Abstract
The aim of this work was to assess the suitability of Miscanthus × giganteus and Spartina pectinata link to Cu, Ni, and Zn phytoremediation. A 2-year microplot experiment with the tested grasses growing on metal-contaminated soil was carried out. Microplots with cement borders, measuring 1 × 1 × 1m, were filled with Haplic Luvisols soil. Simulated soil contamination with Cu, Ni, and Zn was introduced in the following doses in mg kg−1: 0—no metals, Cu1—100, Cu2—200, Cu3—400, Ni1—60, Ni2—100, Ni3—240, Zn1—300, Zn2—600, and Zn3—1200. The phytoremediation potential of grasses was evaluated using a tolerance index (TI), bioaccumulation factor (BF), bioconcentration factor (BCF), and translocation factor (TF). S. pectinata showed a higher tolerance to soil contamination with Cu, Ni, and Zn compared to M. × giganteus. S. pectinata was found to have a high suitability for phytostabilization of Zn and lower suitability of Cu and Ni. M. × giganteus had a lower phytostabilization potential than S. pectinata. The suitability of both grasses for Zn phytoextraction depended on the age of the plants. Both grasses were not suitable for Cu and Ni phytoextraction. The research showed that one-season studies were not valuable for fully assessing the phytoremediation potential of perennial plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals entering the soil due to the increasing industrialization are a significant threat to the environment. They can be toxic to humans, animals, and plants and tend to bioaccumulate in the food chain. Cu, Ni, and Zn are especially harmful for the growth and development of plants (Kabata-Pendias and Mukherjee 2007). Their excessive content in the soil can cause abnormalities in the metabolism of plants and lead to a substantial yield reduction. Mining, metallurgy, fuel combustion, and municipal waste are the main source of contamination of soils with these elements. Soils with excessive metal content require remediation. Numerous remediation techniques are known, yet they are labor-intensive and costly. Phytoremediation, site remediation using plants, is a rapidly growing new technique. Its main advantages are the lack of adverse effects on the structure, biological activity, and fertility of soil, and its lower cost compared to other remediation methods (Grzebisz and Gaj 2001; Mulligan et al. 2001; Padmavathiamma and Li 2007). Phytoextraction and phytostabilization are the two most common phytoremediation techniques. The first one involves extracting metals from the soil by plants by incorporating them in their tissues and then removing them from the field together with the harvested yield. Phytostabilization involves using plants to reduce mobility and bioavailability of contaminants, and thus reducing their negative impact on the environment (Karczewska et al. 2013; Korzeniowska et al. 2011; Padmavathiamma and Li 2007). In the process of phytostabilization, the roots of plants mechanically bind together soil particles, control erosion, reduce seepage of water, and stabilize contamination using root exudates (Meers et al. 2007).
The effectiveness of phytoremediation depends on the tolerance of plants to contamination and the ability to accumulate metals in aboveground organs and roots. In most cases, the adaptation to soils contaminated with heavy metals is connected with a lower uptake of metals, the so-called exclusion mechanism (Baker 1981). However, some plants, called hyperaccumulators, absorb large amounts of metals, far exceeding their concentration in the soil. Distinguishing excluders from accumulators is carried out using bioaccumulation factor (BF), defined as the ratio of the metal concentration in the aboveground biomass to the metal concentration in the soil. The plants called accumulators are characterized by BF > 1 and excluders by BF < 1. Tolerance of plants to heavy metals can be estimated using tolerance index TI developed by Wilkins (1978), which is the ratio of biomass in treatment to the control biomass.
Phytoextraction involves plants which accumulate large amounts of metals in aboveground biomass. Phytostabilization is carried out with the use of plants which accumulate metals in the roots and, at the same time, poorly translocate them from roots to aboveground organs. The ability of plants to extract or stabilize metals in the soil can be determined using three parameters: BF (as above), BCF (bioconcentration factor), and TF (translocation factor). BCF is defined as the ratio of metal concentration in plant roots to the metal in the soil, while TF as the ratio of the metal in aboveground organs to the metal in roots (Sabeen et al. 2013; Stanislawska-Glubiak et al. 2015; Yoon et al. 2006).
Plants with a high bioaccumulation factor (BF > 1) and sufficiently high biomass yield are suitable for phytoextraction (Cheraghi et al. 2011; McGrath and Zhao 2003), while plants with a high bioconcntration factor (BCF > 1) and, at the same time, with a low translocation factor (TF < 1) are suitable for phytostabilization (Cheraghi et al. 2011; Malik et al. 2010; Roccotiello et al. 2010).
Using energy grasses for the phytoremediation could be a profitable solution. The cultivation of these plants on polluted areas could serve both for the remediation and for the production of biomass. Hence, it is important to identify the tolerance of the most common energy grasses to the excess of heavy metals in the soil and to investigate the transfer of metals from the roots to the aboveground organs. Among the grasses, the species such as Miscanthus and Spartina are considered the most promising for renewable energy and phytoremediation purposes (Li et al. 2014; Nsanganwimana et al. 2014; Redondo-Gómez 2013). Although numerous studies were conducted on grasses, most of these works dealt with soil contamination with several elements simultaneously, without comparing the tolerance of grasses to individual metals (Cambrolle et al. 2011; Curado et al. 2014; Ho et al. 2013; Nalla et al. 2012; Weiss et al. 2006). Moreover, the studies were usually carried out with the use of solution culture or pots, where only seedlings or young plants were examined in the first growing season (Li et al. 2014; Mateos-Naranjo et al. 2008, 2011; Nalla et al. 2012; Wanat et al. 2013; Yang et al. 1997). It greatly reduced the possibility of finding out the tolerance of grasses to heavy metals under real soil contamination.
The aim of the study was to compare the tolerance of Miscanthus × giganteus and Spartina pectinata link to the toxicity of Cu, Ni, and Zn and to assess the usefulness of these plants to phytoextraction and phytostabilization in the setting similar to field conditions.
Methodology
Microplot experiment
In the years of 2009–2010, a microplot experiment with two grasses M. × giganteus and S. pectinata was carried out. M. × giganteus is a large grass hybrid of Miscanthus sinensis and Miscanthus sacchariflorus, native to Asia. S. pectinata, known by the common names prairie cordgrass, is native to central North America. Both grasses are perennial plants belonging to Poaceae family and characterized by low nutrient requirements. They are C4 plants, thus exhibit great photosynthetic efficiency and high potential for biomass production. The grasses are used as energy crops for heat and electricity generation.
The experiment was performed using four replications at the Experimental Station Baborowko near Poznan (mid-west Poland). Concrete microplots measuring 1 × 1 × 1 m were filled with Haplic Luvisols soil (the most common type of soil in Poland) from the fields, preserving its natural layering. The characteristics of the used soil are presented in Table 1. The microplots were artificially contaminated with Cu, Ni, and Zn for 3 years before planting grasses in order to obtain level of contamination as stable as possible. It is known that fresh, initially quite high contamination decreases rapidly as a result of metal binding by the various components in the soil (Kabata-Pendias and Pendias 1999).
Soil contamination was achieved according to the following design: 0—the control (no metals), Cu1—100, Cu2—200, Cu3—400, Ni1—60, Ni2—100, Ni3—240, Zn1—300, Zn2—600, and Zn3—1200 mg kg−1. It used 80 microplots at total (2 grasses × 10 treatments × 4 replicates). Selection of the doses was based on our previous studies (Korzeniowska et al. 2011; Stanisławska-Glubiak and Korzeniowska 2014). Metals in the form of sulfates were dissolved in water and applied to microplots down to the depth of 30 cm with hand liquid spreaders. In order to achieve uniform distribution of metals in the soil half of the dose was applied to 15–30 cm layer and mixed and then remaining half was applied to 0–15 cm layer and mixed.
The seedlings of M. × giganteus and S. pectinata were planted in early May 2009, initially in a twofold higher density. After 7 weeks, the plants were thinned, leaving two plants of M. × giganteus and five plants of S. pectinata per plot. In both years of the studies, basic NPK fertilization was applied at the dose 10:2:8 g per plot, respectively. The plants in microplots were weeded manually and watered with deionized water in the periods of insufficient amounts of rainfall.
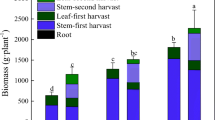
The aboveground part of grasses, leaves, and stems were harvested in the heading stage—one cut in the first year (October 2009) and two cuts in the second year (June and October 2010). At the second cut in year 2010, the belowground organs (rhizomes and roots) were also collected. Plants were dug out, carefully washed with distilled water and separated into above- and belowground biomass. Both parts were weighed having previously dried them in a dryer at 60 °C. Afterwards, they were ground to fine dust and samples for chemical analyses were taken.
In both years, soil samples were taken to determine the concentration of the metals in the soil after harvesting the plants in autumn.
Calculation of tolerance index and metal accumulation parameters
In order to compare the tolerance of M. × giganteus and S. pectinata to the excess of the studied elements, their tolerance index (TI) (resistance to contamination) was calculated. The indices were calculated as the ratio of biomass yield in metal treatment to biomass in control treatment according to the Wilkins (1978) formula in authors’ modification:
In order to compare the accumulation and translocation metals from roots to aboveground organs of grasses, three parameters were calculated: bioaccumulation factor (BF), bioconcentration factor (BCF), and translocation factor (TF), which are expressed by the following formulas (Malik et al. 2010):
Chemical analyses
All the chemical analyses were performed in the Central Laboratory of the Institute of Soil Science and Plant Cultivation in Pulawy, certified by the Polish Centre of Accreditation (certificate no. AB 339) according to PN-EN ISO/IEC 175 17025.
Total organic carbon in soil (TOC) was determined by Tiurin’s method using potassium dichromate (PN-ISO14235: 2003), pH was established potentiometrically in KCl solution (ISO10390: 2005), P and K were determined using Enger-Riehm method (PN-R-04023:1996 and PN-R-04022:1996 adequately), Mg by the Schachtschabel method (PN-R-04020:1994), and texture was evaluated by the aerometric method (PN-R-04033: 1998). Total concentration of Cu, Ni, and Zn in soil was determined by FAAS after mineralization in aqua regia. Heavy metals in plant samples were determined by the FAAS method, after prior dry ashing the material in a muffle furnace at 500 °C and digesting it with 20 % nitric acid (PN-R-04014: 1991). The accuracy of the procedure for metals was estimated by analyzing the two certified reference materials: IPE 952 grass and RTC-CRM026-50G soil.
Statistical analyses
The results for grass biomass and metal concentration were given as means from four replications. For biomass, one-way ANOVAs were conducted. The evaluation of the significance of the data between the groups of the tested parameters was done using Tukey’s test (P < 0.05). Data were first tested for normality with the Kolomogorov-Smirnov test. Calculation of the standard errors (SE), normality, and ANOVA were performed with the Statgraphics v 5.0 software.
Results
The concentration of metals in the soil
The concentration of metals in the soil increased together with increasing their doses (Table 2). In the treatments with the highest doses, the highest increase in the concentration was recorded for copper (50–65-fold), next for nickel (30–42-fold), and the lowest for zinc (16–19-fold) compared to the control.
The concentration of Cu in the soil in the Cu3 treatment exceeded 4-fold the Polish standards for soil contamination with metals, while the concentrations of Zn and Ni in the analogous Zn3 and Ni3 treatments were only 2.5-fold higher than their maximum admissible concentrations in the soil (Regulation of the Minister of Environment 2002).
Biomass production
The biomass of the aboveground organs of M. × giganteus and S. pectinata decreased systematically due to the soil contamination with metals in the both years of the study (Table 3). In the case of S. pectinata, the decrease in yields in the second year was much lower compared to the first year. In 2009, the biomass of S. pectinata in Cu3 treatment decreased by 75 % compared to the control, while in 2010—only by 45 % (Table 3). In the case of M. × giganteus, it was, respectively, by 91 and 82 % lower. Similarly, in the Zn3 treatment, the biomass of S. pectinata decreased in 2009 by 73 %, and in 2010, only by 50 %. M. × giganteus reacted, respectively, with 95 and 97 % yield decrease.
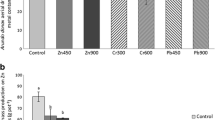
In the both years of the research, the decrease in biomass of aboveground organs of S. pectinata was lower compared to M. × giganteus (Table 3). Averagely for 2 years, a systematic increase in the concentration of Cu caused a 24–60 % decrease of S. pectinata biomass and a 5–87 % decrease of M. × giganteus biomass (Fig. 1). Increasing nickel doses caused the decrease of S. pectinata biomass by 6–75 % and of M. × giganteus by 36–94 %. Increasing soil contamination with Zn caused the decrease of S. pectinata by 27–61 % and of M. × giganteus by 33–94 %. Similarly as in the case of aboveground organs, the highest dose of metals caused a much lower biomass decrease of S. pectinata in comparison with M. × giganteus (Table 3).
For both studied grasses, the decrease of the biomass of belowground organs was lower compared to the aboveground organs. The highest metal dose caused 1.3–1.5-fold higher depression of aboveground organs compared to the belowground organs for S. pectinata and 1.3–2.0-fold higher for M. × giganteus (Table 3).
Tolerance index
Both grasses showed a higher tolerance to the metals in the second growing season compared to the first, which is evidenced by higher values of TIs for aboveground organs in 2010 than in 2009 (Table 4), especially in the case of copper. TI of Cu in the second season was by about 33 % higher for S. pectinata and by 61 % higher for M. × giganteus in comparison to the first growing season.
S. pectinata was characterized by a higher tolerance index to all the three tested metals than M. × giganteus. For the 2 years of research, the average TI for aboveground organs of S. pectinata to copper was higher by approximately 20 %, for nickel by 90 %, and for zinc by up to 130 % compared to M. × giganteus (Table 4). A higher TI for S. pectinata compared to M. × giganteus was also obtained in the case of belowground organs collected in 2010—for copper by approximately 20 %, nickel by 50 %, and zinc by 70 %.
Metal concentration in plants
The concentrations of metals in the aboveground organs of grasses differed in the first and second growing season (Table 5). In 2009, M. × giganteus was characterized by an approximately 1.5–2 higher concentration of all three metals compared to S. pectinata. In 2010, there was a significant decrease in the concentrations of all three metals in M. × giganteus and a slight increase in the concentrations of Cu and Zn in S. pectinata compared to the previous year. As a result, unlike in 2009, S. pectinata accumulated 2–4-fold more Cu and Zn than M. × giganteus, while the Ni concentration was similar in both grasses.
Both tested grasses accumulated more metals in their belowground organs compared to aboveground organs. Greater differences between these organs were recorded for Cu and Ni compared to Zn. On average, in metal treatments, S. pectinata and M. × giganteus accumulated, respectively, by 4- and 7-fold more copper, 9- and 5-fold more nickel, and 1.5-fold more zinc in the belowground organs compared to the aboveground organs.
In both years, the concentration of metals in the aboveground organs of both grasses increased together with increasing soil contamination, but the increase of Cu concentration was much lower than the concentrations of Ni and Zn (Table 5). For example, in 2010, the highest doses of metals caused approximately a 2-fold increase in the concentration of copper, 15–25-fold increase in nickel and 21–29-fold increase in zinc in the aboveground organs of grasses compared to the control. In 2009, the differences between the metals were even higher than in 2010 (Table 5).
While both grasses reacted with a similar increase in the concentration of Cu, Ni, and Zn in the aboveground organs to increased metal content in the soil, in the belowground organs, this increase was different (Table 5). The increase of the concentrations of Ni and Zn in the aboveground organs of M. × giganteus was much lower in comparison with S. pectinata. In the treatments with the highest dose of metals, S. pectinata reacted with 55- and 62-fold, and M. × giganteus with 11- and 5-fold increase in the concentrations of nickel and zinc compared to the control. At the same time, there was only a 5-fold increase of Cu in S. pectinata and a 6-fold increase of this metal in M. × giganteus under the highest doses compared to the control.
The ability of metal accumulation and translocation in plants
BFs and BCFs values presented in Table 6 show that the two studied grasses most easily absorbed and accumulated Zn, then Ni, while the most difficult was Cu. It applied to both aboveground and belowground organs of grasses. BF and BCF values were found to be in the following order: Zn > Ni > Cu. Generally, both parameters increased together with increasing soil contamination.
For both grasses, all values of BF of Cu and Ni were <1 and were at a very low level: 0.01–0.02 for Cu and 0.04–0.19 for Ni. BCF values were not only slightly higher—0.06–0.08 for Cu and 0.19–0.48 for Ni but also did not exceed a critical value of 1, which proves a very low ability of grasses to accumulate Cu and Ni in both aboveground and belowground organs.
BFs and BCFs >1 was obtained only for Zn. In 2009, BF of Zn >1 was observed for both grasses, but higher for M. × giganteus. In the second year of the studies, Zn bioaccumulation by M. × giganteus dropped significantly, but it increased for S. pectinata, in result of which BF > 1 occurred only for S. pectinata. At the same time, the values of BCF of Zn were >1 for both grasses, but they were higher for S. pectinata. The interpretation of the obtained factors for zinc is difficult, as the grasses differed in their reaction to the contamination in the first and second growing season. In the first season, M. × giganteus was a better zinc accumulator in the aboveground organs compared to S. pectinata. In the second growing season, it showed a very low ability to accumulate Zn in the aboveground organs, but it was better in the belowground organs. S. pectinata showed a good Zn accumulation ability in both above- and belowground organs, although in the first growing season, it was much lower compared to M. × giganteus.
For both grasses and three metals, TFs < 1 were obtained, which proves a weak metal translocation from below- to aboveground organs, both with S. pectinata and M. × giganteus (Table 6). Generally, zinc was translocated more effectively in comparison with copper and nickel. There were, however, some differences between the grasses. Higher TFs values of Cu and Zn and lower TFs of Ni were found for S. pectinata than M. × giganteus, which shows a greater ability of S. pectinata to translocate Cu and Zn compared to M. × giganteus, and greater ability of M. × giganteus to translocate Ni from belowground to aboveground organs compared to S. pectinata.
Discussion
Metals tolerance
Both tested grasses showed a higher tolerance to surface soil contamination with metals in the second growing season in relation to the first, which is indicated by their increasing TIs (Table 4). An increase in metals’ tolerance in subsequent growing seasons was also reported by other authors (Kocon and Matyka 2012; Peralta-Videa et al. 2003; Stanislawska-Glubiak et al. 2012).
S. pectinata showed a higher tolerance to Cu, Ni, and Zn in both growing periods than M. × giganteus, despite the fact that in the second season, it accumulated several times more metals both in the aboveground and belowground organs. A higher tolerance of S. pectinata compared to M. × giganteus was clearly indicated by the obtained biomass of grasses and tolerance indices (Tables 3 and 4, Fig. 1).
Among the tested metals, S. pectinata showed a higher tolerance to Zn while M. × giganteus to Cu. The mean tolerance of the grasses to metals from 2 years was in the following order: S. pectinata—Zn > Ni > Cu and M. × giganteus—Cu > Ni > Zn. Although in the second growing season, S. pectinata accumulated significant concentrations of Zn both in aboveground and belowground organs (above 1000 mg kg−1); the TIs of Zn amounted to 77–80 (Table 4). This means only a 20–23 % decrease in the biomass due to the soil contamination with Zn. A high tolerance of S. pectinata sp. to heavy metals was also reported by other authors. Mateos-Naranjo et al. (2008) found that Spartina densiflora was not only capable of tolerating very high and continued exposure to Zn but also demonstrated a high tolerance for other heavy metals such as As, Cu, Mn, Ni, and Pb. Redondo-Gómez (2013) states that a high tolerance to metals by Spartina genus is connected with compartmentation, metal excretion, and chelation mechanisms.
It should be noted that despite a higher soil contamination with copper compared to nickel and zinc (Table 2), M. × giganteus showed a greater tolerance to Cu than the other two metals (Table 4). The opposite results were obtained by Fernando and Oliveira (2004). Their research showed that the tolerance of M. × giganteus was in the following order: Zn > Ni > Cu. These studies, however, were conducted in a single growing season in the pots with very little soil contamination.
Cu and Ni phytoremediation
The usefulness of plants for phytoremediation was assessed on the basis of their tolerance to contamination and BF, BCF, and TF parameters. Both grasses did not show high phytoremediation potential for Cu and Ni. In two consecutive growing seasons, all BFs of Cu and Ni did not exceed 0.2, indicating a complete lack of usefulness of both grasses for phytoextraction (Table 6). Furthermore, both M. × giganteus and S. pectinata were characterized by the values of BCF < 1, with simultaneous values of TF < 1. This proves their partial suitability for phytostabilization. Both species did not, in fact, translocate large amounts of Cu and Ni from the belowground organs of the shoots, but at the same time, they accumulated only small amounts of metals in belowground organs. However, taking into account the relatively high tolerance of S. pectinata to both metals, especially in the second growing season (TI approx. 70), we can conclude that it is to some extent suitable for Cu and Ni phytostabilization.
Zn phytoremediation
The usefulness of the tested grasses for Zn phytoremediation was significantly higher than in the case of Cu and Ni. It should be noted, however, that it was different in the two growing periods, especially for M. × giganteus. Based on the BFs, it can be stated that in the first season, M. × giganteus could be qualified as a plant suitable for Zn phytoextraction (BF of Zn > 1) (Table 6). In the second season, however, the situation radically changed and the BF values of Zn significantly decreased to <1. This excludes the suitability of M. × giganteus to phytoextraction. Also, seasonal changes in the BF for S. pectinata were observed. In the second year of growth, BF of Zn increased, which indicated a much higher suitability of S. pectinata to phytoextraction in the second growing season compared to the first season.
Due to the lack of belowground organs from the first year of the study, it cannot be determined if the suitability of the tested grasses to Zn phytostabilization also changed with time. On the basis of the studies of 2010, S. pectinata can be qualified as a suitable plant for Zn phytostabilization, which was confirmed by the values of BCF of Zn > 1 and TF of Zn < 1. M. × giganteus showed only a partial suitability for phytostabilization. Although the TFs of Zn was lower than 1 and lower than the values found for S. pectinata, BCF of Zn was >1 only at the dose of Zn3 (Table 6).
The literature on the growth of Miscanthus and Spartina genus on the soils contaminated with heavy metals is quite numerous, but it often concerns other species than M. × giganteus and S. pectinata. Moreover, there are no works comparing the phytoremediation potential of both tested species. A lot of authors describe the reaction to heavy metals of such species as M. floridulus (Ho et al. 2013), M. sinensis (Arduini et al. 2006), M. saccharilorus (Li et al. 2014) and S. densiflora (Cambrolle et al. 2011; Mateos-Naranjo et al. 2008, 2011), S. maritima (Curado et al. 2014), and S. alterniflora (Nalla et al. 2012).
Impact of plant age on phytoremediation potential
The study showed changes in the accumulation of metals in the aboveground organs of the grasses and thereby changes in the BFs related to the duration of the experiment. In the second season, M. × giganteus accumulated much less, and S. pectinata more metals than in the first growing season (Table 5). The decrease in the Zn concentration in the shoots of M. × giganteus in consecutive growing seasons was also observed by Pogrzeba et al. (2013) and Kocon and Matyka (2012). Possibly, it was connected with the age and size of plants. It can be assumed that in the second season, the roots of M. × giganteus reached the deeper, uncontaminated soil layers, resulting in a large decrease in metal concentration in the aboveground biomass. In our experiment, metals were introduced into the soil in a layer 0–30 cm, while roots of M. × giganteus can reach a length of 1.5–2.0 m 7 months after planting (Mann et al. 2013).
In contrast, in the second growing season, the most of S. pectinata root biomass remained in the contaminated area, which resulted in an increase of metal accumulation. In the second year after planting, the roots of S. pectinata reach the length of 40 cm (USDA, NRCS 2014).
Soil pollution caused by human activities generally involves surface contamination. The tests carried out under this type of contamination showed that phytoremediation potential of plants may change together with the growth of plants, and therefore, the duration of the experiment. For this reason, the conclusions regarding the suitability of perennial plants for phytoremediation drawn on the basis of short-term studies using solution culture technique or pots may have little practical value.
Conclusions
The 2-year microplot experiments showed that S. pectinata was more tolerant to soil contamination with Cu, Ni, and Zn than M. × giganteus.
Both because of its high tolerance to Zn, as well as ability to accumulate this metal in belowground organs and a small Zn transfer from the belowground to aboveground organs, S. pectinata is very suitable to Zn phytostabilization. M. × giganteus showed a lower Zn phytostabilization potential compared to S. pectinata due to a lower tolerance and a weaker Zn accumulation in the belowground organs.
S. pectinata was found to be suitable for Cu and Ni phytostabilization to some extent. It did not translocate high amounts of Cu and Ni from the belowground organs to the shoot, but at the same time, it accumulated only small amounts of these metals in the belowground organs. However, considering a high tolerance of this grass to both metals, it can be concluded that S. pectinata has some Cu and Ni potential for phytostabilization. The suitability of M. × giganteus to Cu and Ni phytostabilization was found to be lower in comparison with S. pectinata due to its lower tolerance to the soil contaminated with these metals.
The suitability of the studied grasses for phytoextraction of Zn depended on the age of plants. M. × giganteus was found to be suitable for Zn phytoextraction in the first season, but not suitable in the second growing season. The unsuitability of a 2-year plant of M. × giganteus for phytoextraction was related with low metal accumulation in the aboveground organs, which was probably associated with the roots reaching into deeper, uncontaminated soil layers. The suitability of S. pectinata for Zn phytoextraction, though rather small, was higher in the second than in the first growing season.
Both tested grasses were unsuitable for phytoextraction of Cu and Ni due to insufficient accumulation of these metals in aboveground organs.
References
Arduini I, Ercoli L, Mariotti M, Masoni A (2006) Response of Miscanthus to toxic cadmium applications during the period of maximum growth. Environ Exp Bot 55:29–40. doi:10.1016/j.envexpbot.2004.09.009
Baker AJM (1981) Accumulators and excluders strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654
Cambrolle J, Mateos-Naranjo E, Redondo-Gomez S, Luque T, Figueroa ME (2011) The role of two Spartina species in phytostabilization and bioaccumulation of Co, Cr, and Ni in the Tinto–Odiel estuary (SW Spain). Hydrobiologia 671:95–103. doi:10.1007/s10750-011-0706-4
Cheraghi M, Lorestani B, Khorasani N, Yousef N, Karami M (2011) Findings on the phytoextraction and phytostabilization of soils contaminated with heavy metals. Biol Trace Elem Res 144(1–3):1133–1141. doi:10.1007/s12011-009-8359-0
Curado G, Rubio-Casal AE, Figueroa E, Castillo JM (2014) Potential of Spartina maritima in restored salt marshes for phytoremediation of metals in a highly polluted estuary. Int J Phytorem 16:1209–1220. doi:10.1080/15226514.2013.821451
Fernando A, Oliveira JS (2004) Effects on growth, productivity and biomass quality of Miscanthus × giganteus of soils contaminated with heavy metals. 2nd World Conference on Biomass for Energy, Industry and Climate Protection, 10–14 May 2004, Rome, Italy, http://moodle.fct.unl.pt/pluginfile.php/92027/mod_resource/content/0/Fernando_e_Oliveira_2004.pdf
Grzebisz W, Gaj R (2001) Phytoremediation—the agro-technological component of the future strategies of soils contaminated by heavy metals remediation. In: Gworek B, Mocek A (eds) The cycle of elements in nature. Instytut Ochrony Srodowiska, Warszawa, pp 337–344
Ho C-P, Hseu Z-H, Chen N-C, Tsai C-C (2013) Evaluating heavy metal concentration of plants on a serpentine site for phytoremediation applications. Environ Earth Sci 70:191–199. doi:10.1007/s12665-012-2115-z
Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human. Springer Verlag, Heidelberg
Kabata-Pendias A, Pendias H (1999) Biogeochemistry of trace elements. PWN, Warszawa (in Polish)
Karczewska A, Lewinska K, Gałka B (2013) Arsenic extractability and uptake by velvetgrass Holcus lanatus and ryegrass Lolium perenne in variously treated soils polluted by tailing spills. J Hazard Mater 262(15):1014–1102. doi:10.1016/j.jhazmat.2012.09.008
Kocon A, Matyka M (2012) Phytoextractive potential of Miscanthus × giganteus and Sida hermaphrodita growing under moderate contamination of soil with Zn and Pb. J Food Agric Environ 10(2):1253–1256
Korzeniowska J, Stanislawska-Glubiak E, Igras J (2011) Applicability of energy crops for metal phytostabilization of soils moderately contaminated with copper, nickel and zinc. J Food Agric Environ 9(3/4):693–697
Li C, Xiao B, Wang QH, Yao SH, Wu JY (2014) Phytoremediation of Zn- and Cr-contaminated soil using two promising energy grasses. Water Air Soil Pollut 225:2027. doi:10.1007/s11270-014-2027-5
Malik RN, Husain SZ, Nazir I (2010) Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak J Bot 42(1):291–301
Mann JJ, Barney JN, Kyser GB, DiTomaso JM (2013) Root system dynamics of Miscanthus × giganteus and Panicum virgatum in response to rainfed and irrigated conditions in California. Bioenergy Res 6:678–687. doi:10.1007/s12155-012-9287-y
Mateos-Naranjo E, Redondo-Gómez S, Cambrollé J, Luque T, Figueroa ME (2008) Growth and photosynthetic responses to zinc stress of an invasive cordgrass, Spartina densiflora. Plant Biol 10:754–762. doi:10.1111/j.1438-8677.2008.00098.x
Mateos-Naranjo E, Andrades-Moreno L, Redondo-Gomez S (2011) Comparison of germination, growth, photosynthetic responses and metal uptake between three populations of Spartina densiflora under different soil contamination conditions. Ecotoxicol Environ Saf 74:2040–2049. doi:10.1016/j.ecoenv.2011.06.019
McGrath SP, Zhao F-J (2003) Phytoextraction of metals and metalloids from contaminated soils. Curr Opin Biotechnol 14:277–282
Meers E, Vandecasteele B, Ruttens A, Vangronsveld J, Tack FMG (2007) Potential of five willow species (Salix spp.) for phytoextraction of heavy metals. Environ Exp Bot 60:57–68. doi:10.1016/j.envexpbot.2006. 06.008
Mulligan CN, Yong RN, Gibbs BF (2001) Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng Geol 60:193–207
Nalla S, Hardaway CJ, Sneddon J (2012) Phytoextraction of selected metals by the first and second growth seasons of Spartina alterniflora. Instrum Sci Technol 40:17–28. doi:10.1080/10739149.2011.633143
Nsanganwimana F, Pourrut B, Mench M, Douay F (2014) Suitability of Miscanthus species for managing inorganic and organic contaminated land and restoring ecosystem services. A review. J Environ Manag 143:123–134. doi:10.1016/j.jenvman.2014.04.027
Padmavathiamma PK, Li LY (2007) Phytoremediation technology: hyper-accumulation metals in plants. Water Air Soil Pollut 184:105–126. doi:10.1007/s11270-007-9401-5
Peralta-Videa JR, de la Rosaa G, Gonzaleza JH, Gardea-Torresdey JL (2003) Effects of the growth stage on the heavy metal tolerance of alfalfa plants. Adv Environ Res 8:679–685. doi:10.1016/S1093-0191(03)00040-6
Pogrzeba M, Krzyzak J, Sas-Nowosielska A (2013) Environmental hazards related to Miscanthus × giganteus cultivation on heavy metal contaminated soil. In: E3S Web Conference. doi:10.1051/e3sconf/20130129006
Redondo-Gómez S (2013) Bioaccumulation of heavy metals in Spartina. Funct Plant Biol 40:913–921. doi:10.1071/FP12271
Regulation of the Minister of Environment of 9 September 2002 on soil and earth quality standards (2002) Journal of Laws of the Republic of Poland No 165, Item 1359
Roccotiello E, Manfredi A, Drava G, Minganti V, Mariotti M, Berta G, Cornara L (2010) Zinc tolerance and accumulation in the ferns Polypodium cambricum L. and Pteris vittata L. Ecotoxicol Environ Saf 73:1264–1271. doi:10.1016/j.ecoenv.2010.07.019
Sabeen M, Mahmood Q, Irshad M, Fareed I, Khan A, Ullah F, Hussain J, Hayat Y, Tabassum S (2013) Cadmium phytoremediation by Arundo donax L. from contaminated soil and water. BioMed Res Int. doi:10.1155/2013/324830
Stanisławska-Glubiak E, Korzeniowska J (2014) Phytotoxic thresholds for Zn in soil extracted with 1 M HCl. J Food Agric Environ 12(1):146–149
Stanislawska-Glubiak E, Korzeniowska J, Kocon A (2012) Effect of the reclamation of heavy metal-contaminated soil on growth of energy willow. Pol J Environ Stud 21(1):187–192
Stanislawska-Glubiak E, Korzeniowska J, Kocon A (2015) Effect of peat on the accumulation and translocation of heavy metals by maize grown in contaminated soils. Environ Sci Pollut Res 22:4706–4714. doi:10.1007/s11356-014-3706-x
USDA, NRCS (2014) The PLANTS Database. National Plant Data Team, Greensboro, NC 27401–4901 USA. http://plants.usda.gov. Accessed 30 Oct 2014
Wanat N, Austruy A, Joussein E, Soubrand M, Hitmi A, Gauthier-Moussard C, Lenain JF, Vernay P, Munch JC, Pichon M (2013) Potentials of Miscanthus × giganteus grown on highly contaminated technosols. J Geochem Explor 126–127:78–84. doi:10.1016/j.gexplo.2013.01.001
Weiss J, Hondzo M, Biesboer D, Semmens M (2006) Laboratory study of heavy metal phytoremediation by three wetland macrophytes. Int J Phytoremediation 8:245–259. doi:10.1080/15226510600846798
Wilkins DA (1978) The measurement of tolerance to edaphic factors by means of root growth. New Phytol 80:623–633
Yang XE, Baligar VC, Foster JC, Martens DC (1997) Accumulation and transport of nickel in relation to organic acids in ryegrass and maize grown with different nickel levels. Plant Soil 196:271–276
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368:456–464. doi:10.1016/j.scitotenv.2006.01.016
Acknowledgments
The work has been prepared as part of Task 2.6, implemented in the Long-Term Program of Institute of Soil Science and Plant Cultivation-State Research Institute, funded by the Polish Ministry of Agriculture and Rural Development
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Korzeniowska, J., Stanislawska-Glubiak, E. Phytoremediation potential of Miscanthus × giganteus and Spartina pectinata in soil contaminated with heavy metals. Environ Sci Pollut Res 22, 11648–11657 (2015). https://doi.org/10.1007/s11356-015-4439-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4439-1