Abstract
Microalgal lipids can be enhanced through varying nitrogen (N) content, and limited supply of nitrogen source seems to be valuable approach for increased lipid accumulation in microalgae. In this study, Chlorella sp. IM-02 was observed under fluorescence microscope for increased number of lipid bodies under nitrogen scarcity. Fourier transform infrared spectroscopy was used to determine spectral changes due to varying lipid content under nitrogen-starved (N0, without sodium nitrate), nitrogen-limited (N0.1, N0.25, N0.5 and N1.0 representing 0.1, 0.25, 0.5 and 1.0 g/L of sodium nitrate, respectively) and nitrogen-sufficient (N1.5, i.e., 1.5 g/L sodium nitrate) setting. Chlorophyll content was also monitored under these conditions as growth indicator. Various biochemical components viz. total carbohydrates, total proteins and total lipids were also estimated under varying nitrogen levels spectrophotometrically. On fourth day itself, maximum lipid productivity was observed in case of N0.5, which is having one-third of nitrogen concentration present in original growth media, BG-11. This concludes N0.5 as suitable nitrogen provision for better production of lipids in Chlorella sp. IM-02 without much compromising the biomass production as both growth and lipid quantity are key parameters affecting the lipid productivity of any microalgal strain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biofuels are usually considered as the most promising solution to world energy crisis due to their biodegradability, sustainability and environmental benign nature. Microalgae are emerging as most sustainable candidate for biofuel production. Some advantages such as easily cultivable, usage of non-arable land, no competition with food crops for land and water, nutrients uptake from wastewater, ability to produce substantial amount of triacylglycerides (TAG) and shorter doubling time make them better than other available feedstocks (Kiran et al. 2014a; Sharma et al. 2012; Wang et al. 2009).
Microalgae represent rare group of microorganisms that can easily adapt to almost all ecological habitats, with autotrophic, heterotrophic or mixotrophic behavior (John et al. 2011). Autotrophic microalgae can fix carbon dioxide present in the atmosphere in the presence of sunlight through the process of photosynthesis, whereas heterotrophs can utilize organic carbon present in environment for the synthesis of their building blocks. Mixotrophic microalgae can either use inorganic or use organic carbon depending upon availability and requirement.
Microalgal lipid content is dependent on various environmental parameters viz. temperature (Zhu et al. 2009), concentration of nutrients (Gouveia and Oliveira 2009) and light intensity (Yeesang and Cheirsilp 2011). The average lipid content in microalgae is up to 50 % of dry weight, but under stress conditions some species are reported to achieve lipid content up to 70 % (Ma 2006; Xin et al. 2010). Total algal lipids are comprised of both polar (such as phospholipids and glycolipids), and neutral or nonpolar lipids (such as mono-, di- and triacylglycerides. In addition to lipid content, lipid productivity is a key parameter to predict the microalgal performance which is a combination of biomass production and lipid content. Average volumetric productivity (mg/l/d) can be calculated by dividing the product quantity with cultivation time (Breuer et al. 2012). Several studies have shown increased accumulation of lipids under nitrogen-limited conditions (Gouveia and Oliveira 2009; Kiran et al. 2016a; Xin et al. 2010; Yeesang and Cheirsilp 2011). (Sopher and Miller 1949) demonstrated accumulation of lipids up to 85 % in Chlorella pyrenoidosa under nitrogen-limited conditions. Similarly, Xu et al. (2006) reported increased lipid content up to 55 % in Chlorella protothecoides demonstrating the effect of nitrogen source and concentration in growth media on lipid yield. Botham and Ratledge (1979) in their study suggested that lipid accumulation increases due to decreased level of adenosine monophosphate (AMP) under nitrogen-starved condition. Lower level of AMP further restricts citrate metabolism, hence arresting tricarboxylic acid (TCA) cycle. This leads to increased availability of acetyl-CoA for enhanced fatty acid synthesis. But lowering down of microalgal biomass production under nitrogen-limited conditions is a serious concern, which finally decreases the lipid productivity.
Objective of present study is to assess the effect of concentration of nitrate nitrogen present in the cultivation media on growth and lipid production potential of Chlorella sp. IM-02. In previous study, another strain (Chlorella sp. IM-01) had been found to survive quite well in wastewater by utilizing inorganic nutrients for growth simultaneously cleaning the wastewater through the process of bioremediation (Kiran et al. 2014b). But rate of biomass production was affected due to complex nature of wastewater and hindrance caused due to other organic substances. Thus, it is necessary to study systematically the effect of nitrogen concentration on lipids production potential under controlled and simplified nutrient conditions to find out a suitable nitrogen level for enhanced lipid production without compromising the growth. This work is carried out at Indore, M.P. (India), in 2015 to study growth behavior and biochemical composition (proteins, lipids, carbohydrates) of Chlorella sp. IM-02 under varying nitrate concentrations.
Materials and methods
Microorganism, growth medium and cultivation conditions
Chlorella sp. IM-02 was isolated from municipal wastewater collected from Indore city. Pure cultures were maintained in nitrogen-supplemented BG-11 medium (Stanier et al. 1971) in culture room at 27 ± 3 °C temperature under 40 µmol m−2 s−1 light intensity. Microalgal cultures were hand-shaken twice a day to prevent sticking on the walls.
Cultures were inoculated at initial OD680 of 0.05 in 250-mL Erlenmeyer flask containing 100 mL autoclaved BG-11 medium with 1.5 g/L sodium nitrate as nitrogen source (N1.5). Limited conditions of nitrogen were imposed by cultivating microalgal cells (OD680 0.05) in varying sodium nitrate concentrations, i.e., 0, 0.1, 0.25, 0.5 and 1.0 g/L designated as N0, N0.1, N0.25, N0.5, and N1.0, respectively, throughout the study. Cultures were grown at 12 h light: dark period with 40 µmol m−2 s−1 light intensity, and experiments were run for 10 days. Microalgal biomass was harvested on 4, 7 and 10th day to observe the effect on chlorophyll concentration, and biochemical composition with time.
Analytical methods
Chlorophyll concentration was determined through hot extraction method using methanol given by Mackinney (1941). Total proteins were estimated using Lowry’s method through spectrophotometer (Lowry et al. 1951). Standard graph for proteins was obtained using bovine albumin serum (BSA). Total carbohydrates were determined through standard Anthrone method using dextrose as standard (Dubois et al. 1956). Total lipids were extracted with chloroform/methanol solution and analyzed spectrophotometrically (Chen and Vaidyanathan 2012).
Fluorescence microscopy was used to observe neutral lipid accumulation in microalgal cells cultivated under different nitrogen levels. Fluorescent staining of Chlorella sp. IM-02 for neutral lipid induction studies was performed through conventional method as suggested by Chen et al. (2011). Triplicate samples for different treatments were pooled up to single set, and cells were harvested through centrifugation at 3000 rpm for 10 min on 10th day of inoculation after sufficient accumulation of lipids. Microalgal pellets were washed with double-distilled water to remove any salt deposition from growth media. Then, 50 µL of 25 % dimethyl sulphoxide (DMSO) (Analytical Grade) was added to 20 µL microalgal aliquot and properly mixed using vortex for 1 min. After this, 100 µg/mL Nile Red (Sigma-Aldrich) was added to same mixture followed by vortexing for 1 min and finally kept in dark for 10 min for proper staining of cells. Neutral lipid accumulation was observed using fluorescence microscope (Leica 2300) with an excitation and emission wavelength of 530 and 575 nm, respectively.
FTIR spectroscopy was also performed for Chlorella cells growing in nitrogen-starved, nitrogen-limited and unmodified BG -11 medium using potassium bromide pellet. After harvesting and drying of microalgal biomass, 2–3 mg of dried microalgae was mixed with potassium bromide pellet (KBr) and subjected to a pressure of about 8 × 106 Pa in KBr press to obtain clear transparent disk of 16 mm diameter and 1–2 mm thickness (Ponnuswamy et al. 2013). IR spectra were recorded over a wavelength region 3500–400 cm−1 using Fourier transform infrared spectrometer (Tensor 27, BRUKER). Peak attribution was performed according to available literature (Beardall et al. 2012; Meng et al. 2014; Rukminasari 2013). 2800–3000 cm−1 characterizes methyl and methylene groups of lipids where ~2924–~2854 cm−1 are represented by asymmetric and symmetric CH2 and ~1737–~1740 cm−1 are for the presence of –C=O stretching vibration of ester group suggesting the presence of lipids and fatty acyl chains. Frequency bands between ~1650 and 1455 cm−1 represent N–H stretching vibration due to the presence of proteins, whereas primary amines (amide I) and secondary amines (amide II) are represented by frequency bands ranges between ~1724–1585 cm−1 and ~1585–1490 cm−1, respectively. Stretching vibration of methyl groups ranges up to ~1455 cm−1. Frequency range 1200–950 cm−1 characterizes C–O–C stretching of polysaccharides, and spectra of starch displayed intense bands at ~1024, 1150, and 1050 cm−1, which are characteristics of C–O stretching vibrations from carbohydrates.
Calculation for yield parameters
The average volumetric productivity (mg/L/d) was calculated by the amount of product per unit culture volume per unit cultivation time (Breuer et al. 2012).
Average lipid productivity,
where L0 = initial lipid concentration (mg/L) at 0th day, Lt = lipid concentration (mg/L) at time t and t = cultivation time (days).
Average carbohydrate Productivity,
where C0 = carbohydrate concentration (mg/L) at 0th day, Ct = Carbohydrate concentration (mg/L) at time t and t = time (days).
Average protein productivity,
where P0 = initial protein concentration (mg/L) at 0th day, Pt = Protein concentration (mg/L) at any time t and t = time (days).
Statistical analysis
All experiments were conducted in triplicates, and mean values with standard deviation are reported here. Calculations were done using MS-Excel. Additionally, FTIR spectra obtained were initially normalized by a background single-beam spectrum recorded from empty ATR plate. Base line correction, band thickness and absorbance to transmittance conversion were done using OPUS control software.
Results and discussion
Response of Chlorella sp. IM-02 under varying nitrogen conditions
Nitrogen is an important nutrient for growth of microalgae. To observe the effect of varying nitrogen concentration on growth characteristics and to find out the minimum nitrogen level required, Chlorella sp. IM-02 was cultivated in modified BG-11 medium with variable concentrations of sodium nitrate.
Initially till 4th day, chlorophyll concentration remains almost constant at all nitrate levels without much difference and starts increasing 4th day onwards till 10th day in all sodium nitrate treatments from 0.25 to 1.5 g/L (Fig. 1) showing the efficient utilization of nutrients for growth and repair purpose with increased cell density per unit fixed volume. In case of N0 and N0.1, chlorophyll concentration is found to increase till 7th day and after that somewhat decreasing or almost similar to 10th day. This may be due to exhaustion of nutrients (nitrogen) in the aqueous media. This microalga, Chlorella sp. IM-02, is also not able to flourish well in the absence of nitrogen source as presented in Fig. 1 because of deprivation of electron acceptor, i.e., NADP+ at low/zero nitrogen levels resulting in impaired growth. Maximum concentrations are found at N1.5 as 9.4 and 13.8 µg/mL on 7th and 10th day of inoculation, respectively. Similar results had been reported in literature showing decreased chlorophyll synthesis in the absence of a nitrogen source (Pruvost et al. 2011; Da Silva et al. 2009).
Protein productivity has also been found to be dependent on nitrate concentration (Table 1). Maximum protein productivity of 7.9 mg/L/d is observed at highest sodium nitrate concentration (N1.5) on 7th day of cultivation. On the other hand, protein productivity is 6.6 mg/L/d shown on 4th day even at N0.5 level. Among various factors responsible for enhanced lipid accumulation inside microalgal cells, nitrogen levels have shown to be most prominent (Belotti et al. 2013; Breuer et al. 2012; James et al. 2011). Present study showed increased lipid productivity when sodium nitrate level was decreased on 4th day itself, with maximum being found at N0.5 (3.47 mg/L/d) (Table 1). Further decrease in sodium nitrate concentration resulted in decreased accumulation of lipids, and minimum is observed at N0. Maximum lipid productivity observed at N0.5 on 4th day of inoculation shows nitrogen limitation as suitable option as compared to complete nitrogen starvation for induction of lipid accumulation. This result is in agreement with literature suggesting nitrogen deficiency as an efficient trigger for lipid induction in microalgae. With increase in time up to 10th day, lipid productivity is found to decrease or almost constant at all the nitrogen levels from N0.1 to N1.5. Minimum lipid productivity of 0.95 mg/L/d is observed at N0 on 4th day of microalgal inoculation. However, carbohydrate productivity is found to be highest at N0 even on 4th day (3.58 mg/l/d). Similarly, Nigam et al. (2011) studied the effect of different concentrations of potassium nitrate on growth and lipid content of autotrophically cultivated Chlorella pyrenoidosa. It was found that with decreasing nitrate concentration, biomass production also decreased, but lipid content increased and highest lipid accumulation (26 %) was recorded at 0.05 g/L KNO3, which is one-fourth of basal nitrogen source concentration. Results obtained by Yeh and Chang (2011) showed an increase in lipid content from 20.9 to 55.9 % with decreased nitrogen concentration from 1.25 to 0.31 g/L. These observations can be explained by inhibition of carbon assimilation in TCA cycle and utilization of nascent carbon in lipid metabolism through Acetyl-CoA. There are other reports also suggesting the increased accumulation of carbon reserve compounds such as carbohydrates and lipids under nitrogen deficit (Dayananda et al. 2006; Yeesang and Cheirsilp 2011).
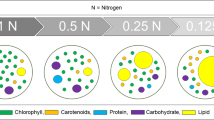
Fluorescence characteristics of Nile red-stained microalga
Nutrient starvation in the medium leads to induction of neutral lipids in microalgal cells which can be observed by staining the cells with Nile red fluorescence dye as shown in Fig. 2. The resultant yellow fluorescent droplets were observed in cells of Chlorella sp. IM-02 on 10th day of inoculation in nitrogen-starved and nitrogen-limited media. Total lipids in algae are comprised of polar and nonpolar/neutral lipids. Biodiesel production of algae is significantly dependent on accumulation of neutral lipids (triacylglycerides). Amassing of neutral lipids in Chlorella sp. cultivated under nitrogen-limited conditions shows positive impact of nitrogen limitation in terms of neutral lipid accumulation.
FTIR studies
FTIR spectra of Chlorella sp. IM-02 cultivated in nitrogen-starved, nitrogen-limited and nitrogen-sufficient conditions are observed with varying time period from 4 to 10th day (Figs. 3, 4, 5, 6, 7, 8). Nitrogen-limited condition resulted in induction of lipids, which has also been confirmed by various researchers. Due to high sensitivity and high throughput means to access carbon allocation in metabolic activity by the cell, FTIR is preferred over spectrophotometric and fluorescent microscopy analysis. Mainly three bands are described here due to relevance to present study, i.e., ~1740 cm−1 for fatty acid, ~1650 cm−1 for proteins and ~1200–950 cm−1 for carbohydrates. Figures 3, 4, 5 show sharp and intense peak at ~1645–1649 cm−1 showing high protein production at N0, N0.1 and N0.25 as compared to other nitrogen levels. Peak at ~1740 cm−1 distinctively characterizes lipids in algal FTIR spectra. Figure 6 shows an intense ester carbonyl band at ~1740 cm−1 as compared to other spectra on 4th day itself, showing maximum lipid accumulation at N0.5. FTIR spectra at other nitrogen levels are showing very small peak for lipids. A prominent band at ~1000 cm−1 in the studied algal strain is due to C–O–C stretching in polysaccharides as observed at N0.1 on 10th day of cultivation, whereas smaller peaks can be noticed at N0.5, N1.0 and N1.5. Generally lipid accumulation in microalgae occurs under limited nutrient resources with appropriate energy (sunlight/fluorescent light) and carbon (carbon dioxide) source. Experimental studies by Xin et al. (2010) and Dean et al. (2010) also showed increase in lipid content under nitrogen-limited and nitrogen-starved conditions. Higher lipid accumulation on 4th day in N0.5 as compared to N0 may be due to high biomass production resulting in early exhaustion of nitrates in the medium. This led to accumulation of lipids under nitrogen-stressed conditions, whereas higher lipid induction on 7th day at N0 suggests the possible utilization of internal nitrogen reservoirs for cell multiplication. At nitrogen-sufficient condition, i.e., N1.5, lipid peak is very small, may be due to high biomass production as compared to lipid synthesis. Studies by Converti et al. (2009) on Chlorella vulgaris showed no significant difference in lipid production when cultivated in nitrogen-starved or nitrogen-limited conditions. Our experimental trail with different nitrogen levels showed less marked cell inhibition with high lipid production at N−0.5. Since there is remarkably higher deterioration rate in cell growth at nitrogen-starved (N0) condition, this condition (N0) cannot be looked upon for efficient biodiesel production. Thus, there is a need to optimize the nitrate supplement for optimal growth and appropriate lipid productivity. This optimization needs not to be fruitful essentially for all microalgal species, but arguments by researchers (Dean et al. 2010; Griffiths and Harrisons 2009; Kiran et al. 2016b) have shown the need for high biomass productivity along with high lipid productivity. Thus, optimizing the nutrient supplementation will not only prevent the early culture death but will also help in enhanced induction of lipids.
Conclusion
The selection of microalgae strain for energy feedstock should be based on the ability of microalgae to efficiently utilize the nutrients available in media. In present study, nitrogen-starved and nitrogen-limited conditions were supplied specifically for lipid induction in Chlorella sp. IM-02. Maximum lipid productivity was found for microalgal cells cultivated under nitrogen-limited condition (N0.5, i.e., 0.5 g/L sodium nitrate) on 4th day itself, without much hindering the protein productivity. The results from FTIR spectroscopy as well as from spectrophotometric analysis are in agreement with each other. This study suggests that cultivation of Chlorella sp. IM-02 could be done sequentially where in first stage initially cultivating in standard nutrient medium to achieve exponential growth, further transferring the culture to nitrogen-limited media instead to nitrogen-starved media for lipid induction without depressing the growth. Furthermore, low retention time and high productivity with limited supply of nutrients for enhanced and cost effective lipid induction can prove to be a novel idea for sustainable biofuel development at commercial level.
References
Beardall J, Berman T, Heraud P, Kadiri MO, Light BR, Patterson G, Roberts S, Sulzberger B, Sahan E, Uehlinger U, Wood B (2012) A comparison of methods for detection of phosphate limitation in microalgae. Aquat Sci 63:107–121
Belotti G, Barvi M, Caprariis DB, Filippis DP, Scarsella M (2013) Effect of nitrogen and phosphorous starvations on Chlorella vulgaris lipid productivity and quantity under different tropic regimens for biodiesel production. Am J Plant Sci 4:44–51
Botham PA, Ratledge C (1979) A biochemical explanation for lipid accumulation in Candida 107 and other oleaginous micro-organisms. J Microbiol 114:361–375
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour Technol 124:217–226
Chen Y, Vaidyanathan S (2012) A simple, reproducible and sensitive spectrophotometric method to estimate microalgal lipids. Anal Chim Acta 724:67–72
Chen W, Zhang C, Song L, Sommerfeld M, Hu Q (2011) Microwave-assisted Nile red method for in vivo quantification of neutral lipids in microalgae. Bioresour Technol 102:134–141
Converti A, Casazza AA, Ortiz EY, Perego P, Borghi MD (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151
Da Silva AF, Lourenco SO, Chalou RM (2009) Effect of nitrogen starvation on the photosynthetic physiology of a tropical marine Rhodomonas sp. (Cryptophyceae). Aquat Bot 91:279–291
Dayananda C, Sarada R, Shamala TR, Ravishankar GA (2006) Influence of nitrogen sources on growth, hydrocarbon and fatty acid production by Botryococcus braunii. Asian J Plant Sci 5:799–804
Dean AP, Sigee DC, Estrada B, Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour Technol 101:4499–4507
Dubois M, Gillies KA, Hamilton JK, Hemers PA, Smith F (1956) Colorimetric method for the determination of sugars and related compound. Anal Chem 28:350–356
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274
Griffiths MJ, Harrisons STL (2009) Lipid productivity is a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
James GO, Hocart CH, Hiller W, Chen H, Kordbacheh F, Price GD, Djordjevic MA (2011) Fatty acid profiling of Chlamydomonas reinhardtii under nitrogen deprivation. Bioresour Technol 102:3343–3351
John RP, Anisha GS, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour Technol 102:186–193
Kiran B, Kumar R, Deshmukh D (2014a) Perspectives of microalgal biofuels as a renewable source of energy. Energy Conserv Manag 88:1228–1244
Kiran B, Pathak K, Kumar R, Deshmukh D (2014b) Cultivation of Chlorella sp. IM-01 in municipal wastewater for simultaneous nutrient removal and energy feedstock production. Ecol Eng J 73:326–330
Kiran B, Pathak K, Kumar R, Deshmukh D (2016a) Growth pattern and biofuel production potential of newly isolated microalga, Chlorococcum sp. IM-03 under nitrogen limited conditions. J Chem Technol Biotechnol 91:1339–1344
Kiran B, Pathak K, Kumar R, Deshmukh D (2016b) Statistical optimization using central composite design for biomass and lipid productivity of microalga: a step towards enhanced biodiesel production. Ecol Eng J 92:73–81
Lowry OH, Rosebrough NJ, Farr LJ, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Ma YL (2006) Microbial oils and its research advance. Chin J Bioprocess Eng 4(4):7–11
Mackinney G (1941) Absoprtion of light by chlorophyll solution. J Biol Chem 140:315–322
Meng Y, Yao C, Xue S, Yang H (2014) Application of Fourier transform infrared (FT-IR) spectroscopy in determination of microalgae composition. Bioresour Technol 151:347–354
Nigam S, Rai MP, Sharma R (2011) Effect of nitrogen on growth and lipid content of Chlorella pyrenoidosa. Am J Biochem Biotechnol 7:124–129
Ponnuswamy I, Madhavan S, Shabudeen S (2013) Isolation and characterization of green microalgae for carbon sequestration, wastewater treatment and bio-fuel production. Int J Biosci Biotechnol 5:17–26
Pruvost J, Vooren G, Gouic B, Mossion A, Legrand J (2011) Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour Technol 102:150–158
Rukminasari N (2013) Effect of temperature and nutrient limitation on the growth and lipid content of three selected microalgae (Dunaliella tertiolecta, Nannochloropsis sp. and Scendesmus sp.) for biodiesel production. Int J Mar Sci 3:135–144
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1555
Sopher HA, Miller WH (1949) The chemical composition of Chlorella; effect of environmental condition. Plant Physiol 24:120–149
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chloroococcales). Bacteriol Rev 35:171–205
Wang L, Min M, Li Y, Chen P, Chen Y, Liu Y, Wang Y, Ruan R (2009) Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol 162:1174–1186
Xin L, Ying HH, Ke G, Xue XY (2010) Effect of different nitrogen and phosphorous concentration on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour Technol 101:5494–5500
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Yeesang C, Cheirsilp B (2011) Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour Technol 103:3034–3040
Yeh KL, Chang JS (2011) Nitrogen starvation strategies and photobioreactor design for enhancing lipid content and lipid production of a newly isolated microalga Chlorella vulgaris ESP-31: implication for biofuels. Biotechnol J 6:1358–1366
Zhu CJ, Lee KL, Chao TM (2009) Effect of temperature and growth phase on lipid and biochemical composition of Isochrysis galbana TK1. J Appl Phycol 9:451–457
Acknowledgments
The authors acknowledge financial assistance provided by Department of Science and Technology, Govt. of India under the INSPIRE Faculty Scheme (IFA12–EAS-01). The funding organization has not played any role in study design, decision to publish or preparation of the manuscript. Authors also acknowledge Dr. Radha Prasanna, IARI, Delhi, for her support in the identification of this strain and Dr. Shrikant Joshi, Veterinary College, Mhow, for giving permission regarding usage of fluorescence microscope in the present study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Kiran, B., Pathak, K., Kumar, R. et al. Influence of varying nitrogen levels on lipid accumulation in Chlorella sp.. Int. J. Environ. Sci. Technol. 13, 1823–1832 (2016). https://doi.org/10.1007/s13762-016-1021-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1021-4