Abstract
A 2-year field experiment was carried out with aim to assess the phytoremediation potential of Miscanthus × giganteus cultivated on the flotation tailings and to evaluate the effects of mineral NPK fertilizer on metal accumulation and plant physiological parameters and growth. Flotation tailings of the mine Rudnik (Serbia) are burdened with Pb, Cu and Zn and cause heavy metal pollution and deterioration of the surrounding ecosystems. In the second year of growth, plants retained the major portion of metals within their roots, with bioconcentration factor > 1 for Cu and Zn and < 1 for Pb. Their translocation factors were far below 1, showing that M. × giganteus acts as excluder of Cu, Zn and especially Pb. Higher amounts of Pb and Zn in leaves reduced the photosynthetic rate and total antioxidative capacity, but increased lipid peroxidation level. Changes at physiological level resulted in pronounced leaf senescence, reduced plant growth rate and annual biomass yield. Fertilization enhanced metal uptake by plant roots, but had no effect on their translocation to leaves. It improved chlorophyll a content, potential efficiency of Photosystem II photochemistry and biomass yield. Overall results indicate that M. × giganteus can be cultivated on the abandoned flotation tailings and that fertilization had positive effects on its physiology and growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining activities produce enormous amounts of fine-particulate metalliferous flotation tailings which represent one of the most hostile waste materials, with persistent negative impact on the surrounding ecosystems (Cross et al. 2017). Flotation tailings are characterized by adverse granulometric structure, sturdiness, high concentrations of residual heavy metals and complete deficiency of organic matter and macronutrients such as nitrogen and phosphorus which are the main constitutional elements of plants. These strong limiting factors are the main constraints to spontaneous growth of native plants. As the result, flotation tailings are devoid of vegetation cover and are consequently prone to wind dispersal and erosion by water, leading to the long-term severe pollution of adjacent ecosystems, and reduction in their health and biodiversity. In order to lessen environmental pollution, it would be necessary to introduce plant species that are resistant to the specific combination of abiotic stressors present in the flotation tailing environment, and can form self-sustainable vegetation cover (Tordoff et al. 2000; Pietrzykowski et al. 2018).
The development of sustainable vegetation cover on the abandoned flotation tailings is one of the main goals of phytostabilization, the phytoremediation technique based on immobilization of metals within the substrate by plants activities, thereby reducing off-site contamination (Seshadri et al. 2015). In phytostabilization, vegetation provides necessary surface stability reducing contaminated particulate dispersal by wind and water; plant roots decrease metal mobility and availability by their precipitation in the rhizosphere, by their adsorption on the root surface and absorption within the roots. Also, the long-term presence of plants and their activities would improve the substrate water regime, increase in the content of organic matter that derives from discarded underground and aboveground plant parts and root exudates, support development of the rhizosphere microbiome and provide more frequent visits of animals.
The potential efficiency of plant species in phytostabilization or phytoextraction, which are both techniques of phytoremediation, can be estimated on the basis of the proportion of heavy metal accumulated within roots and its transfer to aboveground organs. This is achieved by calculation of bioconcentration (BCF) and translocation (TF) factors. BCF represents the ratio between metal concentrations in plant roots and in the respective soil and indicates the role of plant roots in metal immobilization within the soil. TF represents the ratio between heavy metal concentration in plant aboveground and underground organs and indicate the metal extraction potential of the plant species.
Many heavy metals, such as Zn and Cu, perform specific physiological roles in plant organism and are needed in very low concentrations, but when present in excess, they manifest multiple toxic effects on plant functions. On the other hand, some of them, such as Pb, have no physiological role in plant and are toxic at even the lowest concentrations. Heavy metals impair nutrient uptake, respiration and photosynthesis; cause dysfunction of various enzymes and disturb metabolic processes (Aggarwall et al. 2011; Pourrut et al. 2011; Küpper and Andresen 2016). The sum of diverse metal-induced damages in cells finally results in the reduced plant growth and reproductive capacity (Arif et al. 2016).
Photosynthesis is very sensitive to excess heavy metals. They cause damages to different components of photosynthetic apparatus provoking inhibition of Photosystem II (PSII) and activities of enzymes involved in the carbon fixation (Sharma and Dubey 2005; Küpper and Andresen 2016). Therefore, changes in CO2 assimilation rate (A) and chlorophyll a fluorescence parameters are reliable indicators of heavy metal-induced stress in plants.
Many heavy metals in excess induce decline in amounts of photosynthetic pigments, chlorophylls (Chl) and carotenoids (Car), by their direct oxidative damage or by inhibition of enzymes involved in their biosynthesis. Moreover, divalent metal cations of similar radius can substitute Mg2+ in the chlorophyll molecule and reduce its functionality. Carotenoids are structural components of the photosystems with function in supplementing chlorophylls in energy supply or in dissipation of excess energy as heat, as well as in antioxidative defence. Hence, quantification of both groups of pigments can be very valuable tools in the assessment of plant heavy metal intoxication. Much nitrogen and phosphorus are constituents of a large variety of biomolecules involved in the photosynthetic machinery and therefore changes in amounts of Chl, Car, photosynthetic capacity and Chl fluorescence parameters are also valuable indicators of N and P deficiency in plants (Lu and Zhang 2000; Zhang et al. 2014; Carstensen et al. 2018).
Accumulation of transitional metals within the cell increases formation of reactive oxygen species (ROS) which induce oxidative damage to proteins, lipids and DNA. Oxidative degradation of membrane polyunsaturated fatty acids results in formation of reactive malondialdehyde (MDA) which might cause further damage to other cell biomolecules. Accordingly, increase in its production is a good biomarker of oxidative stress and membrane oxidative damages.
Miscanthus × giganteus J. M. Greef & Deuter (Poaceae) is a triploid sterile hybrid native to East-Southeast Asia and originates from natural crossing of M. sinensis and M. sacchariflorus. This perennial grass propagates vegetatively by rhizome cuttings which expand in the soil intensely and develop extensive roots up to 2 m in depth that enable good water supply for the plant. It is characterized by exceptional ecological plasticity manifested as pronounced tolerance to nutrient deficiency, high content of heavy metals and organic pollutants and wide range of temperatures (Naidu et al. 2003; Heaton et al. 2004; Purdy et al. 2013; Wanat et al. 2013; Pavel et al. 2014; Nsanganwimana et al. 2015). It is successfully cultivated worldwide as bioenergy crop, and due to its high ecological adaptability, it produces large biomass even on contaminated and marginal lands in different climate regions.
Flotation tailings are normally deprived in N and P, and application of fertilizer is necessary in order to improve their concentration and availability for plant uptake and support the plant growth. Depending on the type of applied fertilizer and plant species, fertilization can improve or lessen biomass yield, metal absorption and/or its translocation to shoots, as reported for M. × giganteus, Phalaris arundinacea and Spartina pectinata (Rosikoń et al. 2015; Pogrzeba et al. 2018).
In the long-term field experiments, plants experience the complex environment conditions which are often far more variable compared to the controlled laboratory conditions. For the successful phytoremediation, plants should be tolerant not only to the specific stressors, such as excess heavy metal or macronutrient deficiency, but also be highly adaptive to daily and seasonal environmental changes. Having in mind high adaptability of M. × giganteus, we set the field experiment on the flotation tailings rich in Pb, Cu and Zn. Taking into account high heavy metal content and the lack of nutrients in the flotation tailings, as well as the potential beneficial effects of fertilizer on the plant growth, the main aims of this field experiment study were:
- 1.
To examine the effects of applied mineral fertilizer on accumulation and distribution of heavy metals within the plant
- 2.
To examine the effects of accumulated heavy metals and macroelements on several physiological, biochemical and biometric parameters
- 3.
To estimate the growing potential of M. × giganteus on flotation tailings and its potential in phytostabilization and biomass production
To our knowledge, this is the first report on the physiological status and growth parameters of M. × giganteus cultivated on the flotation tailings in the field experiment.
Materials and methods
Site description
A field experiment was set in March 2016 on two localities: (i) on the unpolluted soil at the Institute for Application of Nuclear Energy (INEP), Zemun, Serbia (44°51′N, 20°22′E) and (ii) on the flotation tailings of the mine “Rudnik” (44°06′N, 20°29′E) (Fig. 1a).
Location of the study sites on the map of Serbia (a). Control experimental field on unpolluted chernozem (b). Satellite photograph of the flotation pond and flotation tailings; the experimental field is framed (c). Flotation tailing pond (d). Experimental field on the flotation tailings (e, f) and the flotation tailing substrate (g)
The reference plots with control plants were set in the high-quality luvic chernozem soil (IUSS Working Group WRB 2015) at INEP, unpolluted by heavy metals, fertilizers and pesticides (Fig. 1b).
The mine Rudnik is located in the central Serbia. Since its opening in 1958, over 70% of the total estimated ore has been excavated, which is more than ten million tons. After mechanical crushing of the polymetallic ore (ø = 75 μm) and the flotation process, several metals (lead, copper, zinc) are extracted. The remaining flotation tailings are pumped and discharged with water as mud into a tailing pond, bordered by a natural forest (Fig. 1c–e). Experimental plots were set on the small portion of the temporary abandoned flotation tailings (Fig. 1e–g).
The climate conditions at the experimental fields during plant growth, from March to June 2017, are represented in Table 1.
Plant material
Rhizomes used in this experiment derive from the clonal M. × giganteus that was purchased as rhizomes from Walter Kellner (Austria) in 2007. Since then, the continual cultivation was in uncontaminated fields of INEP.
Experimental setting
Rhizomes, each 10 cm long and with several buds, were planted in March 2016 in both experimental fields. They were planted at depth of 10 cm, with the planting density of 2 rhizomes/m2 (equivalent to 20,000 plants ha−1). Experimental field on flotation tailings was divided into eight plots (2.5 m × 2.0 m = 5 m2 each), four non-fertilized and four fertilized ones, with 2-m distance between one another in a complete randomized block design. Fertilized plots were treated in March 2016 and March 2017 with a single dose of commercial mineral fertilizer (NPK 15:15:15, applied amount 667 kg/ha). Experimental field on the reference site (control chernozem soil) was divided into four non-fertilized plots. The weed that occurred only at the control site was removed by hand when needed, without application of herbicides.
Chemical characterization of the substrate
The control soil and flotation tailings were sampled in March 2017, up to 20 cm at depth. Sampling was performed by Egner’s soil probe sampler prior to fertilization of the flotation tailings. Substrates were homogenized and air dried, then ground with ceramic mortar and pestle and sieved (pore diameter 200 μm).
Determination of the substrate pH
For determination of actual (pHH2O) and exchangeable pH (pHKCl), 10 g of dried substrate was mixed with 25 ml of double-distilled water and 25 ml 1 M KCl, respectively (van Reeuwijk 2002). Samples were stirred for 30 min and the pH was measured directly in the suspension (Iskra MA 5730).
Determination of element concentration in the substrate
For determination of the total element concentration (Pb, Cu, Zn), dried and sieved (< 200 μm) substrate samples were digested in 65% HNO3 (method 3051, USEPA 1998). For determination of available metal concentration, dried and sieved samples were mixed for 2 h in 1 M ammonium acetate and 0.01 M EDTA mixture (pH 7) (Pansu and Gautheyroy 2006). All samples were filtered by quantitative filter paper (Sartorius, grade 391). The absorbance of metals in filtered samples was measured by atomic absorption spectrophotometer (Shimadzu AA-7000). Concentrations of metals were determined by comparison of their absorption values with those of known standards. The accuracy of analytical procedure was evaluated analysing the standard reference material (Soil 90-0115-0106, BIPEA—Bureau Interprofessionnel d ´Etudes Analytiques).
The total nitrogen content (N) was analysed by Kjeldahl digestion (Bremner 1996). Available forms of phosphorus and potassium were determined by the standard AL-method (Egnér et al. 1960). Organic carbon content was determined according to Belčikova (1965) and the percentage of organic matter in the substrate was calculated according to Jackson (1958).
Plant material analysis
Plants were analysed in the second year of plant growth (June 2017), at both the control soil and the flotation tailings, in order to achieve the results of the effects of the long-term exposure to adverse mineral composition in the flotation tailings on plant characteristics. Only the aboveground biomass yield was determined after the harvest in March 2018.
Determination of metal content in plant organs
After sampling, all non-fertilized (control—C; plants cultivated on the flotation tailings—FT) and fertilized plants (plants cultivated on the flotation tailings—FTNPK) were divided in roots, rhizomes, stems and leaves. Plant material was washed carefully in tap water in order to avoid pollution by remaining substrate particles, and then in deionised water. The plant material was oven dried at 105 °C until constant weight, powdered and completely digested in 65% HNO3 according to the method 3051 (USEPA 1998). Concentrations of metals in plant samples were determined by atomic absorption spectrophotometer (Shimadzu AA-7000), comparing the absorption values with those of known standards. For the verification of the analytical procedure, the standard reference material NIST 1515 (apple leaves) was used.
Determination of phytoremediation potential
The phytoremediation potential of M. × giganteus was assessed using bioconcentration factor (BCF) and translocation factor (TF) (Baker 1981):
Photosynthesis and chlorophyll a fluorescence analysis
The measurement of the gas exchange rates and chlorophyll a fluorescence was performed on intact penultimate leaves, using infrared gas analyser (CIRAS 2, PP System, USA) equipped with chlorophyll fluorescence module (CFM). The CO2 assimilation rates (A) for the light response curves (A/PPFD) were obtained at descending PPFD values from 2000 to 0 μmol m−2 s−1, CO2 concentration of 380 μmol mol−1, leaf temperature 25 °C and relative air humidity 60%.
For the measurement of chlorophyll a fluorescence parameters, leaves were pre-darkened for 30 min before exposure to the saturating light. Before the saturating pulse was applied, the minimal level of fluorescence in dark (Fo) was detected. A saturating flash (5100 μmol PPFD m−2 s−1, 0.7 s) was then applied to the leaf in order to obtain the maximal fluorescence yield (Fm). Obtained parameters, Fo and Fm, were used to calculate maximum quantum efficiency of PSII photochemistry (Fv/Fm).
Measurement of leaf pigment content
Chlorophyll a (Chl a), chlorophyll b (Chl b) and total carotenoids (Car) were extracted from leaf using dimethyl sulfoxide solution (Hiscox and Israelstam 1979). The absorbance of pigments was detected at 663 nm, 645 nm and 480 nm by Shimadzu UV-1800 spectrophotometer. Their amounts were determined using equations given by Arnon (1949) and Wellburn (1994) and expressed as mg/g of the leaf dry weight (dw).
Lipid peroxidation determination
Lipid peroxidation level was determined in penultimate leaf by measuring the amount of malondialdehyde (MDA) produced in the reaction with 2-thiobarbituric acid (Heath and Packer 1968). The absorbance of malondialdehyde was measured spectrophotometrically at 450 nm, 532 nm and 600 nm. The MDA content was expressed as μmol g−1dw.
Measurement of the total antioxidative capacity
The total antioxidative capacity of the leaf methanol extract was determined using stable free radical 1,1-diphenyl-2-picrylhydrazyl (DPPH) (Brand-Williams et al. 1995). Spectrophotometric measurements were performed at 517 nm. The total antioxidant capacity of each extract was calculated according the formula: TAC (%) = [(A0 − A1) / A0] × 100, where A0 represents the absorbance of the blank, and A1 is the absorbance of the antioxidant in the sample solution.
Measurement of plant biometric parameters
Before harvesting the plants, several parameters were noted:
Survival rate in the second growing season, expressed as the percentage of plants survived from the initial number of planted rhizomes
Number of shoots per plant
Stem height (cm)
Number of leaves per plant
Percentage of senescent leaves per plant (%)
Biomass yield; the aboveground biomass dry weight (dw) was measured, and expressed as kg/ha
Data analysis
All data are expressed by the mean ± standard deviation (M ± SD) of six replicates per treatment. The obtained results were compared using Mann-Whitney U test (p < 0.05). The Spearman’s rank correlation coefficient was used to analyse relationships between measured parameters. Statistical analyses were performed in R (v3.5.1; R Core Team 2018).
Results
Chemical properties of the control soil and flotation tailings
The pHH2O values of the control soil and flotation tailings were similar and neutral, whereas their pHKCl differed significantly by 1.1 (Table 2). Although flotation tailings contained 2.78% of organic matter, their total N and available K were extremely low, being 24- and 47- fold lower compared to the control soil, respectively, whereas concentration of available P was below the detection limit. Flotation tailings contained significantly higher amounts of total and available metals in comparison with the control soil.
Elemental composition of M. × giganteus plants
Concentrations of analysed metals in all plant organs were significantly higher in plants cultivated on flotation tailings in comparison with those detected in the control (Table 3). Significantly higher amounts of metals were also found in roots (Cu, Zn) and stem (Pb, Zn) of FTNPK compared to those in FT (Table 3). Content of the total N and P in leaves of FT plants was significantly lower than in C and FTNPK.
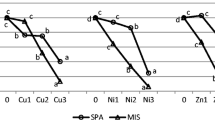
BCFs > 1 were detected for Cu and Zn in all plants grown on the flotation tailings indicating the high level of their accumulation in roots (Fig. 2a). Although fertilized plants showed significantly higher accumulation of Cu and Zn in roots, and higher BCFs (p < 0.05), metal transfer to leaves was not stimulated by fertilization as indicated by their similar concentrations in leaves. TFs for all investigated metals were far below 1, especially for Pb (Fig. 2b). BCFs and TFs between non-fertilized and fertilized plants differed significantly for all metals.
Photosynthetic performance
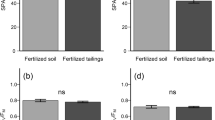
The obtained light response curves (A/PPFD) showed that the control plants C reached the highest photosynthetic activity of 12 μmol CO2 m−2 s−1 at the strongest light intensity of 2000 μmol PPFD m−2 s−1 (Fig. 3). Plants cultivated on flotation tailings, both FT and FTNPK, showed statistically significant reduction in the net photosynthetic rate (p = 0.029, p = 0.005, respectively), with maximum A being 18% and 22.5% lower than in the control plants, respectively. By inspecting the light response curves of FT and FTNPK plants, it can be noticed that the net photosynthetic rates at all applied light intensities were lower in fertilized than in unfertilized plants, although mentioned differences are without statistical significance. The respiration in dark was significantly higher (p = 0.009) in non-fertilized FT plants (A = − 0.80 ± 0.26 μmol CO2 m−2 s−1) compared to the respiration in the control (A = − 0.43 ± 0.05) and FTNPK plants (A = − 0.57 ± 0.15 μmol CO2 m−2 s−1).
Pigments, chlorophyll a fluorescence, lipid peroxidation level and antioxidative capacity in leaves
Non-fertilized plants cultivated on flotation tailings (FT) had significantly reduced concentration of Chl a in comparison with that in both the control (p = 0.005) and FTNPK plants (p = 0.005) (Table 4) and resulted in significantly lower total chlorophyll amount. In plants grown on the flotation tailings, the reduction in Chl a together with slightly but not significantly higher Chl b content resulted also in significantly lower Chl a/b ratio than the control mean value. The content of total carotenoids showed an opposite trend compared to that observed in Chl a, being significantly higher in all metal-stressed plants than in C (p = 0.0046).
Regarding photosynthetic efficiency, there was no significant difference in maximum quantum efficiency of PS II photochemistry (Fv/Fm) between plants cultivated on the flotation tailings and the control. The mean values of lipid peroxidation were significantly higher in FT than in C plants, whereas the total antioxidative capacity in leaves was significantly lower in FT and FTNPK plants compared to the that in the control (p = 0.029, p = 0.005, respectively).
Plant biometric parameters
The adverse elemental composition of flotation tailings significantly reduced most biometric parameters of plants, as shown in Table 5. FT and FTNPK plants had lower survival rate, smaller number of considerably shorter shoots, higher portion of senescent leaves and lower biomass production than in control plants. Their annual biomass yield harvested in March 2018 represented only 2.3% (FT) and 3.5% (FTNPK) of the yield achieved by the control plants (Table 5). Fertilization had a significant effect on aboveground biomass production in FTNPK plants, resulting in 1.5-fold higher yield compared to that of the FT. Fertilized plants had also 1.36-fold higher mean value of the number of shoots per plant than FT, although the mentioned difference was not statistically significant.
Discussion
Chemical properties of the control soil and flotation tailings
The unexpectedly high amount of organic matter (2.78%) detected in the flotation tailings does not originate from natural organic substances because no vascular plants, mosses or lichens were detected on its surface, and no biological remaining was present as a part of its surface layers. It rather originates from the commonly used collector xanthate remained after the flotation process and its different decomposition products. Correspondingly, the amounts of N, P and K in the flotation tailings were far below optimal range necessary for plant nutrition (Marschner 1995). On the other hand, the amounts of total and available metals (Pb, Cu, Zn) in the flotation tailings significantly exceed those typical for unpolluted soils but correspond to concentrations that are commonly found in soils polluted by municipal wastewaters, sewage sludge and metal smelting processes (Kabata-Pendias 2011; Ettler 2016; Kołodziej et al. 2016; Akopyan et al. 2018).
Metal accumulation in plant organs
In the second year of their growth in the flotation tailings, M. × giganteus plants accumulated all metals in roots in far higher amounts comparing to the aboveground organs, showing that they were strongly retained within the root tissues against their transfer to shoots. These results are complement to data previously reported for Zn (Korzeniowska and Stanislawska-Glubiak 2015) and combination of several heavy metals in M. × giganteus (Pidlisnyuk et al. 2018; Nurzhanova et al. 2019), as well as for Cd in three different Miscanthus species cultivated on metal-polluted soils (Guo et al. 2016).
BCFs > 1 were detected for Cu and Zn, whereas BCF for Pb was lower than one due to its very low mobility. Although metal concentrations in roots were in ranges that are toxic to aboveground organs, their critical concentrations in roots can be much higher due to their immobilization in the cell walls and complexation in the vacuoles within the root cortex. Fertilized plants accumulated Cu and Zn in roots in significantly higher amounts comparing to non-fertilized plants. This suggests either the potential promoting effects of fertilization on root development, increase in the root absorption area and efficiency of metal acquisition or decrease in the substrate pH and elevated metal bioavailability.
Although M. × giganteus plants cultivated on flotation tailings demonstrated TFs far lower than 1, they accumulated Zn and Pb in leaves in concentrations that can be harmful for various metabolic processes. Amongst analyzed metals, Zn showed the highest mobility within the plant. In leaves, in both FT and FTNPK plants, it reached concentrations (134 and 135 mg/kg) that are at the lower limit of the range toxic to various plants (100 to 500 mg/kg), but higher than the background concentrations detected in grass and clover (12 to 47 mg/kg) (Macnicol and Beckett 1985; Kabata-Pendias 2011). Concentrations of Cu in leaves were lower than those usually considered toxic to most plants (20 to 100 mg/kg) (Kabata-Pendias 2011). Albeit fertilization with commercial fertilizer increased uptake of both essential elements (Cu and Zn) in roots, the rate of their transport to leaves remained unaffected, which can be associated with their highly regulated transport within the plant (Sinclair and Krämer 2012).
Pb enrichment in M. × giganteus was in the following order, roots > rhizome > stems > leaves, which is similar to that in Ligustrum lucidum seedlings, Armeria maritima, Agrostis tenuis and Cardaminopsis halleri (Dahmani et al. 2000; Zhou et al. 2018). The small portion of its root content was transferred to leaves (1.1% in FT and 2.0% in FTNPK), which is in agreement with its efficiently confined transport toward aboveground organs by the Casparian strip (Seregin et al. 2004). Our results are in accordance with those reported for Lathyrus sativus (Brunet et al. 2009), Zea mays (Gupta et al. 2009), Phaseolus vulgaris (Shahid et al. 2011), Helianthus annuus (Wińska-Krysiak et al. 2015), Ligustrum lucidum (Zhou et al. 2018), etc. Even then, Pb amount in leaves was manifold higher than the background concentrations detected in different plants (Kabata-Pendias 2011).
Photosynthesis, respiration, oxidative stress and growth
The photosynthetic process is one of the prime targets of accumulated metals and therefore gives the good indication of the overall effects of metals on various processes related to photosynthesis. Reduction in the photosynthetic CO2 assimilation capacity in metal-stressed plants (FT, FTNPK) was noticeable along their light response curves and was the most pronounced for the light-saturating photosynthetic rates. Such large reduction in A is highly negatively correlated with elevated Cu (p < 0.01, ρ = − 0.65), Zn (p < 0.05, ρ = − 0.49) and Pb (p < 0.05, ρ = − 0.50) concentrations in leaves indicating their inhibitory effects. The similar data were reported for M. × giganteus exposed to elevated As, Pb, Sb and Zn (Wanat et al. 2013; Andrejić et al. 2018) and for three different Miscanthus species exposed to elevated Cd concentrations (Guo et al. 2016). Divalent metal cations, such as Zn2+ and Cu2+, when accumulated in leaves can substitute Mg2+ from Chl and RuBisCO molecules causing their structural changes. Both replacements lead to functional degradation of photosystem and detectable depression in photosynthetic activity (Küpper et al. 1998, 2002; Küpper and Andresen 2016). Pb accumulated in leaves, even at low concentrations, adversely affects large variety of components of the photosynthetic machinery (Sharma and Dubey 2005; Ahmad et al. 2011; Yang et al. 2015; Zhou et al. 2018).
The metal-induced production of reactive oxygen species (ROS) in FT and FTNPK plants was indicated by significantly higher MDA content, as biochemical marker of free radical-mediated lipid oxidation damage in cells. All three metals are known to enhance ROS generation, directly as redox-active metal ions (Cu2+), or indirectly by metal ion substitution and hampering functionality of different cell processes (Zn, Pb) (Verma and Dubey 2003; Küpper and Andresen 2016). The more prominent MDA accumulation in non-fertilized compared to that in fertilized plants indicate that P starvation was associated with significantly higher oxidative stress in P-deficient plants, as reported for Arabidopsis thaliana exposed to different levels of P starvation (Simancas and Munné-Bosch 2015). The aforementioned increase in membrane oxidative damages in all plants cultivated on the flotation tailings was associated also with significantly reduced ROS scavenging capacity in leaves.
Whereas the net CO2 assimilation rates in FT and FTNPK plants were similar, the amount of Chl a was significantly reduced in non-fertilized plants. Detected decline in Chl a in FT plants was found to be significantly correlated only to P deficiency in leaves (< 1000 μg P g–1 dw) (p < 0.05, ρ = 0.48) which limits Chl a biosynthesis and its content (Zhang et al. 2014). The obtained results were not associated with inhibitory effects of elevated concentrations of Zn and Pb on activity of enzymes involved in chlorophyll biosynthesis (Mukhopadhyay et al. 2013; Andrejić et al. 2018) and point to their efficient immobilization within M. × giganteus leaves.
Decline only in Chl a concentration points to lower sensibility of Chl b and Car biosynthetic pathways to elemental stress. A significant increase in total carotenoids in both FT and FTNPK plants is in line with observed changes in carotenoids aimed at protecting the PSII and stability of photosynthetic processes in several metal-stressed plant species (Simancas and Munné-Bosch 2015; Singh et al. 2017; Zhou et al. 2018), as indicated by high Fv/Fm values.
The potential efficiency of PSII photochemistry (Fv/Fm) is a highly sensitive indicator of plant stress and provides valuable information about the photosynthetic efficiency of the plant and functionality of the PSII (Govindjee 1995). Fv/Fm in the control and FTNPK were similar (0.81) and slightly higher than in FT plants (0.78), being in all treatments within the range that is considered optimal for plants (0.75–0.85) (Björkman and Demmig 1987). Good functionality of PSII indicates that M. × giganteus efficiently immobilized Zn and Pb within the leaf, and complementary pigments (Car) performed a significant role in the protection of the photosystem from oxidative damage and photoinhibition. The significant positive correlation between Fv/Fm and P (p < 0.001, ρ = 0.83) indicates that P shortage in leaves altered maximum efficiency of PSII photochemistry in P-deficient FT plants, as reported for different plant species (Heber et al. 1989; Michelet et al. 2013; Zhang et al. 2014; Frydenvang et al. 2015; Carstensen et al. 2018).
The respiration in dark was more intense in non-fertilized plants. It was positively correlated to P (ρ = 0.49) and negatively correlated to Zn (p < 0.001, r = − 0.77) and Pb content in leaves (p < 0.001, ρ = − 0.74). Exposure to Pb was shown to stimulate respiration rate in intact plants (Burzyński and Kłobus 2004), detached leaves and isolated protoplasts (Parys et al. 1998), albeit the underlying mechanism is not clear (Sharma and Dubey 2005).
Reduced photosynthetic capacity and enhanced rate of oxidative damage in plants cultivated on flotation tailings resulted in pronounced leaf senescence and significantly reduced plant growth and lower annual biomass yield. Fertilization positively contributed to annual biomass production, primarily by lowering oxidative stress and increasing the number of shoots per plant.
Conclusions
In this 2-year field experiment, M. × giganteus showed a remarkable ability to grow in extremely harsh conditions in the flotation tailings and to form a sustainable plant cover without much human interference. Plants retained the major portion of accumulated metals within the roots and showed their low translocation to shoots, which is beneficial in preventing them from entering the food web. In the second year of growth on the flotation tailings, plants showed decline in photosynthetic activity and increase in membrane oxidative damages, which resulted in overall reduced plant growth. Fertilization by single dose of the commercial NPK fertilizer eliminated negative effects of P-deficiency on Chl a content, Fv/Fm and oxidative stress but did not exhibit positive effects on the leaf photosynthetic capacity.
In ecological context, the presence of M. × giganteus plants and their biological activity and gradual accumulation of litter originating from decaying aboveground biomass are expected to ameliorate the extreme environment conditions of the abandoned flotation tailings. Introduction of M. × giganteus, as plant species resistant to multiple stresses, would trigger various slow but positive changes in the flotation tailings surface layers, which will be further guided by living organisms and their specific interaction with the environment.
References
Aggarwall A, Sharma I, Tripathi BN, Munjal AK, Baunthiyal M, Sharma V (2011) Metal toxicity and photosynthesis. In: Itoh S, Mohanty P, Guruprasad KN (eds) Photosynthesis: Overviews on Recent Progress & Future Perspective. IK International Publishing House, New Delhi, pp 229–236
Ahmad MSA, Ashraf M, Tabassam Q, Hussain M, Firdous H (2011) Lead (Pb)-induced regulation of growth, photosynthesis, and mineral nutrition in maize (Zea mays L.) plants at early growth stages. Biol Trace Elem Res 144:1229–1239. https://doi.org/10.1007/s12011-011-9099-5
Akopyan K, Petrosyan V, Grigoryan R, Melkomian DM (2018) Assessment of residential soil contamination with arsenic and lead in mining and smelting towns of northern Armenia. J Geochem Explor 184:97–109. https://doi.org/10.1016/j.gexplo.2017.10.010
Andrejić G, Gajić G, Prica M, Dželetović Ž, Rakić T (2018) Zinc accumulation, photosynthetic gas exchange, and chlorophyll a fluorescence in Zn-stressed Miscanthus × giganteus plants. Photosynthetica 56:1249–1258. https://doi.org/10.1007/s11099-018-0827-3
Arif N, Yadav V, Singh S, Singh S, Ahmad P, Mishra RK, Sharma S, Tripathi DK, Dubey NK, Chauhan DK (2016) Influence of high and low levels of plant-beneficial heavy metal ions on plant growth and development. Front Environ Sci 4:69. https://doi.org/10.3389/fenvs.2016.00069
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidases in Beta vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Baker AJ (1981) Accumulators and excluders-strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654. https://doi.org/10.1080/01904168109362867
Belčikova NP (1965) Determination of the humus of soils by I.V. Tyurin’s method. In: Tyurin IV (ed) Agrochemical methods in study of soils. 4th edn. Nauka, Moscow, pp 75–102
Björkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence at 77 K among vascular plants of diverse origins. Planta 170:489–504. https://doi.org/10.1007/BF00402983
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Bremner JM (1996) Nitrogen – Total. In: Sparks DL (ed) Methods of soil analysis part 3: chemical methods. Soil and Science Society of America, Inc., American Society of Agronomy, Inc., Madison, pp 1085-1122.
Brunet J, Varrault G, Zuily-Fodil Y, Repellin A (2009) Accumulation of lead in the roots of grass pea (Lathyrus sativus L.) plants triggers systemic variation in gene expression in the shoots. Chemosphere 77:1113–1120. https://doi.org/10.1016/j.chemosphere.2009.07.058
Burzyński M, Kłobus G (2004) Changes of photosynthetic parameters in cucumber leaves under Cu, Cd, and Pb stress. Photosynthetica 42:505. https://doi.org/10.1007/S11099-005-0005-2
Carstensen A, Herdean A, Birkelund Schmidt S, Sharma A, Spetea C, Pribil M, Husted S (2018) The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol 177:271–284. https://doi.org/10.1104/pp.17.01624
Cross AT, Stevens JC, Dixon KW (2017) One giant leap for mankind: can ecopoiesis avert mine tailings disasters? Plant Soil 421:1–5. https://doi.org/10.1007/s11104-017-3410-y
Dahmani M, Van Oort HF, Gelie B, Balabane M (2000) Strategies of heavy metal uptake by three plant species growing near a metal smelter. Environ Pollut 109:231–238. https://doi.org/10.1016/S0269-7491(99)00262-6
Egnér H, Riehm H, Domingo WR (1960) Untersuchungen uber die chemische Bodenanalyse als Grundlage fur die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor- und Kaliumbestimmung. Kungliga Lantbrukshögskolans Annaler 26:199–215
Ettler V (2016) Soil contamination near non-ferrous metal smelters: A review. Appl Geochem 64:56–74. https://doi.org/10.1016/j.apgeochem.2015.09.020
Frydenvang J, van Maarschalkerweerd M, Carstensen A, Mundus S, Schmidt SB, Pedas PR, Laursen KH, Schjoerring JK, Husted S (2015) Sensitive detection of phosphorus deficiency in plants using chlorophyll a fluorescence. Plant Physiol 169:353–361. https://doi.org/10.1104/pp.15.00823
Govindjee (1995) Sixty-three years since Kautsky: chlorophyll fluorescence. Aust J Plant Physiol 22:131–160
Guo H, Hong C, Chen X, Xu Y, Liu Y, Jiang D, Zheng B (2016) Different growth and physiological responses to cadmium of the three miscanthus species. PLoS One 11(4):e0153475. https://doi.org/10.1371/journal.pone.0153475
Gupta D, Nicoloso F, Schetinger M, Rossato L, Pereira L, Castro G, Srivastava S, Tripathi R (2009) Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J Hazard Mater 172:479–484. https://doi.org/10.1016/j.jhazmat.2009.06.141
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Heaton E, Voigt T, Long SP (2004) A quantitative review comparing the yields of two candidate C4 perennial biomass crops in relation to nitrogen, temperature and water. Biomass Bioenergy 27:21–30. https://doi.org/10.1016/j.biombioe.2003.10.005
Heber U, Viil J, Neimanis S, Mimura T, Dietz KJ (1989) Photoinhibitory damage to chloroplasts under phosphate deficiency and alleviation of deficiency and damage by photorespiratory reactions. Z Naturforsch C 44(5-6):524–536. https://doi.org/10.1515/znc-1989-5-629
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334. https://doi.org/10.1139/b79-163
IUSS Working Group WRB (2015) World Reference Base for Soil Resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. FAO, Rome
Jackson ML (1958) Soil chemical analysis. Prentice-Hall, Englewood Cliffs
Kabata-Pendias A (2011) Trace elements in soils and plants, 4th edn. Taylor and Francis, Boca Raton
Kołodziej B, Stachyra M, Antonkiewicz J, Bielińska E, Wiśniewski J (2016) The effect of harvest frequency on yielding and quality of energy raw material of reed canary grass grown on municipal sewage sludge. Biomass Bioenergy 85:363–370. https://doi.org/10.1016/j.biombioe.2015.12.025
Korzeniowska J, Stanislawska-Glubiak E (2015) Phytoremediation potential of Miscanthus × giganteus and Spartina pectinata in soil contaminated with heavy metals. Environ Sci Pollut Res 22:11648–11657. https://doi.org/10.1007/s11356-015-4439-1
Küpper H, Andresen E (2016) Mechanisms of metal toxicity in plants. Metallomics 8:269–285. https://doi.org/10.1039/c5mt00244c
Küpper H, Küpper F, Spiller M (1998) In situ detection of heavy metal substituted chlorophylls in water plants. Photosynth Res 58:123–133. https://doi.org/10.1023/A:1006132608181
Küpper H, Šetlík I, Spiller M, Küpper FC, Prášil O (2002) Heavy metal-induced inhibition of photosynthesis: targets of in vivo heavy metal chlorophyll formation. J Phycol 38:429–441. https://doi.org/10.1046/j.1529-8817.2002.01148.x
Lu C, Zhang J (2000) Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci 151:135–143. https://doi.org/10.1016/S0168-9452(99)00207-1
Macnicol RD, Beckett PHT (1985) Critical concentrations of potentially toxic elements. Plant Soil 85:107–129. https://doi.org/10.1007/BF02197805
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, San Diego, New York
Michelet L, Zaffagnini M, Morisse S, Sparla F, Pérez-Pérez ME, Francia F, Danon A, Marchand CH, Fermani S, Trost P, Lemaire SD (2013) Redox regulation of the Calvin-Benson cycle: something old, something new. Front Plant Sci 4:470 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3838966/
Mukhopadhyay M, Das A, Subba P, Bantawa P, Sarkar B, Ghosh PD, Mondal TK (2013) Structural, physiological and biochemical profiling of tea plantlets under zinc stress. Biol Plant 57:474–480. https://doi.org/10.1007/s10535-012-0300-2
Naidu SL, Moose SP, Al-Shoaibi AK, Raines CA, Long SP (2003) Cold tolerance of C4 photosynthesis in Miscanthus × giganteus: adaptation in amounts and sequence of C4 photosynthetic enzymes. Plant Physiol 132:1688–1697. https://doi.org/10.1104/pp.103.021790
Nsanganwimana F, Pourrut B, Waterlot C, Louvel B, Bidar G, Labidi S, Fontaine J, Muchembled J, Lounès-Hadj Sahraoui A, Fourrier H, Douay F (2015) Metal accumulation and shoot yield of Miscanthus × giganteus growing in contaminated agricultural soils: insights into agronomic practices. Agric Ecosyst Environ 213:61–71. https://doi.org/10.1016/j.agee.2015.07.023
Nurzhanova A, Pidlisnyuk V, Abit K, Nurzhanov C, Kenessov B, Stefanovska T, Erickson L (2019) Comparative assessment of using Miscanthus × giganteus for remediation of soils contaminated by heavy metals: a case of military and mining sites. Environ Sci Pollut Res 26:13320–13333. https://doi.org/10.1007/s11356-019-04707-z
Pansu M, Gautheyroy J (2006) Handbook of soil analysis. Mineralogical, organic and inorganic methods. Springer, Berlin
Parys E, Romanowska E, Siedlecka M, Poskuta JW (1998) The effect of lead on photosynthesis and respiration in detached leaves and in mesophyll protoplasts of Pisum sativum. Acta Physiol Plant 20:313–322. https://doi.org/10.1007/s11738-998-0064-7
Pavel PB, Puschenreiter M, Wenzel WW, Diacu E, Barbu CH (2014) Aided phytostabilization using Miscanthus sinensis × giganteus on heavy metal-contaminated soils. Sci Total Environ 479-480:125–131. https://doi.org/10.1016/j.scitotenv.2014.01.097
Pidlisnyuk V, Erickson L, Trögl J, Shapoval P, Davis L, Popelka J, Stefanovska T, Hettiarachchi G (2018) Metals uptake behaviour in Miscanthus x giganteus plant during growth at the contaminated soil from the military site in Sliač, Slovakia. Pol J Chem Technol 20(2):1–7. https://doi.org/10.2478/pjct-2018-0016
Pietrzykowski M, Antonkiewicz J, Gruba P, Pająk M (2018) Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill. Open Chem 16:1143–1152. https://doi.org/10.1515/chem-2018-0129
Pogrzeba M, Rusinowski S, Krzyżak J (2018) Macroelements and heavy metals content in energy crops cultivated on contaminated soil under different fertilization—case studies on autumn harvest. Environ Sci Pollut Res Int 25:12096–12106. https://doi.org/10.1007/s11356-018-1490-8
Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity, and detoxification in plants. Rev Environ Contam Toxicol 213:113–136. https://doi.org/10.1007/978-1-4419-9860-6_4
Purdy SJ, Maddison AL, Jones LE, Webster RJ, Andralojc J, Donnison I, Clifton-Brown J (2013) Characterization of chilling-shock responses in four genotypes of Miscanthus reveals the superior tolerance of M. × giganteus compared with M. sinensis and M. sacchariflorus. Ann Bot 111:999–1013. https://doi.org/10.1093/aob/mct059
R Core Team (2018) R: A language and environment for statistical computing. In: R Foundation for statistical computing. Vienna, Austia. https://www.R-project.org/
Rosikoń K, Fijalkowski K, Kaprzak M (2015) Phytoremediation potential of selected energetic plants (Miscanthus giganteus L. and Phalaris arundinacea L.) in dependence on fertilization. J Environ Sci Eng A 4:587–595. https://doi.org/10.17265/2162-5298/2015.11.004
Seregin IV, Shpigun LK, Ivanov VB (2004) Distribution and toxic effects of cadmium and lead on maize roots. Russ J Plant Physiol 51:525–533. https://doi.org/10.1023/B:RUPP.0000035747.42399.84
Seshadri B, Bolan NS, Naidu R (2015) Rhizosphere-induced heavy metal(loid) transformation in relation to bioavailability and remediation. J Soil Sci Plant Nutr 15(2):524–548. https://doi.org/10.4067/S0718-95162015005000043
Shahid M, Pinelli E, Pourrut B, Silvestre J, Dumat C (2011) Lead-induced genotoxicity to Vicia faba L. roots in relation with metal cell uptake and initial speciation. Ecotoxicol Environ Saf 74:78–84. https://doi.org/10.1016/j.ecoenv.2010.08.037
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17:1–19. https://doi.org/10.1590/s1677-04202005000100004
Simancas B, Munné-Bosch S (2015) Interplay between vitamin E and phosphorus availability in the control of longevity in Arabidopsis thaliana. Ann Bot 116:511–518. https://doi.org/10.1093/aob/mcv033
Sinclair SA, Krämer U (2012) The zinc homeostasis network of land plants. Biochim Biophys Acta 1823:1553–1567. https://doi.org/10.1016/j.bbamcr.2012.05.016
Singh SK, Reddy VR, Fleisher DH, Timlin DJ (2017) Relationship between photosynthetic pigments and chlorophyll fluorescence in soybean under varying phosphorus nutrition at ambient and elevated CO2. Photosynthetica 55:421–433. https://doi.org/10.1007/s11099-016-0657-0
Tordoff GM, Baker AJM, Willis AJ (2000) Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 41:219–228
USEPA Method 3051 (1998) Microwave assisted acid digestion of sediments, sludges and oils. In: In: Test Methods for Evaluating Solid Waste. Environmental Protection Agency, Washington, p SW–846
van Reeuwijk LP (2002) Procedures for soil analysis, 6th edn. Technical Paper / International Soil Reference and Information Centre, Wageningen
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655. https://doi.org/10.1016/S0168-9452(03)00022-0
Wanat N, Austruy A, Joussein E, Soubrand M, Hitmi A, Gauthier-Moussard C, Lenain JF, Vernay P, Munch JC, Pichon M (2013) Potentials of Miscanthus × giganteus grown on highly contaminated Technosols. J Geochem Explor 126-127:78–84. https://doi.org/10.1016/j.gexplo.2013.01.001
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids using various solvents with spectrophotometers of different resolution. J Plant Physiol 144(3):307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Wińska-Krysiak M, Koropacka K, Gawroński S (2015) Determination of the tolerance of sunflower to lead induced stress. J Elem 20:491–502. https://doi.org/10.5601/jelem.2014.19.4.721
Yang YR, Han XZ, Liang Y, Ghosh A, Chen J, Tang M (2015) The combined effects of arbuscular mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PLoS One 10:e0145726. https://doi.org/10.1371/journal.pone.0145726
Zhang K, Liu H, Tao P, Chen H (2014) Comparative proteomic analyses provide new insights into low phosphorus stress responses in maize leaves. PLoS One 9(5):e98215. https://doi.org/10.1371/journal.pone.0098215
Zhou J, Zhang Z, Zhang Y, Wei Y, Jiang Z (2018) Effects of lead stress on the growth, physiology, and cellular structure of privet seedlings. PLoS One 13(3):e0191139. https://doi.org/10.1371/journal.pone.0191139
Acknowledgements
We thank the staff of Mine “Rudnik” for providing us the experimental field on the mine flotation tailings and for the technical support.
Funding
This study was funded by Serbian Ministry of Education, Science and Technological Development (grant number 173030 and TR31057).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andrejić, G., Šinžar-Sekulić, J., Prica, M. et al. Phytoremediation potential and physiological response of Miscanthus × giganteus cultivated on fertilized and non-fertilized flotation tailings. Environ Sci Pollut Res 26, 34658–34669 (2019). https://doi.org/10.1007/s11356-019-06543-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06543-7