Abstract
In this study, the possibility of culturing Dunaliella salina in stickwater (SW) as the main effluent of fishmeal plants was evaluated. D. salina was grown in different media obtained by replacing standard Guillard medium (F/2) with SW at 0% (control), 10%, 25%, 50%, 75%, and 100% ratios. The cell density, pigment contents, proximate composition, saponification value, and fatty acids (FAs) profiles were measured for 14 days. SW was collected from a kilka fishmeal factory in northern Iran, and the characteristics indicated high concentrations of nitrate (242.00 mg L−1) and phosphate (11.13 mg L−1). A significant increase in the cell density was observed in 14 days when 75% SW was used. Moreover, SW significantly affected the pigment contents. The highest contents of chlorophylls, total carotenoids, and β-carotene (3.64 μg mL−1) were calculated in 75% SW. According to the algal proximate composition, the highest and lowest contents of lipid were accumulated in 75% and 100% SW, respectively (p < 0.05). The highest level of saturated FAs was observed in 75% SW compared with the others (p < 0.05). In conclusion, replacing F/2 with SW indicated the capability of D. salina to grow in a treated medium with 75% SW substitution as a bioremediator.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stickwater (SW) is a by-product of the fishmeal industry (Kousoulaki et al. 2009) which has high nutritional value because of its high concentrations of protein and other organic materials (Mahdabi and Hosseini Shekarabi 2018). However, this protein-rich syrup is commonly discharged directly into the environment and creates a strong malodor around processing plants due to the proteolytic microbe’s activities (Kam et al. 2012; Mahdabi and Hosseini Shekarabi 2018). Although SW can provide a considerable amount of nutrients (i.e., nitrogen and phosphorous), there is limited information about incorporating this valuable by-product in the growth medium of microorganisms.
Marine green algae, Dunaliella salina (Polyblepharidacea), is a halophilic flagellate microalga which can grow under various environmental stresses and can be exploited commercially for β-carotene production (Wu et al. 2016). There is a huge demand for the production of D. salina in order to obtain high contents of carotenoids, poly-unsaturated fatty acids (PUFAs), minerals, and vitamins (Gomez et al. 2003; Raja et al. 2007). However, the complexity and cost of the pure standard growth medium for producing microalgae on a large scale are serious problems (Gebremedhin et al. 2018). One effective way to overcome these hindrances is to replace conventional methods of growing microalgae with wastewaters. To date, however, there are no reports of using SW in culturing D. salina as a halophilic microalga.
Some investigators have reported the possibility of using microalgae species to treat wastewaters from different industries (Costa et al. 2004; Park and Craggs 2010). Hence, many different microalgae species, including Chlorella sorokiniana (De Francisci et al. 2017), C. vulgaris (Blanco-Carvajal et al. 2018), Platymonas subcordiformis (Guo et al. 2013), and Scenedesmus obliquus (Gao et al. 2016), have been grown in the wastewater of fish farming to reduce the negative impacts of such aquaculture on the environment. The cultivation of freshwater microalgae species, including Chlorella sorokiniana and C. vulgaris, in dairy wastewaters resulted in more than 80% removal efficiency of the pollutants with the maximum growth performance (Asadi et al. 2019). In one study, the high biological potency for the removal of secondary treated domestic sewage and biodiesel production was observed in the marine microalga Tetraselmis indica (Chandra et al. 2017). Moreover, Kumar et al. (2018) explained the maximum growth and lipid production of the various microalgae species such as C. singularis, C. sorokiniana, and Micractinium pusillum in pretreated human and animal wastes. Therefore, nutrients from some industrial wastewaters can be used in the cultivation of microalgae and to reduce the eutrophication process in aquatic ecosystems (Rawat et al. 2011; Qu et al. 2019).
Kilka species (Clupeonella spp.) are the most abundant and widespread pelagic fish in the Caspian Sea. They provide raw material to fishmeal factories due to their small size and non-marketability for human consumption (Janbakhsh et al. 2018). Therefore, the objective of this study was to evaluate the possibility of using SW from a kilka fishmeal plant as a growth medium for D. salina to examine the algal growth performance, biochemical contents, and fatty acid composition.
Materials and methods
Preparation and physicochemical properties of stickwater
Fresh stickwater was collected at a local kilka fishmeal plant (Fishmeal Company 581 of Babolsar, Mazandaran, Iran) and transported with ice to the Science and Research Laboratory (Tehran). The stickwater was filtered through Whatman No. 1 filter paper, autoclaved (120 °C, 0.1 MPa) for 15 min and then refrigerated at 4 ± 1 °C until further analysis. A spectrophotometer DR/1900 (HACH, Germany) was applied to measure nitrite, nitrate, ammonia, phosphate, potassium, and calcium in the stickwater. Total suspended solids (TSS), total dissolved solids (TDS), total solids (TS), and total volatile solids (TVS) were obtained according to the APHA (1985) method. Also, pH, crude protein (total extracted nitrogen × 6.25), and lipid contents were calculated using the AOAC (1995) method.
Microorganism and experimental design
The initial purified stock solution of D. salina was provided by Zakariya-Razi Laboratory (Tehran, Iran); the microalgae were isolated from the Hoz-e Soltan Salt Lake (Qum province, Iran) using the methods of Tavallaie et al. (2015). Guillard medium (F/2) was used as a control group, and it was replaced by 5 different levels of the stickwater at 10%, 25%, 50%, 75%, and 100% ratios. D. salina was grown in all treatments at 10% NaCl (w/v; 1.7 M NaCl salinity) and 7.5 pH (Tavallaie et al. 2015). The microalgae cultures were maintained in climatic chambers with continuous aeration, at a temperature of 25 ± 1 °C, 55 μmol photons m−2 s−1 light intensity, and 12D:12L (dark/light) regimen for 14 days. All of the chemical materials were procured from Merck Company (Darmstadt, Germany). The initial stocking density of D. salina was 10 mL inoculum of the algae (1 × 104 cells mL−1).
Algal density
A T80 spectrometer (T80+UV/VIS spectrometer PG Instruments Ltd., England) at 680 nm (Rastar et al. 2018) was used to measure the algal density.
Determination of pigments
Chlorophyll (Chl) a, b and carotenoids were extracted using Yang et al. (1998) method with some modifications. The amount of 2 mL of algae sample was mixed with 5 mL of acetone: distilled water (4:1 v/v) and then stirred for 2 min to create a homogenous mixture. The mixture was centrifuged at 2600×g at 20 °C for 5 min (3-30K Sigma, Germany), and the absorption of the supernatants was read at 662, 645, and 470 nm for Chl a, b and carotenoids, respectively.
where A is the absorption read by the spectrophotometer.
The method of Eijckelhoff and Dekker (1997) with slight modification was used to determine the concentration of β-carotene. In short, 1 mL of the algae was centrifuged at 1274×g for 8 min. The precipitates were mixed with acetone 80% (v/v) for an hour and then treated by ultrasonic bath system (Wise Clean, Germany) to extract the pigments. The samples were centrifuged, and the supernatants were measured at 412, 431, 460, and 480 nm. The following equation was used to calculate the β-carotene content:
Proximate chemical composition
After harvesting, the algal biomass was washed three times in deionized water and centrifuged at 6654×g for 15 min to eliminate unwanted materials. Then, the sediment samples were freeze-dried for 24 h using a Christ Alpha 2–4 Plus freeze dryer (Martin Christ Gefriertrocknungsanlagen, Osterode, Germany) and maintained at − 80 °C for a maximum of 2 months before analyzing. Nitrogen contents (N × 6.25) were measured using the Kjeldahl method to determine the crude protein content of the dried samples. The fat content was determined using the method of Folch et al. (1957). Ash content was measured by burning 1 g of the sample over a low flame followed by heating in a muffle furnace at 550 °C for 4 h (AOAC 1995).
Saponification value
The saponification value (SV) was measured based on the method of Sabzi et al. (2018). The extracted oil (3 mL) was mixed with 25 mL of ethanolic KOH. The samples were then enclosed tightly and placed in a water bath at 80 °C for 30 min. Two drops of phenolphthalein were added to the mixture as a pH pointer and titrated with 0.5 M hydrochloric acid to change the color to yellow. A blank sample (all the abovementioned procedures without the oil sample) was also included. The following equation was used to calculate the SV:
where V1 is the volume of ethanolic KOH used in titration of the blank sample (ml), V2 is the volume of ethanolic KOH used in titration of the samples (mL), N is the normality of acid (0.5 N), and W is the weight of the oil samples (g).
Fatty acid profile
The fatty acid (FA) composition of the algae samples cultured in different media was measured using the method by Schierholt et al. (2001). In brief, 1 g of the extracted oil was mixed with 20 mL of potassium methoxide 1% for 25 min. Then, 12 mL of 4-fluorobenzyl bromide was added and the solution was boiled for 10 min. After that, 2 mL of sodium chloride was added and the solution was centrifuged at 2150×g for 10 min. FAs methyl esters (1 μL) were injected into a Nexis 2030 GC-chromatography (Shimadzu, Japan). The GC was equipped with an FID detector and a Dikmacap-2330 capillary column with the length of 60 m and the inner diameter of 0.25 mm. Hydrogen gas with a purity of 99.99% and a velocity of 2 mm per minute was used as the carrier gas. The temperatures of the injection and detector were regulated at 250 °C and 260 °C, respectively. The detection of FAs was measured by comparing their peak areas with the standards.
Statistical analysis
All experiments were conducted in three replicates and data was expressed as mean ± standard error of the mean. SPSS software, version 20.0 (SPSS, Chicago, IL, USA) was used to analyze the obtained data. Data were subjected to a one-way analysis of variance (one-way ANOVA) followed by Tukey’s post-hoc test to identify the significance of differences between the means at a confidence level of 5%.
Results
Physicochemical properties of SW
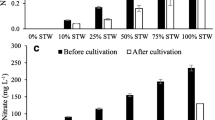
Some physical and chemical characteristics of the SW used in this study are summarized in Table 1. High concentrations of nitrate (242.00 mg L−1) and phosphate (11.13 mg L−1) were measured in SW.
Algal density
As shown in Fig. 1, the density of cultured D. salina was affected by different levels of SW substitution. Although there were significant differences between the treatments on different days, the density of microalgae had an increasing trend during the trial. The highest cell density was observed by replacing F/2 medium with SW up to 75% from 22.05 × 104 to 112.60 × 104 cell mL−1 at the end of the trial (p < 0.05). However, the lowest cell density was calculated in the treatment with 100% replacement by SW (p < 005).
Chlorophyll content
Substitution with SW had a significant impact on Chl a and b contents (Figs. 2 and 3). The highest amount of Chl a was observed at 10 days in 50% SW (4.591 μg mL−1). However, the Chl a concentration on days 12 and 14 of the experiment were at the highest level in 75% SW with 5.741 and 6.540 μg mL−1, respectively. In addition, the minimum content of Chl a was seen in 100% replacement of F/2 medium with SW. At the end of the trial, the highest and lowest Chl b contents were observed in 75% SW (3.334 μg mL−1) and 100% SW (1.639 μg mL−1), respectively (Fig. 3).
Chlorophyll a concentration of Dunaliella salina microalgae grown in different concentrations of stickwater (SW) as a substitute for F/2 medium. The vertical error bars correspond to standard deviation of the mean, and different letters represent statistically significant differences between groups (n = 3, p < 0.05)
Chlorophyll b concentration of Dunaliella salina microalgae grown in different concentrations of stickwater (SW) as a substitute for F/2 medium. The vertical error bars correspond to standard deviation of the mean, and different letters represent statistically significant differences between groups (n = 3, p < 0.05)
Carotenoid concentration
The amount of total carotenoids was increased during the trial in all treatments (Fig. 4). The highest carotenoid concentration was observed on day 14 (5.30 μg mL−1) in 75% SW, while the lowest value (2.33 μg mL−1) was measured in the control group (p < 0.05).
Carotenoid concentration of Dunaliella salina microalgae grown in different concentrations of stickwater (SW) as a substitute for F/2 medium. The vertical error bars correspond to standard deviation of the mean, and different letters represent statistically significant differences between groups (n = 3, p < 0.05)
The β-carotene contents of D. salina in different concentrations of SW are shown in Fig. 5. The highest and lowest β-carotene levels were observed in 75% SW (3.64 μg mL−1) and the control group (0.51 μg mL−1), respectively (p < 0.05).
β-Carotene concentration of Dunaliella salina microalgae grown in different concentrations of stickwater (SW) as a substitute for F/2 medium. The vertical error bars correspond to standard deviation of the mean, and different letters represent statistically significant differences between groups (n = 3, p < 0.05)
Approximate chemical composition
The amount of moisture showed no significant differences among treatments (Table 2). The highest and lowest levels of protein were observed in 75% SW (59.18%, dry basis) and the control (46.63%, dry basis), respectively (p < 0.05). The highest fat and ash contents were calculated in 75% SW (16.3%, dry basis) and 10% SW (55.31%, dry basis), respectively (p < 0.05). The lowest fat (14.8%, dry basis) and ash (39.17%, dry basis) contents were seen in 100% SW (p < 0.05; Table 2).
Saponification value of the extracted oil
The highest SV was obtained in 75% SW (195.000 mg KOH g−1), while the lowest value was observed in 100% SW (177 mg KOH g−1) (p < 0.05; Fig. 6).
Saponification value of Dunaliella salina microalgae grown in different concentrations of stickwater (SW) as a substitute for F/2 medium. The vertical error bars correspond to standard deviation of the mean, and different letters represent statistically significant differences between groups (n = 3, p < 0.05)
Fatty acid composition
Table 3 shows the FA composition of the extracted oil from D. salina which was grown in different concentrations of SW. The highest myristic acid (C14:0) was obtained in the control group (5.84%), while the minimum level was measured in 100% SW (1.31%) (p < 0.05). In all samples, palmitic acid (C16:0) was the main FAs and had the highest value in 75% SW (33.59%) compared with the others (p < 0.05). The highest and lowest contents of pentadecanoic acid (C15:1) as a dominant mono-unsaturated FAs (MUFAs) were in 10% SW (13.16%) and 100% SW (0.29%) treatments, respectively (p < 0.05). Also, the highest value of vaccenic acid (C18:1t) was achieved in the 100% SW group, while the minimum level was seen in the 75% SW treatment (p > 0.05). The highest amount of oleic acid (C18:1c) was observed in 100% SW compared with the control group (p < 0.05). The maximum amount of linoleic acid (C18:2c) was measured in the control group. The content of other n-6 FAs family members, including gamma linolenic (GLA, C18:3), was changed in a parallel manner, but reached the minimum of 4.84% in 100% SW. There were no significant differences among experimental treatments for eicosapentaenoic acid (EPA, C20:5) content (p > 0.05).
The amounts of MUFAs, saturated FAs (SFAs), and PUFAs were significantly varied among the treatments, and the highest level of SFAs (40.470%) was found in the 75% SW group among other groups (p < 0.05). However, the control group showed the highest contents of MUFA (30.445%) and PUFA (27.395%) (p < 0.05).
Discussion
The main cost of microalgae production is related to the ingredients in the growth media. However, it is possible to produce a cheaper microalga by replacing the culture medium with low-cost wastewaters, like stickwater from fishmeal factories. Growth is the most important way to express the ecological success or the ability of adaptation of an algae species to the environmental conditions. An increase in the density of D. salina microalgae in treated media with partial replacement with SW showed the compatibility of these microalgae to growing in wastewater to become a potential bioremediator. Therefore, SW is a liquid nutrient substance that probably can provide a high concentration of nutrients for the microbial biomass.
This study showed that the standard cultivation medium of D. salina can be replaced with SW up to 75% without negative effects. However, the microalgae growth performance in a treatment which is totally replaced with SW was significantly decreased possibly due to the inability to properly meet the needs of the algae. Various factors have been proposed to reduce the growth rate of algae, including salinity changes, optical limitations, carbon dioxide or mineral carbon limitations, and undesirable ratios of nitrogen to phosphorus (Tam et al. 1994). Similarly, Kam et al. (2012) explained that the standard growth mediums of Chlorella sp., Pseudomonas aeruginosa, and Saccharomyces cerevisiae could be partially replaced by SW from a kilka fishmeal plant. Spirulina platensis was also cultured in agro-industrial wastewaters, and the results showed that producing the microalgal biomass was increased by increasing the nitrogen concentration (Markou and Georgakakis 2011). A study conducted by Lv et al. (2010) showed that low nitrogen concentrations (0.2–0.3 mM) led to a significant reduction in Chlorella vulgaris cell growth, while a high concentration of nitrogen (up to 0.5 mM) improved the growth performance. Safafar et al. (2016) examined industrial process water (IPW) as a C. vulgaris cultivation medium and indicated that the algal biomass which had been cultured in 67% of IPW was higher than 100% of ICW (internal circulation water of sludge tower reactor). Similar to the current results, the high growth rate and biomass productivity of C. sorokiniana were reported when it was grown in the secondary treated effluent of dairy plants (Asadi et al. 2019).
The photosynthetic pigments are specific markers for microalgae cells which are mostly affected by the availability of essential nutrients (Kaya et al. 1995). The present study showed that by increasing the substitution level of F/2 with SW up to 75%, chlorophyll concentrations were increased. The main reason for this increasing trend was the high levels of nitrogen and phosphorus in SW (Mahdabi and Hosseini Shekarabi 2018). Kiran et al. (2016) examined the effects of various concentrations of nitrogen on Chlorella growth performance and showed that the algae could not increase the amount of chlorophyll pigments properly under conditions with nitrogen deficiency due to the reduction in electron receptors such as NADP+ at low nitrogen levels (Da Silva et al. 2009; Pruvost et al. 2011; Kiran et al. 2016). Carotenoids are produced in the chloroplasts of microalgae and reach the highest concentrations under stress environmental conditions (Zhu and Jiang 2008). β-Carotene also plays a vital role in protecting chlorophyll from the destructive effects of oxidation and optical suppression in the microorganisms (Ben-Amotz 1981). D. salina is a proper candidate for producing natural β-carotene on an industrial scale (Ribeiro et al. 2011). In the current study, total carotenoids and β-carotene were increased by increasing the replacement of cultivation medium of the algae with SW. Although this increase in 100% stickwater treatment was lower than other SW treatments, it was higher than the control group. This could be due to stressful conditions for the algal biomass (Nikokar et al. 2004; Ak et al. 2008). There are many probable reasons to explain the highest amount of pigments in 75% SW treatment including (1) cell growth and multiplication (Kaya et al. 1995; Tam and Wang 2000); (2) decreased incident light by immobilization, self-shading of light, and screen effect by gel, thus increasing the synthesis of pigments (Vichez and Vega 1994); and (3) immobilization which promotes the algal anabolism and physiological activity leading to enhanced removal efficiency of inorganic nutrients (Yan et al. 1995).
In general, microalgae produce protein, lipid, and carbohydrates by consuming the energy in the photosynthesis process (Liu et al. 2012). The present study showed the highest and lowest amounts of protein in D. salina in 75% SW and control treatments, respectively. The positive relation between the protein content of algae and the nitrogen concentration of the growth media has been proven by many investigations (Lv et al. 2010; He et al. 2013; Guccione et al. 2014). This may mean that appropriate amounts of nitrogen in the growth medium can lead to stabilized carbon and synthesis of nitrogen-based compounds such as proteins by the cells in the photosynthesis process (Griffiths et al. 2014). Because stickwater has a high level of nitrogen, it uptakes during metabolic activities and increases the nitrogen concentration of the microalgae cells in parallel by increasing the content of cell protein (Djaghoubi et al. 2015; Venckus et al. 2017). The amount of lipid storage in microalgae can be changed by manipulating some environmental conditions such as light (Yeesang and Cheirsilp 2011), nutrients availability (Gouveia and Oliveira 2009), and temperature (Zhu et al. 2009; Bellou and Aggelis 2012). In this study, the highest content of fat accumulation was observed in 75% SW on day 14. Similar results have been reported regarding the direct effect of nitrogen levels on the fat storage of Ellipsoidion sp. and Isochrysis zhangjiangensis microalgae (Xu et al. 2001; Feng et al. 2011). Generally, lipid storage level in algae species is estimated to be about 50% of their dry weight, but some types of algae can store fat up to 70% of their dry weight in stressful conditions (Xin et al. 2010). Increases in the nitrogen concentration (NaNO3) of the growth medium lead to increases in the cellular fat accumulation of Chlorella. However, fat storage in Nannochloropsis gaditana is doubled in comparison with Chlorella under nutrient-replete conditions (Ajjawi et al. 2017). Therefore, the response of algae cells to accumulating lipids in order to change the nitrogen budget (increase or decrease) differs among algae species (Sreedharan et al. 2018).
Since microalgae include high levels of essential fatty acids, it is possible to be soaped (El-Mashad et al. 2008). In this study, the highest soap number was seen in the 75% SW treatment (414.1 mg KOH g−1). High levels of SV indicate a higher quality of biofuels (Predojević et al. 2012). Kirk and Sawyer (1991) stated that high levels of SV are mostly due to the high contribution of carbon in short-chain fatty acids. In other words, a lower soap number indicates a longer chain fatty acid (Nielson 1994).
Fatty acids play a crucial role in the algal cell membrane function, metabolism, and adaptation processes against environmental changes (Zhukova and Titlyanov 2006; Sabzi et al. 2018). One protective mechanism of algae for overcoming environmental fluctuations is the synthesis and accumulation of lipids in the form of saturated FAs (Stansell et al. 2011). Stickwater is a rich source of nitrogen and phosphate, which are needed to grow microalgae (Shi et al. 2000). However, kilka fish have non-protein nitrogenous compounds (NPNs), and the stickwater may possibly have high amounts of NPNs. In fact, the NPNs could not be fully absorbed by the algae and negatively impacted the fatty acid composition of the microalgae. The types and concentrations of NPNs differ in various aquatic organisms and generally include free amino acids, peptides, nucleotides, guanidine, quaternary ammonium, and urea (Gram and Huss 1996). Some researchers have shown that microalgae can store high levels of NPN compounds (Dortch et al. 1984), and most marine microalgae species can store 15–30% of the total intracellular nitrogen as NPNs (Lourenço et al. 2004; González López et al. 2010). The possible amount of nitrogen available to D. salina algae is decreased because of the high amount of NPNs. By increasing the replacement of the culture medium with SW up to 100%, cell density, pigments, and lipid accumulation were markedly reduced.
In this research, C16:0 level was the major FAs in the 75% SW treatment, a higher rate than those of Botryococcus braunii, Scenedesmus sp., and Chlorella sp. (Lee et al. 2010; Matsumoto et al. 2010). The major FAs in D. salina are C16:0 and C18:0 in the standard growth medium (Akter et al. 2016). For biodiesel production, C16:0 is one of the greatest thermal and oxidative stability FAs components among SFAs, which can delay the deterioration of its fuel in high-temperature conditions or long-term storage (Hoekman et al. 2012). The highest SFA content was measured in the 75% SW treatment, while the control group showed the highest amounts of MUFAs and PUFAs. In agreement with the current results, Chandra et al. (2017) reported the highest amount of SFAs from T. indica which was grown in secondary treated domestic sewage for 14 days.
The results showed that the lowest GLA content was obtained in 100% SW in comparison with the other treatments. This reduction may be explained by the fact that the microalgae were not able to meet their nutritional demands in order to produce bioactive compounds from SW when F/2 was replaced with 100% SW. GLA from PUFAs plays a key role as the precursor of eicosanoids that are implicated in many physiological functions of biological systems (Bellou et al., 2016). High GLA content is found in most commercial microbial oils of some fungi species (Ratledge 2013). Producing these FAs is highly interesting for nutritional and pharmaceutical applications due to its anti-inflammatory properties and therapeutic values (Gunstone 1992). The quantity and quality of microalgae FAs could be changed and promoted by manipulating the media and environmental conditions (Bougaran et al. 2012; Draaisma et al. 2013; Vinayak et al. 2015). In fact, algae produce FAs as building blocks in their plastids to form different types of fat for construction of the cell membrane or to be stored in its cells (Ohlrogge and Browse 1995).
Conclusion
Stickwater is produced in fishmeal plants, and, despite the high concentrations of nutrients, it is directly discharged into the environment. In this study, replacing D. salina microalgae standard growth medium (F/2) with different concentrations of SW showed the highest cell density, chlorophyll, beta-carotene, carotenoid, lipid accumulation, and protein contents in the treated medium with 75% SW. Moreover, the highest amounts of fat storage and SFA composition were observed in the 75% SW treatment. However, complete substitution (100%) of F/2 with SW negatively influenced the microalgae growth performance and biochemical compositions. Therefore, D. salina growth medium can be replaced with 75% SW for the production of biofuel and some bioactive compounds, like carotenoids, with many economic and environmental advantages. Further supplementary studies are needed to assess how effective D. salina is in removing the nutrients of SW.
References
Ajjawi I, Verruto J, Aqui M, Soriaga LB, Coppersmith J, Kwok K, Peach L, Orchard E, Kalb R, Xu W, Carlson TJ, Francis K, Konigsfeld K, Bartalis J, Schultz A, Lambert W, Schwartz AS, Brown R, Moellering ER (2017) Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nat Biotechnol 35:647–652. https://doi.org/10.1038/nbt.3865
Ak I, Cirik S, Goksan T (2008) Effect of light intensity, salinity and temperature on growth in Camalti strain of Dunaliella viridis Teodoresco from Turkey. J Biol Sci 8(8):1356–1359. https://doi.org/10.3923/jbs.2008.1356.1359
Akter R, Hossain S, Zhe W, Kermanee P, Juntawong N (2016) Enhanced lipid production in Dunaliella salina grown under high light intensity by shifting the culture from high to low nitrogen concentration. Adv Environ Biol 10(7):18–28
AOAC (Association of Official Analytical Chemists) (1995) Official methods for analysis, 16th edn. AOAC, Washington, D.C.
APHA (American Public Health Association) (1985) Standard methods for the examination of water and wastewater, 10th edn. American Public Health Association, Washington DC
Asadi P, Rad HA, Qaderi F (2019) Comparison of Chlorella vulgaris and Chlorella sorokiniana pa.91 in post treatment of dairy wastewater treatment plant effluents. Environ Sci Pollut Res:1–17. https://doi.org/10.1007/s11356-019-06051-8
Bellou S, Aggelis G (2012) Biochemical activities in Chlorella sp. and Nannochloropsis salina during lipid and sugar synthesis in a lab-scale open pond simulating reactor. J Biotechnol 164(2):318–329. https://doi.org/10.1016/j.jbiotec.2013.01.010
Bellou S, Triantaphyllidou IE, Aggeli D, Elazzazy AM, Baeshen MN, Aggelis G (2016) Microbial oils as food additives: recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr Opin Biotechnol 37:24–35. https://doi.org/10.1016/j.copbio.2015.09.005
Ben-Amotz A (1981) Effect of irradiance and nutrient deficiency on the chemical composition of Dunaliella bardawil Ben-Amotz and Avron (Volvocales, Chlorophyta). Plant Physiol 131(5):479–487. https://doi.org/10.1016/S0176-1617(87)80290-0
Blanco-Carvajal E, Sánchez-Galvis E, González-Delgado AD, JBG M, Barajas-Solano AF (2018) Cultivation of Chlorella vulgaris in aquaculture wastewater for protein production. Contemp Eng Sci 11(2):93–100. https://doi.org/10.12988/ces.2018.712203
Bougaran G, Rouxel C, Dubois N, Kaas R, Grouas S, Lukomska E, Le Coz JR, Cadoret JP (2012) Enhancement of neutral lipid productivity in the microalga Isochrysis affinis galbana (T-Iso) by a mutation-selection procedure. Biotechnol Bioeng 109(11):2737–2745. https://doi.org/10.1002/bit.24560
Chandra R, Ghosh UK, Nayak JK (2017) Phycoremediation potential of marine microalga Tetraselmis indica on secondary treated domestic sewage for nutrient removal and biodiesel production. Environ Sci Pollut Res 24(26):20868–20875. https://doi.org/10.1007/s11356-017-9734-6
Costa RAAM, Koening DML, Macedo SJD (2004) Urban secondary sewage: an alternative medium for the culture of Tetraselmis chuii (Prasinophyceae) and Dunaliella viridis (Chlorophyceae). Braz Arch Biol Technol 47(3):451–459. https://doi.org/10.1590/S1516-89132004000300016
Da Silva AF, Lourenco SO, Chalou RM (2009) Effect of nitrogen starvation on the photosynthetic physiology of a tropical marine Rhodomonas sp. (Cryptophyceae). Aquat Bot 91(4):279–291. https://doi.org/10.1016/j.aquabot.2009.08.001
De Francisci D, Su Y, Iital A, Angelidaki I (2017) Evaluation of microalgae production coupled with wastewater treatment. Environ Technol 10(5):581–592. https://doi.org/10.1080/09593330.2017.1308441
Djaghoubi A, Daddi Bouhoun M, Hadj Saida S, Saggaï A, Sobti S, Hamdi Aissa B (2015) Growth and nitrogen removal efficiency as protein content of Spirulina from tertiary municipal wastewater in Ouargla (Algerian Bas-Sahara). Energy Procedia 74:1402–1409. https://doi.org/10.1016/j.egypro.2015.07.786
Dortch Q, Clayton JR, Thoresen SS, Ahmed SI (1984) Species differences in accumulation of nitrogen pools in phytoplankton. Mar Biol 81(3):237–250. https://doi.org/10.1007/BF00393218
Draaisma RB, Wijffels RH, Slegers PM, Brentner LB, Roy A, Barbosa MJ (2013) Food commodities from microalgae. Curr Opin Biotechnol 24(2):169–177. https://doi.org/10.1016/j.copbio.2012.09.012
Eijckelhoff C, Dekker JP (1997) A routine method to determine the chlorophyll a, pheophytin a and β-carotene contents of isolated photosystem II reaction center complexes. Photosyn Res 52(1):69–73. https://doi.org/10.1023/A:1005834006985
El-Mashad HM, Zhang R, Avena-Bustillos RJ (2008) A two-step process for biodiesel production from salmon oil. Biosyst Eng 99(2):220–227. https://doi.org/10.1016/j.biosystemseng.2007.09.029
Feng D, Chen Z, Xue S, Zhang W (2011) Increased lipid production of the marine oleaginous microalgae Isochrysis zhangjiangensis (Chrysophyta) by nitrogen supplement. Bioresour Technol 102(12):6710–6716. https://doi.org/10.1016/j.biortech.2011.04.006
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226(1):497–509
Gao F, Li C, Yanga Z-H, Zenga G-M, Feng LJ, Liub JZ, Liub M, Cai HW (2016) Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol Eng 92:55–61. https://doi.org/10.1016/j.ecoleng.2016.03.046
Gebremedhin M, Mishra S, Mohanty K (2018) Augmentation of native microalgae based biofuel production through statistical optimization of campus sewage wastewater as low-cost growth media. J Environ Chem Eng 6(5):6623–6632. https://doi.org/10.1016/j.jece.2018.08.061
Gomez P, Barriga A, Cifuentes AS, Gonzalez M (2003) Effect of salinity on the quantity and quality of carotenoids accumulated by Dunaliella salina (strain CONC-007) and Dunaliella bardawil (strain ATCC 30861) Chlorophyta. Biol Res 36:185–192. https://doi.org/10.4067/S0716-97602003000200008
González López CV, CerónGarcía MC, Acién Fernández FC, Bustos CS, Chisti Y, Fernández Sevilla JM (2010) Protein measurements of microalgal and cyanobacterial biomass. Bioresour Technol 101(19):7587–7591. https://doi.org/10.1016/j.biortech.2010.04.077
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36(2):269–274. https://doi.org/10.1007/s10295-008-0495-6
Gram L, Huss HH (1996) Microbiological spoilage of fish and fish products. J Food Microbiol 33:121–137. https://doi.org/10.1016/0168-1605(96)01134-8
Griffiths MJ, van Hille RP, Harrison STL (2014) The effect of nitrogen limitation on lipid productivity and cell composition in Chlorella vulgaris. Appl Microbiol Biotechnol 98(5):2345–2356. https://doi.org/10.1007/s00253-013-5442-4
Guccione A, Biondi N, Sampietro G (2014) Chlorella for protein and biofuels: from strain selection to outdoor cultivation in a Green Wall Panel photo bioreactor. Biotechnol Biofuels 7:84. https://doi.org/10.1186/1754-6834-7-84
Gunstone FD (1992) Gamma linolenic acid-occurrence and physical and chemical properties. Prog Lipid Res 31:145–161. https://doi.org/10.1016/0163-7827(92)90007-6
Guo Z, Liu Y, Guo H, Yan S, Mu J (2013) Microalgae cultivation using an aquaculture wastewater as growth medium for biomass and biofuel production. J Environ Sci 1:S85–S88. https://doi.org/10.1016/S1001-0742(14)60632-X
He PJ, Mao B, Shen CM (2013) Cultivation of Chlorella vulgaris on wastewater containing high levels of ammonia for biodiesel production. Bioresour Technol 129:177–181. https://doi.org/10.1016/j.biortech.2012.10.162
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sust Energ Rev 16(1):143–169. https://doi.org/10.1016/j.rser.2011.07.143
Janbakhsh S, Hosseini Shekarabi SP, Shamsaie Mergan M (2018) Nutritional value and heavy metal content of fishmeal from the Southwest Caspian Sea. Caspian J Environ Sci 16(4):307–317
Kam S, Abedian Kenari A, Younesi H (2012) Production of single cell protein in stickwater by Lactobacillus acidophilus and Aspergillus niger. J Aquat Food Prod T 21(5):403–417. https://doi.org/10.1080/10498850.2011.605539
Kaya VM, Goulet J, dela Noüe J, Picard G (1995) A comparative study of four systems for tertiary wastewater treatment by Scenedesmus bicellularis: new technology for immobilization. J Appl Phycol 7(1):85–95. https://doi.org/10.1007/BF00003556
Kiran B, Pathak K, Kumar R, Deshmukh D, Rani N (2016) Influence of varying nitrogen levels on lipid accumulation in Chlorella sp. Int J Environ Sci Technol 13(7):1823–1832. https://doi.org/10.1007/s13762-016-1021-4
Kirk R, Sawyer R (1991) Pearson’s composition and analysis of foods, 9th edn. Addison Wesley longman Ltd., London
Kousoulaki K, Albrektsen S, Langmyhr E, Olsen HJ, Campbell P, Aksnes A (2009) The water soluble fraction in fish meal (stickwater) stimulates growth in Atlantic salmon (Salmo salar L.) given high plant protein diets. Aquaculture 289(1-2):74–83. https://doi.org/10.1016/j.aquaculture.2008.12.034
Kumar V, Kumar A, Nanda M (2018) Pretreated animal and human waste as a substantial nutrient source for cultivation of microalgae for biodiesel production. Environ Sci Pollut Res 25(22):22052–22059. https://doi.org/10.1007/s11356-018-2339-x
Lee JY, Yoo C, Jun SY, Ahn CY, Oh HM (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 101(1):75–77. https://doi.org/10.1016/j.biortech.2009.03.058
Liu W, Huang Z, Li P, Xia J, Chen B (2012) Formation of triacylglycerol in Nitzschia closterium f. minutissima under nitrogen limitation and possible physiological and biochemical mechanisms. J Exp Mar Biol Ecol 418:24–29. https://doi.org/10.1016/j.jembe.2012.03.005
Lourenço SO, Barbarino E, Lavín PL, Marquez UML, Aidar E (2004) Distribution of intracellular nitrogen in marine microalgae: calculation of new nitrogen-to-protein conversion factors. Eur J Phycol 39(1):17–32. https://doi.org/10.1080/0967026032000157156
Lv JM, Cheng LH, Xu XH (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 101(17):6797–6804. https://doi.org/10.1016/j.biortech.2010.03.120
Mahdabi M, Hosseini Shekarabi SP (2018) A comparative study on some functional and antioxidant properties of kilka meat, fishmeal, and stickwater protein hydrolysates. J Aquat Food Prod Technol 27(7):844–858. https://doi.org/10.1080/10498850.2018.1500503
Markou G, Georgakakis D (2011) Cultivation of filamentous cyanobacteria (blue-green algae) in agro industrial wastes and wastewaters: a review. Appl Energ 88(10):3389–3401. https://doi.org/10.1016/j.apenergy.2010.12.042
Matsumoto M, Sugiyama H, Maeda Y, Sato R, Tanaka T, Matsunaga T (2010) Marine diatom, Navicula sp. strain JPCC Da0580 and marine green alga, Chlorella sp. strain NKG400014 as potential sources for biodiesel production. Appl Biochem Biotechnol 161(1-8):483–490. https://doi.org/10.1007/s12010-009-8766-x
Nielson SS (1994) Introduction to the chemical analysis of foods. Chapman and Hall, New York
Nikokar K, Moradshahi A, Kharati M (2004) Influence of salinity on the growth, pigmentation and ascorbate peroxidase activity Dunaliella salina isolated from Maharlu salt lake in Shiraz. Iran J Sci Technol 28(1):117–125
Ohlrogge J, Browse J (1995) Lipid biosynthesis. Plant Cell 7(7):957–970. https://doi.org/10.1105/tpc.7.7.957
Park JBK, Craggs RJ (2010) Wastewater treatment and algal production in high rate algal ponds with carbon dioxide addition. Wat Sci Tech 61:633–639. https://doi.org/10.2166/wst.2010.951
Predojević Z, Škrbić B, Durišić-Mladenović N (2012) Transesterification of linoleic and oleic sunflower oils to biodiesel using CaO as a solid base catalyst. J Serb Chem Soc 77(6):815–832. https://doi.org/10.2298/JSC110824206P
Pruvost J, Van Vooren G, Le Gouic B, Couzinet-Mossion A, Legrand J (2011) Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour Technol 102(1):150–158. https://doi.org/10.1016/j.biortech.2010.06.153
Qu Z, Duan P, Cao X, Liu M, Lin L, Li M (2019) Comparison of monoculture and mixed culture (Scenedesmus obliquus and wild algae) for C, N, and P removal and lipid production. Environ Sci Pollut Res:1–8. https://doi.org/10.1007/s11356-019-05339-z
Raja R, Hemaiswarya S, Rengasamy R (2007) Exploitation of Dunaliella for β-carotene production. Appl Microbiol Biotechnol 74(3):517–523. https://doi.org/10.1007/s00253-006-0777-8
Rastar M, Hosseini Shekarabi SP, Shamsaie Mehrgan M, Sabzi S (2018) Effects of iron and zinc concentrations on growth performance and biochemical composition of Haematococcus pluvialis: a comparison between nanoparticles and their corresponding. J Algal Biomass Util 9(2):59–67
Ratledge C (2013) Microbial oils: an introductory overview of current status and future prospects. Ocl 20(6):1–7. https://doi.org/10.1051/ocl/2013029
Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2011) Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energ 88(10):3411–3424. https://doi.org/10.1016/j.apenergy.2010.11.025
Ribeiro BD, Barreto DW, Coelho MAZ (2011) Technological aspects of β-carotene production. Food Bioprocess Tech 4(5):693–701. https://doi.org/10.1007/s11947-011-0545-3
Sabzi S, Shamsaie Mehrgan M, Rajabi Islami H, Hosseini Shekarabi SP (2018) Changes in biochemical composition and fatty acid accumulation of Nannochloropsis oculata in response to different iron concentrations. Biofuels:1–7. https://doi.org/10.1080/17597269.2018.1489672
Safafar H, Nørregaard PU, Ljubic A, Møller P, Holdt SL, Jacobsen C (2016) Enhancement of protein and pigment content in two Chlorella species cultivated on Industrial process water. J Mar Sci Engin 4(4):1–15. https://doi.org/10.3390/jmse4040084
Schierholt A, Rucker B, Becker C (2001) Inheritance of high oleic acid mutations in winter oilseed rape (Brassica napus). J Trop Crop Sci 41(5):1444–1449. https://doi.org/10.2135/cropsci2001.4151444x
Shi XM, Zhang X, Chen F (2000) Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzyme Microb Technol 27(3-5):312–318. https://doi.org/10.1016/S0141-0229(00)00208-8
Sreedharan A, Velmurugan N, Suresh A (2018) Nitrogen replete stress condition for enhanced lipid accumulation in microalgae Chlorella sp. J Algal Biomass Util 9:37–43
Stansell G, Gray VM, Sym S (2011) Microalgal fatty acid composition: implications for biodiesel quality. J Appl Phycol 24(4):791–801. https://doi.org/10.1007/s10811-012-9824-2
Tam NFY, Wang YS (2000) Effect of immobilized microalgal bead concentration on wastewater nutrient removal. Environ Pollut 107(1):145–151. https://doi.org/10.1016/S0269-7491(99)00118-9
Tam NFY, Lau PS, Wong YS (1994) Wastewater inorganic N and P removal by immobilized Chlorella vulgaris. Wat Sci Tech 30(6):369–374
Tavallaie S, Emtyazjoo M, Rostami K, Kosari H, Assadi MM (2015) Comparative studies of β-carotene and protein production from Dunaliella salina isolated from Lake Hoze-soltan, Iran. J Aquat Food Prod Technol 24(1):79–90. https://doi.org/10.1080/10498850.2012.759634
Venckus P, Kostkeviciene J, Bendikiene V (2017) Green algae Chlorella vulgaris cultivation in municipal wastewater and biomass composition. J Environ Eng Landsc 25(1):56–63. https://doi.org/10.3846/16486897.2016.1245661
Vichez C, Vega JM (1994) Nitrate uptake by Chlamydomonas reinhardtii cells immobilized in calcium alginate. Appl Microbiol Biotechnol 41(1):137–141. https://doi.org/10.1007/BF00166096
Vinayak V, Manoylov KM, Gateau H, Blanckaert V, Herault J, Pencreac’h G, Marchand J, Gordon R, Schoefs B (2015) Diatom milking: a review and new approaches. Mar Drugs 13(5):2629–2665. https://doi.org/10.3390/md13052629
Wu Z, Duangmanee P, Zhao P, Juntawong N, Ma C (2016) The effects of light, temperature, and nutrition on growth and pigment accumulation of three Dunaliella salina strains isolated from saline soil. Jundishapur J Microbiol 9(1):1–9. https://doi.org/10.5812/jjm.26732
Xin L, Ying HH, Ke G, Xue XY (2010) Effect of different nitrogen and phosphorous concentration on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour Technol 101(14):5494–5500. https://doi.org/10.1016/j.biortech.2010.02.016
Xu N, Zhang X, Fan X, Han L, Zeng C (2001) Effects of nitrogen source and concentration on growth rate and fatty acid composition of Ellipsoidion sp. (Eustigmatophyta). J Appl Phycol 13(6):463–469. https://doi.org/10.1023/A:1012537219198
Yan GA, Li YJ, Wang ZJ (1995) Immobilization of Scenedesmus for sewage purification and change of its physiological characteristics. J Environ Sci 1:10–13
Yang CM, Chang KW, Yin MH, Huang HM (1998) Methods for the determination of the chlorophylls and their derivatives. Taiwania 43:116–122. https://doi.org/10.6165/tai.1998.43(2).116
Yeesang C, Cheirsilp B (2011) Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour Technol 102(3):3034–3040. https://doi.org/10.1016/j.biortech.2010.10.013
Zhu YH, Jiang JG (2008) Continuous cultivation of Dunaliella salina in photobioreactor for the production of β-carotene. Eur Food Res Technol 227(3):953–959. https://doi.org/10.1007/s00217-007-0789-3
Zhu CJ, Lee KL, Chao TM (2009) Effect of temperature and growth phase on lipid and biochemical composition of Isochrysis galbana TK1. J Appl Phycol 9(5):451–457. https://doi.org/10.1023/A:100797331
Zhukova NV, Titlyanov EA (2006) Effect of light intensity on fatty acid composition of dinoflagellates symbiotic with hermatypic corals. J Botanica 49(4):339–346. https://doi.org/10.1515/BOT.2006.041
Acknowledgements
The authors would like to thank the members of the Science and Research Laboratory staff for their helpful and technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hadizadeh, Z., Mehrgan, M.S. & Shekarabi, S.P.H. The potential use of stickwater from a kilka fishmeal plant in Dunaliella salina cultivation. Environ Sci Pollut Res 27, 2144–2154 (2020). https://doi.org/10.1007/s11356-019-06926-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06926-w