Abstract
Oleaginous microalgae and yeast are the two major propitious factories which are sustainable sources for biodiesel production, as they can accumulate high quantities of lipids inside their bodies. To date, various microalgal and yeast species have been exploited singly for biodiesel production. However, despite the ongoing efforts, their low lipid productivity and the high cost of cultivation are still the major bottlenecks hindering their large-scale deployment. Co-culturing of microalgae and yeast has the potential to increase the overall lipid productivity by minimizing its production cost as both these organisms can utilize each other’s by-products. Microalgae act as an O2 generator for yeast while consuming the CO2 and organic acids released by the yeast cells. Further, yeast can break complex sugars in the medium, which can then be utilized by microalgae thereby opening new options for copious and low-cost feedstocks such as agricultural residues. The current review provides a historical and technical overview of the existing studies on co-culturing of yeast and microalgae and elucidates the crucial factors that affect the symbiotic relationship between these two organisms. Furthermore, the review also highlighted the advantages and the future perspectives for paving a path towards a sustainable biodiesel product.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Insatiable appetite for industrialization and urbanization by mankind has led to an increase in global demand for transportation fuels, which poses a threat to fossil fuel reserves and environmental and economic security of the world. Currently, fossil fuels fulfill 80% of the world’s primary energy requirements in which 58% is consumed by the transportation sector (Mardhiah et al. 2017). Renewable energy derived from sustainable feedstocks can reduce the load on the fossil fuels and curb the greenhouse gas (GHG) emissions. To this end, biofuels specifically biodiesel production have increased (2.8 billion gallons in 2016) due to its renewability, reduced carbon emissions, unburned hydrocarbons, and particulate emissions than petro diesel engines (Thliveros et al. 2014; http://biodiesel.org/). Biodiesel is a mixture of fatty acid methyl esters (FAMEs) derived from plant oils, animal fats, and waste cooking oils. However, among these sources especially plant oil-derived biodiesel cannot be sustainable, as edible oils compete with the food consumption (Rulli et al. 2016). Further, non-edible oils and waste cooking oils have high amount of free fatty acids (FFA), which are undesirable for biodiesel production and also demand large areas of land reserves and water resources, thus competing with food crops (Pourzolfaghar et al. 2016). Animal fats are cheap alternatives but result in biodiesel with high pour point, viscosity, and flash point (Gürü et al. 2009).
Microbial oils have emerged as an attractive alternative for lipid production/biodiesel generation as oleaginous (lipid producing) microorganism such as bacteria, fungi, yeast, and algae can accumulate up to 60–70% lipids of their dry cell weight in response to metabolic stress (Thliveros et al. 2014). Although some of the bacterial strains accumulate high lipid content (Arthrobacter sp., Acinetobacter calcoaceticus, Bacillus alcalophilus, Gordonia sp., Rhodococcus opacus), these lipids majorly comprise of phospholipids (30–60%) and galactolipids as opposed to triacylglycerols (TAGs), which is the major feedstock for biodiesel (Feofilova et al. 2010). A TAG basically comprises three fatty acids attached to glycerol backbone. These fatty acids can be classified as saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs), based on the number of double bonds, along the acyl chain (Knothe 2008). SFAs contain no double bond, whereas MUFA and PUFA contain single and more than two double bonds, respectively. Similarly, filamentous fungi, on the other hand, accumulate high lipid content intracellularly; however, they produce specific lipids such as docosahexaenoic acid, ɤ linolenic acid, and eicosapentaenoic acid as compared to TAGs. These specific lipids indeed increase the viscosity of the growth medium making the oxygen transfer difficult, thus limiting the total biomass/lipid content (Azocar et al. 2010; Meeuwse et al. 2013). Although bacterial strains and fungi are capable of accumulating high lipid contents under defined conditions, due to their inherent lipid characteristics, these organisms have a very limited scope in sustainable biodiesel production. On the other hand, oleaginous yeasts belonging to Yarrowia, Candida, Rhodotorula, Rhodosporidium, Cryptococcus, Trichosporon, and Lipomyces genera have been reported to accumulate discrete bodies (40–70%) of neutral lipids (mainly as triacylglycerol) intracellularly (Table 1) (Ageitos et al. 2011; Meng et al. 2009; Patel et al. 2016; Zhao et al. 2014). These yeasts have an inherit capability to survive and utilize various cheap and copious carbon sources such as industrial and agricultural wastes making them a promising substrate for biodiesel production (Patel et al. 2016). The major fatty acids present in these oleaginous yeasts are myristic acid (C14:0), palmitic acid (C16:0), palmitoleic acid (C16:1), oleic acid (C18:1), and linoleic acid (C18:2), respectively (Beopoulos et al. 2011; Meng et al. 2009). However, the fatty acid composition can vary with culture conditions such as temperature, pH, and carbon source and also with a strain of species (Sitepu et al. 2014). These factors will be discussed in detail in the “Factors affecting the lipid productivity in co-culturing system” section.
The other promising sustainable source of biodiesel is the microalgae. They are unicellular photosynthetic organisms that require water, sunlight, and CO2 for generating biomass. Also, they have shorter generation time, require less land, high photosynthetic ability, and biomass production as compared to energy crops such as rapeseed and soybean (Mubarak et al. 2015). Furthermore, they have a unique ability to adapt to various environments ranging from fresh to marine water and even wastewater along with the mitigation of 1.83 t of CO2 (1-t algal biomass) (Huo et al. 2011). Under adverse conditions such as nutrient limitation, high or low temperature, high concentrations of heavy metals, pH, and light intensity, microalgae can accumulate up to 40–60% of lipids (dry cell weight) making them as desirable feedstocks for biodiesel production (Table 1) (Sharma et al. 2012). These stored lipids or TAGs contain fatty acids ranging from C12 to C24 that are identical to plant oils (jatropha, palm, and soya).
Despite the advantages associated with microbial oils, the major impediment in their large-scale production is the low lipid productivity and high production cost (Meng et al. 2009). Oleaginous microalgae and yeast increase their lipid content in response to environmental stresses (physiological or chemical). However, researchers observed that the enhanced lipid content is accompanied with reduced growth rate leading to diminishing of overall low lipid productivity (Sharma et al. 2012). Various strategies have been deployed to address the low lipid productivity in these microorganisms such as two-stage cultivation (generate sufficient biomass and then induce stress), metabolic/genetic engineering (overexpression/under expression of regulatory genes), and co-culturing (symbiosis) (De Bhowmick et al. 2015; Dias et al. 2015; Ghosh et al. 2016; Levering et al. 2015; Singh et al. 2016). These strategies have resulted in increasing the lipid productivity in both microalgae and yeast cultures (Levering et al. 2015, Singh et al. 2016).
Among these techniques, co-culturing oleaginous yeast and microalgae have been studied extensively in the recent years for enhancing lipid productivity by utilizing minimal resources. Co-culturing is similar to mixed cultures in terms of cultivating two or more species together in the same medium, where the organisms can mutually exploit each other’s metabolic pathways (Goers et al. 2014). However, in case of co-cultures, the quality, quantity, and type of organism involved are well defined unlike in mixed cultures (Goers et al. 2014). Indeed, co-cultivation technique has been widely used for various industrial processes such as wastewater treatment, biogas production, soil remediation, and production of cheese, yoghurt, pickles, whisky, etc. as listed in Table 2.
The current review highlights the metabolic links between the lipid biosynthesis pathway of yeast and microalgae. It addresses the historical developments and recent advances in the field of co-culturing oleaginous microalgae and yeast for augmented lipid productivity. Further, it also details the compilation of the co-culture studies on these microorganisms that lead to successful optimization of growth conditions, thus paving a path for economically viable biofuels. Moreover, various key factors affecting the TAG productivity in microalgae and yeast such as strain selection, cultivation media, seed ratio, light intensity and photoperiod, carbon-to-nitrogen ratio, cultivation time, pH agitation speed, and temperature have been discussed in detail. The present review also sheds light on key technological advances applicable in co-culturing strategy and the future innovations that are essential for improving the lipid/biodiesel productivity.
Biosynthetic mechanism of triacylglycerol biosynthesis in oleaginous microalgae and yeast
Oleaginous microorganisms have the ability to synthesize both simple lipids (fatty acids, sterols, and acylglycerols) and complex lipids (glycerophospholipids and glycosphingolipids) (Fahy et al. 2005). Among these, TAG is the feedstock for biodiesel, which comprises of an ester with three fatty chains and glycerol as backbone of the molecule. Under adverse conditions, TAG serves as an energy molecule (carbon storage) and maintains intracellular homoeostasis, membrane structure, and cellular functions aiding cell survival (Zhao et al. 2014). The fatty acid synthesis is achieved via three major lipid synthesis pathways. They include (a) de novo fatty acid synthesis, (b) lipid recycling, and (c) ex novo synthesis (Bellou et al. 2014).
De novo fatty acid synthesis
De novo fatty acid synthesis in microalgae starts in the plastids by conversion of CO2 to glycerate-3-phosphate (GP) and then to pyruvate followed by the formation of acetyl-CoA, which acts a precursor for fatty acid synthesis (Fig. 1a) (Bellou et al. 2014; Lenka et al. 2016). In the case of oleaginous yeasts, under stress conditions (such as nitrogen limitation), the excess carbon source present in the medium is converted into fatty acids by activating nitrogen-scavenging enzymes such as adenosine monophosphate (AMP) deaminase which catalyzes the conversion of AMP to inosine 5′-monophosphate (IMP), freeing ammonia which can be then utilized by the yeast cell for its growth (Fig. 1b) (Evans and Ratledge 1984). This enzyme has been reported to be absent in the case of non-oleaginous yeasts, thus signifying the difference between the two species (Papanikolaou 2012). The decrease in AMP in turn results in the inactivation of isocitrate dehydrogenase (ICDH), hindering the conversion of isocitrate to oxoglutarate in tricarboxylic acid cycle (TCA), and leads to the accumulation of citrate inside the mitochondria (Evans et al. 1983). The citrate is then transported to the cytosol via malate/citrate translocase system where adenine triphosphate (ATP) citrate lyase (ACL) cleaves the citrate yielding acetyl-CoA and oxaloacetate (Fig. 1b) (Ratledge and Wynn 2002; Zhao et al. 2014).
Overview of the biosynthetic mechanism fatty acid production. a Microalgae. b Yeast. c Triacylglycerol synthesis in microalgae and yeast. 3PG, 3-phosphoglyceric acid; ACCase, acetyl-CoA carboxylase; ACP, acyl carrier protein; ACL-ATP, citrate lyase; CoA, coenzyme A; DHAP, dihydroxyacetone phosphate; DAGAT, diacylglycerol acyltransferase; ENR, enoyl-ACP reductase; FAT, fatty acyl-ACP thioesterase; G6P, glucose 6 phosphate; G3PDH, gycerol-3-phosphate dehydrogenase; GPAT, glycerol-3-phosphate acyltransferase; HD, 3-hydroxyacyl-ACP dehydratase; ICDH, isocitrate dehydrogenase; KAS, 3-ketoacyl-ACP synthase; KAR, 3-ketoacyl-ACP reductase; LPAAT, lysophosphatidic acid acyltransferase; LPAT, lysophosphatidylcholine acyltransferase; MAT, malonyl-CoA:ACP transacylase; PDH, pyruvate dehydrogenase complex; TAG, triacylglycerol
After the formation of acetyl-CoA, both microalgae and yeast share common fatty acid synthesis and TAG synthesis (Kennedy pathway) as depicted in Fig. 1c. The formation of malonyl-CoA is the first committing step towards the lipid biosynthesis as it is formed by carboxylation of acetyl-CoA which is catalyzed by acetyl-CoA carboxylase (ACCase) and enters in a series of condensation, reduction, and dehydration reactions (Bellou et al. 2014). Two forms of ACCase (homomeric and heteromeric), depending on the origin of plastids, are present in microalgae. A few microalgae have two genes for ACCase, one located in the plastid (ACC1) and the other in cytosol (ACC2), respectively (Bellou et al. 2014). Malonyl-CoA is then transferred to acyl carrier protein (ACP) with the help of fatty acid synthase (FAS) complex (Dias et al. 2015). This results in the formation of malonyl-ACP which is then converted to 3-keto acyl-ACP synthase followed by the formation of 3-keto butyryl-ACP, 3-hydroxybutyrl-ACP, butyryl-ACP, and finally to 3-keto acyl-ACP (Fig. 1c) (Bellou et al. 2014). This cycle of reactions halts when a carbon length of C16:0 and C18:0 is achieved. After this, the elongation of fatty acids is terminated either by removal of acyl group from ACP by acyl-ACP thioesterase or by acyltransferases in the chloroplast for microalgae (Bellou et al. 2014). However in the case of yeasts, thioesterases that are specific to saturated fatty acids release the fatty acids from the ACP (Probst et al. 2016). Further, in order to generate unsaturated fatty acids, yeast cells utilize ∆9 desaturase for the formation of palmitoleic acid (C16:1) and oleic acid (C18:1 n-9) while ∆12 desaturase to form linoleic acid (C18: 2n-6) and w3-desaturase for linolenic acid (C18: 3n-3), respectively (Probst et al. 2016).
These fatty acids are then transported to the cytosol and then to the endoplasmic reticulum (ER) for further processing and conversion to TAGs (Fig. 1c). In the ER, fatty acids are transferred from ACP to glycerol-3-phosphate (G3P) by acyl-ACP thioesterase, then converted to lysophosphatidic acid (LPA)-phosphatidic acid (PA)-diacylglycerol (DAG), and finally to TAG (Lenka et al. 2016). These reactions are catalyzed by glycerol-3-phosphate acyltransferase (GPAT), lysophosphatidic acid acyl transferase (LPAAT), lysophosphatidic acid acyltransferase (LPAT), and acyl-CoA:DAG acyl transferases (DGAT), respectively (Fig. 1c).
Alternate pathways
Apart from the de novo pathways, microalgae and yeasts have lipid recycling pathway (Fig. 2a). This alternative pathway is also known as phospholipid:diacylglycerol acyl transferase (PDAT) pathway, which aids in the conversion of membrane lipids present on the plastid envelope/ER to TAGs (Zienkiewicz et al. 2016). Phosphatidylcholine (PC) acts as an acyl donor, while sin-1,2,-diacylglycerol accepts the acyl group (Fig. 2a). The reaction is catalyzed by PDAT which channelizes the bilayer fatty acids such as ricinoleic and vernolic acid from the PC onto the TAG pool (Bellou et al. 2014). The lipid bodies that are formed using either of the two pathways are then packed into simple spherical organelles, surrounded by phospholipid monolayer followed by excretion from the ER into the cytosol.
Interestingly, oleaginous yeasts can incorporate free fatty acids, TAGs, sterols, and esters present in the growth media (Fig. 2b). Once in the cell, the free fatty acids are degraded by β-oxidation, generating shorter acyl-CoA and acetyl-CoA, which can then be used for nicotinamide adenine dinucleotide hydrogen/nicotinamide adenine dinucleotide phosphate hydrogen (NADH/NADPH) generation or incorporated as lipid bodies for storage (Probst et al. 2016). This kind of TAG synthesis is termed as ex novo fatty acid biosynthesis. In the case of Yarrowia lipolytica, it secretes extracellular lipase (Lip 2p) which is encoded by LIP2 gene, which catalyzes the synthesis of pre-pro-mature protein with a Lys-Arg (KK) cleavage site and other intracellular lipases such as Lip 7p and Lip 8p. These specific lipases released into the medium specifically cleave oleate (C18:1), caproate (C6:0), and caprate (C10:0) which are then transported inside the yeast cell (Beopoulos et al. 2009).
Variations in TAG composition with physiological and culture conditions
The fatty acid composition of intracellular TAG in microalgae and yeast is variable in terms of length of the carbon chain, the degree of unsaturation/saturation, and the number of double bonds in the chain. These variations influence the final quality of biodiesel produced as will be discussed in detail in the “Assessment of fatty acid profiles and biodiesel properties of lipids obtained under co-culturing technique” section. The composition depends on the species, growth phase, environmental conditions, substrates, and media components (Sitepu et al. 2013). Under optimal conditions, the fatty acid composition in microbial lipids ranges from lauric acid (C12:0) to docosahexaenoic acid (C22:6) with C16:0, C18:0, C18:1, and C18:2 constituting the largest fractions (Subramaniam et al. 2010). The neutral lipid portion typically comprises of ~ 25–45% SFAs, while ~ 50–55% are unsaturated fatty acids (UFAs), i.e., a ratio of 1:2 of SFA/UFA as similar to that of plant oils (such as palm). However, this fatty acid proportion is modulated when microalgae or yeast is cultivated under stress conditions. An increase in temperature results in the accumulation of more saturated fatty acids to polyunsaturated fatty acids, as saturated fatty acids maintain the fluidity of cell membrane (Renaud et al. 2002). Under low temperatures and carbon (organic carbon for yeast and CO2 for microalgae) conditions, there is an apparent increase in PUFA, while low nutrients (especially nitrogen and phosphorous) and alkaline pH cause an increase in SFAs (Juneja et al. 2013). Also, the content of MUFAs especially C18:1 increases under stress conditions as the conversion from C18:0 to C18:1 requires large amounts of NAD(P)H and oxygen, which can contribute to the reduction of reactive oxygen species (ROS) inside the cells and aids in cell survival (Patel et al. 2016). Further, it was reported that when yeast cells are in an exponential phase, C18:2 seems to be the major fatty acid present, while upon reaching the stationary phase, C18:0 and C18:1 are the dominant fatty acids (Sitepu et al. 2013). Indeed, all these details provided here regarding the fatty acid profiles belong to the monocultures of either yeast or microalgae (Juneja et al. 2013; Patel et al. 2016; Sitepu et al. 2013; Subramaniam et al. 2010). In such a scenario, under a co-culturing scheme, depending on the several factors that influence the culturing conditions, the total fatty acid content/individual fatty acid profiles of these microorganisms can substantially modulate. In the following sections, we will discuss the co-cultivation phenomenon of these oleaginous microorganisms and the physico-chemical aspects that influence their growth and hence the total lipid content produced.
Co-culturing of microorganisms: a historical perspective
In natural ecosystems, microorganisms exist in complex and dynamic communities which are beneficial for their survival (Rajendran and Hu 2016). This synergy between microorganisms has been extensively exploited for the production of various valuable industrial products (Table 2). For example, the production of propionic acid was enhanced by co-cultivating propionic acid-producing bacteria (Propionibacterium shermanii) and lactic acid-producing bacteria (Lactobacillus acidophilus) as compared to the monoculture of P. shermanii (Liu and Moon 1982). This enhanced yield of propionic acid was due to the lactic acid produced by lactic acid-producing bacteria which acts as a substrate for P. shermanii (Liu and Moon 1982). Later, Tang et al. reported the mixed culture of homolactic- (Streptococcus lactis) and homoacetic-producing bacteria (Clostridium formicoaceticum) for improving the yield of acetic acid using lactose and whey permeate (Tang et al. 1988). In the year 1993, co-culture of non-Saccharomyces yeasts along with Saccharomyces strains were utilized by Bisson and Kunkee to improve the chemical and sensory properties of wine (Bisson and Kunkee 1991; Ciani et al. 2010). Co-culturing of Candida utilis and Aspergillus niger was also used for increasing the protein content in apple pomace (residue left after extraction of apple juice) (Bhalla and Joshi 1994). The authors reported that mold secretes cellulases and xylanases that hydrolyse the cellulose and hemicellulose of the apple pomace, while the yeast uses the resultant sugars which are not feasible with monocultures. Simultaneously, Wolfaart et al. reported increased removal (36% higher) of diclofop methyl when algae-bacterium consortium was utilized (Wolfaardt et al. 1994). Co-culturing also holds a great promise for efficient mitigation of toxic chlorinated compounds such as dichloroethane, vinyl chloride, and even phenols (Bradley 2003). Further, the first demonstration of symbiotic interaction of microalgae and bacteria for biological oxygen demand (BOD) removal in wastewater ponds was made by Oswald et al. in the year 1953 (Oswald et al. 1953). Later, co-cultivation of two aerobic bacteria (Pseudomonas diminuta and Pseudomonas vesicularis) and two microalgae (Scenedesmus bicellularis and Chlorella sp.) led to an increase in the growth rate of the algal strains (Mouget et al. 1995).
Co-culture of microalgae and yeast for bioenergy and biofuel production has great application in providing potential feedstocks by hydrolysis of lignocellulose biomass (agricultural residues, forest, paper, municipal and solid wastes) (Huo et al. 2011). Bioenergy is a term referred to every form of chemical energy stored in biological materials. It comprises both biohydrogen and biofuel (bioethanol and biodiesel) (Moghtaderi et al. 2007). Co-culturing of microorganisms offers an upper hand to simultaneously hydrolyze and degrade cellulose, hemicellulose, and lignin present in the recalcitrant lignocellulose biomass (Zuroff and Curtis 2012). The concept of co-culture to breakdown different cellulosic material for bioethanol production was first demonstrated in the year 1983 by Panda et al. by co-culturing Trichoderma reesei D1-6 and Aspergillus wentii Pt 2804 (Bhatia et al. 2012; Duff et al. 1985). T. reesei is able to produce cellobiohydrolase (CBH) and endoglucanases (EG) which then act on cellulose to degrade them to soluble cellulose and cello-oligosaccharides, while β-glucosidase secreted by A. niger hydrolyses cello-oligosaccharides to glucose (Bhatia et al. 2012).
From a biofuel perspective, Abate et al. first reported the increase in ethanol production during co-culturing of Zymomonas mobilis and Saccharomyces sp. on xylose and rice straw hydrolysate as compared to pure culture of either of the microorganisms (Abate et al. 1996). Breakdown of different cellulosic material for bioethanol production has also been extensively demonstrated using various co-cultures of yeast/mold species such as Saccharomyces cerevisiae and Fusarium oxysporum, Kluyveromyces marxianus and Talaromyces emersonii, S. cerevisiae, Pachysolen tannophilis, and recombinant Escherichia coli (Huo et al. 2011; Meersman et al. 2010). Several of the above-listed co-culturing studies reported an increase in bioethanol production, which is associated to improved enzyme production and metabolic degradation of inhibiting substances (Meersman et al. 2010). Co-culturing of anaerobic bacteria Clostridia sp. with aerobic Bacillus sp. resulted in an increase in the biohydrogen production (Pachapur et al. 2015). Recently, co-cultures of adaptive strains S. cerevisiae and Pichia argophorae were used to produce enhanced bioethanol production (Sunwoo et al. 2017). These adaptive strains are capable of efficiently utilizing galactose and mannitol that were obtained from enzymatic hydrolysis of seaweed, indicating their upper hand for biofuel production over non-adaptive strains. Further, Zhang et al. developed an innovative technology to bioflocculate microalgae (Chlorella vulgaris) by co-culturing it with a filamentous fungus (Mucor circinelloides) which has the potential to reduce the algal biofuel cost (Gultom and Hu 2013; Xia et al. 2011). On a similar note, it was also reported that co-culturing of microalgae C. vulgaris and fungi Agaricus blazei resulted in an increase in extra polysaccharide (EPS), which are considered to be emerging sources of bioactive value-added compounds (Angelis et al. 2012).
All these co-culturing studies emphasize the importance of symbiotic relationship of the participating microorganisms for enhancing the production of target material. The present review focuses on the importance of co-culture particularly microalgae and yeast for enhanced lipid production. Considering the biochemical composition of individual species, and the mutual beneficiary nature of the microorganisms under provided growth conditions, yeast and microalgae emerged as promising partners for enhanced production of lipids/biodiesel (Kitcha and Cheirsilp 2014; Ling et al. 2014; Xue et al. 2010; Zhang et al. 2014). Indeed, selection of yeast and microalgae for co-culturing is of utmost importance, since a stable symbiotic relationship has to be maintained for increasing the overall lipid productivity. The holistic overview on the integration of microalgae and yeast cultivation and key factors affecting this synergy has been detailed in the sections below.
Integrating microalgae and yeast cultivation
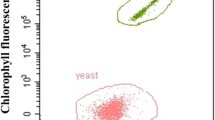
Cultivating microalgae and yeast will mean that the O2 released by the microalga will be utilized by the yeast and the CO2 released by the yeast will be taken up by the microalga (Ling et al. 2014). Further, the organic acids that are released by the yeast which are inhibitory for its growth at later stages will be taken up by the microalga for its growth (Kitcha and Cheirsilp 2014; Xue et al. 2010; Zhang et al. 2014). In turn, yeast can metabolize/break down various complex sugars into simple sugars, which can be then be utilized by microalgae for its cell division. Microalgae convert the CO2 present in the medium to bicarbonate, which is then consumed by it, releasing OH− ions making the medium alkaline (Xue et al. 2010). On the contrary, the growth of the yeast cells results in acidic medium, which eventually hinders its growth. This interplay of metabolites can result in balancing of the intrinsic O2/CO2, pH, and dissolved oxygen in the media leading to an overall increase in the growth rate of both the species (Fig. 3).
Various studies on the co-culturing of different microalga and yeast utilizing different feedstocks have been listed in Table 3. The maximum lipid productivity of 1.54 g/L/day with 41.27% lipid content was attained in the co-culture of Chlorella pyrenoidosa and Rhodotorula glutinis utilizing 40 g/L of cassava bagasse hydrolysate under fed-batch cultivation mode (Liu et al. 2018b). Co-culture of C. vulgaris and R. glutinis also showed a high lipid content of 62.20% and lipid productivity of 920 mg/L/day when they were grown together utilizing seafood processing effluent and water (1:1) (Cheirsilp et al. 2011).
Factors affecting the lipid productivity of co-culturing system
In order to efficiently cultivate microalgae and yeast together for enhanced lipid content, certain physiological parameters need to be optimized. The important parameters that govern the balanced growth of yeast and microalgae in a medium are strain selection, cultivation media/feedstock, seed ratio of microalgae:yeast (M:Y), light intensity, cultivation time, carbon/nitrogen ratio (C/N), pH, agitation speed, temperature, etc. In the following paragraphs, the influence of these parameters on lipid productivity under co-culturing conditions is elucidated in detail.
Strain selection
The first step towards successful co-culturing is the strain selection of respective microalgae and yeast that can propagate together thereby maintaining a symbiotic interaction with each other. Previously, researchers have listed certain key characteristics for selecting microalgae or yeasts for biodiesel production. These characteristics include rapid growth rate, high lipid content, capable of growing extreme conditions, tolerance to contamination and extreme environmental conditions, and large cell size to ease the harvesting of the biomass. (Griffiths and Harrison 2009; Kitcha and Cheirsilp 2012). However, a particular species cannot have all the above-listed properties; thus, selection of species requires prioritization, which mainly depends on the culture medium and local environment conditions prevailing in the culture zone. In the co-culture scenario, the foremost criteria for selection depend on the compatibility of the two strains, i.e., should have comparable growth temperature range and doubling time so that they can be cultivated together (discussed below). Followed by an attempt to reduce the cost of the cultivation and yeasts and microalgae that can grow on lignocelluosic biomass (biomass composed of cellulose, hemicellulose, and lignin), wastewaters can be targeted followed by high lipid-accumulating strains (Kitcha and Cheirsilp 2012). Finally, species with high growth rate are crucial as they increase the yield per harvest volume, decrease cost, and reduce the risk of contamination so as to outplay the slow-growing counterparts. To date, there is no data available on the selection method of microalgae and yeast for co-culturing. Hence, a thorough research investigation needs to be probed.
Cultivation media/feedstock
Variation in the cultivation media greatly influences the growth rate and lipid production in the microalgae and yeast. Macronutrients such as nitrogen and phosphorous affect the growth rate as they are the building blocks for the synthesis of nucleic acids and enzymes (Converti et al. 2009). They also play vital roles in several signal transduction/cellular process (Converti et al. 2009; Liang et al. 2012; Zhang et al. 2010). Yeasts have a unique capability to grow on various complex sugar sources such as lignocellulosic biomass and agricultural wastes, while microalgae can adapt to heterotrophic (dark) and mixotrophic (light +dark) growth modes. To establish a co-culture, the medium should contain optimum nitrogen, phosphorous, and carbon source. Previous co-culture studies have utilized various carbon sources such as glucose, sucrose, and glycerol (Table 3). Utilization of glucose (20 g/L) for co-culturing C. vulgaris and R. glutinis resulted in 20.8% lipid content, while replacing the microalga with Scenedesmus obliquus increased the lipid content to 24%, respectively (Zhang et al. 2014). Similarly, 1% addition of sucrose in BG-11 media resulted in 30% lipid content in C. pyrenoidosa and R. glutinis culture, while crude glycerol in the growth media resulted in maximum lipid content of 40.81% in C. vulgaris and Trichosporonoides spathulata, respectively (Table 3). Presence of organic carbon sources in the medium can activate de novo fatty acid synthesis during the exponential phase of cells, while lipid recycling is more prominent when the nutrients get depleted and cells enter stationary phase (Sakthivel 2011).
However, the addition of carbon sources (glucose, sucrose) in the growth media increases the cost of production as it has been estimated that these organic carbon substrates account up to 80% of the cost of cultivation medium (Bhatnagar et al. 2011; Patel et al. 2016). To combat this, inexpensive and abundant organic sources such as crude glycerol from biodiesel production, non-edible lignocellulosic biomass, and industrial, agricultural, and domestic wastes have been explored (Patel et al. 2016). It is interesting to note the utilization of various low-cost feedstocks such as liquid digestate, starch, cassava bagasse hydrolysate, food waste hydrolyzed broth, crude glycerol, aged seawater, monosodium wastewater, distillery + local municipal wastewater, winery wastewater, and seafood production waste effluent for co-culturing microalgae and yeast (Table 3). All of the above low-cost substrates resulted in high lipid productivity as compared to basal growth media’s such as BG-11/BBM.
Integrating wastewater remediation with biodiesel production has already been reported as one of the sustainable methods for large-scale deployment (Pittman et al. 2011; Xie et al. 2013). Secondary and tertiary wastewaters containing large amount of nutrients which can be utilized by microalgae and yeast for their growth will significantly decrease the load on freshwater reserves by avoiding high-nutrient wastewater discharge (Xie et al. 2013). However, monocultures of microalgae can efficiently remove nitrogen, phosphorous, and CO2 from the wastewater streams but are not effective to remove organic matter with chemical oxygen demand (COD) over 5 g/L due to retarded growth (Ling et al. 2014). On the other hand, yeast can easily grow in wastewaters high in chemical oxygen demand ranging from 15 to 50 g/L but are inefficient at removing nitrogen and phosphorus. Thus, co-culturing the microalgae and yeast can effectively recycle nitrogen, phosphorous, COD, and TOC from the wastewaters as compared to monocultures along with the provision of green energy (Ling et al. 2014). The above statement has been validated in co-culture studies including removal efficiencies of C. pyrenoidosa and Rhodosporidium toruloides (95.34% COD, 51.18% TN, 89.29% TP), C. vulgaris and R. glutinis (79% COD, 33% TN), C. vulgaris and Y. lipolytica (99% COD, 88.30% TN, 100% TN), and Spirulina platensis and R. glutinis (73% COD, 94% reducing sugars, 35% ammonia), where the removal was 10–15% higher than the monocultures of microalgae and yeast from distillery and local domestic wastewater, seafood processing effluent, and monosodium glutamate wastewater, respectively (Qin et al. 2018b; Shu et al. 2013; Xue et al. 2010; Zhang et al. 2014). These wastewaters also contain fatty acids, which could activate the ex novo fatty acid synthesis along with de novo fatty acid synthesis in yeasts leading to a high lipid accumulation. However, to throw light onto the mechanistic aspects of lipid synthesis in co-cultures, comprehensive proteomic/transcriptomic studies are essential. These studies will estimate the level of gene/protein expression of PDAT, DGAT, LIP2, and extracellular lipases and delineate the correlation between high lipid accumulation and activation of their respective fatty acid synthetic pathways.
Microalgae: yeast seed ratio
Under the co-cultivating scenario of microalgae, yeast normally is the dominant species in the start (24–48 h) due to its faster growth rate, then microalga gradually takes over with time (Cai et al. 2007; Cheirsilp et al. 2011; Shu et al. 2013). This initial slow growth of microalgae could be due to the inhibitory effect of CO2 released by yeast in the medium (Yen et al. 2015), slow cell division rate, or lack of light penetration due to dense yeast growth (Kitcha and Cheirsilp 2014). However, after 24–48 h (depends on the microalga and yeast species), microalgae get acclimated to the environment and start growing at a faster rate while yeast has already reached its stationary phase as the nutrients (mainly organic carbon) get exhausted (Yen et al. 2015).
In order to increase the microalgal growth during the early stages of co-culture, various researchers have used different M:Y seed ratios ranging from as high as 40:1 to as low as 1:2 (Cheirsilp et al. 2011; Jiang et al. 2018; Ling et al. 2014; Qin et al. 2018b; Shu et al. 2013; Yen et al. 2015; Zhang et al. 2014). A summary of the impact of initial seed ratio on lipid productivity is given in Table 3. The optimum ratio of 1:1 to 3:1 resulted in maximum lipid content. Indeed, the M:Y seed ratio depends on the growth rate of the selected microalgae/yeast which enables equilibrium between the two species.
Light intensity and photoperiod
Light intensity, duration of the photoperiod or light-to-dark ratio, and light wavelength may all influence lipid production, particularly in microalgae. Natural or artificial light sources are the basic energy source for photosynthesis in microalgae. During photosynthesis, electrons pass from water to nicotinamide adenine dinucleotide phosphate (NADP+) generating ATP (Wahidin et al. 2013). Low light intensity plays a crucial role, as it can lead to retarded photosynthetic rates while high illumination results in damage of the photosynthetic pigments causing photoinhibition (Wahidin et al. 2013; Zuroff and Curtis 2012). The light intensity also modulates the microalgal biochemical composition specifically lipids and carbohydrates. A high illumination can lead to the induction of stress in microalgal cells, hence resulting in the accumulation of TAGs intracellularly (Huo et al. 2011). It is therefore required to optimize the light intensity in the co-cultures so that microalgal cells can maintain their photosynthetic receptors along with high lipid production. Previous co-culture studies have tested light intensity from 27 to 108 μmol/m2/s and observed an increase in the lipid content up to 67.5 μmol/m2/s, followed by a decrease in the lipid productivity after this limit (Cheirsilp et al. 2011; Kitcha and Cheirsilp 2014). Further, during co-cultivation, microalgal cells appear yellow than green which could be due to the heterotrophic mode of cultivation in the presence of organic carbon sources such as glycerol or due to the shading effect due to the high density of yeast cells (Kitcha and Cheirsilp 2014).
Light duration or photoperiod (light: dark hours) plays a vital role in increasing the light-harvesting efficiency of microalgae as prolonged dark periods with high light intensity allow photosynthetic machinery to fully utilize captured photons and convert them into chemical energy (starch, lipids) by avoiding the effect of photoinhibition (Juneja et al. 2013). In addition to photoperiod, the spectral composition also affects the overall lipid productivity (using the same photon flux density) for stimulating growth and lipid content (Blair et al. 2014). It has been reported that blue light (470 nm) stimulates the growth and lipid production in microalgae as compared to red light (680 nm) (Das et al. 2011). This could be due to the shorter wavelength of the blue photons which have a higher probability of striking at the light harvesting complex (LHC) as compared to the red photons (Das et al. 2011). Moreover, blue light is responsible for the chloroplast development and controls the expression of key photosynthetic genes including RubPCase, NADP-dependent GAPDH, and enzymes involved in chlorophyll and carotenoid synthesis (Ruyters 1984). Further, it was reported that the damage caused by the prolonged exposure of red light to chloroplasts can be repaired by the addition of blue light (Ruyters 1984). However, till date, the above phenomena have been tested only in microalgae monocultures and no detailed studies have been carried out on analyzing the effects of light duration and spectral composition for co-cultures and its correlation to attenuation of lipid quantity and quality, leading to variable biodiesel properties. In general, co-cultivation of yeast and microalgae should be done keeping in mind the outdoor cultivations so that a more realistic data could be attained. These conditions include using diurnal cycle (photoperiod depending on the season), with fluctuating in the light intensities and light wavelengths between 400 and 700 nm, respectively.
Carbon-to-nitrogen ratio
As described above, the lipid metabolism in oleaginous yeast and microalgae is controlled by carbon-to-nitrogen ratio (C/N). A high C/N ratio leads to enhanced lipid accumulation due to the depletion of nitrogen, which is a growth-limiting nutrient (Braunwald et al. 2013; Silaban et al. 2014). This high C/N ratio causes activation of nitrogen-scavenging enzymes, which decreases the level of AMP thereby inhibiting isocitrate dehydrogenase thus hindering the citric acid cycle (EVANS et al. 1983). This results in the accumulation of citrate in the mitochondria, which is then transported to cytosol for its subsequent conversion to acetyl-CoA, the precursor of triacylglycerol synthesis (Somashekar and Joseph 2000). Based on the previous studies on monocultures, an optimum C/N ratio ranging from 50 to 100 for yeast and < 17 for microalgae has been reported to increase the lipid accumulation (Braunwald et al. 2013; Daliry et al. 2017; Sattur and Karanth 1989). A recent study evaluated the effect of C/N ratio ranging from 16 to 64 on the biomass and lipid accumulation capacity when C. pyrenoidosa and R. glutinis were co-cultured in BBM supplemented with 10 g/L of glucose (Liu et al. 2018a). The authors reported an improvement in the biomass from 2.92 to 6.12 g/L when C/N ratio was increased from 16 to 64 with augmentation in lipid content from 25% to 40.55%, respectively. Further, maximum lipid content (4.6 g/L) was observed in seafood processing effluent which could be due to high C/N ratio (Cheirsilp et al. 2011). Thus, utilization of low-cost feedstocks having high C/N ratio not only reduces the cost of the growth medium but also significantly increases lipid content. Indeed, a balance of C/N ratio which can range from 20 to 60 is imperative in co-cultures that enable efficient growth of both microalgae and yeast.
Cultivation time
Oleaginous microalgae and yeast accumulate most of the lipids in the early stationary phase. Upon entering into late stationary phase, although TAG is synthesized, the lipid peroxidation pathway also gets activated leading to a decrease in TAG content (Sitepu et al. 2013). Therefore, cultivation time plays an important parameter for deciding the maximum lipid accumulation phase (LAP) and thereby optimizing the harvesting time point. The LAP in the co-culture can vary according to the microalgae and yeast division time, environmental conditions, and media composition. Fast growing species can reach LAP early as compared to slow-growing organisms; similarly, lack of nutrients or stressful conditions can reduce the cell division leading to a quick LAP. On examination of the previous co-culture studies, time required for onset of LAP was around 5–6 days for Chlorella protothecoides and R. toruloides when cultivated individually, whereas co-culture of both took only 15 h (minimum) for the accumulation of 26.9% and 27.9% lipid, respectively, when cultivated in nitrogen-limited media. While Chlorella KKU-S2 with Trichosporon globose YUS/2 and Isochrysis galbana 8701 with Ambrosiozyma cicatricosa took a cultivation time of 7 days (maximum) when grown in sugarcane juice and aged seawater supplemented with 2 g/L seawater, respectively (Table 3). Optimizing LAP is crucial to enable maximum lipid productivity at the time of harvesting and thus more studies need to be undertaken focusing on the characterization of yeast and microalgae in terms of growth rate and lipid accumulation under co-culture and its comparison with monocultures.
pH, agitation speed, and temperature
pH, agitation speed, and temperature are the other important parameters that significantly influence the overall lipid productivity. The pH of the medium determines the solubility and availability of CO2 and essential nutrients (Juneja et al. 2013). It has been reported the optimum pH for yeast is 4–6.5, while for microalgae, it is 6.5–9, respectively (Simosa 2016; Yalcin and Ozbas 2008). High pH limits the availability of free CO2 in the medium by lowering its affinity to microalgae, thereby suppressing the growth of microalgal cells leading to lipid accumulation (Huo et al. 2011; Juneja et al. 2013). To date, only two co-culture studies have been done to analyze the effect of pH on lipid accumulation (Cheirsilp et al. 2011; Zhang et al. 2014). A decrease in pH from 6.0 to 3.2 was recorded initially (36 h) mainly due to rapid growth of yeast cells, while after 36 h, an increase in pH from 3.2 to 4.9 was recorded, attributing to the microalgal growth at later growth stages (Zhang et al. 2014). Similarly, a pH of 5 was reported as optimum for balanced microalgal and yeast growth, as the growth rate of the yeast declines with an increase in pH from 5 to 8 (Cheirsilp et al. 2011).
Agitation speed controls the mass transfer rate thereby modulating the exchange of O2 and CO2 between the microalga and yeast. Increasing agitation speed from 100 to 150 rpm enhanced the biomass and lipid production in the co-culture of C. vulgaris and R. glutinis. But a further increase in revolutions per minute did not significantly affect the growth rate of microalgae or yeast suggesting that the optimal agitation speed should be ~ 150 rpm (Cheirsilp et al. 2011). Till date, only one study has been carried out to examine the effect of agitation speed on the lipid accumulation in co-culture; more research is warranted.
Cultivating the cells at optimum temperature results in fast growth rate with efficient nutrient uptake (Juneja et al. 2013). In the case of microalgae, when cultivated under low temperatures, the CO2 fixation rate decreases leading to a slow electron transport. Moreover, increasing or decreasing temperatures, above or below the optimum range, result in the inhibition of photosystem II (PS II) as it causes degradation of D1 protein, which impedes the repair mechanism (Juneja et al. 2013). Furthermore, fluctuations (increase or decrease) in temperatures (ranging from 10 to 40 °C) result in an increase in lipid/protein ratio in both microalgae and yeast (Juneja et al. 2013; Vanhercke et al. 2013). Exposure to high and low temperature causes stressful conditions leading to an unbalanced energy equilibrium, excess production of free radicals, and inhibition in growth rate, respectively (Juneja et al. 2013, Vanhercke et al. 2013). It is therefore essential to optimize co-culturing temperature, so that both the species grow at equal growth rates with a subsequent increase in lipid accumulation. In general, due to the fast doubling time of yeast, it outcompetes microalgae in co-culture, and only after yeast reaches stationary phase that microalgae growth starts. This inadequacy in the growth rates of the two species may result in lipid degradation in the yeast in the late stationary phase thereby decreasing the lipid productivity and also increasing the cultivation time leading to an increase in the cost of production. In the previously reported co-culture studies, a range of 25–30 °C was taken as all the microalgae and yeasts strains used were mesophilic organisms (Table 3). An interesting aspect could be co-culturing psychrophiles (cold-tolerant with optimal growth temperature < 15 °C), thermophiles (temperature tolerant 41 to 122 °C), or halotolerant (salt tolerance < 2.5 M of salt) microalgae and yeast so that they could be grown in cold/hot climatic conditions without the need of maintaining the temperature thereby decreasing the production cost.
Effect of extracellular metabolites on symbiotic environment
Analysis of the extracellular metabolites released and their pattern of exchange during symbiotic relationship of two or more microorganisms can provide insights into the complex interactions (Ding et al. 2015). For example, one of the most obvious strategies of bacterial populations to communicate with each is via release of pheromones which aid in cell-cell signaling (Williams et al. 2007). Bacterial cells release a number of small extracellular metabolites including antibiotics, siderophores, and metabolic end products that aid in growth and defense from other invaders (Williams et al. 2007). Apart from this, a symbiotic relationship between Ketogulonicigenum vulgare and Bacillus megaterium enhanced the production of 2-ketogluonic acid (a precursor of vitamin C) by stimulating the growth of K. vulgare by active exchange of extracellular metabolites such as amino acids, erythrose, erythritol, guanine, and inositol (Ma et al. 2011). Moreover, the well-documented example of vitamin B12 exchange from bacteria to microalgae emphasizes on the importance of extracellular metabolites for maintaining symbiotic relationships (Croft et al. 2005). In a recent study, co-culture of Tetradesmus obliquus and actinomycetes resulted in enhanced growth and lipid production of the microalgae due to the release of indole acetic acid (growth hormone) by the bacteria (Kumsiri et al. 2018).
Extracellular metabolites can be characterized by using techniques such as gas chromatography-mass spectroscopy (GC-MS), high performance liquid chromatography (HPLC), or nuclear magnetic resonance (NMR). Among these techniques, HPLC and GC-MS are rapid, sensitive, selective, and require expensive reagents and derivatization of samples, while NMR is a straightforward and robust technique that does not require derivatization of samples but is comparatively less sensitive (Dunn and Ellis 2005). The differential profile of the extracellular metabolites excreted in the medium in the case of microalga-yeast co-cultures as compared to monocultures was also reported using HPLC and GC-MS (Xue et al. 2010; Zhang et al. 2014). The levels of glycine and proline increased, while propionic acid, pyruvic acid, acetic acid, and palmitic acid decreased in co-cultures indicating that the microalgal cells (C. vulgaris) consumed the organic acids released by the yeast (R. glutinis), which was also evidenced by the pH fluctuations (less acidic as compared to monocultures) (Zhang et al. 2014). Further, in yeast monocultures, the presence of glycerol and acetic acid was detected, while algal cultures showed the presence of glycinamide and acetamide (Zhang et al. 2014). Similar results were reported by Xue et al. while co-culturing S. platensis and R. glutinis in monosodium glutamate wastewater (Xue et al. 2010). Nevertheless, comprehensive investigations on metabolite profiling of these microorganisms under various experimental conditions using NMR/GC-MS are imperative to throw a light on the mechanistic and signaling aspects of the inherent cellular pathways that are involved in tunable lipid production.
Key technological aspects of co-cultivation for enhanced production of TAGs
Mode of cultivation plays a crucial role in enhanced biomass production for example trophic status, batch versus continuous culture, and solution phase versus gel immobilization on gels. Zhang et al. evaluated the autotrophic (obtain carbon from CO2 or inorganic carbon sources) and heterotrophic (obtain carbon from organic carbon sources) nature of strains for co-culturing (Zhang et al. 2017). They used the co-culture of C. vulgaris with R. glutinis in winery effluent, which showed the enhanced biomass and lipid productivity in autotrophic conditions as compared to heterotrophic conditions (Zhang et al. 2017). Indeed, winery wastewater contains various waste organic carbon sources thereby converting the autotrophic mode into mixotrophy which has been reported to boost biomass and lipid accumulation in microalgae (Patel et al. 2016). Mixotrophic mode is an amalgamation of autotrophic and heterotrophic mode, thereby assimilating both CO2 and organic carbon simultaneously (Patel et al. 2016).
In another study, batch and semicontinuous cultivation was compared for the co-culture of sucrose-secreting cyanobacterium CscB+ Synechococcus elongates PCC7942 and R. glutinis (Li et al. 2017). Batch culture operated for 4 days showed maximum biomass accumulation (0.8 g/L) as compared to the semicontinuous culture (0.6 g/L) in 21 days. Authors also evaluated the impact of co-culturing with R. glutinis on S. elongates growth and hydrogen peroxide (H2O2) alleviation which is formed due to light exposure and chemicals present in the growth medium. Interestingly, R. glutinis not only facilitated the growth of the S. elongates but also progressively scavenged H2O2 from the culturing media (Li et al. 2017).
Co-immobilization of microalga and yeast in gel beads (sodium alginate, carrageenan, gelatin, etc.) has been reported to increase the lipid accumulation, as it could efficiently enhance the interaction (enhanced gas and metabolite transfer) between the two microorganisms thereby alleviating the problem of mass transfer (Kitcha and Cheirsilp 2014). For example, culture of C. pyrenoidosa with immobilization S. cerevisiae resulted in an increase in lipid content (29.70%) as compared to monocultures of the microalga (Wang et al. 2016). Authors demonstrated that the immobilized yeast can be reused up to three times, after which the mechanical strength is weakened. In another study, immobilization of C. vulgaris and T. spathulata resulted in a total lipid content of 40.4% thus attaining a lipid productivity of 768 mg/L/day (Kitcha and Cheirsilp 2014). Co-cultivation of S. obliquus with Candida tropicalis leads to higher biomass production (4.3 g/L) as compared to the co-culture of S. obliquus with S. cerevisiae (3.4 g/L), the latter was almost equivalent to the monoculture of S. obliquus (Wang et al. 2016). Such an increase in biomass of S. obliquus and C. tropicalis co-culture was attributed to the filament nature of C. tropicalis, which facilitates the microalgal attachment and thereby improved the gas and substance exchange between the two microorganisms (Wang et al. 2016).
In summary, immobilization of the microbial cells is technically advantageous over free cells, as entrapment of cells can protect microorganisms from inhibitory by-products present in the growth medium and thereby yielding high cell density and lipid productivity (Table 3) (Park and Chang 2000; Rathore et al. 2013). Further, immobilization can significantly reduce the harvesting cost, as the settling of gel beads (alginate) aids in recovery process from the medium with no cell flocculation. Hence, the biomass can then be extracted from the beads by dissolving the beads in sodium carbonate without any further centrifugation (Kitcha and Cheirsilp 2014).
Assessment of fatty acid profiles and biodiesel properties of lipids obtained under co-culturing technique
Enhancement of lipid/biomass production is a pre-requisite for co-culturing although this property alone is not sufficient to make this technique viable. Evaluation of biodiesel properties obtained from these microbial lipids is required to qualify it as a commercial fuel. For commercial use of the biodiesel, it should comply with the specifications listed by American Society for Testing and Materials (ASTM) D6751 and EN 14214 in Europe. In general, the lipids thus produced by these microorganisms will be converted into biodiesel via a process known as transesterification in which the microbial lipid reacts 1 mol of triacylglyceride to 3 mol of alcohol (3:1) to form 1 mol of glycerol and 3 mol of the respective fatty acid alkyl esters (Ramos et al. 2009). The alcohol could be methanol or ethanol and can be catalyzed either by homogenous catalyst (acid or base) or heterologous catalysts (acid, base, or enzyme) (Stansell et al. 2011). Among the alcohols, methanol is more frequently used due to its low cost and fast reactivity with the catalyst (Leung et al. 2010). On the other hand, homogenous alkali catalyst such as NaOH or KOH enhances the rate and conversion of the transesterification reaction as compared to acid catalysts (HCl, H2SO4). The reaction is generally operated at temperatures 50–60 °C for < 90 min in a closed glass vial but can vary according to the oil used (Leung et al. 2010). Higher reaction temperatures can reduce the viscosity of the biodiesel and shorten the reaction time. Increasing the temperatures above optimal level can decrease the biodiesel yield as it leads to saponification of triglycerides (Leung et al. 2010).
The physical properties such as ignition quality, heat of combustion, cold flow, oxidative stability, viscosity, and lubricity of biodiesel fuel are determined by the composition and structure of fatty acids (Table 4) (Knothe 2005). The two most important properties of fatty acids that affect the fuel properties as listed above are (a) length of the carbon chain and (b) number of double bonds (Stansell et al. 2011). Ideally, a good quality biodiesel should have maximum C16:1 and C18:1 with other FAMEs should be as low as possible (Knothe 2008; Stansell et al. 2011). An increase in fatty acid length and degree of saturation increases the cetane number and lowers the NOx emissions (Saraf and Thomas 2007). Cetane number is a dimensionless indicator of ignition quality of diesel fuel. According to ASTM D6751 and EN 14214, it should be higher than 47 and 51, respectively (Knothe 2008). Further, the degree of unsaturation in the fatty acids affects the oxidative stability of the biodiesel with SFAs being the most stable followed by MUFAs compared to the least stable PUFAs, respectively (Ashraful et al. 2014). High PUFA content in the biodiesel decreases the cetane number thereby increasing the NOx emissions and lowering the lubricity leading to gum formation in the engines. However, a high PUFA content improves the cold flow properties due to their low melting points making the diesel operable in cold climates (Knothe 2008). On the other hand, an increase in short and unsaturated fatty acids decreases the kinematic viscosity (KV) of the biodiesel, the property that controls the fuel flow in the engine. A low KV favors the smooth engine flow by appropriate mixing of fuel with air (Ashraful et al. 2014). Thus, a thorough analysis of FAME composition and biodiesel properties is essential for selecting yeast and microalgal species for co-culture.
In order to study the variations in the fatty acid profiles of different co-cultures, we have analyzed the results of various researchers and represented in the form of a histogram (Fig. 4). The nomenclature and details of all the fatty acids that are common constituents of biodiesel are given in Table 5. FAMEs majorly comprised of C14:0 (myristic acid), C16:0 (palmitic acid), C18:0 (stearic acid), C18:1 (oleic acid), and C18:2 (linoleic acid) (Fig. 4). In general, the monocultures of yeast irrespective of the species showed 7–20% of C16:0, 0.1–0.8% of C16:1, 3–12% of C18:0, and 4–9% of C18:3 with a more variation in the C18:1 (28–85%) and C18:2 (5–20%), respectively (Cheirsilp et al. 2011; Kitcha and Cheirsilp 2014; Liu et al. 2018a; Liu et al. 2018b; Santos et al. 2013; Wang et al. 2018b; Yen et al. 2015). On the other hand, microalgae irrespective of the species composed of 12–40% C16:0 and 0.1–1% of C16:1 showing a variation in C18:0 (2–32%), C18:1 (21–71%), and C18:3 (0–10%) content. Interestingly, co-culturing yeast and microalgae resulted in an optimum balance of essential fatty acids rich in SFA (approximately double) while decrease in PUFA as compared to monocultures. Further, co-culturing significantly reduced the C18:3 content indicating blending yeast and microalgae oils can be beneficial keeping in mind the 12% limit of 18:3 in biodiesel by EN (Cheirsilp et al. 2011; Kitcha and Cheirsilp 2014; Santos et al. 2013; Yen et al. 2015). Moreover, the co-culture of C. vulgaris and T. spathulata showed an increase in C22:1 from 0.38% to 8.89%. Among the reported fatty acid profiles, the maximum SFA was obtained in the co-culture of C. vulgaris and T. spathulata, while MUFA content was highest in Xanthophyllomyces dendrorhus and Chromochloris zofigiensis (Jiang et al. 2018) and C. pyrenoidosa and R. glutinis (Wang et al. 2016) co-culture (Cai et al. 2007; Kitcha and Cheirsilp 2014).
Comparison of fatty acid profile of different co-cultures of microalga and yeast for biodiesel production. 1, Chlorella sp. and S. cerevisiae (Shu et al. 2013); 2, C. vulgaris and T. spathulata (Kitcha and Cheirsilp 2014); 3, I. galbana and A. cicatricose (Cai et al. 2007); 4, C. protothecoides and R. toruloides (Santos et al. 2013); 5, C. vulgaris and R. glutinis (Cheirsilp et al. 2011); 6, S. obliquus and C. tropicalis (Wang et al. 2016); 7, C. vulgaris and R. glutinis (Zhang et al. 2017); 8, X. dendrorhous and C. zofingiensis (Jiang et al. 2018); 9, C. pyrenoidosa and S. fibuligera (Wang et al. 2018a); 10, C. pyrenoidosa and R. glutinis (Liu et al. 2018b); 11, C. pyrenoidosa and S. cerevisiae (Wang et al. 2018b)
To throw light on the properties of biodiesel obtained using co-culturing, we performed a theoretical comparative analysis of the biodiesel physical properties using empirical formulas (Table 4) and compared with ASTM D6751 and EN 14214 biodiesel standards (Table 6) (Patel et al. 2016). All the co-cultures showed a high cetane number (CN) and low iodine value (IV), i.e., within the acceptable limit of both the biodiesel standards with the exception of C. protothecoides and R. toruloides (CN-37 and IV-146 gI2/100 g) and C. protothecoides and R. glutinis (CN-45 and IV-126 gI2/100 g). Iodine number measures the degree of unsaturation and heating of oils. High unsaturated fatty acids lead to the formation of glycerides leading to deposits and deterioration of lubricating oil (Francisco et al. 2010). This high IV (146 gI2/100 g) could be due the presence of high amounts of C18:3 in the co-cultures making the biodiesel more prone to oxidative degradation, and an decrease in the cetane number could be responsible for ignition delay (Islam et al. 2013). As most of the co-cultures showed high CN (62–88) values, the biodiesel thus obtained can be used directly or blended with conventional petro diesel. This makes its usage easier as the set range of CN for utilization is ~ 40–50 in case of diesel engines (Francisco et al. 2010; Islam et al. 2013). The FAME-derived high heating values (HHV) of all the biodiesel lies between 39 and 44 MJ/kg which complies with the set range of HHV for standard biodiesel and close to the conventional diesel HHV (46 MJ/kg) (Islam et al. 2013; Ramírez-Verduzco et al. 2012).
Besides the above properties, the degree of unsaturation (DU), which is the sum of monounsaturated and polyunsaturated fatty acids, also affects the stability of the biodiesel. A high DU decreases the oxidative stability (shelf life of biodiesel) thereby increasing the NOX emissions. The values varied from 25% to 127% with co-culture of Chlorella sp. and S. cerevisiae showing lowest DU, while C. protothecoides and R. toruloides had highest DU indicating a high PUFA content in its fatty acid profile. The presence of unsaturated fatty acids decreases the viscosity of the biodiesel thereby aiding smooth engine operation and increasing the efficiency of the diesel engine (Francisco et al. 2010). The density and kinematic viscosity of all the co-culture-derived biodiesel were within the acceptable range of biodiesel standards. Lastly, the cold filter plugging properties decides the low temperature flow of biodiesel is influenced by the saturated fatty acids which can crystallize inside the engines. If the engine is operated at low temperature, these crystals can grow rapidly and agglomerate, clogging fuel lines and filters, thus causing major operational problems (Francisco et al. 2010; Ramos et al. 2009). Biodiesel derived from C. vulgaris and T. spathulata showed the highest (26.7 °C) cold filter plugging property (CFPP), while C. pyrenoidosa and R. glutinis showed the lowest CFPP (− 9.49 °C) indicating that the biodiesel from the latter can be used in cold climates (Table 5). These values of CFPP are positively correlated to the long chain saturation factor (LCSF); the longer the carbon chain, the poor is the low temperature operability. Thus, in order to use the obtained biodiesel, additives must be added to improve the CFPP values (Francisco et al. 2010). Based on the above results, the biodiesel derived from co-culturing microalgae and yeast could be suggested for hot regions as it has low IV, high CN, and high oxidative stability (Table 5). Furthermore, it should be noted that the FAMEs that result in high CN and low IV can also cause poor CFPP. It is therefore necessary to achieve a balance between the C16:1 and C18:1 in the biodiesel (Knothe 2008). Keeping in mind the ideal biodiesel C16:1 and C18:1, only Chlorella sp. and S. cerevisiae co-culture-derived biodiesels as the CN, IV, OS, and CFPP were within the acceptable range due to the presence of maximum C16:1 (~ 23%) and C18:1 (~ 45%) content as compared other co-cultures (Shu et al. 2013). However, a ratio of 5:4:1 of C16:1, C18:1, and C14:0 is recommended for a good quality biodiesel which was not observed in any of the co-culture-derived FAMEs (Fakhry and Maghraby 2013). Further studies are required to optimize the co-culture conditions to gain desired fatty acid profile that can enhance the quality of co-cultivated microbial biodiesel (Francisco et al. 2010).
Concluding remarks
Despite the limited information available on the co-culture of yeast and microalgae for augmenting lipid productivity, the reported studies illustrated that these two can be the ideal partners as compared to monocultures, which could lead to sustainable and greener diesel fuels in the near future. This inherent property of surviving together under environmental perturbations can be explained by the efficient use of the inhibitory products of each other such as CO2, organic acids by the microalgae, and O2 by the yeast, alleviating the growth inhibition leading to a stable environment (maintained pH and DO). The current review explicitly described the various biological, growth features, and physico-chemical factors (such as strain selection, pH, seed ratio, feedstocks, temperature, light intensity/photoperiod, cultivation time, and C/N ratio) that are crucial for enhanced lipid synthesis. Based on the literature survey, the most promising yeast and microalgal species for co-culture belonged to the genera of Rhodotorula sp. and Chlorella sp. as they showed comparatively high lipid productivity as to the combinations of other yeast and microalgal. The other abiotic parameters that enhanced the lipid productivity were mixotrophic mode of cultivation, 1:1 microalgae/yeast ratio, culturing at 25–28 °C with initial pH of the media to be neutral. Further, co-culturing microalgae and yeast in low-cost feedstocks specifically wastewater effluents can result in not only reducing the cost of the cultivation media but also mitigating the pollutants (nitrate, phosphate, organic content) and chemical oxygen demand thereby making the overall process greener and viable. The review also delineated the technological aspects and symbiotic requirements that paved a path for efficient co-cultivation of oleaginous microalgae and yeast for boosting TAG accumulation. Indeed, the review comprehensively provided the cellular mechanisms of TAG accumulation in both the oleaginous species and also presented the overall qualitative and quantitative aspects of biodiesel production (FAME profiles) by analyzing the research data produced across the globe on TAG synthesis using algal/yeast co-cultures.
Future perspectives
For large-scale use of microalgal and yeast co-culture for the production of biodiesel to be viable, more research is required. This includes the development of cost-effective feedstocks, harvesting strategies along with generation of robust data for studying the effects of various physiological parameters (pH, temperature, CO2, seed ratio, light intensity, etc.), which could be potentially exploited for cost-competitive conversion of biomass to biodiesel. Other fascinating aspects that need to be focused is the molecular changes occurring in the microalgae and yeast cultured together as compared to their monocultures by studying as transcriptomics, proteomics, and metabolomics. These molecular studies can deepen our understanding on the interplay of various genes, proteins, and metabolites that are crucial for maintaining this symbiotic relationship and enhancing the lipid production. Eventually, an interesting future aspect could be co-culturing of genetic modified or high lipid accumulating mutants of microalgae and yeast, which can grow at the same growth rate and not compete with each other. The above-stated innovative engineering solutions can potentially lead to successful implementation of co-culture for biodiesel production.
References
Abate C, Callieri D, Rodríguez E, Garro O (1996) Ethanol production by a mixed culture of flocculent strains of Zymomonas mobilis and Saccharomyces sp. Appl Microbiol Biotechnol 45:580–583
Ageitos JM, Vallejo JA, Veiga-Crespo P, Villa TG (2011) Oily yeasts as oleaginous cell factories. Appl Microbiol Biotechnol 90:1219–1227
Ahmed RA, He M, Aftab RA, Zheng S, Nagi M, Bakri R, Wang C (2017) Bioenergy application of Dunaliella salina SA 134 grown at various salinity levels for lipid production. Sci Rep 7:8118
Angelis S, Novak AC, Sydney EB, Soccol VT, Carvalho JC, Pandey A, Noseda MD, Tholozan JL, Lorquin J, Soccol CR (2012) Co-culture of microalgae, cyanobacteria, and macromycetes for exopolysaccharides production: process preliminary optimization and partial characterization. Appl Biochem Biotechnol 167:1092–1106
Angerbauer C, Siebenhofer M, Mittelbach M, Guebitz G (2008) Conversion of sewage sludge into lipids by Lipomyces starkeyi for biodiesel production. Bioresour Technol 99:3051–3056
Arora N, Patel A, Pruthi PA, Pruthi V (2016) Synergistic dynamics of nitrogen and phosphorous influences lipid productivity in Chlorella minutissima for biodiesel production. Bioresour Technol 213:79–87
Ashraful AM, Masjuki HH, Kalam MA, Rizwanul Fattah IM, Imtenan S, Shahir SA, Mobarak HM (2014) Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: a review. Energy Convers Manag 80:202–228
Azocar L, Ciudad G, Heipieper HJ, Navia R (2010) Biotechnological processes for biodiesel production using alternative oils. Appl Microbiol Biotechnol 88:621–636
Bellou S, Baeshen MN, Elazzazy AM, Aggeli D, Sayegh F, Aggelis G (2014) Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol Adv 32:1476–1493
Beopoulos A, Cescut J, Haddouche R, Uribelarrea J-L, Molina-Jouve C, Nicaud J-M (2009) Yarrowia lipolytica as a model for bio-oil production. Prog Lipid Res 48:375–387
Beopoulos A, Nicaud JM, Gaillardin C (2011) An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl Microbiol Biotechnol 90:1193–1206
Bertrand S, Bohni N, Schnee S, Schumpp O, Gindro K, Wolfender JL (2014) Metabolite induction via microorganism co-culture: a potential way to enhance chemical diversity for drug discovery. Biotechnol Adv 32:1180–1204
Bhalla T, Joshi M (1994) Protein enrichment of apple pomace by co-culture of cellulolytic moulds and yeasts. World J Microbiol Biotechnol 10:116–117
Bhatia L, Johri S, Ahmad R (2012) An economic and ecological perspective of ethanol production from renewable agro waste: a review. AMB Express 2:65
Bhatnagar A, Chinnasamy S, Singh M, Das KC (2011) Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl Energy 88:3425–3431
Bisson L, Kunkee R (1991): Microbial interactions during wine production. Mixed cultures in biotechnology. McGraw-Hill, New York, pp 37–68
Blair MF, Kokabian B, Gude VG (2014) Light and growth medium effect on Chlorella vulgaris biomass production. Journal of Environmental Chemical Engineering 2:665–674
Bradley PM (2003) History and ecology of chloroethene biodegradation: a review. Bioremediation Journal 7:81–109
Braunwald T, Schwemmlein L, Graeff-Honninger S, French WT, Hernandez R, Holmes WE, Claupein W (2013) Effect of different C/N ratios on carotenoid and lipid production by Rhodotorula glutinis. Appl Microbiol Biotechnol 97:6581–6588
Cai S, Hu C, Du S (2007) Comparisons of growth and biochemical composition between mixed culture of alga and yeast and monocultures. J Biosci Bioeng 104:391–397
Chakraborty S, Mohanty D, Ghosh S, Das D (2016) Improvement of lipid content of Chlorella minutissima MCC 5 for biodiesel production. J Biosci Bioeng 122:294–300
Cheirsilp B, Suwannarat W, Niyomdecha R (2011) Mixed culture of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for lipid production from industrial wastes and its use as biodiesel feedstock. New Biotechnol 28:362–368
Chen C-Y, Chang HY (2016) Lipid production of microalga Chlorella sorokiniana CY1 is improved by light source arrangement, bioreactor operation mode and deep-sea water supplements. Biotechnol J 11:356–362
Chi Z, Zheng Y, Jiang A, Chen S (2011) Lipid production by culturing oleaginous yeast and algae with food waste and municipal wastewater in an integrated process. Appl Biochem Biotechnol 165:442–453
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Ciani M, Comitini F, Mannazzu I, Domizio P (2010) Controlled mixed culture fermentation: a new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res 10:123–133
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process Process Intensif 48:1146–1151
Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG (2005) Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438:90–93
Daliry S, Hallajsani A, Mohammadi Roshandeh J, Nouri H, Golzary A (2017) Investigation of optimal condition for Chlorella vulgaris microalgae growth. Glob J Environ Sci Manag 3:217–230
Das P, Lei W, Aziz SS, Obbard JP (2011) Enhanced algae growth in both phototrophic and mixotrophic culture under blue light. Bioresour Technol 102:3883–3887
De Bhowmick G, Koduru L, Sen R (2015) Metabolic pathway engineering towards enhancing microalgal lipid biosynthesis for biofuel application—a review. Renew Sust Energ Rev 50:1239–1253
Dias C, Sousa S, Caldeira J, Reis A, Lopes da Silva T (2015) New dual-stage pH control fed-batch cultivation strategy for the improvement of lipids and carotenoids production by the red yeast Rhodosporidium toruloides NCYC 921. Bioresour Technol 189:309–318
Ding Y, Tian Y, Li Z, Zuo W, Zhang J (2015) A comprehensive study into fouling properties of extracellular polymeric substance (EPS) extracted from bulk sludge and cake sludge in a mesophilic anaerobic membrane bioreactor. Bioresour Technol 192:105–114
Duff SJ, Cooper DG, Fuller OM (1985) Cellulase and beta-glucosidase production by mixed culture of Trichoderma reesei rut C30 and Aspergillus phoenicis. Biotechnol Lett 7:185–190
Dunn WB, Ellis DI (2005) Metabolomics: current analytical platforms and methodologies. TrAC Trends Anal Chem 24:285–294
Evans CT, Ratledge C (1984) Effect of nitrogen source on lipid accumulation in oleaginous yeasts. Microbiology 130:1693–1704
EVANS CT, SCRAGG AH, RATLEDGE C (1983) A comparative study of citrate efflux from mitochondria of oleaginous and non-oleaginous yeasts. FEBS J 130:195–204
Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH Jr, Murphy RC, Raetz CR, Russell DW, Seyama Y, Shaw W, Shimizu T, Spener F, van Meer G, VanNieuwenhze MS, White SH, Witztum JL, Dennis EA (2005) A comprehensive classification system for lipids. J Lipid Res 46:839–861
Fakhry EM, Maghraby DME (2013) Fatty acids composition and biodiesel characterization of Dunaliella salina. J Water Resour Prot 05:894–899
Feofilova EP, Sergeeva YE, Ivashechkin AA (2010) Biodiesel-fuel: content, production, producers, contemporary biotechnology (review). Appl Biochem Microbiol 46:369–378
Francisco ÉC, Neves DB, Jacob-Lopes E, Franco TT (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403
Gao Q, Cui Z, Zhang J, Bao J (2014) Lipid fermentation of corncob residues hydrolysate by oleaginous yeast Trichosporon cutaneum. Bioresour Technol 152:552–556
Ghosh S, Padmanabhan B, Anand C, Nagaraja V (2016) Lysine acetylation of the Mycobacterium tuberculosis HU protein modulates its DNA binding and genome organization. Mol Microbiol 100:577–588
Goers L, Freemont P, Polizzi KM (2014) Co-culture systems and technologies: taking synthetic biology to the next level. J R Soc Interface 11:20140065
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Gultom S, Hu B (2013) Review of microalgae harvesting via co-pelletization with filamentous fungus. Energies 6:5921–5939
Gürü M, Artukoğlu BD, Keskin A, Koca A (2009) Biodiesel production from waste animal fat and improvement of its characteristics by synthesized nickel and magnesium additive. Energy Convers Manag 50:498–502
Gutierrez-Correa M, Tengerdy RP (1998) Xylanase production by fungal mixed culture solid substrate fermentation on sugar cane bagasse. Biotechnol Lett 20:45–47
Hamedi S, Mahdavi MA, Gheshlaghi R (2012) Lipid content and biomass production of Chlorella vulgaris is affected by growth conditions. 2012 Second Iranian Conference on Renewable Energy and Distributed Generation. IEEE, pp. 65-68
Herrera-Valencia VA, Contreras-Pool PY, López-Adrián SJ, Peraza-Echeverría S, Barahona-Pérez LF (2011) The green microalga Chlorella saccharophila as a suitable source of oil for biodiesel production. Curr Microbiol 63:151–157
Huang C, Zong M-h, Wu H, Q-p L (2009) Microbial oil production from rice straw hydrolysate by Trichosporon fermentans. Bioresour Technol 100:4535–4538
Huang C, Wu H, L-p L, W-y L, M-h Z (2012) Effects of alcohol compounds on the growth and lipid accumulation of oleaginous yeast Trichosporon fermentans. PLoS One 7:e46975
Huo S, Dong R, Wang Z, Pang C, Yuan Z, Zhu S, Chen L (2011) Available resources for algal biofuel development in China. Energies 4:1321–1335
Iassonova DR, Hammond EG, Beattie SE (2008) Oxidative stability of polyunsaturated triacylglycerols encapsulated in oleaginous yeast. J Am Oil Chem Soc 85:711–716
Illman A, Scragg A, Shales S (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzym Microb Technol 27:631–635
Islam M, Magnusson M, Brown R, Ayoko G, Nabi M, Heimann K (2013) Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies 6:5676–5702
Jiang X, Liu L, Chen J, Wei D (2018) Effects of Xanthophyllomyces dendrorhous on cell growth, lipid, and astaxanthin production of Chromochloris zofingiensis by mixed culture strategy. J Appl Phycol 30:3009–3015
Juneja A, Ceballos R, Murthy G (2013) Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies 6:4607–4638
Kapoor A, Kumar R, Kumar A, Sharma A, Prasad S (1998) Application of immobilized mixed bacterial culture for the degradation of phenol present in oil refinery effluent. J Environ Sci Health A 33:1009–1021
Kitcha S, Cheirsilp B (2012) Enhancing lipid production from crude glycerol by newly isolated oleaginous yeasts: strain selection, process optimization, and fed-batch strategy. BioEnergy Research 6:300–310
Kitcha S, Cheirsilp B (2014) Enhanced lipid production by co-cultivation and co-encapsulation of oleaginous yeast Trichosporonoides spathulata with microalgae in alginate gel beads. Appl Biochem Biotechnol 173:522–534
Kleerebezem R, van Loosdrecht MC (2007) Mixed culture biotechnology for bioenergy production. Curr Opin Biotechnol 18:207–212
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86:1059–1070
Knothe G (2006) Analyzing biodiesel: standards and other methods. J Am Oil Chem Soc 83:823–833
Knothe G (2008) “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energy Fuel 22:1358–1364
Kraisintu P, Yongmanitchai W, Limtong S (2010) Selection and optimization for lipid production of a newly isolated oleaginous yeast, Rhodosporidium toruloides DMKU3-TK16. Kasetsart J (Nat Sci) 44:436–445
Kumsiri B, Pekkoh J, Pathom-aree W, Lumyong S, Pumas C (2018) Synergistic effect of co-culture of microalga and actinomycete in diluted chicken manure digestate for lipid production. Algal Res 33:239–247
Lamacka M, Sajbidor J, Bohov P (1998) Lipid isolation and fatty acid analysis in Saccharomyces cerevisiae. Comparison of different methods. Biotechnol Tech 12:621–625
Lenka SK, Carbonaro N, Park R, Miller SM, Thorpe I, Li Y (2016) Current advances in molecular, biochemical, and computational modeling analysis of microalgal triacylglycerol biosynthesis. Biotechnol Adv 34:1046–1063
Leung DYC, Wu X, Leung MKH (2010) A review on biodiesel production using catalyzed transesterification. Appl Energy 87:1083–1095
Levering J, Broddrick J, Zengler K (2015) Engineering of oleaginous organisms for lipid production. Curr Opin Biotechnol 36:32–39
Li T, Li C-T, Butler K, Hays SG, Guarnieri MT, Oyler GA, Betenbaugh MJ (2017) Mimicking lichens: incorporation of yeast strains together with sucrose-secreting cyanobacteria improves survival, growth, ROS removal, and lipid production in a stable mutualistic co-culture production platform. Biotechnology for biofuels 10:55
Liang MH, Jiang JG (2013) Advancing oleaginous microorganisms to produce lipid via metabolic engineering technology. Prog Lipid Res 52:395–408
Liang K, Zhang Q, Gu M, Cong W (2012) Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J Appl Phycol 25:311–318
Lin J, Shen H, Tan H, Zhao X, Wu S, Hu C, Zhao ZK (2011) Lipid production by Lipomyces starkeyi cells in glucose solution without auxiliary nutrients. J Biotechnol 152:184–188
Ling J, Nip S, Cheok WL, de Toledo RA, Shim H (2014) Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresour Technol 173:132–139
Liu JA, Moon NJ (1982) Commensalistic interaction between Lactobacillus acidophilus and Propionibacterium shermanii. Appl Environ Microbiol 44:715–722
Liu L, Chen J, Lim P-E, Wei D (2018a) Dual-species cultivation of microalgae and yeast for enhanced biomass and microbial lipid production. J Appl Phycol 30:2997–3007
Liu L, Chen J, Lim PE, Wei D (2018b) Enhanced single cell oil production by mixed culture of Chlorella pyrenoidosa and Rhodotorula glutinis using cassava bagasse hydrolysate as carbon source. Bioresour Technol 255:140–148
Ma Q, Zhou J, Zhang W, Meng X, Sun J, Yuan YJ (2011) Integrated proteomic and metabolomic analysis of an artificial microbial community for two-step production of vitamin C. PLoS One 6:e26108
Mardhiah HH, Ong HC, Masjuki HH, Lim S, Lee HV (2017) A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew Sust Energ Rev 67:1225–1236
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Meersman F, Atilgan C, Miles AJ, Bader R, Shang W, Matagne A, Wallace BA, Koch MH (2010) Consistent picture of the reversible thermal unfolding of hen egg-white lysozyme from experiment and molecular dynamics. Biophys J 99:2255–2263
Meeuwse P, Sanders JPM, Tramper J, Rinzema A (2013) Lipids from yeasts and fungi: tomorrow's source of biodiesel? Biofuels Bioprod Biorefin 7:512–524
Meng X, Yang J, Xu X, Zhang L, Nie Q, Xian M (2009) Biodiesel production from oleaginous microorganisms. Renew Energy 34:1–5
Moghtaderi B, Ness J, Spero C, Cohen D, Cetin E, Corderoy B (2007) Coal-biomass cofiring handbook. Cooperative Research Centre for Coal in Sustainable Development, Callaghan, NSW
Mouget J-L, Dakhama A, Lavoie MC, de la Noüe J (1995) Algal growth enhancement by bacteria: is consumption of photosynthetic oxygen involved? FEMS Microbiol Ecol 18:35–43
Mubarak M, Shaija A, Suchithra TV (2015) A review on the extraction of lipid from microalgae for biodiesel production. Algal Res 7:117–123
Oswald WJ, Gotaas H, Ludwig HF, Lynch V (1953) Algae symbiosis in oxidation ponds: III. Photosynthetic oxygenation. Sewage Ind Wastes 25(6):692–705
Pachapur VL, Sarma SJ, Brar SK, Le Bihan Y, Buelna G, Verma M (2015) Biological hydrogen production using co-culture versus mono-culture system. Environmental Technology Reviews 4:55–70
Papanikolaou S (2012) Oleaginous yeasts: biochemical events related with lipid synthesis and potential biotechnological applications. Fermentation Technology. https://doi.org/10.4172/2167-7972.1000e103
Papone T, Kookkhunthod S, Leesing R (2012) Microbial oil production by monoculture and mixed cultures of microalgae and oleaginous yeasts using sugarcane juice as substrate. World Acad Sci Eng Technol 64:1127–1131
Park J, Chang H (2000) Microencapsulation of microbial cells. Biotechnol Adv 18:303–319
Parmar A, Singh NK, Pandey A, Gnansounou E, Madamwar D (2011) Cyanobacteria and microalgae: a positive prospect for biofuels. Bioresour Technol 102:10163–10172
Patel A, Arora N, Sartaj K, Pruthi V, Pruthi PA (2016) Sustainable biodiesel production from oleaginous yeasts utilizing hydrolysates of various non-edible lignocellulosic biomasses. Renew Sust Energ Rev 62:836–855
Pittman JK, Dean AP, Osundeko O (2011) The potential of sustainable algal biofuel production using wastewater resources. Bioresour Technol 102:17–25
Pourzolfaghar H, Abnisa F, Daud WMAW, Aroua MK (2016) A review of the enzymatic hydroesterification process for biodiesel production. Renew Sust Energ Rev 61:245–257
Probst KV, Schulte LR, Durrett TP, Rezac ME, Vadlani PV (2016) Oleaginous yeast: a value-added platform for renewable oils. Crit Rev Biotechnol 36:942–955
Qin L, Liu L, Wang Z, Chen W, Wei D (2018a) Efficient resource recycling from liquid digestate by microalgae-yeast mixed culture and the assessment of key gene transcription related to nitrogen assimilation in microalgae. Bioresour Technol 264:90–97
Qin L, Wei D, Wang Z, Alam MA (2018b) Advantage assessment of mixed culture of Chlorella vulgaris and Yarrowia lipolytica for treatment of liquid digestate of yeast industry and cogeneration of biofuel feedstock. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-018-2854-8
Rajendran A, Hu B (2016) Mycoalgae biofilm: development of a novel platform technology using algae and fungal cultures. Biotechnol Biofuels 9:112
Ramírez-Verduzco LF, Rodríguez-Rodríguez JE, del Rayo J-JA (2012) Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 91:102–111
Ramos MJ, Fernandez CM, Casas A, Rodriguez L, Perez A (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268
Rathore S, Desai PM, Liew CV, Chan LW, Heng PWS (2013) Microencapsulation of microbial cells. J Food Eng 116:369–381
Ratledge C, Wynn JP (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51:1–52
Renaud SM, Thinh L-V, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211:195–214
Rulli MC, Bellomi D, Cazzoli A, De Carolis G, D'Odorico P (2016) The water-land-food nexus of first-generation biofuels. Sci Rep 6:22521
Ruyters G (1984) Effects of blue light on enzymes. In: Blue light effects in biological systems. Springer, Berlin, pp 283–301
Sakthivel R (2011) Microalgae lipid research, past, present: a critical review for biodiesel production, in the future. J Exp Sci 2:10
Salama el S, Kim HC, Abou-Shanab RA, Ji MK, Oh YK, Kim SH, Jeon BH (2013) Biomass, lipid content, and fatty acid composition of freshwater Chlamydomonas mexicana and Scenedesmus obliquus grown under salt stress. Bioprocess Biosyst Eng 36:827–833
Santos CA, Caldeira ML, Lopes da Silva T, Novais JM, Reis A (2013) Enhanced lipidic algae biomass production using gas transfer from a fermentative Rhodosporidium toruloides culture to an autotrophic Chlorella protothecoides culture. Bioresour Technol 138:48–54
Saraf S, Thomas B (2007) Influence of feedstock and process chemistry on biodiesel quality. Process Saf Environ Prot 85:360–364
Sattur A, Karanth N (1989) Production of microbial lipids: II. Influence of C/N ratio—model prediction. Biotechnol Bioeng 34:868–871
Senthilvelan T, Kanagaraj J, Panda RC, Mandal AB (2013) Biodegradation of phenol by mixed microbial culture: an eco-friendly approach for the pollution reduction. Clean Techn Environ Policy 16:113–126
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553
Shu C-H, Tsai C-C, Chen K-Y, Liao W-H, Huang H-C (2013) Enhancing high quality oil accumulation and carbon dioxide fixation by a mixed culture of Chlorella sp. and Saccharomyces cerevisiae. J Taiwan Inst Chem Eng 44:936–942
Silaban A, Bai R, Gutierrez-Wing MT, Negulescu II, Rusch KA (2014) Effect of organic carbon, C:N ratio and light on the growth and lipid productivity of microalgae/cyanobacteria coculture. Engineering in Life Sciences 14:47–56
Silva-Benavides AM, Torzillo G (2011) Nitrogen and phosphorus removal through laboratory batch cultures of microalga Chlorella vulgaris and cyanobacterium Planktothrix isothrix grown as monoalgal and as co-cultures. J Appl Phycol 24:267–276
Simosa AE (2016) Factors affecting algal biomass growth and cell wall destruction
Singh P, Kumari S, Guldhe A, Misra R, Rawat I, Bux F (2016) Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew Sust Energ Rev 55:1–16
Sitepu IR, Sestric R, Ignatia L, Levin D, German JB, Gillies LA, Almada LA, Boundy-Mills KL (2013) Manipulation of culture conditions alters lipid content and fatty acid profiles of a wide variety of known and new oleaginous yeast species. Bioresour Technol 144:360–369
Sitepu IR, Garay LA, Sestric R, Levin D, Block DE, German JB, Boundy-Mills KL (2014) Oleaginous yeasts for biodiesel: current and future trends in biology and production. Biotechnol Adv 32:1336–1360
Smid EJ, Lacroix C (2013) Microbe-microbe interactions in mixed culture food fermentations. Curr Opin Biotechnol 24:148–154
Somashekar D, Joseph R (2000) Inverse relationship between carotenoid and lipid formation in Rhodotorula gracilis according to the C/N ratio of the growth medium. World J Microbiol Biotechnol 16:491–493
Stansell GR, Gray VM, Sym SD (2011) Microalgal fatty acid composition: implications for biodiesel quality. J Appl Phycol 24:791–801
Subashchandrabose SR, Ramakrishnan B, Megharaj M, Venkateswarlu K, Naidu R (2011) Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol Adv 29:896–907
Subramaniam R, Dufreche S, Zappi M, Bajpai R (2010) Microbial lipids from renewable resources: production and characterization. J Ind Microbiol Biotechnol 37:1271–1287
Sunwoo IY, Kwon JE, Nguyen TH, Ra CH, Jeong GT, Kim SK (2017) Bioethanol production using waste seaweed obtained from Gwangalli Beach, Busan, Korea by co-culture of yeasts with adaptive evolution. Appl Biochem Biotechnol 183:966–979
Tang I-C, Yang S-T, Okos MR (1988) Acetic acid production from whey lactose by the co-culture of Streptococcus lactis and Clostridium formicoaceticum. Appl Microbiol Biotechnol 28:138–143
Taniguchi M, Tanaka T (2004) Clarification of interactions among microorganisms and development of co-culture system for production of useful substances. Adv Biochem Eng Biotechnol 90:35–62
Thevenieau F, Nicaud J-M (2013) Microorganisms as sources of oils. Ocl 20:D603
Thliveros P, Uckun Kiran E, Webb C (2014) Microbial biodiesel production by direct methanolysis of oleaginous biomass. Bioresour Technol 157:181–187
Tsigie YA, Wang C-Y, Truong C-T, Ju Y-H (2011) Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate. Bioresour Technol 102:9216–9222
Vanhercke T, El Tahchy A, Shrestha P, Zhou XR, Singh SP, Petrie JR (2013) Synergistic effect of WRI1 and DGAT1 coexpression on triacylglycerol biosynthesis in plants. FEBS Lett 587:364–369
Wahidin S, Idris A, Shaleh SR (2013) The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour Technol 129:7–11
Wang S, Wu Y, Wang X (2016) Heterotrophic cultivation of Chlorella pyrenoidosa using sucrose as the sole carbon source by co-culture with Rhodotorula glutinis. Bioresour Technol 220:615–620
Wang S-K, Wang X, Tian Y-T, Tao H-H, Sun X-S (2018a) Direct utilization of starch for heterotrophic cultivation of Chlorella pyrenoidosa by co-culture with immobilized Saccharomycopsis fibuligera. Algal Res 33:406–411
Wang SK, Wang X, Tao HH, Sun XS, Tian YT (2018b) Heterotrophic culture of Chlorella pyrenoidosa using sucrose as the sole carbon source by co-culture with immobilized yeast. Bioresour Technol 249:425–430
Williams P, Winzer K, Chan WC, Camara M (2007) Look who's talking: communication and quorum sensing in the bacterial world. Philos Trans R Soc London Sers B Biol Sci 362:1119–1134
Wolfaardt G, Lawrence J, Robarts R, Caldwell D (1994) The role of interactions, sessile growth, and nutrient amendments on the degradative efficiency of a microbial consortium. Can J Microbiol 40:331–340
Wu H, Miao X (2014) Biodiesel quality and biochemical changes of microalgae Chlorella pyrenoidosa and Scenedesmus obliquus in response to nitrate levels. Bioresour Technol 170:421–427
Xia C, Zhang J, Zhang W, Hu B (2011) A new cultivation method for microbial oil production: cell pelletization and lipid accumulation by Mucor circinelloides. Biotechnology for biofuels 4:15
Xie S, Sun S, Dai SY, S.Yuan J (2013): Efficient coagulation of microalgae in cultures with filamentous fungi. Algal Res 2, 28–33
Xue F, Miao J, Zhang X, Tan T (2010) A new strategy for lipid production by mix cultivation of Spirulina platensis and Rhodotorula glutinis. Appl Biochem Biotechnol 160:498–503
Yalcin SK, Ozbas ZY (2008) Effects of pH and temperature on growth and glycerol production kinetics of two indigenous wine strains of Saccharomyces cerevisiae from Turkey. Braz J Microbiol 39:325–332
Yen H-W, Chen P-W, Chen L-J (2015) The synergistic effects for the co-cultivation of oleaginous yeast-Rhodotorula glutinis and microalgae-Scenedesmus obliquus on the biomass and total lipids accumulation. Bioresour Technol 184:148–152
Yong-Hong L, Bo L, Zong-Bao Z, Feng-Wu B (2006) Optimization of culture conditions for lipid production by Rhodosporidium toruloides. Chin J Biotechnol 22:650–656
Yucel S, Terzioglu P, Bogoclu ME, Celikkol M (2016) Changes in the cell growth, lipid content and lipid profile of chlorella protothecoides under different mediums. Sigma J Eng Nat Sci 34:183–190
Zhan J, Lin H, Shen Q, Zhou Q, Zhao Y (2013) Potential utilization of waste sweet potato vines hydrolysate as a new source for single cell oils production by Trichosporon fermentans. Bioresour Technol 135:622–629
Zhang H-l, Wu S-h, Tao Y, L-q Z, Z-q S (2010) Preparation and characterization of water-soluble chitosan nanoparticles as protein delivery system. J Nanomater 2010:1
Zhang Z, Ji H, Gong G, Zhang X, Tan T (2014) Synergistic effects of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for enhancement of biomass and lipid yields. Bioresour Technol 164:93–99
Zhang K, Zheng J, Xue D, Ren D, Lu J (2017) Effect of photoautotrophic and heteroautotrophic conditions on growth and lipid production in Chlorella vulgaris cultured in industrial wastewater with the yeast Rhodotorula glutinis. J Appl Phycol 29:2783–2788
Zhao P, Yu X, Li J, Tang X, Huang Z (2014) Enhancing lipid productivity by co-cultivation of Chlorella sp. U4341 and Monoraphidium sp. FXY-10. J Biosci Bioeng 118:72–77
Zienkiewicz K, Du ZY, Ma W, Vollheyde K, Benning C (2016) Stress-induced neutral lipid biosynthesis in microalgae—molecular, cellular and physiological insights. Biochim Biophys Acta 1861:1269–1281
Zuroff TR, Curtis WR (2012) Developing symbiotic consortia for lignocellulosic biofuel production. Appl Microbiol Biotechnol 93:1423–1435
Acknowledgements
Authors are thankful for the support of the Department of Biotechnology (DBT) through BioCare Program and SRF to NA. KMP acknowledges the Research Support of BEST Pvt. Ltd., NMCG-MoWR, and SERB Extra mural research grant from GOI. The authors express their special gratitude to Ms. Shweta Tripathi, IIT-Roorkee for providing critical technical suggestions and invaluable support during the revision stage of the review article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Gerald Thouand
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Research Highlights:
• Symbiotic relationship between oleaginous microalgae and yeast.
• Enhanced lipid productivity in co-culture as compared to monocultures.
• Biodiesel production using low-cost feedstocks.
• Synergistic approach for sustainable biofuel production.
Rights and permissions
About this article
Cite this article
Arora, N., Patel, A., Mehtani, J. et al. Co-culturing of oleaginous microalgae and yeast: paradigm shift towards enhanced lipid productivity. Environ Sci Pollut Res 26, 16952–16973 (2019). https://doi.org/10.1007/s11356-019-05138-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05138-6