents using single cell microalgae Chlamydomonas reinhardtii. Asian J Chem 26(15):4801–4806
Zaharia, C, Suteu, D (2012) Textile organic dyes—characteristics, polluting effects and separation/elimination procedures from industrial effluents—a critical overview. Organic Pollutants Ten Years After the Stockholm Convention. Environmental and Analytical Update, Dr. Tomasz Puzyn (Ed.), InTech. https://doi.org/10.5772/32373
Zaharia C, Suteau D (2013) Coal fly ash as adsorptive material for treatment of a real textile effluent: operating parameters and treatment efficiency. Environ Sci Pollut Res 20:2226–2235. https://doi.org/10.1007/s11356-012-1065-z
Zocolo GJ, dos Santos GP, Vendemiatti J, Vacchi FI, Umbuzeiro G, de A, Zanoni MVB (2015) Using SPE–LC–ESI–MS/MS analysis to assess disperse dyes in environmental water samples. J Chromatogr Sci 53:1257–1264. https://doi.org/10.1093/chromsci/bmu221
Zöllner N, Kirsch K (1962) Microdetermination of lipids by the sulphophospho vanillin reaction. Z die Gesamte Exp Med Einschl Exp Chir 135:545–561
Zucker E (1985) Hazard Evaluation Division—standard evaluation procedure: acute toxicity test for freshwater fish (540/9-85-006). USEPA Publication, Washington, USA
Acknowledgements
Miriam Hernández-Zamora received a postdoctoral scholarship from the Consejo Nacional de Ciencia y Tecnología, and thanks the Escuela Nacional de Ciencias Biológicas (IPN) for providing the support and facilities to conduct the present study. Fernando Martínez-Jerónimo acknowledges the Secretaría de Investigación y Posgrado - IPN, and the Comisión de Operación y Fomento de Actividades Académicas del IPN for the support provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hernández-Zamora, M., Martínez-Jerónimo, F. Exposure to the azo dye Direct blue 15 produces toxic effects on microalgae, cladocerans, and zebrafish embryos. Ecotoxicology 28, 890–902 (2019). https://doi.org/10.1007/s10646-019-02087-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-019-02087-1