Abstract
The rupture of Fundão dam was the biggest environmental disaster of the worlds’ mining industry, dumping tons of iron ore tailings into the environment. Studies have shown that the Fundão dam’s tailings are poor in nutrients and have high Fe and Mn concentration. In this context, our objective was to evaluate the growth performance of two native tree species (Bowdichia virgilioides and Dictyoloma vandellianum) in two treatments: fertilized soil and fertilized tailings. We hypothesize that the high concentrations of iron and manganese in the tailings can impair the growth performance of plants by interfering with the absorption of nutrients made available through fertilization. Soil and tailings samples were collected in the municipality of Barra Longa (MG, Brazil), and then fertilized with mixed mineral fertilizer (“Osmocote Plus 15–9-12” at 7.5 g L−1). The experiment was conducted for 75 days in a greenhouse using 180 cm3 tubes. We evaluate chlorophyll content, maximal PSII quantum yield, root length, shoot length, root:shoot ratio, leaf area, specific leaf area and leaf area ratio, dry mass, macro- and micronutrients concentration in the tissues, and metal translocation factor. Although assuring the adequate levels of the main nutrients to plant growth (N, P, K, Ca, and Mg), the fertilization did not reverse the negative effect of tailing on these species. The high concentration of Fe in the tissues associated with less biomass production, lower plant height, smaller leaf area, bigger specific leaf area, and reduced chlorophyll content indicates a probable phytotoxic effect of iron present in the tailings for D. vandellianum. Our results base further field evaluations and longer experiments, which will facilitate the understanding of the performance of tree species submitted to tailings with fertilization. So far, this study suggests that B. virgilioides are more tolerant to excess Fe from the tailings of Fundão dam than D. vandellianum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
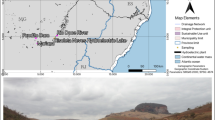
Brazil is the world’s second largest producer of iron ore (IBRAM 2012). Despite being an indispensable activity for human society, mining can generate major impacts on the environment. Brazil had more than 80 mine-related environmental disasters and it is estimated that at least 126 mining dams in the country are vulnerable to failure in the forthcoming years (Nazareno and Vitule 2016; Garcia et al. 2017). At the end of 2015, the Fundão dam, operated by Samarco Mining Company and located in the municipality of Mariana, Minas Gerais (Brazil), collapsed, launching tons of iron ore tailings into the environment, which traveled over 600 km of watercourses from the Doce River basin until reaching the Atlantic Ocean (IBAMA 2015; Samarco 2016).
Considering the volume of tailings dumped (~ 50 million m3) and the magnitude of the socioenvironmental damages, the rupture of Fundão dam was the biggest environmental disaster of the worlds’ mining industry (IBAMA 2015; Carmo et al. 2017). This accident reached the Brazilian Atlantic Forest which given its species richness and endemism levels, it is considered one of the world’s biodiversity hotspots, making its restoration considerably important (Myers et al. 2000; Ribeiro et al. 2009; Massad et al. 2011). The ore tailings from Fundão dam flooded and destroyed native riparian vegetation, i.e., 457.6 ha of Atlantic Forest along the river course (Omachi et al. 2018).
The contents of the potential toxic elements (Ag, Al, As, Cd, Co, Cr, Cu, Hg, Mn, Ni, Pb, Rb, Ti, Zn, Zr) in the tailings released from the Fundão dam rupture were below the threshold levels defined by Brazilian environmental law (EMBRAPA 2015). However, according to Segura et al. (2016), some days after the disaster, the sediments in suspension in the river water affected by the tailings contained iron (Fe) and manganese (Mn) at concentrations approximately 4 (Fe) and 1.5(Mn)-fold higher than the maximum Brazilian limit for water bodies quality assessment. Furthermore, the concentration of Fe and Mn found in the tailing samples were very high compared to the recommended concentration for plant growth in Minas Gerais’ soils (Zago et al. 2019) and were above the values allowed by the CONAMA (National Environment Council) (Esteves et al. 2020a). Despite Fe and Mn are mainly associated with less bioavailable fractions (oxides and hydroxides), environmental physical–chemical conditions and plant activity may solubilize these forms, thus releasing their associated metals and increasing its bioavailability (Andrade et al. 2018; Queiroz et al. 2018). Additionally, the tailings are deficient in nutrients, poor in organic matter, and have less clay content and less cation exchange capacity than the regions’ natural soils (SEDRU 2016; Segura et al. 2016; Cruz et al. 2020). Therefore, the tailings have the potential to affect soil over time, making it difficult to recover and develop previously established plant species (IBAMA 2015).
It is difficult to estimate how many years it will take for the affected flora to recover (Lyra 2019). An accident of this magnitude, where the long-lasting effects are still unknown, requires studies to mitigate environmental damage and restore the functioning of ecosystems (Segura et al. 2016; Hatje et al. 2017). Revegetation plans, erosion control, and restoration of the ciliary strip aiming at the recovery of the affected areas (EMBRAPA 2015) are demanded by the Federal Public Ministry of Brazil. One of the main issues of a reforestation project is the diversity of plant species (Massad et al. 2011). Studies have shown that plant species perform better in mixed plots than in monoculture, and, therefore, biodiversity may improve productivity in tropical reforestation (Montagnini 2001; Erskine et al. 2006; Potvin and Gotelli 2008). Indeed, it is very important to choose the correct species for the success of the restoration process (Balestrin et al. 2019). In addition to support biodiversity, the chosen species may tolerate the adverse conditions to be effectively established in the recovering area. In this context, the study of the performance of potential species to be used in areas affected by the Fundão dam tailings is a priority.
There are some studies on the performance of plants in Samarco’s mining tailings. In a field study at impacted areas by the Fundão tailings, Santos et al. (2019) related the mortality of tree species due to the presence of high amounts of sodium and ammonium and low nutrient contents in the tailings. The deleterious effects in plant growth were associated to nutrient deficiency as well as to physical properties (high density and silt percentage) of the Fundão tailings (Andrade et al. 2018; Esteves et al. 2020a). In that context, the nutritional amendment of tailings improved biomass production of three species of aromatic grasses (Chrysopogon zizanioides, Cymbopogon citratus, and Cymbopogon winterianus) (Zago et al. 2019), three agronomic species (Pennisetum glaucum L., Sorghum bicolor, and Zea mays L.) (Esteves et al. 2020b), and five tree species (Albizia polycephala, Cybistax antisyphilitica, Handroanthus heptaphyllus, Handroanthus impetiginosus, and Peltophorum dubium) (Cruz et al. 2020).
The initial coverage with grasses and legumes and the planting of native tree species are two measures included in the Integrated Environmental Recovery Plan (PRAI), prepared by the Renova Foundation—the organization responsible for conducting environmental restoration measures in the Doce River Basin (Renova 2017). The best performance of this initial vegetation cover was observed in areas where inorganic fertilizer was used in comparison to areas without fertilization (Renova 2017). According to the Renova Foundation (Renova 2020), the tailings from the rupture of the Fundão dam are inert (composed mainly of dirt, iron, and manganese) and its removal from the places where it was deposited would cause a second wave of environmental impacts. The next steps focus on direct planting of seedlings of native tree species, which include items such as irrigation, ant control, and fertilization (Renova 2020).
Given the presence of iron ore tailings in some sites to be reforested, the fact that the region’s soil and the tailings deposited are poor in nutrients (EMBRAPA 2006; Coelho 2009; SEDRU 2016) and fertilization is a practice adopted in projects for the recovery of degraded areas, it is necessary to select species to revegetate the affected area. Bowdichia virgilioides Kunth [“sucupira preta,” Fabaceae] and Dictyoloma vandellianum Adr. Juss. [“tingui,” Rutaceae] were the two tree species selected for this study. Both are naturally occurring in the region affected by the collapse dam (Lombardi and Gonçalves 2000; Lopes et al. 2002) and recommended in programs for recover of degraded areas (Montagnini 2001; Flavio and Paula 2010; Lima et al. 2013; Filho and Sartorelli 2015; Colmanetti et al. 2016). Bowdichia virgilioides is classificated as late secondary (Oliveira et al. 2011) and D. vandellianum as a pioneer species in ecological succession (Balestrin et al. 2019). Furthermore, these species are found in ferruginous ecosystems (Messias et al. 2012; Pereira et al. 2013; Andrino et al. 2020), known as canga, which have high concentrations of oxides and hydroxides of iron (Simmons 1960; Maciel et al. 2017). These metal-rich environments such as canga are expected to harbor metal-tolerant and hyperaccumulator plant species (Jacobi et al. 2007).
Therefore, the objective of this study was to evaluate the growth performance of these two native tree species (Bowdichia virgilioides and Dictyoloma vandellianum) in two treatments: fertilized soil and fertilized tailings. We hypothesize that the high concentrations of iron and manganese in the tailings can impair the growth performance of plants by interfering with the absorption of nutrients made available through fertilization. For this purpose, we evaluate several physiological parameters: chlorophyll content, maximal PSII quantum yield (FV/FM), root length, shoot length, root:shoot ratio, leaf area, specific leaf area and leaf area ratio, dry mass, macro- and micronutrients concentration in the tissues, and metal translocation factor. The present research contributes to a physiological understanding of tree species that are potentially be used in restoration programs in areas affected by iron ore tailings, as in the case of the Fundão dam rupture.
Material and methods
Soil and iron ore tailings sampling
Substrates were collected (20 cm deep) on the riverside of the Carmo River (20° 21′ 26.45″ S, 43°7′ 4.69″ W), in the municipality of Barra Longa (MG, Brazil) in April 2017, 18 months after the rupture of the Fundão dam. Soil samples (dystrophic red-yellow latosols/argisols) were obtained from not affected area (with no tailing at 5 m from the tailings collection site, under the same transect) and tailings samples from area with iron ore tailings deposition. After sampling, the substrates were homogenized and packed in plastic bags until the experiments were carried out. Soil and tailings properties and bioavailability of chemical elements of the collected substrates can be seen in our previous studies (Cruz et al. 2020).
Conditions of plant growth
Bowdichia virgilioides and D. vandellianum seeds were provided by the Instituto Espinhaço, Biodiversidade, Cultura e Desenvolvimento Socioambiental (Gouveia, MG, Brazil). Physical dormancy imposed by hard seed coat in B. virgilioides was broken by chemical scarification with 7-min immersion in sulfuric acid (Albuquerque et al. 2007). Physical or physiological dormancy was not reported for D. vandellianum seeds, and, therefore, no breaking dormancy treatment was applied. Afterwards, seeds of each species were placed to germinate in transparent Gerbox boxes containing filter paper (Whatman No. 1) moistened with distilled water (Brasil 2009). Freshly germinated (emergence of 2 mm of the primary root) seeds were transplanted into 180 cm3 polyvinyl chloride tubes containing one of the two treatments: fertilized soil or fertilized tailings. One replicate was defined as one tube containing one plant, totaling 24 replicates per treatment for each species.
Substrates were fertilized with mixed mineral fertilizer applied to the soil/tailings via “Osmocote Plus 15–9-12” (15% N, 9% P2O5, 12% K2O, 1.3% Mg, 6% S, 0.05% Cu, 0.46% Fe, 0.06% Mn, and 0.02% Mo; Dublin, USA—with a nutrient release duration of 6 months). The concentration used was 7.5 g L−1, as suggested by the manufacturer for native tree species. “Osmocote” fertilizer was used because it is a slow-release fertilizer capable of increasing the efficiency of nutrient use by plants and reducing losses by physical processes (Shaviv and Mikkelsen 1993), as well as being practical to handle and assuring the possibility of a single application.
The growth experiment was conducted for 75 days in a greenhouse located at the Espinhaço Institute–Biodiversity, Culture and Socioenvironmental Development, situated at the Federal University of São João Del Rey, Sete Lagoas campus (MG, Brazil). Plants were arranged in a complete randomized design for each species (2 substrate treatments × 24 replicates). During the experiment, the average humidity was 82.4% and the temperature ranged between 19.6 and 28.8 °C, averaging at 23.4 °C. The plants were exposed to natural sunlight with a 50% shade screen (from November to February) and irrigated by micro-sprinkler irrigation for 15 min, four times a day. Invasive plants were removed manually every week, when necessary.
Photosynthetic pigment and chlorophyll a fluorescence
We measured the photosynthetic pigment in the leaves using a SPAD-502 portable chlorophyll meter (Minolta, Japan). These measurements were performed on fully expanded leaves in five plants per treatment. The result for each plant was the average of four readings on three different physiologically mature leaves from each repetition.
The minimal fluorescence level (F0) and the maximal fluorescence yield (FM) were determined in five plants per treatment using a pulse-amplitude modulation (PAM) fluorometer (model PAM-2500, WALZ, Effeltrich, Germany). We measured chlorophyll a fluorescence on the first, second, and third fully expanded leaves (physiologically mature leaves), for a total of three measurements per plant. Before these measurements, the leaves of the plants were adapted to darkness for 30 min to completely re-oxidize the primary electron acceptors of photosystem II (PSII). The maximal PSII quantum yield (FV/FM) was evaluated following Butler (1978):
where FM is the dark-adapted maximal fluorescence and F0 is the minimal fluorescence.
Growth parameters
After 75 days, ten plants of each treatment were collected and washed with water. The shoot and main root length were measured with a measuring tape (cm). For leaf area (LA) determination, all leaves of ten plants per treatment were scanned and then the LA (in cm2) was calculated using the ImageJ software. Subsequently, the leaf, stem, and root dry biomass production were assessed after drying plant organs in an oven with forced air circulation at 65 °C until constant weight. Biomass was weighed in an analytical scale (0.001 g precision). To evaluate biomass partition among root and shoot, the root/shoot ratio were calculated following Hunt (1982):
where WR is the root dry weight; WS is the stem dry weight; and WL is the leaf dry weight.
The specific leaf area (SLA) and the leaf area ratio (LAR) were calculated according to the following equations (Beadle 1985):
where LA is the leaf area; WL is the leaf dry weight; and WT is the total dry weight.
Concentration of nutrients in the plant tissues
Shoots or roots of six plants from each treatment were mixed, constituting one replicate, with a total of three replicates/treatment (fertilized soil of D. vandellianum has four replicates). The dry plant material was sent to the Laboratory of Soil, Vegetable Tissue and Fertilizer analysis at Universidade Federal de Viçosa (Minas Gerais, Brazil). For nitrogen, samples were digested at 350 °C using sulfuric acid and after, they have been cooled, filtered, and completed with ultra-pure water, and then total N was determined by the Kjeldahl method. For P, K, Ca, Mg, Fe, Cu, Zn, and Mn evaluations, plant tissues (roots or shoots) were digested at 200 °C using 10 ml of the 4:1 nitric acid + perchloric acid mixture (Sarruge and Haag 1974). After digesting and filtering the extracts (Whatman n° 40 filter paper), phosphorus concentration was measured colorimetrically using the ascorbic acid method (Braga and Defelipo 1974). The other nutrients (Ca, Mg, Fe, Cu, Zn, and Mn) were determined using an inductively coupled plasma optical emission spectrometry (ICP-OES PerkinElmer model Optima 8300 DV). The limit of quantification (LOQ) was 1.5 mg kg−1 for Mg, 2 mg kg−1 for Fe, and 0.5 mg kg−1 for Ca, Cu, Mn, and Zn.
Translocation factor
Translocation factor (TF) was calculated as the ratio between the metal (Ca, Cu, Fe, K, Mg, Mn, and Zn) concentration in shoots and roots (Bao et al. 2009).
Statistical analyses
Generalized linear models (GLMs) were created to assess differences in growth parameters (dry biomass, root length, shoot length, root:shoot ratio, leaf area, specific leaf area, leaf area ratio), chlorophyll content, nutrient concentration, and translocation factor (all Gaussian distribution) between treatments (fertilized tailings and fertilized soil) for each species and organs (nutrition data) separately. A GLM was also made for the maximal PSII quantum yield (FV/FM), which has a quasi-binomial distribution. Tests with P values lower than 0.05 were considered significantly different. Statistical analyses were performed using R software (R Development Core Team 2020).
Results
Chlorophyll concentration was 15% lower in D. vandellianum plants growing in the fertilized tailings compared to fertilized soil (P < 0.01; Fig. 1). For the two species, maximal photochemical efficiency of PSII (FV/FM) was not significantly different (P > 0.05) between treatments (Fig. 1). Compared to fertilized tailings, D. vandellianum plants grown in the fertilized soil were 4.9% higher (P < 0.001) and no difference in main root length was observed between the treatments for both species (Fig. 2). Bowdichia virgilioides had 60.9% higher root/shoot ratio in the fertilized tailings than in fertilized soil (P < 0.001; Fig. 2).
Chlorophyll content (SPAD) and the maximal PSII quantum yield (FV/FM) of two tree species grown in different substrates (fertilized soil and fertilized tailings). (a), (b) = Bowdichia virgilioides and (c), (d) = Dictyoloma vandellianum. Bars represent means ± SD of five replicates (n = 10). The symbols *, **, or *** indicate significant differences between treatments at P < 0.05, P < 0.01, and P < 0.001, respectively
Root length, shoot length, and root:shoot ratio of two tree species grown in different substrates (fertilized soil and fertilized tailings). (a), (b), (c) = Bowdichia virgilioides and (d), (e), (f) = Dictyoloma vandellianum. Bars represent means ± SD of ten replicates (n = 20). The symbols *, **, or *** indicate significant differences between treatments by analysis of variance at P < 0.05, P < 0.01, and P < 0.001, respectively
Lower total leaf area was observed in plants of both species growing under the fertilized tailing treatment (P < 0.001; Fig. 3). The leaf area of D. vandellianum plants grown in the fertilized tailings was 52.2% smaller than that found in the plants in the fertilized soil, whereas B. virgilioides plants had a 41.1% smaller leaf area in fertilized tailings than in the fertilized soil. Dictyoloma vandellianum plants grown in the fertilized tailings showed high specific leaf area (P < 0.01; Fig. 3). While for B. virgilioides plants, the leaf area ratio was higher in the fertilized soil (P < 0.05), for D. vandellianum, it was greater in plants growing in the fertilized tailings (P < 0.01) (Fig. 3). Greater dry biomass was observed for both species growing in the fertilized soil than in the fertilized tailings (P < 0.01 for B. virgilioides; P < 0.001 for D. vandellianum; Fig. 4). The plants of the species B. virgilioides accumulated 50% more biomass in the fertilized soil than in the fertilized tailings and those of the species D. vandellianum 160% more in the fertilized soil than in the fertilized tailings.
Leaf area (cm2), specific leaf area, and leaf area ratio of two tree species grown in different substrates (fertilized soil and fertilized tailings). (a), (b), (c) = Bowdichia virgilioides and (d), (e), (f) = Dictyoloma vandellianum. Bars represent means ± SD of ten replicates (n = 20). The symbols *, **, or *** indicate significant differences between treatments by analysis of variance at P < 0.05, P < 0.01, and P < 0.001, respectively
Dry mass of two tree species grown with fertilization in different substrates (fertilized soil and fertilized tailings). (a), (b) = Bowdichia virgilioides and (c), (d) = Dictyoloma vandellianum. Bars represent means ± SD of ten replicates (n = 20). The symbols *, **, or *** indicate significant differences between treatments by analysis of variance at P < 0.05, P < 0.01, and P < 0.001, respectively. The scale bar in the photos is 5 cm
The N, P, and K concentrations found in the shoots and roots did not significantly differ between treatments for both species (P > 0.05; Figs. 5 and 6). Only D. vandellianum showed a difference between treatments in the concentration of Ca, where plants grown in the fertilized tailings had 86.5% higher shoot concentration of Ca than those grown in the fertilized soil. A 32.1% and 37.5% lower root Mg concentration (P < 0.05; Fig. 5) was observed in D. vandellianum and B. virgilioides plants, respectively, grown in the fertilized tailings compared to fertilized soil. Similarly, shoot Mg concentration was 28.1% lower in D. vandellianum plants grown in fertilized tailings (P < 0.05; Fig. 6). Shoot Fe and Mn concentrations were greater in plants growing in the fertilized tailings (P < 0.05; Fig. 6). Dictyoloma vandellianum shoots from the fertilized tailings had a 10 times higher concentration of iron and five times higher of manganese compared to the shoots from the fertilized soil treatment. For B. virgilioides, this difference was less expressive, three times higher iron concentration and 3.75 times more manganese in the plants grown in the tailings compared to those grown in the soil. Similar results were observed for root Fe and Mn concentration of D. vandellianum, while in B. virgilioides roots, it differs only for iron root concentration and not for manganese (Fig. 5). There was no significant difference (P > 0.05) in copper concentration, neither in the roots nor in the shoots, for both species. In the shoots, the zinc concentration was 34.3% lower for D. vandellianum and 47.4% lower for B. virgilioides in plants grown on fertilized tailings compared to fertilized soil (Fig. 6). Similarly, the zinc concentration in the roots was 2.9 and 4.6 times higher (D. vandellianum and B. virgilioides, respectively) in the plants grown in the fertilized soil (Fig. 5).
Macro- and micronutrients concentration in the roots of two tree species (Bowdichia virgilioides and Dictyoloma vandellianum) grown in different substrates (FS = fertilized soil and FT = fertilized tailings). Bars represent means ± SD of three replicates (n = 6). The symbols *, **, or *** indicate significant differences between treatments by analysis of variance at P < 0.05, P < 0.01, and P < 0.001, respectively
Macro- and micronutrients concentration in the shoots of two tree species (Bowdichia virgilioides and Dictyoloma vandellianum) grown in different substrates (FS = fertilized soil and FT = fertilized tailings). Bars represent means ± SD of three replicates (n = 6). The symbols *, **, or *** indicate significant differences between treatments by analysis of variance at P < 0.05, P < 0.01, and P < 0.001, respectively
The Ca, Cu, Fe, K, Mg, and Mn translocation factor did not significantly differ (P > 0.05) between treatments and Zn translocation factor was higher in the fertilized tailings for B. virgilioides plants (Table 1). In contrast, for D. vandellianum, the plants exposed to fertilized tailings showed Ca, Cu, Fe, Mn, and Zn translocation factor greater than those from the fertilized soil (1.62, 2.46, 2.56, 1.84, and 2.03 times as much, respectively) (P < 0.01; Table 1).
Discussion
Lower biomass production of D. vandellianum and B. virgilioides plants was observed in fertilized tailings than in fertilized soils. The difference between treatments were more accentuated for D. vandellianum in which total dry mass was 2.6 times greater in fertilized soil in relation to fertilized tailings, while for B. virgilioides, this relation was of 1.5 times. The lack of nutrients is the main factor responsible for the negative effect of iron ore tailings from the rupture of the Fundão dam to plant growth (Andrade et al. 2018; Cruz et al. 2020). Although physical and chemical characteristics such as particles size (silt 44.1% and clay 6.9%), organic matter, and cation exchange capacity are unfavorable in the tailings compared to the soil (Cruz et al. 2020), there was no difference in the concentrations of N, P, K, Ca, and Mg in the shoots of the B. virgilioides plants grown on both fertilized substrates. The same occurred for D. vandellianum, except for Mg, which was present in higher concentrations in plants grown in the fertilized soil. These results demonstrate that even assuring the levels of important nutrients to plants (N, P, K, Ca, and Mg), the fertilization did not reverse the negative effect of tailing on plant growth. The absorption of these macronutrients (except Mg for D. vandellianum) was not affected by iron and manganese as hypothesized. This does not mean that one of these micronutrients may not have impaired plant growth, as discussed below.
Manganese and iron concentrations were higher in plants grown in fertilized tailings. Manganese is required for the activity of several enzymes and participates in the photochemical release of O2 in photosynthesis (Millaleo et al. 2010). Compared to macronutrients, micronutrients, such as Mn, are needed in small amounts, and at excessive levels, they could become toxic to plants (Nagajyoti et al. 2010). The concentrations of mineral elements in plant tissues vary widely, depending on the species, soil characteristics, and climate (Smith 1962; Han et al. 2011). The usual Mn concentration found in terrestrial plants varies from 5 to 2000 mg kg−1 of dry plant (Sheoran et al. 2010). The total concentration of Mn in plants grown in the fertilized tailings was 2217.83 mg kg−1 for B. virgilioides and 1858.13 mg kg−1 for D. vandellianum, values close to the usual range. Indeed, the Mn concentration in the shoots, 899.52 mg kg−1 for B. virgilioides and 769 mg kg−1 for D. vandellianum, is below the critical toxicity concentrations of Mn in the shoots of other species such as sweet potato (1380 mg kg−1), sunflower (5300 mg kg−1), and Eucalyptus camaldulensis (6510 mg kg−1) (Reichman et al. 2004; Marschner 2011). Moreover, typical visual symptoms of Mn toxicity, such as chlorosis and necrotic brown spotting on leaves (Millaleo et al. 2010; Reichman 2002), were not observed in the plants of this study. Manganese toxicity is accompanied by induced deficiencies of other nutrients such as Ca, Mg, Fe, and Zn and the concentration of these elements in the species of this study was above or within the levels for adequate growth (3–10, 1–3, 100, and 0.01–05 g kg−1 for Ca, Mg, Fe, and Zn, respectively) (Ronquim 2010; Marschner 2011). Enhanced formation of auxiliary shoots (“witches’ broom”) is another symptom of Mn toxicity (Bañados et al. 2009) that was not observed in the plants. Therefore, it is most likely that Mn did not cause phytotoxicity in the studied species.
In contrast, excess of Fe on plant tissues could explain the decreased growth of plants grown on fertilized tailings in relation to those grown in fertilized soil. Generally, the critical iron toxicity concentrations are above 500 mg Fe kg−1 dry weight (Marschner 2011). Both species accumulate Fe in the shoots (820.98 mg kg−1 for B. virgilioides and 2797.17 mg kg−1 for D. vandellianum) in phytotoxic concentrations (Pugh et al. 2002; Kuki et al. 2008; Santana et al. 2014). Zago et al. (2019) also observed high concentrations of iron (1200–1400 mg kg−1) in plants grown in the tailings from the rupture of the Fundão dam. There is no study about the limit of Fe toxicity for the B. virgilioides and D. vandellianum; however, both species are found in an iron-saturated environment (Versieux et al. 2011; Teixeira and Filho 2013; Guimarães et al. 2019). Nevertheless, the species in this study had higher iron concentration in the shoots (820.98 mg kg−1 and 2797.17 mg kg−1) than found by Schettini et al. (2018) in leaves of 27 native plants from ferruginous ecosystems (90.6 to 696.5 mg kg −1).
Iron has a central role in redox reactions (Guerinot and Yi 1994). It is found in components of proteins and catalyzes redox reactions in several fundamental processes such as photosynthesis, respiration, nitrogen metabolism, hormone biosynthesis, and defense against pathogens (Hänsch and Mendel 2009; Nagajyoti et al. 2010). It is necessary for chloroplast development and chlorophyll biosynthesis and is part of several complexes in the electron transport chain of photosynthesis (Hell and Stephan 2003; Nagajyoti et al. 2010). However, iron excess can cause the reduction in the accumulation of dry matter and lower plant height during the vegetative stages (Becker and Asch 2005; Li et al. 2012; Casierra-Posada et al. 2014). Also, Fe excess can increase the production and accumulation of reactive oxygen species (ROS), causing oxidative stress and consequent cellular damage (Kuki et al. 2008; Neves et al. 2009; Jucoski et al. 2013). Once accumulated, ROS can react with chlorophylls leading to their degradation (Gomes et al. 2016), and similarly to D. vandellianum plants grown in the fertilized tailings, several studies have observed that Fe excess decreased the chlorophyll content in plants (Xing et al. 2010; Pereira et al. 2013; Xu et al. 2015). Iron concentrations above the adequate physiological level negatively affect photosynthesis, decreasing the electron transport rate, plant net photosynthetic rate, and FV/FM, which culminate in reduction of the plant dry mass production (Nenova 2006; Neves et al. 2009; Pereira et al. 2013; Xu et al. 2015). We, however, did not observe significant differences between treatments for FV/FM in both species. Similarly, FV/FM was not affected in maize, millet, and sorghum plants grown in the Fundão tailings, although Fe concentrations had exceeded its limit toxicity in these agronomic species (Esteves et al. 2020a, b). Although FV/FM is normally useful to attest stress condition to plants (Baker and Rosenqvist 2004), it is important to note that it does not reflect the performance at which the PSII is operating under ambient light (Baker 2008). Therefore, more sensitive chlorophyll a fluorescence parameters such as effective PSII quantum yield and electron transport rate must be used when assessing the impact of high Fe concentration on the leaves of plants grown in the tailings.
The leaf area ratio (LAR) was higher in the fertilized soil treatment for B. virgilioides and higher in the fertilized tailings for D. vandellianum. LAR expresses the ratio between the area of leaf and the total plant biomass and reflects the leafiness of a plant or amount of leaf area formed per unit of biomass. As for LAR value, B. virgilioides plants grown in fertilized soil showed bigger leaf area showing their great investment in shoot biomass. On the other hand, for D. vandellianum, although the total leaf area of the plant was also higher in plants grown in fertilized soil, the value of LAR as well as SLA was higher in plants grown in the fertilized tailings, which indicate the production of thinner leaves in plants growing in tailings. The specific leaf area (SLA) measures the ratio of leaf area to leaf dry mass of plants, i.e., light-capture area deployed per unit leaf mass. Thinner leaves (high SLA) have been observed in susceptible cultivars of rice submitted to iron toxic levels (Audebert and Sahrawat 2000), which corroborate our hypothesis of Fe toxicity in plants grown in tailings.
Bowdichia virgilioides had higher root/shoot ratio in the fertilized tailings, indicating a higher biomass allocation to roots. The preferential biomass allocation to roots in B. virgilioides plants grown in fertilized tailings also corroborates to the smaller leaf area observed in these plants and consequently, less light interception and less growth. Under conditions of high iron levels, plants allocate proportionally more biomass in the root than in the shoot to tolerate the stressor (Casierra-Posada et al. 2014). The greater allocation of biomass in the root occurs because they become thicker in metal excess, which represents a great metal chelation surface and restrain of their transport to shoots (Gomes et al. 2011). However, this process of root thickening caused less absorption of magnesium by the roots grown in the iron tailing, considering that the root thickening process does not guarantee greater root absorption, which is done by the thin roots (Gomes et al. 2011). In the case of D. vandellianum, this species also had a lower Mg concentration in the shoots of the plants grown in the fertilized tailings, which explains the lower chlorophyll concentration (Guo et al. 2016). Magnesium acts as a cofactor for several enzymes and in the stabilization of nucleotides and nucleic acids, besides being the central element in the chlorophyll molecule (Maathuis 2009).
D. vandellianum transferred large amounts of Fe to the leaves as evidenced by the iron translocation factor. Translocation factor indicates the efficiency of plants to transfer metals from root to shoots. However, for both species, the iron TF (regardless of the substrate where plant grown) is considered low (< 1; Nouri et al. 2011). Bowdichia virgilioides may have mechanisms that avoid the translocation of Fe to shoots with the aim of reducing the impairment of their physiological performance. In several species, the root system accumulated higher Fe concentrations in relation to shoots (Becker and Asch 2005; Nouri et al. 2009; de Araújo et al. 2014; Santana et al. 2014; Cruz et al. 2020). A greater transfer of calcium and consequent greater Ca accumulation in the shoot were observed in D. vandellianum plants grown in the fertilized tailings. This can evidence that Ca is involved in alleviation mechanism of iron-induced toxicity to plants. It has been suggested that calcium participates in mechanisms to decrease toxicity caused by arsenic (Rahman et al. 2015), cadmium (Ahmad et al. 2015), nickel (Mozafari 2013), and zinc (Gai et al. 2017). Possible Ca mechanisms to protect plants against metal stress are as follows: controlling membrane permeability and movements of divalent cations across cell membrane, scavenging reactive oxygen species, promoting antioxidant system, competing for transporter sites, as well as participating in Ca-dependent signaling transduction under metal stress (Hirschi 2004; Farzadfar et al. 2013; Huang et al. 2017).
In summary, even assuring the adequate levels of important nutrients to plant growth (N, P, K, Ca, and Mg), the fertilization did not reverse the negative effect of tailing on B. virgilioides and D. vandellianum. We emphasize that fertilization techniques in areas affected by iron ore tailings must consider both the needs and tolerances of the species and the level of nutrients already present in the tailings. In addition, it is important to consider the age of the plants; here, recently germinated plants were exposed to the tailings; however, older plants might respond differently. For now, the high concentration of Fe in the tissues associated with less biomass production, lower plant height, smaller leaf area, bigger specific leaf area, and reduced chlorophyll content indicates a probable phytotoxic effect of iron present in the tailings for D. vandellianum. We observed, however, less sensitivity of B. virgilioides plants to Fe excess on tailings. The results of this study base further field evaluations and longer experiments, which will facilitate the understanding of the performance of tree species submitted to tailings with fertilization. So far, this study suggests that B. virgilioides are more tolerant to excess Fe from the tailings of Fundão dam than D. vandellianum.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, Tran LSP (2015) Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS One 10:e0114571. https://doi.org/10.1371/journal.pone.0114571
Albuquerque KS, Guimarães RM, Almeida ÍFD, Clemente ADCS (2007) Métodos para a superação da dormência em sementes de sucupira-preta (Bowdichia virgilioides Kunth.). Ciênc Agrotec 31:1716–1721. https://doi.org/10.1590/S1413-70542007000600017
Andrade GF, Paniz AP, Martins AC Jr, Rocha BA, Lobato AKS, Rodrigues JL, Cardoso-Gustavson P, Masuda HP, Batista BL (2018) Agricultural use of Samarco’s spilled mud assessed by rice cultivation: a promising residue use? Chemosphere 193:892–902. https://doi.org/10.1016/j.chemosphere.2017.11.099
Andrino CO, Barbosa-Silva RG, Lovo J, Viana PL, Moro MF, Zappi DC (2020) Iron islands in the Amazon: investigating plant beta diversity of canga outcrops. PhytoKeys 165:1. https://doi.org/10.3897/phytokeys.165.54819
Audebert A, Sahrawat KL (2000) Mechanisms for iron toxicity tolerance in lowland rice. J Plant Nutr 23:1877–1885. https://doi.org/10.1080/01904160009382150
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113. https://doi.org/10.1146/annurev.arplant.59.032607.092759
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621. https://doi.org/10.1093/jxb/erh196
Balestrin D, Martins SV, Schoorl JM, Lopes AT, Andrade CF (2019) Phytosociological study to define restoration measures in a mined area in Minas Gerais, Brazil. Ecol Eng 135:8–16. https://doi.org/10.1016/j.ecoleng.2019.04.023
Bañados MP, Ibáñez F, Toso AM (2009) Manganese toxicity induces abnormal shoot growth in ‘O’Neal’ blueberry. Acta Hortic 810: 509–512. https://doi.org/10.17660/ActaHortic.2009.810.67
Bao T, Sun L, Sun T, Zhang P, Niu Z (2009) Iron-deficiency induces cadmium uptake and accumulation in Solanum nigrum L. Bull Environ Contam Toxicol 82:338–342. https://doi.org/10.1007/s00128-008-9573-8
Beadle L (1985) Plant growth analysis. In: Techniques in bioproductivity and photosynthesis, 2nd edn. Pergamon International Library of Science, Technology, Engineering and Social Studies, pp 20–25
Becker M, Asch F (2005) Iron toxicity in rice - conditions and management concepts. J Plant Nutr Soil Sci 168:558–573. https://doi.org/10.1002/jpln.200520504
Braga JM, Defelipo BV (1974) Determinação espectrofotométrica do fósforo em extrato de solo e plantas. Rev Ceres 21:73–85
Brasil (2009) Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária, Brasília
Butler WL (1978) Energy distribution in the photochemical apparatus of photosynthesis. Annu Rev Plant Physiol 29:345–378. https://doi.org/10.1146/annurev.pp.29.060178.002021
Carmo FF, Kamino LHY, Junior RT, Campos IC, Carmo FF, Silvino G, Castro KJSX, Mauro ML, Rodrigues NUA, Miranda MPS, Pinto CEF (2017) Fundão tailings dam failures: the environment tragedy of the largest technological disaster of Brazilian mining in global context. Perspect Ecol Conser 15:145–151. https://doi.org/10.1016/j.pecon.2017.06.002
Casierra-Posada F, Blanke MM, Guerrero-Guío JC (2014) Iron tolerance in Calla Lilies (Zantedeschia aethiopica). Gesunde Pflanzen 66:63–68. https://doi.org/10.1007/s10343-014-0316-y
Coelho ALN (2009) Bacia hidrográfica do Rio Doce (MG/ES): uma análise socioambiental integrada. Geografares 7
Colmanetti MAA, Barbosa LM, Shirasuna RT, Couto HTZD (2016) Phytosociology and structural characterization of woody regeneration from a reforestation with native species in southeastern Brazil. Rev Árvore 40:209–218. https://doi.org/10.1590/0100-67622016000200003
Cruz FVS, Gomes MP, Bicalho EM, Torre FD, Garcia QS (2020) Does Samarco’s spilled mud impair the growth of native trees of the Atlantic Rainforest? Ecotox Environ Saf 189:110021. https://doi.org/10.1016/j.ecoenv.2019.110021
de Araújo TO, de Freitas-Silva L, Santana BVN, Kuki KN, Pereira EG, Azevedo AA, da Silva LC (2014) Tolerance to iron accumulation and its effects on mineral composition and growth of two grass species. Environ Sci Poll Res 21(4):2777–2784. https://doi.org/10.1007/s11356-013-2201-0
EMBRAPA (2006) Centro nacional de Pesquisa de Solos. Rio de Janeiro, RJ. Sistema brasileiro de classificação de solos 306 Rio de Janeiro 2. Ed
EMBRAPA (2015) Relatório Técnico - Avaliação dos Impactos Causados ao Solo pelo Rompimento de Barragem de Rejeito de Mineração em Mariana, MG: Apoio ao Plano de Recuperação Agropecuária. http://www.consultaesic.cgu.gov.br/busca/dados/Lists/Pedido/Attachments/566237/RESPOSTA_PEDIDO_Relatorio%20Tecnico_Avaliacao%20dos%20Impactos_Acidente%20Mariana_Embrapa.pdf. Accessed 15 July 2020
Erskine PD, Lamb D, Bristow M (2006) Tree species diversity and ecosystem function: can tropical multi-species plantations generate greater productivity? Forest Ecol Manag 233:205–210. https://doi.org/10.1016/j.foreco.2006.05.013
Esteves GF, Bressanin LA, Souza KRD, Silva AB, Mantovani JR, Marques DM, … Souza TC (2020a) Do tailings from the Mariana, MG (Brazil), disaster affect the initial development of millet, maize, and sorghum? Environ Sci Pollut Res 27:38662–38673. https://doi.org/10.1007/s11356-020-10013-w
Esteves GF, Souza KRDS, Bressanin LA, Andrade PCC, Júnior VV, Reis PE, Silva AB, Mantovani JR, Magalhães PC, Pasqual M, Souza TC (2020b) Vermicompost improves maize, millet and sorghum growth in iron mine tailings. J Environ Manag 264:110468. https://doi.org/10.1016/j.jenvman.2020.110468
Farzadfar S, Zarinkamar F, Modarres-Sanavy SAM, Hojati M (2013) Exogenously applied calcium alleviates cadmium toxicity in Matricaria chamomilla L. plants. Environ Sci Pollut Res 20:1413–1422. https://doi.org/10.1007/s11356-012-1181-9
Flavio JJP, Paula RC (2010) Accelerated aging and electrical conductivity tests in Dictyoloma vandelianum A. Juss Seeds Sci for 38:391–399
Filho EMC, Sartorelli AR (2015) Guia de identificação de espécies-chave para restauração florestal na região do Alto Pires, Mato Grosso. The Nature Conservancy, São Paulo
Gai APC, Santos DS, Vieira EA (2017) Effects of zinc excess on antioxidant metabolism, mineral content and initial growth of Handroanthus impetiginosus (Mart. ex DC.) Mattos and Tabebuia roseoalba (Ridl.) Sandwith. Environ Exp Bot 144:88–99. https://doi.org/10.1016/j.envexpbot.2017.09.006
Garcia LC, Ribeiro DB, Roque FO, Ochoa-Quintero JM, Laurance WF (2017) Brazil’s worst mining disaster: Corporations must be compelled to pay the actual environmental costs. Ecol Appl 27:5–9. https://doi.org/10.1002/eap.1461
Gomes MP, Nogueira MDOG, Castro EMD, Soares ÂM (2011) Ecophysiological and anatomical changes due to uptake and accumulation of heavy metal in Brachiaria decumbens. Sci Agric 68:566–573. https://doi.org/10.1590/S0103-90162011000500009
Gomes MP, Le Manac’h SG, Maccario S, Labrecque M, Lucotte M, Juneau P (2016) Differential effects of glyphosate and aminomethylphosphonic acid (AMPA) on photosynthesis and chlorophyll metabolism in willow plants. Pestic Biochem Phys 130:65–70. https://doi.org/10.1016/j.pestbp.2015.11.010
Guerinot ML, Yi Y (1994) Iron: nutritious, noxious, and not readily available. Plant Physiol 104:815. https://doi.org/10.1104/pp.104.3.815
Guimarães AF, Querido LAC, Coelho PA, Santos PF, Santos RM (2019) Unveiling neotropical serpentine flora: a list of Brazilian tree species in an iron saturated environment in Bom Sucesso, Minas Gerais. Acta Sci Biol Sci 41. https://doi.org/10.4025/actascibiolsci.v41i1.44594
Guo W, Nazim H, Liang Z, Yang D (2016) Magnesium deficiency in plants: an urgent problem. Crop J 4:83–91. https://doi.org/10.1016/j.cj.2015.11.003
Han WX, Fang JY, Reich PB, Ian Woodward F, Wang ZH (2011) Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol Lett 14:788–796. https://doi.org/10.1111/j.1461-0248.2011.01641.x
Hänsch R, Mendel RR (2009) Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr Opin Plant Biol 12:259–266. https://doi.org/10.1016/j.pbi.2009.05.006
Hatje V, Pedreira RM, Rezende CE, Schettini CAF, Souza GC, Marin DC, Hackspacher PC (2017) The environmental impacts of one of the largest tailing dam failures worldwide. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-11143-x
Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216:541–551. https://doi.org/10.1007/s00425-002-0920-4
Hirschi KD (2004) The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol 136:2438–2442. https://doi.org/10.1104/pp.104.046490
Huang D, Gong X, Liu Y, Zeng G, Lai C, Bashir H, Zhou L, Wang D, Xu P, Cheng M, Wan J (2017) Effects of calcium at toxic concentrations of cadmium in plants. Planta 245(5):863–873. https://doi.org/10.1007/s00425-017-2664-1
Hunt R (1982) Plant Growth Curves: the Functional Approach to Plant Growth Analysis. Edward Arnold, London
IBAMA (2015) Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis, Ministério do Meio Ambiente. Diretoria de Proteção Ambiental - DIPRO e Coordenação Geral de Emergências Ambientais - CGEMA. Laudo Técnico Preliminar: impactos ambientais decorrentes do desastre envolvendo o rompimento da barragem de Fundão, em Mariana, Minas Gerais, Novembro de 2015. http://www.ibama.gov.br/phocadownload/barragemdefundao/laudos/laudo_tecnico_preliminar_Ibama.pdf. Accessed 15 July 2020
IBRAM (2012) Instituto Brasileiro de Mineração. Information and analysis of the Brazilian mineral economy. IBRAM, 7ed., Brasília
Lima RAF, Pinheiro IG, Aguirre AG, Pinheiro IG (2013) Guia de árvores para a restauração do Oeste da Bahia. Instituto Interamericano de Cooperação para a Agricultura (IICA)
Jacobi CM, Carmo FF, Vincent RC, Stehmann JR (2007) Plant communities on ironstone outcrops: a diverse and endangered Brazilian ecosystem. Biodivers Conserv 16:2185–2200. https://doi.org/10.1007/s10531-007-9156-8
Jucoski GO, Cambraia J, Ribeiro C, Oliveira JA, Paula SO, Oliva MA (2013) Impact of iron toxicity on oxidative metabolism in young Eugenia uniflora L. plants. Acta Physiol Plant 35:1645–1657. https://doi.org/10.1007/s11738-012-1207-4
Kuki KN, Oliva MA, Pereira EG, Costa AC, Cambraia J (2008) Effects of simulated deposition of acid mist and iron ore particulate matter on photosynthesis and the generation of oxidative stress in Schinus terebinthifolius Radii and Sophora tomentosa L. Sci Total Environ 403:207–214. https://doi.org/10.1016/j.scitotenv.2008.05.004
Li X, Ma H, Jia P, Wang J, Jia L, Zhang T, Yang Y, Chen H, Wei X (2012) Responses of seedling growth and antioxidant activity to excess iron and copper in Triticum aestivum L. Ecotox Environ Saf 86:47–53. https://doi.org/10.1016/j.ecoenv.2012.09.010
Lombardi JA, Gonçalves M (2000) Composição florística de dois remanescentes de Mata Atlântica do sudeste de Minas Gerais, Brasil. Braz J Bot 23:255–282. https://doi.org/10.1590/S0100-84042000000300003
Lopes WDP, Silva AFD, Souza ALD, Meira Neto JAA (2002) Estrutura fitossocióloga de um trecho de vegetação arbórea no Parque Estadual do Rio Doce-Minas Gerais, Brasil. Acta Bot Bras 16:443–456. https://doi.org/10.1590/S0102-33062002000400007
Lyra MG (2019) Challenging extractivism: activism over the aftermath of the Fundão disaster. Extract Ind Soc 6:897–905. https://doi.org/10.1016/j.exis.2019.05.010
Massad TJ, Chambers JQ, Rolim SG, Jesus RM, Dyer LA (2011) Restoration of pasture to forest in Brazil’s Mata Atlântica: The roles of herbivory, seedling defenses, and plot design in reforestation. Restor Ecol 19:257–267. https://doi.org/10.1111/j.1526-100X.2010.00683.x
Maathuis FJ (2009) Physiological functions of mineral macronutrients. Curr Opin Plant Biol 12:250–258. https://doi.org/10.1016/j.pbi.2009.04.003
Maciel JML, Carmo FF, Kamino LHY, Moreira LM (2017) Cangas ferruginosas: proposta pedagógica sobre a necessidade de conservação de um ecossistema ameaçado. Revista Brasileira de Pos-Graduação 13: 32. https://doi.org/10.21713/2358-2332.2016.v13.1011
Marschner H (2011) Marschner's mineral nutrition of higher plants. Academic press
Messias MCTB, Tonaco AC, Meira Neto JAA, Leite MGP (2012) Levantamento florístico de um campo rupestre ferruginoso na Serra de Antônio Pereira, Ouro Preto, Minas Gerais. MG Biota, v. 5, p. 4–18. http://www.ief.mg.gov.br/images/stories/mg_biota/2014/mg.biota%20v.5%20n.3.pdf. Accessed 15 March 2021
Millaleo R, Reyes-Díaz M, Ivanov AG, Mora ML, Alberdi M (2010) Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. J Soil Sci Plant Nutr 10:470–481. https://doi.org/10.4067/S0718-95162010000200008
Montagnini F (2001) Strategies for the recovery of degraded ecosystems: experiences from Latin America. Interciencia 26: 498–503. Available in: https://www.redalyc.org/pdf/339/33906113.pdf Accessed 20 July 2020
Mozafari H (2013) Calcium and L-histidine interaction on growth improvement of three tomato cultivars under nickel stress. Acta Biol Szeged 57: 131–144. http://abs.bibl.u-szeged.hu/index.php/abs/article/view/2804. Accessed 20 July 2020
Myers N, Mittermeier RA, Mittermeie CG, Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853. https://doi.org/10.1038/35002501
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216. https://doi.org/10.1007/s10311-010-0297-8
Nazareno AG, Vitule JRS (2016) Too many mining disasters in Brazil. Nature 531:580–580. https://doi.org/10.1038/531580e
Nenova V (2006) Effect of iron supply on growth and photosystem II efficiency of pea plants. Gen Appl Plant Physiol 32:81–90
Neves NR, Oliva MA, Centeno DC, Costa AC, Ribas RF, Pereira EG (2009) Photosynthesis and oxidative stress in the restinga plant species Eugenia uniflora L. exposed to simulated acid rain and iron ore dust deposition: potential use in environmental risk assessment. Sci Total Environ 407:3740–3745. https://doi.org/10.1016/j.scitotenv.2009.02.035
Nouri J, Khorasani N, Lorestani B, Karami M, Hassani AH, Yousefi N (2009) Accumulation of heavy metals in soil and uptake by plant species with phytoremediation potential. Environ Earth Sci 59:315–323. https://doi.org/10.1007/s12665-009-0028-2
Nouri J, Lorestani B, Yousefi N, Khorasani N, Hasani AH, Seif F, Cheraghi M (2011) Phytoremediation potential of native plants grown in the vicinity of Ahangaran lead–zinc mine (Hamedan, Iran). Environ Earth Sci 62:639–644. https://doi.org/10.1007/s12665-010-0553-z
Oliveira LS, Marangon LC, Feliciano ALP, Lima AS, Cardoso MDO, Silva VF (2011) Florística, classificação sucessional e síndromes de dispersão em um remanescente de Floresta Atlântica, Moreno-PE. Revista Brasileira De Ciências Agrárias 6:502–507
Omachi CY, Siani SMO, Chagas FM, Mascagni ML, Cordeiro M, Garcia GD, Thompson CC, Siegle E, Thompson FL (2018) Atlantic Forest loss caused by the world´ s largest tailing dam collapse (Fundão Dam, Mariana, Brazil). Remote Sens Appl Soc Environ 12:30–34. https://doi.org/10.1016/j.rsase.2018.08.003
Pereira EG, Oliva MA, Rosado-Souza L, Mendes GC, Colares DS, Stopato CH, Almeida AM (2013) Iron excess affects rice photosynthesis through stomatal and non-stomatal limitations. Plant Sci 201:81–92. https://doi.org/10.1016/j.plantsci.2012.12.003
Potvin C, Gotelli NJ (2008) Biodiversity enhances individual performance but does not affect survivorship in tropical trees. Ecol Lett 11:217–223. https://doi.org/10.1111/j.1461-0248.2007.01148.x
Pugh RE, Dick DG, Fredeen AL (2002) Heavy metal (Pb, Zn, Cd, Fe, and Cu) contents of plant foliage near the Anvil Range lead/zinc mine, Faro, Yukon Territory. Ecotoxicol Environ Saf 52:273–279. https://doi.org/10.1006/eesa.2002.2201
Queiroz HM, Nóbrega GN, Ferreira TO, Almeida LS, Romero TB, Santaella ST, Bernardino AF, Otero XL (2018) The Samarco mine tailing disaster: a possible time-bomb for heavy metals contamination? Sci Total Environ 637:498–506. https://doi.org/10.1016/j.scitotenv.2018.04.370
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rahman A, Mostofa MG, Alam M, Nahar K, Hasanuzzaman M, Fujita M (2015) Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. Biomed Res Int 2015:340812. https://doi.org/10.1155/2015/340812
Reichman SM (2002) The responses of plants to metal toxicity: a review focusing on copper, manganese & zinc. Australian Minerals & Energy Environment Foundation, Melbourne
Reichman SM, Menzies NW, Asher CJ, Mulligan DR (2004) Seedling responses of four Australian tree species to toxic concentrations of manganese in solution culture. Plant Soil 258:341–350. https://doi.org/10.1023/B:PLSO.0000016564.14512.eb
Renova (2017) Atualização do Plano de Recuperação Ambiental Integrado PRAI. Fundação Renova. https://www.fundacaorenova.org/wp-content/uploads/2017/01/prai_renova_jan17_rev03.pdf. Accessed 20 July 2020
Renova (2020) Relatório anual de atividades Ano 2019 – Janeiro 2020. Fundação Renova. https://www.fundacaorenova.org/wp-content/uploads/2020/02/relatorio-mensal-de-atividades-fevereiro-20-ref-janeiro-20.pdf. Accessed 21 October 2020
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implic Conserv Biol Conserv 142:1141–1153. https://doi.org/10.1016/j.biocon.2009.02.021
Ronquim CC (2010) Conceitos de fertilidade do solo e manejo adequado para as regiões tropicais. Embrapa Territorial-Boletim de Pesquisa e Desenvolvimento (INFOTECA-E)
Samarco (2016) Relatório Bienal 2015–16. https://www.samarco.com/wp406content/uploads/2017/09/Samarco_Relatorio-Bienal-2015_16-08092017.pdf. Accessed 15 July 2020
Santana BVN, Araújo TO, Andrade GC, Freitas-Silva L, Kuki KN, Pereira EG, Azevedo AA, Silva LC (2014) Leaf morphoanatomy of species tolerant to excess iron and evaluation of their phytoextraction potential. Environ Sci Pollut Res 21:2550–2562. https://doi.org/10.1007/s11356-013-2160-5
Santos OSH, Avellar FC, Alves M, Trindade RC, Menezes MB, Ferreira MC, França GS, Cordeiro J, Sobreira FG, Yoshida IM, Moura PM, Baptista MB, Scotti MR (2019) Understanding the environmental impact of a mine dam rupture in Brazil: Prospects for remediation. J Environ Qual 48:439–449. https://doi.org/10.2134/jeq2018.04.0168
Sarruge JR, Haag HP (1974) Análise química de plantas. Escola Superior de Agricultura Luiz de Queiroz, Piracicaba
Schettini AT, Leite MG, Messias MCT, Gauthier A, Li H, Kozovits AR (2018) Exploring Al, Mn and Fe phytoextraction in 27 ferruginous rocky outcrops plant species. Flora 238:175–182. https://doi.org/10.1016/j.flora.2017.05.004
SEDRU (2016) Relatório: Avaliação dos efeitos e desdobramentos do rompimento da Barragem de Fundão em Mariana-MG. Secretaria De Estado De Desenvolvimento Regional Política Urbana e Gestão Metropolitana. http://www.agenciaminas.mg.gov.br/ckeditor_assets/attachments/770/relatorio_final_ft_03_02_2016_15h5min.pdf. Accessed 13 July 2020
Segura FR, Nunes EA, Paniz FP, Paulell ACC, Rodrigues GB, Braga GÚL, Batista BL (2016) Potential risks of the residue from Samarco’s mine dam burst (Bento Rodrigues, Brazil). Environ Pollut 218:813–825. https://doi.org/10.1016/j.envpol.2016.08.005
Shaviv A, Mikkelsen RL (1993) Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation-a review. Fertil Res 35:1–12. https://doi.org/10.1007/BF00750215
Sheoran V, Sheoran AS, Poonia P (2010) Role of hyperaccumulators in phytoextraction of metals from contaminated mining sites: a review. Crit Rev Environ Sci Technol 41:168–214. https://doi.org/10.1080/10643380902718418
Simmons GC (1960) Origin of certain cangas of the "Quadrilátero Ferrífero" of Minas Gerais, Brazil. Boletim da Sociedade Brasileira de Geologia 9:37–59. http://www.ppegeo.igc.usp.br/index.php/BSBG/article/view/12670. Accessed 20 March 2021
Smith PF (1962) Mineral analysis of plant tissues. Annu Rev Plant Physiol 13:81–108. https://doi.org/10.1146/annurev.pp.13.060162.000501
Teixeira WA, Lemos Filho JP (2013) A flórula rupestre do Pico de Itabirito, Minas Gerais, Brasil: lista das plantas vasculares. Boletim de Botânica 31: 199–230. https://doi.org/10.11606/issn.2316-9052.v31i2p199-230
Versieux LM, Medeiros MCMP, Spósito TCS, Stehmann JR (2011) Characterization of the tree component in a semideciduous forest in the Espinhaço Range: a subsidy to conservation. Rev Caatinga 24:85–94
Xing W, Huang W, Liu G (2010) Effect of excess iron and copper on physiology of aquatic plant Spirodela polyrrhiza (L.) Schleid. Environ Tox 25:103–112. https://doi.org/10.1002/tox.20480
Xu S, Lin D, Sun H, Yang X, Zhang X (2015) Excess iron alters the fatty acid composition of chloroplast membrane and decreases the photosynthesis rate: a study in hydroponic pea seedlings. Acta Physiol Plant 37:212. https://doi.org/10.1007/s11738-015-1969-6
Zago VCP, Dores NC, Watts BA (2019) Strategy for phytomanagement in an area affected by iron ore dam rupture: A study case in Minas Gerais State, Brazil. Environ Pollut 249:1029–1037. https://doi.org/10.1016/j.envpol.2019.03.060
Acknowledgements
We thank Dr. Fabio Vieira for helping in the selection of areas and substrate sampling campaigns and Instituto Espinhaço–Biodiversidade, Cultura e Desenvolvimento Socioambiental for providing seeds and logistic support. This research was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES), Finance Code 001, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) through a strategic grant (REDES—Remediation of the Rio Doce Basin: potential of the aquatic and terrestrial biota; grant number 88881.118082/2016-01). Q.S. Garcia and M.P. Gomes received research productivity scholarship from CNPq.
Funding
This research was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES), Finance Code 001, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) through a strategic grant (REDES—Remediation of the Rio Doce Basin: potential of the aquatic and terrestrial biota; grant number 88881.118082/2016–01). Q.S. Garcia and M.P. Gomes received research productivity scholarship from CNPq.
Author information
Authors and Affiliations
Contributions
FVSC performed the study conception, formal analysis, investigation, and writing (original draft). MPG contributed with the conceptualization, supervision, and writing (review and editing). EMB contributed with the investigation and data collection. QSG was responsible for the acquisition of the financial support, conceptualization, and supervision. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cruz, F.V., Gomes, M.P., Bicalho, E.M. et al. Fertilization assures mineral nutrition but does not overcome the effects of Fe accumulation in plants grown in iron ore tailings. Environ Sci Pollut Res 29, 18047–18062 (2022). https://doi.org/10.1007/s11356-021-16989-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16989-3