Abstract
Global consumption of synthetic dyes is roughly 7 × 105 tons per year, of which the textile industry expends about two-thirds. Consumption of synthetic dyes produces large volumes of wastewater discharged into aquatic ecosystems. Colored effluents produce toxic effects in the hydrobionts, reduce light penetration, and alter the photosynthetic activity, causing oxygen depletion, among other effects. Some dyes, such as Congo red (CR), are elaborated with benzidine, a known carcinogenic compound. Information regarding dye toxicity in aquatic ecosystems is scarce; therefore, our study was aimed at evaluating the toxicity of CR on a battery of bioassays: the microalga Pseudokirchneriella subcapitata, the cladocerans Daphnia magna and Ceriodaphnia rigaudi, and the zebrafish Danio rerio. P. subcapitata was the most sensitive species to CR (IC50, 3.11 mg L−1); in exposed individuals, population growth was inhibited, but photosynthetic pigments and macromolecule concentrations were stimulated. D. magna was tolerant to high dye concentrations, the determined LC50 (322.9 mg L−1) is not an environmentally relevant value, but for C. rigaudi, LC50 was significantly lower (62.92 mg L−1). In zebrafish embryos, exposure to CR produced yolk sac edema, skeletal deformities, and stopped larvae hatching; lack of heart beating was the only observed lethal effect. CR affected organisms of different trophic levels diversely. Particularly, the effects observed in microalgae confirm the vulnerability of primary producers to dye-polluted wastewaters, because dyes produced toxic effects and interfered with photosynthesis. Different cladoceran species displayed different acute effects; thus, species sensitivity must also be considered when toxicity of dyes is assessed. Inhibition of fish larvae hatching is a significant effect not previously reported that warns about the toxicity of dyes in fish population dynamics. Synthetic azo colorants should be considered as emerging pollutants because they are discharged into the aquatic environment and are not currently included in the environmental regulation of several countries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial wastewater discharges cause water pollution and pose significant environmental problems. The textile dyeing and finishing industries contribute significantly to water pollution. According to Kant (2012), approximately 17 to 20% of industrial wastes correspond to dye discharges. Effluents containing azo dyes induce water coloration; in addition to the aesthetic effects, dyes reduce light penetration and affect photosynthesis of phytoplankton and aquatic plants (Hernández-Zamora et al. 2014), reducing dissolved oxygen concentration and negatively affecting the biota in the aquatic ecosystem (Xu et al. 2005). Another concern is raised because many of the dyes are made from carcinogenic compounds, such as benzidine (Golka et al. 2004).
Benzidine production was banned in 1997 (Pielesz 1999), but the use and manufacture of this compound continue due to its low cost. Congo red is a benzidine-based anionic diazo dye prepared by coupling tetrazotized benzidine with two molecules of naphthionic acid (Purkait et al. 2007). This dye is highly water soluble and has a stout affinity to cellulose fibers; for this reason, it is used in textile, paper, rubber, and plastic industries (Mahapatra et al. 2013).
Removal of textile dyes from wastewater becomes of fundamental importance for the protection of the aquatic environment. Physical, biological, and chemical methods like adsorption, membrane filtration, ion exchange, coagulation-flocculation, and advanced oxidation processes have been used for the removal of dyes (Qu et al. 2015; Buthiyappan et al. 2016). However, some degradation products could be more toxic than the colorant; for example, benzidine is a toxic metabolite produced during the degradation of the azo dye Direct Red 28 (Sponza and Isik 2005).
The environmental impact caused by the discharge of textile dye effluents has been scarcely studied, and information about concentrations in the aquatic environment has been determined only in recent years. Environment Canada and Health Canada (2009, 2013a, 2013b) estimated, in Canadian waters, concentrations of disazo dyes, azo solvent dyes (e.g., Sudan Red G), and azo disperse dyes (e.g., Disperse Yellow 7) of 3.7, 0.40–10.8, and 0.12–102.7 μg L−1, respectively. In South America, Vacchi et al. (2016) studied the occurrence of Disperse Red 1 dye in a river in Sao Paulo State, Brazil, reporting concentrations in a range of 50–500 μg L−1. No other data is available for other regions of the world, although colored waters have been documented profusely.
Some basic, acid, and direct azo dyes can be classified as toxic or very toxic to fish, crustaceans, algae, and bacteria, whereas reactive azo dyes are toxic only at very high concentrations (> 100 mg L−1) (Novotný et al. 2006). The evaluation of the toxic effects of pollutants on freshwater ecosystems is conducted through the controlled exposure of selected test organisms, which should be preferably representative of the main communities in aquatic ecosystems (Hernández-Zamora et al. 2016), including algae, cladocerans, and fish.
Green algae are the primary producers in aquatic ecosystems and are also bioindicator organisms. Microalgae are used to evaluate water quality and the toxicity of chemical pollutants (Wen et al. 2011; Xu et al. 2013), including metals, herbicides, insecticides, and other xenobiotics (Levy et al. 2007; Qian et al. 2008; Jena et al. 2012).
Due to their ecological relevance, cladocerans are often used as test organisms because they are one of the main components of the zooplankton community and act as a fundamental link between primary producers and secondary consumers (Martínez-Jerónimo et al. 2000). Daphnia magna and Ceriodaphnia dubia are reference test organisms for ecotoxicological studies (Blaise and Férard 2005). Specifically, D. magna has been used to assess the toxicity of dyes and metals (Heugens et al. 2003; Bae and Freeman 2007; Croce et al. 2017).
Zebrafish has been widely used as a vertebrate model organism for assessing the toxicity of chemicals (Selderslaghs et al. 2012; Gu et al. 2010; Rekha et al. 2008). Zebrafish embryos enable the identification of different endpoints and allow elucidation of mechanisms of toxicity (Hill et al. 2005). Zebrafish has also been used to determine the toxicity of some dyes (Parrott et al. 2016; Rodrigues de Oliveira et al. 2016).
Toxic effects of synthetic dyes have not been deeply studied. Frequently, more attention is focused on the unpleasant aesthetic impact of colored waters, but the toxicity on aquatic biota has not been fully documented. Because toxic responses are expected to be different in the different communities in aquatic ecosystems, the use of single species toxicity tests would provide limited information; consequently, an array of species representing different trophic levels undoubtedly could provide evidence that enables a better understanding of the negative impact of synthetic dyes in hydrobionts.
We evaluated the toxic effect of the azo dye Congo red on a battery of bioassays, including a primary producer (Pseudokirchneriella subcapitata), two primary consumers (the reference species Daphnia magna and the tropical cladoceran Ceriodaphnia rigaudi), and a zooplankton feeder fish (Danio rerio). The aim was to determine the effects of CR in different representants of aquatic biota in freshwater environments to establish the adverse effects expected when this dye pollutes water.
Material and methods
Dye
Congo red (CR) azo dye (C32H22N6O6S2Na2, also called Direct Red 28, molecular weight 696.7 g mol−1) was obtained from Sigma-Aldrich®. For all the experiments, the dye was prepared as a stock solution at 1000 mg L−1 with deionized water and this solution was stored protected from light at 4 °C.
Test organisms
Experiments were conducted with the chlorophycean microalga Pseudokirchneriella subcapitata grown in batch cultures under aseptic conditions. The strain was obtained from the Laboratorio de Hidrobiología Experimental of the Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Mexico City, Mexico.
P. subcapitata was axenically cultured in 250 mL of Bold’s basal medium (250 mg L−1 NaNO3, 25 mg L−1 CaCl2•2H2O, 75 mg L−1 MgSO4•7H2O, 75 mg L−1 K2HPO4, 175 mg L−1 KH2PO4, 25 mg L−1 NaCl, 4.98 mg L−1 of FeSO4•7H2O, 0.001 mL L−1 H2SO4, 11.42 mg L−1 H3BO3, 50 mg L−1 EDTA, 31 mg L−1 KOH, 8.82 mg L−1 ZnSO4•7H2O, 1.44 mg L−1 MnCl2•4H2O, 0.71 mg L−1 MoO3, 1.57 mg L−1 CuSO4•5H2O, 0.49 mg L−1 Co(NO3)2•6H2O (Stein 1973), using 500-mL, flat-sided glass bottles. The incubation conditions were as follows: 24 ± 1 °C; 120 μmol m−2 s−1 light intensity, continuous illumination (no photoperiod), and continuous airflow bubbling (200 mL min−1).
The clonal strains of cladocerans used in experiments were obtained from the collection of the Laboratorio de Hidrobiología Experimental of the Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Mexico City, Mexico. Daphnia magna and Ceriodaphnia rigaudi were cultured in ISO medium (2 mM CaCl2•2H2O, 0.5 mM MgSO4•7H2O, 0.75 mM NaHCO3, 0.07 mM KCl) (ISO 1996) diluted 5:1 with deionized water. Both cladocerans were maintained at 25 + 1 °C in environmental chambers with a photoperiod of 16:8 h (light/darkness).
According to USEPA (2002), cultures were fed daily with 1 × 106 cells mL−1 of P. subcapitata to maintain the organisms in optimum condition to provide maximum reproduction.
Controlled batches of known age females of each species were implemented for neonate production (< 24-h-old) (USEPA 2002). Once reproduction began, adult parthenogenetic females were separated, and the released progeny was carefully collected by filtering through a 125-μm sieve; neonates obtained from the third brood and after that were used for the tests (Hernández-Zamora et al. 2015; Martínez-Jerónimo et al. 2008).
Adult male and female zebrafish were obtained from the fish collection of the Laboratorio de Hidrobiología Experimental of the Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Mexico City, Mexico, where they have been successfully maintained through controlled cultivation for over 8 years. The protocol proposed by OECD (2013) Guideline 236 was followed to obtain fertilized eggs. Briefly, the day before the test, adult zebrafish, males and females (ratio 2:1), were placed in spawning tanks few hours before the onset of darkness. Mating, spawning, and fertilization took place within 30 min after the onset of light in the morning. The zebrafish eggs were collected and cleaned in ISO medium. All eggs were examined under a stereomicroscope, and unfertilized eggs were discarded; live embryos were used for the toxicity tests.
Determination of the median inhibitory concentration (IC50) of microalgae exposed to Congo red
The median inhibitory concentration (IC50) was calculated according to the OECD protocol 201 (OECD 2011) to determine the toxic effects of CR on microalgae. For this purpose, bioassays with axenic inocula of P. subcapitata were exposed to different concentrations of CR (4, 8, 16, 32, and 64 mg L−1) in OECD medium (pH 8.1); initial inoculum was 1 × 104 cells mL−1. Cell density was determined using a Neubauer’s hemocytometer. Incubation conditions for the tests were 24 ± 1 °C and continuous fluorescent light of 120 μmol m−2 s−1. The test period was 96 h; samples were obtained every 24 h to evaluate cellular density in a Neubauer hemocytometer. The average IC50 value was obtained from three independent assays, with three replicates for each bioassay.
Sub-inhibitory toxicity test in P. subcapitata
Once IC50 was determined, P. subcapitata was grown for 96 h in OECD medium containing the CR concentrations equivalent to the IC1, IC10, and IC30. Test vessels were incubated at 24 ± 1 °C, continuous illumination of 120 μmol m−2 s−1, and constant bubbling with an airflow of 200 mL min−1.
Experiments were performed in flat-sided glass bottles with a total capacity of 500 mL and a test volume of 250 mL. The cell density was determined daily. After 96 h of exposure, the biomass was centrifuged at 3500 rpm for 10 min. Immediately after, the microalgae pellet was washed three times using OECD medium to eliminate all the dye remnants and the dye possibly adsorbed to the cell wall, following the centrifugation procedure previously described. Lastly, the cells were resuspended in OECD medium and stored in darkness at 4 °C to determine pigments and macromolecules.
Photosynthetic pigments
Photosynthetic pigments were determined as follows: (i) samples of 500 μL were centrifuged at 14,000 rpm for 3 min at room temperature; (ii) supernatant was discarded and the pellet was added 600 μL of dimethyl sulfoxide (DMSO); (iii) sample was vortexed for 20 s and centrifuged at 14,000 rpm for 3 min; (iv) samples were kept for 2 min under dark conditions at room temperature, and after this centrifuged at 14,000 rpm for 3 min; (v) supernatant was separated, and the absorbance of chlorophyll (Chl) a, Chl b, and total carotenoids were read at 665, 649, and 470 nm respectively; and finally, (vi) the concentration of photosynthetic pigments were determined according to the equations of Wellburn (1994):
Proteins
Soluble protein of P. subcapitata cells was extracted as follows: (i) samples of 500 μL was centrifuged at 14,000 rpm for 3 min and the supernatant was discarded; (ii) pellet was added 300 μL of 1 N NaOH solution and vortex for 20 s; (iii) sample was heated on a hot plate at 90 °C for 15 min; (iv) sample was centrifuged at 14,000 rpm for 5 min; (vi) 100 μL of supernatant was used to determinant the amount of protein by the Lowry method (Lowry et al. 1951). Bovine serum albumin was used as a standard solution. Absorbance was read at 590 nm.
Carbohydrates
Carbohydrates were extracted as follows: (i) 500 μL of sample was centrifuged at 14,000 rpm for 3 min and the supernatant was discarded; (ii) pellet was added 400 μL of solution 2 N HCl, and vortexed during 20 s; (iii) sample was heated on a hot plate at 90 °C for 1 h; (iv) sample was cooled at room temperature and centrifuged at 14,000 rpm for 5 min; (v) 100 μL of supernatant was used to quantify total carbohydrates. Glucose was used as a standard solution. The concentration of carbohydrates was determined according to Dubois et al. (1951) and the absorbance was read at 490 nm.
Lipids
Lipids were quantified as follows: (i) 500 μL of sample was centrifuged at 14,000 rpm for 3 min and the supernatant was discarded; (ii) pellet was added 400 μL of chloroform and 200 μL of methanol, and vortexed for 20 s; (iii) 500 μL of deionized water was added to samples and centrifuged at 14,000 rpm for 5 min; (iv) organic phase was used to quantify lipids. The concentration of lipids was determined according to Zöllner and Kirsch (1962), using cholesterol as a standard. Absorbance was read at 525 nm.
Determination of the median lethal concentration (LC50) for D. magna and C. rigaudi exposed to CR
The acute toxic effects of Congo red in cladocerans were determined at 48 h, following the procedure suggested by USEPA (2002) and Hernández-Zamora et al. (2016). After a range-finding test, final assayed CR concentrations were 25, 50, 100, 200, 300, and 400 mg L−1. ISO medium and deionized water (1:5) was used as dilution water; test volume was 33 mL in each of three replicates. Ten neonates per replicate were transferred to each test vessel. Incubation was in an environmental chamber (24 ± 1 °C, 16:8 h light: darkness). No food was supplied during the assays. The number of affected, immobilized, and dead (no heartbeat, through observation at the stereomicroscope) organisms was recorded at 24 and 48 h. The average LC50 was obtained from three independent bioassays for each cladoceran.
Zebrafish toxicity test
Two sets of CR concentrations were tested; the first one was 6.25, 12.5, 25, 50, 100 mg L−1. Because reduced toxic effects were observed, a second range was assayed: 100, 200, 300, 400, and 500 mg L−1. Congo red was dissolved in ISO medium: deionized water (1:5). 3,4-Dichloroaniline (4 mg L−1) was used as positive control, and ISO medium: deionized water (1:5) was the negative control. We followed the procedures described in the OECD guideline 236 (OECD 2013) and by Busquet et al. (2014).
The test started with 2-hpf (hours post-fertilization) embryos. One egg was distributed in each well of 24-well plates, containing 2 mL of freshly prepared test solution (final volume per well). Test organisms were distributed as follows: (a) 20 eggs for each concentration of RC (one plate for each concentration); (b) 20 eggs as positive control on one plate; (c) 4 eggs as negative, internal control on each of the above-described plates; and (d) 24 eggs as negative external control in a different plate. The tests were incubated at 26 ± 1 °C for 144 h with a photoperiod of 16:8 h (light: darkness) in an environmental chamber. Renewal of test solution was as described in the OECD guideline (OECD 2013).
Endpoints of lethal and sublethal effects in early life stages (ELS) of D. rerio
Toxic effects in ELS of zebrafish were determined according to the criteria described in the OECD guideline 236 through observations at 24, 48, 72, and 96 h, but extended observations were performed at 120 and 144 hpf. Lethal endpoints were coagulation, no tail detachment, no somite formation, and no heartbeat (this last one measured after 48 h). Table 1 shows the endpoints evaluated in the present study, according to Rodrigues de Oliveira et al. (2016). The criteria established in the OECD guideline 236 were followed to distinguish between normal and abnormal embryonic development parameters. The four endpoints that indicated acute toxicity and consequently death of the embryos are egg coagulation, no tail detachment, no somite formation, and no heartbeat. The sublethal endpoints used to assess the effects of the RC on zebrafish development included deformities in the yolk sac, gas bladder, and skeleton, as well as a delay in larval hatching rate.
Statistical analysis
The median inhibitory concentration (IC50) and the median lethal concentration (LC50) with the corresponding 95% confidence intervals were calculated via the Probit method, using RA software (Risk Assessment, Hazard Assessment Tools, v. 1.0). To compare differences among treatments, one-way analysis of variance (ANOVA) was performed followed by Dunnett’s comparison test to identify significant differences between treatments and the control, and by Tukey’s pairwise comparison test to make multiple comparisons among all treatments, including the control. For all the analyses, the software Sigma Plot ver. 11.0 was used.
Results
Effect of Congo red on the microalgal population growth
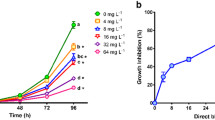
P. subcapitata growth was affected by exposure to CR in all the tested concentrations, as observed in Fig. 1a. ANOVA test evidenced significant differences (P < 0.05) for population density values among treatments, determined at 96 h. The Dunnett’s test demonstrated that cell densities in the five CR concentrations were significantly lower than that observed in the control. The average IC50 value was 3.11 mg L−1 (95% limits 1.24–5.13 mg L−1).
Population growth of P. subcapitata exposed during 96 h to a different concentrations of Congo red for the determination of the IC50, and b sub-inhibitory concentrations of Congo red. Symbols represent the mean values for three bioassays with three replicates ± standard error. Asterisk indicates significant differences (P < 0.05) with respect to control (Dunnett’s test). Different letters indicate significant differences (P < 0.05) after Tukey’s pairwise comparisons
Sub-inhibitory toxicity test
The three CR concentrations selected to assess sub-inhibitory effects were 0.04, 0.27, and 1.15 mg L−1, equivalent to IC1, IC10, and IC30, respectively. ANOVA for 96 h cell densities showed highly significant differences (P < 0.001) (Fig. 1b). The population growth inhibition was of 67, 69, and 80%, respectively, for IC1, IC10, and IC30, compared to control. According to Dunnett’s test, P. subcapitata densities were significantly lower than that observed in the control (P < 0.001).
Figure 2a shows the concentration of photosynthetic pigments quantified at the end of the assay (96 h). ANOVA showed highly significant differences (P < 0.001). The chlorophyll a and b contents in the cells exposed to 0.27 and 1.15 mg L−1 were significantly higher than the control (Dunnett’s test, P < 0.05). Furthermore, carotenoids in cells exposed to all CR concentrations were significantly higher than the control by Dunnett’s test. The differences among treatments determined by Tukey’s post hoc multiple comparisons test are shown in Fig. 2a; the concentration of chlorophyll a and b was significantly different at the highest tested concentration (1.15 mg L−1). The carotenoid content per cell increased with increasing CR concentrations.
Biomarkers in cells of P. subcapitata exposed to sub-inhibitory concentrations of Congo red during 96 h. a Concentration of photosynthetic pigments. b Concentration of macromolecules. Bars represent the mean values for three replicates ± standard error bars. Asterisk indicates significant difference (P < 0.05) with respect to control (Dunnett’s test). Different letters above the bars indicate significant differences (P < 0.05) for each set of pigments or macromolecule data, after Tukey’s pairwise comparisons
The effect of CR on the content of macromolecules (proteins, carbohydrates, and lipids) measured at the end of the assay (96 h) is shown in Fig. 2b. Regarding proteins, ANOVA demonstrated highly significant differences among treatments (P < 0.001). Dunnett’s test evidenced that proteins in cells exposed to 0.04 mg L−1 CR concentration was significantly higher than that measured in the control series (P < 0.05). Protein concentration in the control and cells exposed to 0.27 and 1.15 mg L−1 were not significantly different among them (Tukey’s test, P < 0.05).
The concentration of carbohydrates per cell was significantly different among treatments (ANOVA, P < 0.05). In all CR concentrations, the concentration of carbohydrates was significantly higher (2, 2.4, and 2.7 times for 0.04, 0.27, and 1.15 mg L−1 CR, respectively) than that observed in the control (Dunnett’s test, P < 0.05).
The concentration of lipids per cell was significantly different among treatments and control (ANOVA, P < 0.001); as observed in Fig. 2b, lipid content was higher in all the algae exposed to CR (Dunnett’s test, P < 0.05); the highest values were recorded with 0.27 and 1.15 mg L−1 CR (Tukey test, P < 0.05).
Effect of Congo red on cladocerans D. magna and C. rigaudi
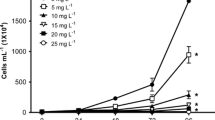
Survival percentages of D. magna decreased significantly (P < 0.05) in organism exposed to 300 and 400 mg L−1. Survival for C. rigaudi was significantly lower (P < 0.05) in all tested CR concentrations, and decreased as CR concentration increased (P < 0.05). Regarding this endpoint, D. magna was more tolerant than C. dubia to Congo red dye.
The 48-h LC50 (Probit analysis), determined as the average of three independent bioassays, was 345.27 mg L−1 (95% confidence limits, 290.65–382.52 mg L−1) for D. magna, whereas for C. rigaudi, the value was 62.92 mg L−1 (95% confidence limits, 47.05–79.94 mg L−1). This result confirms the higher sensitivity of C. rigaudi compared to that of the reference species D. magna.
Effect of Congo red on zebrafish embryos
Hatching rate of zebrafish larvae was measured at two different ranges of CR concentrations: 0–100 mg L−1 and 0–500 mg L−1. In the negative controls, hatching started at 72 h, and 100% hatched larvae were observed at 96 h. With all the CR concentrations, in both sets of experiments, no hatching was recorded, despite that larvae looked well developed and had movements inside the egg.
Figure 3 shows the development of the 96 hpf zebrafish embryos exposed to 6.25, 100, and 500 mg L−1 CR. At 96 h, exposure to CR did not induce lethal responses (identified as coagulation, no tail detachment, no somite formation, and no heartbeat) or sublethal effects (yolk sac edema, gas bladder deformities, or skeletal malformations). Nevertheless, concentration-related accumulation of unidentified, tightly adhered debris covering the chorion in the concentrations of 100 to 500 mg L−1 of the dye, was observed at 48 hpf.
At 144 hpf, exposure to CR produced no heartbeats in embryos as the only lethal effect observed. Other effects, as delayed pulse and yolk sac edema, increased significantly as the concentration of CR augmented (P < 0.05), but a reverse tendency was observed for the percentage of embryos with curved tail (P < 0.05), as shown in Fig. 4.
No heartbeat, delayed pulse, curved tail, and yolk sac edema in zebrafish larvae after 144 hpf exposure to different concentrations of CR. Bars represent the mean values for three replicates ± standard error bars. Asterisk indicates significant difference (P < 0.05) with respect to control (Dunnett’s test). Different letters above the bars indicate significant differences (P < 0.05) for each effect, after Tukey’s pairwise comparisons
No hatching was observed after 144 hpf of exposure to CR concentrations. Because of this, the chorion was mechanically disrupted to release the individuals to determine if the larvae were alive. The chorion in all the CR treatments was hardened and broke into irregular fragments because of its solidified structure. In all cases, released larvae were alive but had reduced motility, and most of them had yolk sac edema, as observed in Figs. 5b–c. Also, larvae exposed to 500 mg L−1 of CR had the tail curved and red pigmentation in the tip of the tail (Fig. 5c), indicating that part of the dye penetrated the chorion. The debris covering the chorion could be deposition of Congo red salts, and was apparently responsible for hardening the chorion, impeding in this way the hatching of larvae; the reduced movement observed in these individuals could indicate that energy was unsuccessfully invested trying to break the chorion.
Discussion
Results demonstrate that CR reduced the population growth and produced sub-inhibitory effects in the chlorophycean P. subcapitata at concentrations as low as 0.04 mg L−1. Lim et al. (2010) have reported that the growth of the microalga C. vulgaris decreases with increasing concentration of textile wastewater containing azo dyes. Cheriaa et al. (2009) observed that the growth and biomass production in Chlorella were inhibited by exposure to 10 mg L−1 of indigo dye. Also, Chia and Musa (2014) showed that the indigo dye adversely affects cell density and dry weight, causing inhibition of the growth of Scenedesmus quadricauda. Nevertheless, there are reports of high susceptibility and high tolerance in algae exposed to dyes. For example, Novotný et al. (2006) showed that P. subcapitata was very sensitive to Disperse Blue 3 because this alga could only grow in 0.5 mg L−1 of dye. On the other hand, C. vulgaris could grow without apparent damage exposed to 400 mg L−1 of Tectilon Yellow 2G (Acuner and Dilek 2004).
There have been very few attempts to explain what causes cellular growth inhibition resulting from exposure to azo dyes. Ogawa et al. (1989) reported the inhibitory effect of textile dyes; they found that inhibition was caused by intercalation of dye molecules between DNA base pairs, thus preventing DNA transcription, interfering with the enzymatic activity and cell replication. Ganesh et al. (1994) reported inhibition of biomass in a wastewater system from an active dye treatment, but they suggested that this effect was caused by the products of dye degradation rather than the dye itself. Other studies have shown that growth inhibition is a common response of freshwater microalgae to the toxic effect of textile effluents because they reduce photosynthesis and cell division (Roy et al. 2010). Hernández-Zamora et al. (2014) reported that one of the possible causes of the growth inhibition is related to a decrease in metabolic activity, particularly with the alteration of photosynthetic activity at the electron donor stage of photosystem II.
The sensitivity of P. subcapitata to the dye was established through the mean inhibitory concentration (IC50, 3.11 mg L−1). In another study, Hernández-Zamora et al. (2016) reported an IC50 of 5.19 mg L−1 for C. vulgaris exposed to Congo red after 96 h of incubation. Novotný et al. (2006) reported a similar result for Selenastrum capricornutum after 96 h of exposure to Congo red (EC50, 4.8 + 1.0 mg L−1). Other studies have reported that the sensitivity of microalgae to dyes is very high; for example, Vacchi et al. (2016) mentioned that Raphidocelis subcapitata exposed to Disperse Red 1 for 72 h had an EC50 of 102 mg L−1. Chia and Musa (2014) reported an EC50 of 162.52 mg L−1 for Scenedesmus quadricauda exposed to indigo dye. Based on the results of this research and the published information, we can assume that the sensitivity of microalgae to azo dyes could be related mainly to their chemical structure and concentration, although the response could also be species-specific.
Determination of chlorophyll a, b, and carotenoid concentrations could indirectly evaluate photosynthetic function. CR exposure stimulated the content of photosynthetic pigments, as observed in Fig. 2a. There are few reports on the effect of dyes on the content of photosynthetic pigments. Hernández-Zamora et al. (2014) mention that the total chlorophyll (a + b) of C. vulgaris exposed to Congo red decreased significantly compared to the control, as the dye concentration increased; this effect was opposite to the results found in the present study. Studies with other toxicants have found that when the microalga P. subcapitata is exposed to 18.23 μmol L−1 of tylosin (antibiotic) for 4 days, the total chlorophyll and carotenoid content increased 185 and 165%, respectively, compared to the control (Guo et al. 2016). Petit et al. (2010) reported that the total chlorophyll content in the microalga Chlamydomonas reinhardtii increased 121% compared with the control when it was exposed to polyamidoamine (0.76 μmol L−1).
Regarding the increase in the concentration of carotenoids (Fig. 2a), these pigments may be acting as protectors against the chlorophyll photooxidation, as observed in the marine microalga Dunaliella salina (Marín et al. 1998). Kebede (1997) also reported the role of carotenoids in protecting chlorophyll from photooxidation. Furthermore, Richardson et al. (2002) reported that in microalgae under stress situations, the carotenoid content increased. Carotenoids are antioxidant molecules counterbalancing reactive oxygen species in cells.
The macromolecules (proteins, carbohydrates, and lipids) were affected by the exposure to CR (Fig. 2b). Proteins are important molecules that are mainly involved in structures, transport, and enzymatic activity. Cells of P. subcapitata had soluble protein reduction only with the CR concentration of 0.04 mg L−1. Carfagna et al. (2013) observed protein reduction in C. vulgaris exposed to toxic metals, like lead and cadmium.
Carbohydrates and lipid concentrations (Fig. 2b) significantly increased in P. subcapitata exposed to CR; both these molecules are importantly involved in the biosynthesis of storage products in microalgae (George et al. 2014). Carbohydrates are relevant for the structure of the cell wall and represent an important form of energy readily used; they are also intermediaries in the photosynthetic and respiratory pathways. On the other hand, lipids are important energy storage products and are also essential for cell and organelle membranes. If microalgae are under stress conditions, lipid synthesis is frequently favored (George et al. 2014; Han et al. 2011); thus, an increase in lipids could be observed as a chemical stress response.
George et al. (2014) mentioned that under unfavorable conditions, microalgal growth is arrested, photosynthetic activity decreases, and the excess energy could be stored as carbohydrates and lipids, as observed in the present study (Fig. 2b). Stress conditions, such as inadequate values of light (quality and quantity), CO2 concentration, pH, nutrient deficiency, as well as some chemical pollutants, promote the synthesis of carbohydrates and lipids (Cheng and He 2014; George et al. 2014; Chen et al. 2013; Sharma et al. 2012; Yu et al. 2011).
Regarding cladocerans, C. rigaudi was more sensitive than D. magna to CR (Fig. 3). This sensitivity was confirmed with the values of EC50; C. rigaudi was approximately 50 times more sensitive than D. magna to CR (62.92 and 322.9 mg L−1 of dye, respectively). Although we determined the LC50 at non-environmentally relevant concentrations for D. magna, we observed that the digestive tract of test organisms was remarkably red colored; hence, toxic effects could be expected at sub-chronic exposures.
On the effect of Congo red on cladocerans, Hernández-Zamora et al. (2015) reported that the EC50 of CR (dissolved in Bold’s basal medium) for D. magna and C. dubia were 17.0 and 3.32 mg L−1, respectively. Wong et al. (2006) evaluated the acute toxicity of CR in Moina macrocopa at 4 and 7 days, reporting LC50 values of 0.16 and 0.07 mg L−1, respectively. The reported values in published papers are below the one found in our study; however, test conditions were rather different regarding exposure time, dilution water, and cladoceran species, factors that could help to explain these differences.
Dye toxicity to cladocerans reported by different authors denoted higher LC50 values than those we observed. Verma (2008) mentioned that the 48-h EC50 values for two azo dyes in D. magna were 47 mg L−1 and 55 mg L−1, respectively, for Remazol Golden Yellow and Remazol Parrot Green. Meric et al. (2005) reported in D. magna, exposed to the dye Remazol Black B, an EC50 value of 75 mg L−1. In contrast, lower LC50 values have also been reported; Bae and Freeman (2007) studied the toxicity of different dyes in D. magna and reported that Direct Blue 218 was very toxic for daphnids, obtaining LC50 values of 1–10 mg L−1. In addition, de Luna et al. (2014) observed that the anthraquinone dye, Vat Green 3, was highly toxic to Daphnia similis (EC50, 6.9 mg L−1) and C. dubia (EC50, 0.5 mg L−1). Other studies demonstrated that two xanthene dyes exerted different toxicities on Daphnia pulex; the 48-h LC50 for phloxine B was 0.423 mg L−1, whereas for fluorescein, it was 337 mg L−1 (Walthall and Stark 1999).
Few studies reported dye toxicity below 1 mg L−1. Artal et al. (2011) demonstrated that the azo dye, Disperse Red 1, caused immobility in the copepod Nitroka sp., with an EC50 (96 h) of 0.14 mg L−1. Ferraz et al. (2010) determined that the dyes Disperse Red 1 and Disperse Red 13 are highly toxic to D. similis, with EC50 values of 127 and 18.7 μg L−1, respectively.
Differences in the values reported in the literature and those found in this study can be attributed not only to the type of dye but also to species-specific responses, indicating that there are species of zooplankton that are more sensitive. For this reason, it is important to classify the toxicity of dyes correctly, taking into account the differences in sensitivity that zooplankton species could have. Based on the EC50 values, emerging contaminants can be classified as harmful (EC50, 10–100 mg L−1), toxic (EC50, 1–10 mg L−1), or very toxic (EC50, < 1 mg L−1) (Bhaskar et al. 2015). According to this classification, some of the most common azo dyes could be categorized as toxic to very toxic.
All concentrations of CR precluded hatching of zebrafish embryos during the normal exposure time (96 h) and up to 144 hpf (Fig. 4a–b), as the extended incubation time. This effect on hatching was the consequence of the inability of the embryos to break the chorion as also observed by Du et al. (2012). Rodrigues de Oliveira et al. (2016) reported no significant mortality in hatched larvae exposed to Reactive Orange 16 at 144 hpf; however, from 96 to 120 hpf, the Reactive Orange 16 significantly delayed or inhibited the ability of zebrafish embryos to hatch at the highest concentration tested (100 mg L−1). This effect on hatching rate was similar to that observed in our study with Congo red dye.
Exposure to CR did not induce lethal or sublethal effects in the development of embryos in the tested conditions. It is likely that, during the embryo stage, the chorion protected the embryo during the first 5 days of exposure (Parrott et al. 2016). Apparently, the Congo red dye did not cross over the chorion barrier but produced an accumulation of CR debris adhered to the chorion at highest CR concentrations, inhibiting larval hatching. Nevertheless, the embryonic development was not affected at 96 hpf and the sole lethal effect observed in zebrafish embryos was no heartbeat. The inclusion of sublethal endpoints in toxicity tests is important, because such effects may occur in non-target organisms, and significantly impact on population or ecosystem structure (Lammer et al. 2009).
Exposure to CR induced sublethal effects in embryos in the tested conditions. Such damages included yolk sac edema (Figs. 4 and 5b–c), skeletal deformities (Figs. 4 and 5b–c), and delayed or precluded hatching. Yolk sac provides vital nutritive materials during larval development, playing an important role in the early development of zebrafish; yolk sac edema is a deformity produced by chemical stress and is an important toxicological endpoint (Li et al. 2011). Development malformations like yolk sac edema and curved tail were observed at 144 hpf (Fig. 5b–c).
Congo red dye is not acutely toxic to zebrafish embryos (LC50 of 476.84 mg L−1), but it induces swimming bladder deflation at concentrations ranging from 28.8 to 351 mg L−1 (Soni et al. 2006); hence, sublethal effects could be expected during chronic exposure. Rodrigues de Oliveira et al. (2016) reported that the Direct Black 38 (DB38) dye produced swim bladder deflation at 6.25 mg L−1 after 96 hpf and that larvae exposed to DB38 at 25 mg L−1 presented noninflated gas bladder and curved tail at 120 and 144 hpf. The dye Vat Green 3 at 100 mg L−1 did not cause lethal effects in zebrafish embryos, but sublethal effects like yolk sac edema and swim bladder deflation occurred at 120 hpf. Jang et al. (2009) observed blood circulation defects, including slow heartbeat, heart edema, blood accumulation near the heart region, and a big yolk; these effects were evident after 72 hpf in D. rerio embryos exposed to Malachite Green (150 mgL−1).
The toxicity of six different textile dyes (Basic Blue 159, Remazol Red RR, Cibacron Red FN-3G, Cibacron Blue FNR, Basic Red 46, and Reactive Blue 21) has been evaluated in Xenopus laevis embryos. The authors observed that low concentrations of all of these dyes produced tail flexure as well as yolk and head edemas (Birhanli and Ozmen 2005).
Zebrafish embryos exposed to azo dyes displayed a wide range of effects from deformities to hatching failures. Although in some studies the dye concentration required to produce an effect could be not environmentally relevant, for some other dyes, effective concentrations were very low, so the chemical structure of the colorant is a factor that should be considered when ecotoxicity is determined. Furthermore, hardening of the chorion is an important observation, as an effect that avoids larval hatching; with all the ecological consequences, this phenomenon could have in natural oviparous fish populations.
In an overall analysis of the toxic effects of CR on different representatives of a freshwater community, we observed that the effect-response was quite dissimilar, depending on the ecological position (primary producer, primary consumer, secondary consumer). Besides, in the same group, two zooplankters displayed significantly different sensitivities, reflecting that it is difficult to generalize potential toxic effects even at the same trophic level. Nevertheless, assaying toxic effects with test organisms representative of aquatic communities is an approach that enables defining risk scenarios in a wider context. Effects of synthetic azo dyes on aquatic biota is a matter of concern at present. First, because of the significant water volumes used in the textile activity and the amounts of dye remnants discharged without treatment, and secondly due to the potential biological damages that can be produced to hydrobionts. Our results alert about the potential toxic effects expected in aquatic ecosystems.
Conclusions
The negative effects observed in P. subcapitata confirm the vulnerability of primary producers to Congo red dye because this synthetic colorant inhibits population growth, altering the photosynthetic pigment concentration and the macromolecule content.
CR exposure produced different acute toxic effects in two zooplankters, indicating differences in the sensitivity of similar organisms.
Congo red hindered Danio rerio embryos of hatching after 144 hpf, and although they were alive at that time, they presented yolk sac edema and curved tail. This effect warns about possible negative consequences of CR exposure on oviparous fish.
The toxic effects found in primary producers, primary consumers, and secondary consumers evidence the potential damage that azo dyes could cause to aquatic ecosystems receiving discharges containing Congo red dye remnants. Azo dyes should be considered emerging pollutants because they are frequently discharged into the aquatic environment and are not currently included in environmental monitoring and regulation programs in many countries.
References
Acuner A, Dilek FB (2004) Treatment of tectilon yellow 2G by Chlorella vulgaris. Process Biochem 39:623–631. https://doi.org/10.1016/S0032-9592(03)00138-9
Artal A, Albuquerque A, Lotufo G, Umbuzeiro G (2011) The toxicity of commercial dye disperse red 1 and his main fraction to estuarine copepod. In: 32nd Society of Environmental Toxicology and Chemistry (North America) Meeting, Boston, USA. http://www.setac.org/store/download.asp?id=D8FB98CE-3B61-4528-98A3-202D0D761E24
Bae JS, Freeman HS (2007) Aquatic toxicity evaluation of copper-complexed direct dyes to the Daphnia magna. Dyes Pigments 73(1):126–132. https://doi.org/10.1016/j.dyepig.2005.10.019
Bhaskar S, Kuldeep B, Faizal B (2015) Developments in applied phycology 7: algae and environmental sustainability. Springer, New Delhi, p 194. https://doi.org/10.1007/978-81-322-2641-3 ISBN 978-81-322-2639-0
Birhanli A, Ozmen M (2005) Evaluation of the toxicity and teratogenity of six commercial textile dyes using the frog embryo teratogenesis assay-Xenopus. Drug Chem Toxicol 28:51–65. https://doi.org/10.1081/DCT-39689
Blaise C, Férard JF (2005) Small-scale freshwater toxicity investigations. Vol. 1–toxicity test methods. Springer, Berlin, p 422 ISBN 978-1-4020-3120-5
Busquet F, Strecker R, Rawlings JM, Belanger SE, Braunbeck T, Carr GJ, Cenijne P, Fochtmanf P, Gourmelong A, Hübler N, Kleensanga A, Knöbeli M, Kussatz C, Legler J, Lillicrap A, Martínez-Jerónimo F, Polleichtner C, Rzodeczko H, Salinas E, Schneider KE, Scholz S, van den Brandhof E-J, van der Ven LTM, Walter-Rohde S, Weigt S, Witters H, Halder M (2014) OECD validation study to assess intra- and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul Toxicol Pharmacol 69:496–511. https://doi.org/10.1016/j.yrtph.2014.05.018
Buthiyappan A, Aziz ARA, Daud WMAW (2016) Recent advances and prospects of catalytic advanced oxidation process in treating textile effluents. Rev Chem Eng 32(1):1–47. https://doi.org/10.1515/revce-2015-0034
Carfagna S, Lanza N, Salbitani G, Basile A, Sorbo S, Vona V (2013) Physiological and morphological responses of lead or cadmium exposed Chlorella sorokiniana 211-8K (Chlorophyceae). Springerplus 2(1):1–7. https://doi.org/10.1186/2193-1801-2-147
Chen CY, Zhao XQ, Yen HW, Ho SH, Cheng CL, Lee DJ, Bai FW, Chang JS (2013) Microalgae-based carbohydrates for biofuel production. Biochem Eng J 78:1–10. https://doi.org/10.1016/j.bej.2013.03.006
Cheng D, He Q (2014) Assessment of environmental stresses for enhanced microalgal biofuel production – an overview. Front Energy Res 2(26):1–8. https://doi.org/10.3389/fenrg.2014.00026
Cheriaa J, Bettaieb F, Denden I, Bakhrouf A (2009) Characterization of new algae isolated from textile wastewater plant. J Food Agric Environ 7(3&4):700–704
Chia MA, Musa RI (2014) Effect of indigo dye effluent on the growth, biomass, production and phenotypic plasticity of Scenedesmus quadricauda (Chlorococcales). An Acad Bras Cienc 86(1):419–428. https://doi.org/10.1590/0001-3765201420130225
Croce R, Ciná F, Lombardo A, Crispeyn G, Cappelli CI, Vian M, Maiorana S, Benfenati E, Baderna D (2017) Aquatic toxicity of several textile dye formulations: acute and chronic assays with Daphnia magna and Raphidocelis subcapitata. Ecotoxicol Environ Saf 37(10):2145–2151. https://doi.org/10.1016/j.ecoenv.2017.05.046
de Luna LA, Silva TH, Nogueira RF, Kummrow F, Umbuzeiro GA (2014) Aquatic toxicity of dyes before and after photo-Fenton treatment. J Hazard Mater 276:332–338. https://doi.org/10.1016/j.jhazmat.2014.05.047
Du M, Zhang D, Yan C, Zhang X (2012) Developmental toxicity evaluation of three hexabromocyclododecane diastereoisomers on zebrafish embryos. Aquat Toxicol 112–113:1–10. https://doi.org/10.1016/j.aquatox.2012.01.013
Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F (1951) A colorimetric method for the determination of sugars. Nature 168(4265):167. https://doi.org/10.1038/168167a0
Environment Canada and Health Canada (2009) Screening assessment for the challenge: five disazo dyes
Environment Canada and Health Canada (2013a) Draft screening assesment-aromatic azo and benzidine-based substance grouping-certain azo disperse dyes
Environment Canada and Health Canada (2013b) Draft screening assesment-aromatic azo and benzidine-based substance grouping- certain azo solvent dyes
Ferraz ERA, Umbuzeiro GA, de-Alameida G, Caloto-Oliveira A, Chequer FMD, Zanoni MVB, Dorta DJ, Oliveira DP (2010) Differential toxicity of Disperse Red 1 and Disperse Red 13 in the Ames test, HepG2 cytotoxicity assay, and Daphnia acute toxicity test. Environ Toxicol 26(5):489–497. https://doi.org/10.1002/tox.20576
Ganesh R, Boardman GD, Michelson D (1994) Fate of azo dyes in sludges. Water Res 28:1367–1376. https://doi.org/10.1016/0043-1354(94)90303-4
George B, Pancha I, Desai C, Choskshi K, Paliwal C, Ghosh T, Mishra S (2014) Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus –a potential strain for bio-fuel production. Bioresour Technol 171:367–374. https://doi.org/10.1016/j.biortech.2014.08.086
Golka K, Kopps S, Myslak WZ (2004) Carcinogenicity of azo colorants: influence of solubility and bioavailability. Toxicol Lett 15:203–210. https://doi.org/10.1016/j.toxlet.2003.11.016
Gu A, Shi X, Yuan C, Ji G, Zhou Y, Long Y, Song L, Wang S, Wang X (2010) Exposure to fenvalerate causes brain impairment during zebrafish development. Toxicol Lett 197:188–192. https://doi.org/10.1016/j.toxlet.2010.05.021
Guo J, Selby K, Boxall ABA (2016) Effects of antibiotics on the growth and physiology of chlorophytes, cyanobacteria and diatom. Arch Environ Contam Toxicol 71(4):589–602. https://doi.org/10.1007/s00244-016-0305-5
Han Y, Wen Q, Chen Z, Li P (2011) Review of methods used for microalgal lipid-content analysis. Energy Procedia 12:944–950. https://doi.org/10.1016/j.egypro.2011.10.124
Hernández-Zamora M, Perales-Vela HV, Flores-Ortiz CM, Cañizares-Villanueva RO (2014) Physiological and biochemical responses of Chlorella vulgaris to Congo Red. Ecotoxicol Environ Saf 108:72–77. https://doi.org/10.1016/j.ecoenv.2014.05.030
Hernández-Zamora M, Cristiani-Urbina E, Martínez-Jerónimo F, Perales-Vela HV, Ponce-Noyola T, Montes-Horcasitas M, Cañizares-Villanueva RO (2015) Bioremoval of the azo dye Congo Red by the microalga Chlorella vulgaris. Environ Sci Pollut Res Int 14(22):10811–10823. https://doi.org/10.1007/s11356-015-4277-1
Hernández-Zamora M, Martínez-Jerónimo F, Cristiani-Urbina E, Cañizares-Villanueva RO (2016) Congo red dye affects survival and reproduction in the cladoceran Ceriodaphnia dubia. Effects of direct and dietary exposure. Ecotoxicology 25(10):1832–1840. https://doi.org/10.1007/s10646-016-1731-x
Heugens EH, Jager T, Creyghton R, Kraak MH, Hendriks AJ, Van Straalen NM, Admiraal W (2003) Temperature-dependent effects of cadmium on Daphnia magna: accumulation versus sensitivity. Environ Sci Technol 37(10):2145–2151
Hill AJ, Teraoka H, Heideman W, Peterson RE (2005) Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 86(1):6–19. https://doi.org/10.1093/toxsci/kfi110
International Organization for Standardization (1996) ISO-7346-1: water quality: determination of the acute lethal toxicity of substances to a freshwater fish (Brachidanio rerio Hamilton: Buchanan [Teleostei, Cyprinidae]): part 1: static method. Geneva, Switzerland. http://www.iso.org/iso/catalogue_detail.htm?csnumber=14026
Jang GH, Park IS, Lee SH, Huh TL, Lee YM (2009) Malachite green induces cardiovascular defects in developing zebrafish (Danio rerio) embryos by blocking VEGFR-2 signaling. Biochem Biophys Res Commun 382(3):486–491. https://doi.org/10.1016/j.bbrc.2009.01.118
Jena S, Acharya S, Mohapatra PK (2012) Variation in effects of our OP insecticides on photosynthetic pigment fluorescence of Chlorella Vulgaris Beij. Ecotoxicol Environ Saf 80:111–117. https://doi.org/10.1016/j.ecoenv.2012.02.016
Kant R (2012) Textile dyeing industry an environmental hazard. Nat Sci 4:22–26. https://doi.org/10.4236/ns.2012.41004
Kebede E (1997) Response of Spirulina platensis (=Arthrospira fusiformis) from Lake Chitu, Ethiopia, to salinity stress from sodium salts. J Appl Phycol 9:551–558. https://doi.org/10.1023/A:1007949021786
Lammer E, Carr GJ, Wendler K, Rawlings JM, Belanger SE, Braunbeck T (2009) Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp Biochem Physiol C 149:196–209. https://doi.org/10.1016/j.cbpc.2008.11.006
Levy JL, Stauber JL, Jolley DF (2007) Sensitivity of marine microalgae to copper: the effect of biotic factors on copper adsorption and toxicity. Sci Total Environ 387(1–3):141–154. https://doi.org/10.1016/j.scitotenv.2007.07.016
Li J, Liang Y, Zhang X, Lu J, Zhang J, Ruan T, Zhou Q, Jiang G (2011) Impaired gas bladder inflation in zebrafish exposed to a novel heterocyclic brominated flame retardant tris (2, 3-dibromopropyl) isocyanurate. Environ Sci Technol 45(22):9750–9757. https://doi.org/10.1021/es202420g
Lim SL, Chu WL, Phang SM (2010) Use of Chlorella vulgaris for bioremediation of textile wastewater. Bioresour Technol 101(19):7314–7322. https://doi.org/10.1016/j.biortech.2010.04.092
Lowry OH, Rosebrough NJ, Farr A, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mahapatra A, Mishra BG, Hota G (2013) Adsorptive removal of Congo res dye from wastewater by mixed iron oxide-alumina nanocomposites. Ceram Int 39:5443–5441. https://doi.org/10.1016/j.watres.2003.12.009
Marín N, Morales F, Tamigneaux E, Lodeiros C (1998) Effects of nitrate concentrations on the growth and pigment synthesis of a wild strain of Dunaliella salina preadapted to different salinities and cultivated under low illumination. J Appl Phycol 10:405–411. https://doi.org/10.1023/A:1008017928651
Martínez-Jerónimo F, Espinosa-Chávez F, Villaseñor-Córdova R (2000) Effect of culture volume and adult density on the neonate production of Daphnia magna, as test organisms for aquatic toxicity test. Environ Toxicol 15:155–159. https://doi.org/10.1002/1522-7278(2000)
Martínez-Jerónimo F, Rodríguez-Estrada J, Martínez-Jerónimo L (2008) Daphnia exilis Herrick, 1895 (Crustacea: Cladocera). Una especie zooplanctónica potencialmente utilizable como organismo de prueba en bioensayos de toxicidad aguda en ambientes tropicales y subtropicales. Rev Int Contam Ambient 24(4):153–159
Meric S, Selçuk H, Belgiorno V (2005) Acute toxicity removal in textile finishing wastewater by Fenton’s oxidation, ozone and coagulation-flocculation processes. Water Res 39(6):1147–1153. https://doi.org/10.1016/j.watres.2004.12.021
Novotný N, Dias A, Kapanen K, Malachová M, Vándrovcová M, Itävaara N, Lima N (2006) Comparative use of bacterial, algal and protozoan tests to study toxicity of azo and anthraquinone dyes. Chemosphere 63:1436–1442. https://doi.org/10.1016/j.chemosphere.2005.10.002
Ogawa T, Fuj H, Kawai K, Yatome C, Idaka E (1989) Growth inhibition of Bacillus subtilis upon interaction between basic dyes and DNA. Bull Environ Contam Toxicol 42:402–408
Organisation for Economic Co-operation and Development, OECD (2011) Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. https://doi.org/10.1787/9789264069923-en
Organisation for Economic Co-operation and Development, OECD (2013) Test No. 236: Fish embryo acute toxicity (FET) test. OECD Guidelines for the Testing of Chemicals. Paris, France. https://doi.org/10.1787/9789264203709-en
Parrott JL, Bartlett AJ, Balakrishnan VK (2016) Chronic toxicity of azo and anthracenedione dyes to embryo-larval fathead minnow. Environ Pollut 210:40–47. https://doi.org/10.1016/j.envpol.2015.11.037
Petit AN, Eullaffroy P, Debenest T, Gagne F (2010) Toxicity of PAMAM dendrimers to Chlamydomonas reinhardtii. Aquat Toxicol 100:187–193. https://doi.org/10.1016/j.aquatox.2010.01.019
Pielesz A (1999) The process of the reduction of azo dyes used in dyeing textiles on the basis of infrared spectroscopy analysis. J Mol Struct 511-512:337–344. https://doi.org/10.1016/S0022-2860(99)00176-3
Purkait MK, Maiti A, Das Gupta S, De S (2007) Removal of Congo Red using activated carbon and its regeneration. J Hazard Mater 145(1–2):287–295. https://doi.org/10.1016/j.jhazmat.2006.11.021
Qian HF, Chen W, Sheng GD, Xu XY, Liu WP, Fu ZW (2008) Effects of glufosinate on antioxidant enzymes, subcellular structure, and gene expression in the unicellular green alga Chlorella vulgaris. Aquat Toxicol 88:301–307. https://doi.org/10.1016/j.aquatox.2008.05.009
Qu R, Xu B, Meng L, Wang L, Wang Z (2015) Ozonation of indigo enhanced by carboxylated carbon nanotubes: performance optimization, degradation products, reaction mechanism and toxicity evaluation. Water Res 68:316–327. https://doi.org/10.1016/j.watres.2014.10.017
Rekha RD, Amali AA, Her GM, Yeh YH, Gong HY, Hu SY, Lin GH, Wu JL (2008) Thioacetamide accelerates steatohepatitis, cirrhosis and HCC by expressing HCV core protein in transgenic zebrafish Danio rerio. Toxicology 243(1–2):11–22. https://doi.org/10.1016/j.tox.2007.09.007
Richardson AD, Duigan SP, Berlyn GP (2002) An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol 153(1):185–194. https://doi.org/10.1046/j.0028-646X.2001.00289.x
Rodrigues de Oliveira GA, de Lapuente J, Teixidó E, Porredón C, Borràs M, de Oliveira DP (2016) Textile dyes induce toxicity on zebrafish early life stages. Environ Toxicol Chem 35(2):429–434. https://doi.org/10.1002/etc.3202
Roy R, Fakhruddin ANM, Khatun R, Islam MS, Ahsan MA, Neger AJMT (2010) Characterization of textile industrial effluents and its effects on aquatic macrophytes and algae. Bangladesh J Sci Ind Res 45(1):79–84. https://doi.org/10.3329/bjsir.v45i1.5187
Selderslaghs IWT, Blust R, Witters HE (2012) Feasibility study of the zebrafish assay as an alternative method to screen for developmental toxicity and embryotoxicity using a training set of 27 compounds. Reprod Toxicol 33:142–154. https://doi.org/10.1016/j.reprotox.2011.08.003
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5(5):532–1553. https://doi.org/10.3390/en5051532
Soni P, Sharma S, Sharma S, Kumar S, Sharma KP (2006) A comparative study on the toxic effects of textile dye wastewaters (untreated and treated) on mortality and RBC of a freshwater fish Gambusia affinis (Baird and Gerard). J Environ Biol 27:623–628
Sponza DT, Isik M (2005) Toxicity and intermediates of C.I. Direct Red 28 dye through sequential anaerobic/aerobic treatment. Process Biochem 40:2735–2744. https://doi.org/10.1016/j.procbio.2004.12.016
Stein JR (1973) Handbook of phycological methods. Culture Methods and Growth Measurements. Cambridge University Press, London, pp 7–24
US Environmental Protection Agency (2002) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organism. EPA-821-R-02-012. 5th Ed., Office of Research and Development, Cincinnati, OH. https://www3.epa.gov/region6/water/npdes/wet/wet_methods_manuals/atx.pdf
Vacchi FI, Von der Ohe PC, Albuquerque AF, Vendemiatti JA, Azevedo CC, Honório JG, Silva BF, Zanoni MV, Henry TB, Nogueira AJ, de Umbuzeiro GA (2016) Occurrence and risk assessment of an azo dye - the case of disperse red 1. Chemosphere 156:95–100. https://doi.org/10.1016/j.chemosphere.2016.04.121
Verma Y (2008) Acute toxicity assessment of textile dyes and textile and dye industrial effluents using Daphnia magna bioassay. Toxicol Ind Health 24(7):491–500. https://doi.org/10.1177/0748233710380218
Walthall WK, Stark JD (1999) The acute and chronic toxicity of two xanthene fluorescein sodium salt and phloxine B, to Daphnia pulex. Environ Pollut 104(2):207–215. https://doi.org/10.1016/S0269-7491(98)00189-4
Wellburn AR (1994) The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Wen YZ, Chen H, Shen CS, Zhao MR, Liu WP (2011) Enantioselectivity tuning of chiral herbicide dichlorprop by copper: roles of reactive oxygen species. Environ Sci Technol 45:4778–4784. https://doi.org/10.1021/es2003793
Wong K, Liu XJ, Lee AOK, Wong PK (2006) Effect of azo dyes on survivorship, oxygen consumption rate, and filtration rate of the freshwater cladoceran Moina macrocopa. Hum Ecol Risk Assess 12:89–300. https://doi.org/10.1080/10807030500531604
Xu XR, Li HB, Wang WH, Gu JD (2005) Decolorization of dyes and textile wastewater by potassium permanganate. Chemosphere 59:893–898. https://doi.org/10.1016/j.chemosphere.2004.11.013
Xu D, Li C, Chen H, Shao B (2013) Cellular response of freshwater green algae to perfluorooctanoic acid toxicity. Ecotoxicol Environ Saf 88:103–107. https://doi.org/10.1016/j.ecoenv.2012.10.027
Yu WL, Ansari W, Schoepp NG, Hannon MJ, Mayfield SP, Burkart MD (2011) Modifications of the metabolic pathways of lipid and triacylglycerol production in microalgae. Microb Cell Factories 10:91–101. https://doi.org/10.1186/1475-2859-10-91
Zöllner N, Kirsch K (1962) Microdetermination of lipids by the sulphophosphovanillin reaction. Zeitschrift fur die Gesamte Experimentelle Medizin einschliesslich Experimenteller Chirurgie 135:545–561
Acknowledgments
Miriam Hernández-Zamora received a postdoctoral scholarship from the Consejo Nacional de Ciencia y Tecnología, and thanks to the Escuela Nacional de Ciencias Biológicas for providing the support and facilities to conduct the present study. Fernando Martínez-Jerónimo acknowledges the Secretaria de Investigación y Posgrado I. P. N., and the Comisión de Operación y Fomento de Actividades Académicas del I. P. N. for the support provided.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Cinta Porte
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hernández-Zamora, M., Martínez-Jerónimo, F. Congo red dye diversely affects organisms of different trophic levels: a comparative study with microalgae, cladocerans, and zebrafish embryos. Environ Sci Pollut Res 26, 11743–11755 (2019). https://doi.org/10.1007/s11356-019-04589-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04589-1