Abstract
Marine diatom, strain JPCC DA0580, and marine green microalga strain NKG400014 were selected as high neutral lipid-producers from marine microalgal culture collection toward biodiesel production. These strains were tentatively identified as Navicula sp. and Chlorella sp., respectively, by 18S rDNA analysis. Growth and lipid accumulation conditions of both strains were analyzed by changing nutrient concentrations in growth media and initial illuminance intensity. The highest productivity of fatty acid methyl ester (FAME) reached to 154 mg/L/week for NKG400014 and 185 mg/L/week for JPCC DA0580. Gas chromatography/mass spectrometry analysis indicates that FAME fraction from NKG400014 mainly contained 9-12-15-octadecatrienoate (C18:3) and that from JPCC DA0580 mainly contained methyl palmitate (C16:0) and methyl palmitoleate (C16:1). Furthermore, calorimetric analysis revealed that the energy content of strain was 4,233 ± 55 kcal/kg (i.e., 15.9 ± 0.2 MJ/kg) for NKG400014 and 6,423 ± 139 kcal/mg (i.e., 26.9 ± 0.6 MJ/kg) for JPCC DA0580, respectively. The value from JPCC DA0580 was equivalent to that of coal. The strains NKG400014 and JPCC DA0580 will become a promising resource that can grow as dominant species in the open ocean toward production of both liquid and solid biofuels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae, the largest primary biomass, have been considered as a source of high-lipid material for the production of biofuel because photosynthetic conversion is an efficient and alternative process, they do not compete with food crops, and carbon dioxide released is fixed. Recent progress in elucidating microalgal feedstock production revealed that candidate strains have been devoted to several microalgal genus among diatoms, green algae, Eustigmatophytes, Prymnesiophytes, and red algae [1]. They accumulate triglyceride as a storage lipid under specific culture conditions. One of the promising approaches to improve the productivity of triglycerides from microalgae is the genetic manipulation technology. However, metabolic engineering approach toward the biodiesel production had made a little progress by the lack of appropriate methodology in most microalgae except for specific model strains [2, 3] and the complexity of lipid accumulation metabolism associating with the stress response [4], though several trial had been demonstrated [5]. Therefore, selection of candidate strains among specific microalgal genus producing high amount of lipid has been still placed as a major challenge for biodiesel production. Especially, marine microalgae with seawater requirement for growth have several advantages in practical application due to low land costs and free seawater and repressing contaminant microorganisms from preferentially growing.
Until now, possible candidates for biodiesel production, such as Chlorella sp., Tetraselmis sp., Isochrysis sp., and Scenedesmus sp., have been reported by several literature reviews [6–8]; however, limited information was available for biodiesel production in marine microalgae, i.e., seawater-requiring strains. The marine genus Nannochloropsis, which was one of the best candidates for biodiesel production, was solo exception [1].
Following screening of high neutral lipid producers from marine microalgal culture collection in our group [9], a green microalga, strain NKG400014, and a diatom, strain JPCC DA0580, were selected as high neutral lipid producers by Nile red screening. In this study, the biomass and neutral lipid productivity, and fatty acid compositions in both strains, were investigated. Furthermore, the lyophilized cells were subjected to calorimetric measurements to compare the calorific value of dried biomass with that of fossil fuels.
Materials and Methods
Microalgal Strains
Two microalgal strains were selected as high neutral lipid-producers form marine microalgal culture collection (1,393 strains of marine microalgae) using Nile red screening. Strain NKG400014 (tentatively identified as Chlorella sp.) [9] was used as one of the highest neutral lipid producers among green algae. Marine diatom, strain JPCC DA0580, was newly isolated from the junction of Sumiyo River and Yakugachi River, Kagoshima, Japan (28° east longitude and 129° north latitude), as the highest neutral lipid producer. Strain NKG 400014 was maintained on an IMK medium (200 mg NaNO3, 1.4 mg Na2HPO4, 5 mg K2HPO4, 2.68 mg NH4Cl, 5.2 mg Fe-EDTA, 0.332 mg Mn-EDTA, 37.2 mg Na2-EDTA, 0.023 mg ZnSO4 · 7H2O, 0.014 mg CoSO4 · 7H2O, 7.3 μg Na2MoO4 · 2H2O, 2.5 μg CuSO4 · 5H2O, 0.2 mg Thiamin-HCl, 1.5 μg biotin, 1.5 μg vitamin B12, and 0.18 mg MnCl2 · 4H2O) dissolved in a liter of artificial seawater. Strain JPCC DA0580 was maintained on f/2 medium (75 mg NaNO3, 6 mg Na2HPO4 · 2H2O, 0.5 μg vitamin B12, 0.5 μg biotin, 100 μg Thiamine HCl, 10 mg Na2SiO3 · 9H2O, 4.4 mg Na2-EDTA, 3.16 mg FeCl3 · 6H2O, 12 μg CoSO4 · 5H2O, 21 μg ZnSO4 · 7H2O, 0.18 mg MnCl2 · 4H2O, 70 μg CuSO4 · 5H2O, and 7 μg Na2MoO4 · 2H2O) dissolved in a liter of artificial seawater. The artificial seawater prepared according to manufacturer's instructions was defined as 100% artificial seawater.
Growth Conditions
Microalagal strains (NKG 400014 and JPCC DA0580) were cultured in appropriate media under nutrient-deficiency or nutrient-limitation conditions using the diluted media (0%, 10%, 20%, 30%, or 100%) with artificial seawater. The cultures were bubbled with a sterile air. Especially, two-phase cultivation of strain NKG 400014 cells was carried out for efficient accumulation of neutral lipids. The NKG400014 was incubated in 100% IMK medium for 1 week under 100 μmol/m2/s (5,000 lx) illumination and collected by centrifugation at 5,800×g for 10 min. After washing twice, the cells were suspended in the 0%, 10%, 25% IMK medium diluted with artificial seawater and incubated for another 1 week under 100 μmol/m2/s (5,000 lx) illumination. The continuous illumination was applied to flat-shaped flask using a cool fluorescent lamp from one direction and illumination intensity was measured using illuminometer. Initial illumination intensity was defined as the average of the intensity at the both face of flat-shaped flask. These cultures were inoculated in flat-shaped flask (500 ml). Growth of experimental cultures was monitored by measuring the absorbance (ABS) at 750 nm. The cultured cells in 500 ml medium were collected by centrifugation and lyophilized to measure the dry weight. The biomass productivity was calculated from the dry cell weight using the following formula:
18S rDNA Analysis
Marine microalgal strain was identified based on 18S rDNA sequences. Microalgal cells were disrupted by sonication for 1 min three times. The 18S rDNA was amplified by PCR using disrupted microalgal cells as a template and universal primers: forward primer 5′-GGTGATCCTGCCAGTAGTCATATGCTTG-3′ (ss5) and reverse primer 5′-GATCCTTCCGCAGGTTCACCTACGGAAACC-3′ (ss3). PCR products were separated by electrophoresis. Sequences of 18S rDNA were determined by the ABI PRISM 3100 DNA Sequencer (Applied Biosystems). The similarity to sequences was analyzed by BLAST [10].
GC/MS Analysis
The lyophilized microalgal cells (20 mg) were suspended in 5% HCl–methanol (2 ml) and heated at 100 °C for 1 h. After esterification, n-hexane (1.5 ml) and purified water (0.5 ml) were added. The mixture was centrifuged (8,500×g, 5 min), and the supernatant was collected. Purified water was added to the supernatant and centrifuged again. The dehydration was conducted using 10 mg of sodium sulfate. The evaporated fraction (lipid fraction) was resuspended in 1 ml of n-hexane. The lipid components were identified by GC/MS (QP2010; Shimadzu Co.). GC conditions was set as the following column: FAMEWAX (30 m, 0.25 mm ID, 0.25 µm, RESTEK), carrier gas: helium, oven: 90 °C (5 min) → 7 °C/min → 220 °C.
Calorimetry
The calorific values of dried cells were determined using an automatic adiabatic bomb calorimeter (Yoshida seisakusyo Co., Ltd. Tokyo, Japan). The calorific value was calculated by measurement of elevated water temperature due to the heat of combustion of a particular reaction. Appropriate dry weight of strain NKG400014 and JPCC DA0580 were wrapped with paper and combusted in the bomb calorimeter. The calorific value of wrapping paper without biomass was also measured to subtract from raw calorific values of biomass samples to remove background value.
Results and Discussion
Selection of Neutral Lipid Producers from Marine Microalgal Culture Collection
Nile red staining of microalgal cells cultured for more than 2 months was performed for the detection of intracellular lipid droplets by fluorescence microscopy. Neutral lipids including hydrocarbons and triglycerides are stained in yellow, while polar lipids are stained in red. Among 1,393 marine microalgal strains, two strains of NKG400014 and JPCC DA0580 showed obvious yellow fluorescence, i.e., neutral lipid accumulation.
PCR amplification and subsequent DNA sequencing allowed the determination of approximately 1.6 kbp of the 18S rDNA gene for NKG400014 and approximately 1.8 kbp for JPCC DA0580. Strain NKG400014 and JPCC DA0580 displayed the highest 18S rDNA sequence relatedness with a green alga, Chlorella sp. (the closest relative: Chlorella sp. KAS012; accession No. AB176666, 99% similarity), and a diatom, Navicula pelliculosa (the closest relative; N. pelliculosa strain CCMP543, accession No. AY485444, 99% similarity), respectively.
Growth Characteristics
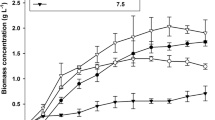
Figure 1 shows the growth curves of strain NKG400014 in IMK medium and strain JPCC DA0580 in f/2 medium. Cell growth of each strain was monitored by measuring ABS750 nm [1, 11, 12]. The cells reached plateau of growth from more than approximately 300 h for NKG400014 and approximately 200 h for JPCC DA0580 (Fig. 1). Growth rates between both strains did not change significantly. Although final optical density of NKG400014 and JPCC DA0580 was different from each other, the difference in the biomass productivity (mg/L/week) was not markedly observed (see Table 1 and Fig. 2). In this study, the biomass productivity was expressed as final dry cell weight per liter divided by culture time, i.e. (mg/L/week). A significant difference in growth times was observed in both strains (Fig. 1), so net biomass productivities (i.e., mg-dry cell weight/L-culture) were 626 mg/L for NKG400014 and 346 mg/L for JPCC DA05080. These results indicate that NKG400014 showed approximately 1.8-fold net biomass productivity as compared with JPCC DA0580. On the other hand, the final optical density of NKG400014 was approximately 1.9-fold higher than that of JPCC DA0580 (OD = 1.8 for NKG400014, and 0.96 for JPCC DA0580). This tendency agrees with the difference in net biomass productivity.
Lipid Accumulation of Chlorella sp. Strain NKG400014
To evaluate the effect of seawater on growth, strain NKG400014 was cultured in the IMK media in the presence or absence of artificial sea salts. Approximately 1.6 times higher biomass was obtained in the presence of artificial sea salt, although NKG400014 could grow without sea salt. This result suggests that strain NKG400014 requires seawater for efficient growth. The highest biomass productivity and FAME productivity were 335 and 92 mg/L/week, respectively (Table 1).
It is well known that nutrient-starved Chlorella sp. can accumulate neutral lipids in its biomass [1, 13–15]. To improve the productivity of FAME, the nutrient deprivation experiment was conducted. Following a week incubation using IMK medium, cultured cells were collected and resuspended into the dilution series of IMK nutrients (0%, 10%, and 25%) with artificial seawater. After 1 week incubation, biomass and FAME productivity were evaluated (Table 1). As expected, higher FAME accumulations were observed under nutrient-deficiency or nutrient-limitation conditions as compared with no starving conditions, although a proportional relation between IMK nutrient concentrations and FAME productivities was not obtained. While biomass productivity decreased along with the nutrient dilution, the highest FAME productivity of 154 mg/L/week was obtained at 10% IMK nutrient concentration. These results indicate that two-phase cultivation was useful for higher FAME production in Chlorella sp. strain NKG400014.
Lipid Accumulation in N. pelliculosa Strain JPCC DA0580
The effect of sea salts on Navicula sp. strain JPCC DA0580 growth was examined in the same manner with strain NKG400014. Growth of Navicula sp. strain JPCC DA0580 was significantly affected by seawater concentrations, and little growth was observed in 0% sea salt conditions. This result suggests that strain JPCC DA0580 has a halophilic behavior requiring at least 2% to 3% of sodium salt for maximal growth. The highest biomass productivity as 0.46 g/L/week was obtained under 100% sea salt conditions. On the other hand, no significant change in neutral lipid contents determined by Nile red staining was observed under nutrient starvation conditions. These results agree with a previous report in diatom, indicating no increasing the lipid content under nutrient starvation conditions [16]. Furthermore, the illumination conditions did not affect on the FAME productivity in JPCC DA0580 regardless of the biomass productivity (Fig. 2). The highest FAME productivity was 185 mg/L/week when 7,000 lx of initial illumination intensity was applied. The decreasing of the biomass productivity at more than 8,000 lx was expected to be due to the photoinhibition.
FAME Compositions in NKG400014 and JPCC DA0580
Major components of FAME by GC-MS analysis were summarized in Table 2. The major FAME in NKG400014 was methyl 9-12-15-octadecatrienoate (C18:3). On the other hand, the major fatty acid methyl esters in JPCC DA0580 were methyl palmitate (C16:0) and methyl palmitoleate (C16:1). European biodiesel standards EN 14214 determined the maximum limits of fatty acid contents, i.e., methyl linolenate (C18:3) (<12% (w/w)) and FAME with more than four double bounds (<1% (w/w)) because the unsaturation grade affects the cold flow, stability, and ignition quality of diesel fuel [17, 18]. Therefore, the FAME from NKG400014 was expected to be not suitable for biodiesel production because of higher methyl linolenate (C18:3) as a major content. Hydration process should be required for fatty acid saturation. On the other hand, the FAME from JPCC DA0580 mainly contained methyl palmitate (C16:0) and methyl palmitoleate (C16:1), which could be suitable for biodiesel production.
Calorific Values
The direct use of dried microalgal biomass is one of promising approaches for a fuel supplement in a diesel engine [14, 15] because it was employed to eliminate the lipid extraction and specific conversion processes and to reduce costs. The lyophilized cells of each microalgae were subjected to calorimetry to estimate the calorific value of dried biomass. The calorific values were 4,233 ± 55 kcal/kg (i.e., 15.9 ± 0.2 MJ/kg) for NKG400014 and 6,423 ± 139 kcal/mg (i.e., 26.9 ± 0.6 MJ/kg) for JPCC DA0580. In previous work, a novel biofuel producer, Scenedesmus sp. strain JPCC GA0024 had enough high calorific value of 6,160 kcal/kg (i.e., 25.8 MJ/kg), which was equaled to that of coal (approximately 27 MJ/kg) [9]. The calorific value of strain JPCC DA0580 was similar or higher level to those in Scenedesmus sp. strain JPCC GA0024 and other candidate of biodiesel producers (18 to 29 MJ/kg) [9, 15]. Therefore, strain JPCC DA0580 could be a potential candidate for the direct use for a fuel supplement in a diesel engine.
Conclusions
Marine microalgae, NKG400014 and JPCC DA0580 (tentatively Chlorella sp. and Navicula sp., respectively) were identified as high neutral lipid producers among marine microalgal culture collection. Both Chlorella sp. NKG400014 and Navicula sp. JPCC DA0580 showed the best growth under seawater-based conditions. The highest FAME productivities were 154 mg/L/week for Chlorella sp. NKG400014 and 185 mg/L/week for Navicula sp. JPCC DA0580. The FAME productivities were comparable to those of non-seawater requiring microalgae. These results suggests that microalgae, strain NKG400014 and strain JPCC DA0580, will become a promising biofuel resources that can grow as dominant species in seawater.
References
Rodolfi, L. Chini Zittelli, G. Bassi, N. Padovani, G. Biondi, N. Bonini, G. et al. (2009). Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnology and Bioengineering, 102, 100–112.
Leon-Basares, R. Gonzalez-Ballester, D. Galvan, A. & Fernandez, E. (2004). Transgenic microalgae as green cell-factories. Trends in Biotechnology, 22, 45–52.
Walker, T. L. Purton, S. Becker, D. K. & Collet, C. (2005). Microalgae as bioreactors. Plant Cell Reports, 24, 629–641.
Hu, Q. Sommerfeld, M. Jarvis, E. Ghirardi, M. Posewitz, M. Seibert, M. et al. (2008). Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant Journal, 54, 621.
Rosenberg, J. N. Oyler, G. A. Wilkinson, L. & Betenbaugh, M. J. (2008). A green light for engineered algae: Redirecting metabolism to fuel a biotechnology revolution. Current Opinion in Biotechnology, 19, 430–436.
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25, 294–306.
Gouveia, L. & Oliveira, A. C. (2009). Microalgae as a raw material for biofuels production. Journal of Industrial Microbiology and Biotechnology, 36, 269–274.
Li, Q. Du, W. & Liu, D. (2008). Perspectives of microbial oils for biodiesel production. Applied Microbiology and Biotechnology, 80, 749–756.
Matsunaga, T. Matsumoto, M. Maeda, Y. Sugiyama, H. Sato, R. & Tanaka, T. (2009). Characterization of marine microalga, Scenedesmus sp. strain JPCC GA0024 toward biofuel production. Biotechnology Letters, 31, 1367–1372.
Altschul, S. F. Gish, W. Miller, W. Myers, E. W. & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
Magnusson, M. Heimann, K. & Negri, A. P. (2008). Comparative effects of herbicides on photosynthesis and growth of tropical estuarine microalgae. Marine Pollution Bulletin, 56, 1545–1552.
Feng, F. Y. Yang, W. Jiang, G. Z. Xu, Y. N. & Kuang, T. Y. (2005). Enhancement of fatty acid production of Chlorella sp.(Chlorophyceae) by addition of glucose and sodium thiosulphate to culture medium. Process Biochemistry, 40, 1315–1318.
Xiong, W. Li, X. Xiang, J. & Wu, Q. (2008). High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Applied Microbiology and Biotechnology, 78, 29–36.
Scragg, A. H. Morrison, J. & Shales, S. W. (2003). The use of a fuel containing Chlorella vulgaris in a diesel engine. Enzyme and Microbial Technology, 33, 884–889.
Illman, A. M. Scragg, A. H. & Shales, S. W. (2000). Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme and Microbial Technology, 27, 631–635.
Shifrin, N. S. & Chisholm, S. W. (1981). Pytoplankton lipids: Interspecific differences and effectws of nitrate, silicate and light-dark cycle 1. Journal of Phycology, 17, 374–384.
Durrett, T. P. Benning, C. & Ohlrogge, J. (2008). Plant triacylglycerols as feedstocks for the production of biofuels. Plant Journal, 54, 593.
Knothe, G. (2006). Analyzing biodiesel: Standards and other methods. Journal of the American Oil Chemists' Society, 83, 823–833.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumoto, M., Sugiyama, H., Maeda, Y. et al. Marine Diatom, Navicula sp. Strain JPCC DA0580 and Marine Green Alga, Chlorella sp. Strain NKG400014 as Potential Sources for Biodiesel Production. Appl Biochem Biotechnol 161, 483–490 (2010). https://doi.org/10.1007/s12010-009-8766-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8766-x