Abstract
Microalgae are ubiquitous in nature that grow rapidly and thrive in harsh conditions due to their single cellular or simple multicellular structure. Microalgae are characterized as oleaginous, as they accumulate appreciable quantity of lipids ranging between 20 and 70% on dry weight basis depending on the surrounding environmental conditions. Extensive research has been performed on different aspects of microalgal lipids, as they are a source of potential compounds that have wide applications in food, chemical, pharmaceutical, and cosmetology industries. Oil-accumulating algae have the potential to enable the commercial-scale biodiesel production. This chapter discusses the current knowledge in microalgae lipids and their metabolism and various approaches and biotechnological applications for the enhancement of lipid content. Different environmental stress conditions such as nutrients (nitrogen and phosphorus) limitation, temperature, light, salinity, and heavy metals that lead to alteration of the lipid biosynthetic pathways toward the neutral lipids (20–50% DCW) formation and accumulation, have been exploited by researchers to obtain high lipid-accumulating strains for biodiesel production. Supplementation of CO2 and phytohormones has also been used to improve the microalgae biomass/lipid productivity. Recently, genetic and metabolic engineering tools have been used for a characterization of genes encoding lipid biosynthesis enzymes and further develop highly efficient and potent strains that enable the algae-based biodiesel production feasible. In addition, microalgae have the ability to uptake CO2 and grow on wastewaters that can be directed toward development of an eco-friendly and economically feasible strategy to produce biomass for biodiesel generation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
1.1 Sources of Biofuel
In the last few years, energy consumption has dramatically increased due to the ever increasing world population, and the global energy demand has been estimated to grow by >85% by 2040 (Parsaeimehr et al. 2015). The world’s energy requirements are majorly satisfied by the fossil fuels that currently serve as the primary energy source. The depletion of the fossil fuels due to the exponentially increasing energy demand has alarmed the research communities to discover alternative energy sources. In view of the above, biofuels generated from renewable resources could be a more effective and sustainable option. Depending on the source, biofuels have been classified as first generation (produced from edible plant substrates such as oilseeds and grains), second generation (produced from nonedible plants or nonedible parts of the plant such as straw, wood, and biomass) and third generation (biofuels derived from algae) (Mohr and Raman 2013). First- and second-generation biofuels have commercial limitations as they require arable land and add to the food crisis faced by today’s society. Third-generation biofuels have emerged as a viable option since they do not require arable land. Recently, fourth-generation biofuels have been characterized that use genetically modified organisms (particularly algae) to attain sustainable production of biofuels. Microalgal lipids have been recognized as a high-energy, low cost, and renewable feedstock for biodiesel production (Borowitzka and Moheimani 2013; Gupta et al. 2014; Guldhe et al. 2014; Ansari et al. 2015).
1.2 Advantages of Algae Biofuel
Algae fuel has been recognized by several energy experts to significantly decrease the dependency on fossil fuels and reduce the greenhouse gases (GHG) emissions. Microalgae are tiny autotrophs that are capable of growing in extreme conditions and produce substantial amount of lipids that can easily be converted into the biofuels by bio-/thermochemical methods. Particularly, the neutral lipids–triacylglycerides (TAGs) which serve as energy storage for microalgae are converted into biodiesel through transesterification process (Chisti 2008; Chen 2011). Several advantages of algal biofuel have been identified by researchers. Demirbas and Demirbas (2011) reported that as per estimates, 20,000–80,000 L algae oil can be produced per acre which is 30 times higher than oil crops such as palm oil. Parker et al. (2008) suggested that the microalgae are responsible for the global carbon fixation (more than 40%) through the efficient utilization of carbon dioxide. Algae are able to thrive in nutrient-rich waste sources including animal wastes, domestic wastewaters (sewage), and some industrial effluents, which can be exploited to develop an integrated process for the treatment of waste sources with simultaneous production of biomass suitable for biofuels production (Abdel-Raouf et al. 2012). In addition, microalgae biomass can be used in aquaculture, as animal feed (Granados et al. 2012) and for extraction of high-value-added bio-products (Lacerda et al. 2011). Mata et al. (2010) and Cuellar-Bermudez et al. (2014) reported that the microalgal compounds including triglycerides, antioxidants, pigments, beta-carotene, polysaccharides, fatty acids, and vitamins are widely used in different industrial sectors (e.g., biofuels, functional foods, nutraceuticals, pharmaceuticals, cosmetics, aquaculture) as bulk commodities. Moreover, microalgae containing lipids and fatty acids, including omega (ω3 and ω6) families, have received attention due to the health benefits upon consumption (Spolaore et al. 2006). However, commercialization of microalgae-based processes is bound to certain limitations (Chisti 2013). Hannon et al. (2010) also revealed that there are number of challenges in the economic cultivation of algae to enhance the oil extraction and fuel process, so that it can compensate petroleum and consequently mitigate CO2 release. Other major challenges include strain isolation and selection, resources (i.e., nutrient and water) and utilization, harvesting, fuel extraction, refining, utilization of residual algal biomass and production, and coproduct development and management.
2 Biology and Biochemical Composition of Microalgae
2.1 Major Biochemical Groups and Their Function
Determination of the biochemical composition of microalgae biomass provides an insight of the organisms behavior and its adaptational response to changes in its environment (Chen and Vaidyanathan 2013). Especially, microalgae ecophysiology is very essential to understand and optimize the large-scale biomass production for biofuel generation (Chia et al. 2013). The biochemical components in microalgae primarily include proteins, carbohydrates, fats, and nucleic acids. The quantity of the components varies with the type of species (Table 1) and is significantly influenced by the environmental conditions including light intensity, temperature, pH, and nutrients availability. The values of the components range as follows: proteins (10–50%), carbohydrates (10–40%), and lipids (20–80%). Gatenby et al. (1997) reported that the biochemical composition variation, due to growth stage, can be related to the age of culture and nutrient depletion, particularly if an organism grows in batch culture. Proteins make up a large fraction (sometimes even more than carbohydrates and lipids) of the actively growing microalgae having both structural and metabolic functions. It is also involved in the photosynthesis apparatus, CO2 fixation, and cell growth machinery. Several algae with high-protein fraction are an ideal source of nutrients for production of functional foods, food additives, and nutraceuticals that have been commercialized in the food and feed markets. Recently, certain amino acid fractions from the algal proteins have been identified as a suitable feedstock for production of higher alcohols (Lan and Liao 2013; Eldalatony et al. 2016). Carbohydrates are the significant products derived from photosynthetic process and the carbon fixation metabolism (Ho et al. 2011). Chlorella, Dunaliella, Scenedesmus, and Chlamydomonas have been reported more than 50% of starch accumulated based on their dry cell weight (Ueda et al. 1996). The carbohydrates in green algae mainly include starch (storage component) in chloroplasts and cellulose/polysaccharides (structural components) in the cell walls. Both polysaccharides and starch can be converted into sugars for the consequent bioethanol production through microbial fermentation (Wang et al. 2011; Choi et al. 2011a, b; Jeon et al. 2013). Microalgae lipids can be divided into two categories: (a) the storage lipids (neutral or nonpolar lipids) and (b) structural lipids (membrane or polar lipids). Storage lipids mostly include TAGs which are predominantly saturated fatty acids and some unsaturated fatty acids that can be converted to biodiesel by transesterification, while structural lipids contain maximum content of polyunsaturated fatty acids. These PUFAs are essential for the nutrition of humans and aquatic animals. Sterols and polar lipids are the key structural components of cell membranes, providing the matrix for different metabolic processes. It also acts as key intermediates in cell signaling pathways.
Schorken and Kempers (2009) reported that the different types of fatty acid from triacylglycerols are the main targets for the development of biotechnological products. Glycolipids, phospholipids, sphingolipids, carotenoids, sterols, and other lipid-soluble compounds from algae are being utilized for the production of bioactive molecules for nutrition, cosmetics, and pharmaceuticals. Kumar et al. (2015) suggested that microalgae biomass with appropriate proportions of unsaturated fatty acids (linoleic (18:2), palmitoleic (16:1), oleic (18:1), and linolenic acid (18:3)) and saturated (stearic (18:0) and palmitic (16:0)) fatty acids could be a suitable biodiesel feedstock.
2.2 Sustainable Energy Sources of Microalgae
The second-generation biomass resources (food crops) are not an appropriate option for biofuel generation due to their inefficiency and unsustainability. On the other hand, microalgae are the most sustainable source of biofuel and positive toward food security and their environmental impact, which encouraged researchers to develop technologies related to microalgal biomass production (Ahmad et al. 2011). Lim et al. (2012) suggested that the microalgal sources are believed to be a sustainable option in biofuel production and the ability of meeting the global demand for sustainable transport fuels. Waltz (2009) also suggested energy density, which can be harvested from microalgae, and it is higher than that of the chief oil-producing crops. Moreover, it does not hold a competing tap into the global food supply chain, and this technology makes it economically viable and feasible for large-scale cultivation and harvesting purposes.
3 Lipid Biochemistry in Microalgae
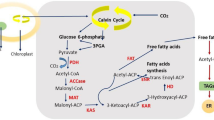
Microalgae have been characterized as oleaginous, as they are capable of accumulating appreciable quantity of lipids. The algal lipid content is considerably influenced due to environmental conditions of the habitat and has been observed to range between 5 and 70% (Table 2). Membrane bilayer constitutes the major fraction of algal lipids, other than triacylglycerol (TAG), hydrocarbons, wax esters, sterols, and prenyl derivatives (Yu et al. 2011). Recent reviews have well documented the biochemistry of lipid synthesis in algae (De Bhowmick et al. 2015; Bellou et al. 2014) and have been presented in Fig. 1. Photosynthesis converts CO2 to glycerate-3-phosphate (G3P) that is a starting material of storage compounds including lipids and carbohydrates. Lipid biosynthetic pathway initiates by transformation of G3P to pyruvate and subsequently to acetyl-CoA in the plastid. The pathway that converts polysaccharides to lipids also yields Acetyl-CoA (Bellou et al. 2012), which is usually used for sugar assimilation by the oleaginous heterotrophs (Bellou et al. 2014). The storage polysaccharides are fragmented via glycolysis in the cytosol and further via citric acid cycle in the mitochondrion. Environmental stress conditions, however, interfere with the citric acid cycle and lead to citrate accumulation in the mitochondrion followed by its transfer in the cytosol. Further, the citrate is sequentially converted to oxaloacetate and acetyl-CoA by cytosolic ATP-dependent citrate lyase. Cytosolic acetyl-CoA carboxylase catalyzes the conversion of acetyl-CoA to malonyl-CoA that is utilized for elongation of fatty acids in the endoplasmic reticulum (ER) membrane (Mühlroth et al. 2013). The aforementioned mechanism was specifically demonstrated in Nannochloropsis salina and Chlorella sp. (Bellou et al. 2012), but it is perhaps similar in oleaginous strains that can thrive under heterotrophic conditions.
Outline of lipid synthesis in microalgae (Adopted from Bellou et al. 2014). Abbreviations: ACC acetyl-CoA carboxylase, ACP acyl-carrier protein, LACS long-chain acyl-CoA synthetase, ATP:CL ATP-dependent citrate lyase, CoA coenzyme A, DGAT diacylglycerol acyltransferase, ER endoplasmic reticulum, FAS fatty acid synthase, FAT fatty acyl-ACP thioesterase, G3P glycerate-3-phosphate, GPAT glycerol-3-phosphate acyltransferase, KAS 3-ketoacyl-ACP synthase, LPAAT lysophosphatidic acid acyltransferase, LPCAT lysophosphatidylcholine acyltransferase, PDC pyruvate dehydrogenase complex, TAG triacylglycerol
In the plastid, malonyl-CoA:ACP transacetylase facilitates the transfer of malonyl-CoA to the acyl-carrier protein (ACP) of the fatty acid synthase (FAS) complex (Blatti et al. 2012). The 3-ketoacyl-ACP synthase catalyzes the generation of ketobutyryl-ACP by condensing the acetyl group with malonyl-ACP. Further, consecutive reduction–dehydration–reduction reactions converts the ketobutyryl-ACP to butyryl-ACP, and such repeated cycles lead to formation of palmitoyl-ACP. Addition of two carbon skeleton from acetyl-CoA leads to formation of stearoyl-ACP. Desaturation of stearoyl-ACP leads to formation of oleoyl-ACP (Yu et al. 2011; Mühlroth et al. 2013). The fatty acids bound to ACP are released and further activated into acyl-CoA by acyl-CoA synthetase situated in the chloroplast envelope and eventually shifted to the cytosol for lipid synthesis. Polyunsaturated fatty acids (PUFAs) are obtained by esterification of the acyl-CoA chains with the structural phospholipids of the ER. Fatty acids are utilized as precursors for generation of TAG in the ER through Kennedy pathway.
The Kennedy pathway includes acylation of G3P by glycerol-3-phosphate acyltransferases followed by lysophosphatidic acid acylation by lysophosphatidate acyltranferase to generate phosphatidic acid (PA). Dephosphorylation of PA leads to generation of diacylglycerol (DAG), which is the primary starting material for synthesis of membrane and storage lipids (TAG) occurring in the chloroplast (Mühlroth et al. 2013). The synthesized TAGs are later deposited in the form of lipid droplets in the cytosol (Martin and Parton 2006). Lipid droplets promote the distribution and recirculation of neutral lipids, phospholipids, lysophospholipids, and acyl groups (De Bhowmick et al. 2015). The disclosure and understanding of lipid synthesis mechanism in algae has opened a path for the metabolic engineering to obtain highly potent strains for biodiesel production, which has been discussed in later section.
4 Recent Common Approaches for Enhanced Lipid Production
Commercialization of algae oil-derived biodiesel requires high lipid productivity of the desired and rapidly growing algae. Optimal growth conditions lead to production of huge amounts of algal biomass but with comparatively low lipid contents. Enhancement of microalgal lipids could improve the economics of biodiesel, and considering this fact, lot of efforts have been focused in developing the strategies in order to improve the biomass and lipid contents (Fig. 2). Microalgae biomass and triacylglycerols (TAGs) compete for the photosynthetic assimilate, and modulation of biochemical pathways is needed to improve lipid biosynthesis. Unfavorable growth conditions such as light, temperature, nutrients (nitrogen and phosphorus) limitation, salinity, and heavy metals modulate the lipid biosynthetic pathways in many microalgae species, leading to the generation and accumulation of neutral lipids (20–50% DCW), majorly as TAG, supporting the microalgae to survive under such adverse conditions (Fig. 3). Such ability of algae to overcome unfavorable environmental conditions by modulating their metabolic pathways has been exploited by researchers to obtain high lipid-accumulating strains for biodiesel production.
Induction of algae lipids under unfavorable stress conditions (Adopted from Sharma et al. 2012)
4.1 Nutrient Limitation
Microalgae growth and lipid composition are significantly affected by the nutrients availability. Under nutrients limitation conditions, the cell division rate gets declined, but active fatty acid biosynthesis is maintained in certain species of algae under sufficient light and CO2 availability for photosynthesis (Thompson 1996). As algal growth declines, synthesis of new membrane compounds is not required, diverting fatty acids into TAG that serve as a defensive mechanism during stress conditions. The ATP and NADPH obtained from photosynthetic reactions are utilized for generation of biomass, regenerating ADP and NADP+ as acceptor molecules for continued photosynthesis under favorable growth conditions. Nutrient limitation conditions lead to depletion of NADP+ pool for photosynthesis due to reduced cell growth and proliferation. Under such conditions, NADPH is consumed in fatty acid biosynthesis, thus regenerating the pool of NADP+ and protecting the cells by continuation of photosynthesis under light conditions (Hu et al. 2008).
Nitrogen is a crucial macronutrient for microalgae, as it influences the growth and lipid metabolism, and is a crucial constituent of the cell organization (Sharma et al. 2012). Nitrogen accounts for 1% to more than 10% of biomass (Costa et al. 2001) and can be used as NO3 −, NO2 −, or NH4 + and also as N2. Nitrogen limitation leads to accumulation of lipids in different microalgae species (Table 3). It decreases the cellular proportion of thylakoid membrane, activates acyl hydrolase, and stimulates phospholipid hydrolysis, which together increases the intracellular fraction of fatty acid acyl-CoA. Nitrogen limitation also activates diacylglycerol acyltransferase, which further catalyzes the conversion of the accumulated acyl-CoA to TAG (Xin et al. 2010).
Under favorable conditions, a small amount of TAG is biosynthesized, and carbon can be fixed not only by photosynthesis but also from acetate (Deng et al. 2011) (Fig. 4a). Nitrogen and sulfur are crucial for protein synthesis, and their insufficiency leads to inhibition of the citric acid cycle and photosynthesis due to inadequacy of the proteins that constitute the photosystem reaction center and photosynthetic electron transport. This leads to reduction in photosynthesis and induction of acetate assimilation. Numerous intermediate metabolites formed during the acetate assimilation are pooled toward Kennedy pathway for generation of TAGs (Fig. 4b).
Synthesis of TAG using (A) photosynthesis and acetate assimilation intermediates under nitrogen and sulfur sufficiency, and (B) acetate assimilation intermediates under nitrogen and sulfur deficiency (Adapted from Deng et al. 2011)
Phosphorus significantly influences the energy transfer and signal transduction mediating cellular metabolic processes, photosynthesis, and respiration. Phosphorus limitation causes defect in cell division, leading to halt of cell growth. The absence of phosphorus also impairs phospholipids synthesis, which promotes the synthesis of TAGs (Deng et al. 2011). Enhanced accumulation of lipids under phosphorus limitation has been reported in different microalgae species (Table 3). In addition, deprivation of silicon also leads to accumulation of lipids in several algal strains (Table 3).
4.2 Light Irradiation and Temperature Stress
Light is an indispensable factor for the survival and growth of autotrophic organisms. Light intensity affects the algae growth by influencing photosynthesis (Stockenreiter et al. 2013). Algae can grow under varying light intensities and show notable alteration in their gross chemical composition and photosynthetic activity (Hu et al. 2008). The algal growth rate is highest at saturation intensity, and it declines with a shift of light intensity from the saturation (Sorokin and Krauss 1958). The photoadaptation process leads to alteration of the algal cell properties depending on the light intensity, which includes alteration in profiles of pigments, growth rate, and the availability of essential fatty acids (Juneja et al. 2013). The lipid metabolism is modulated under influence of different light intensities leading to alteration of the lipid profile (Table 4). Lower light intensities induce the generation of chloroplast bound membrane polar lipids, while under higher light intensities, the total content of polar lipids gets decreased with a concurrent enhancement in neutral lipids, primarily TAGs (Sharma et al. 2012).
The light cycles and the incident light spectral composition also affect the growth of algae. Light/dark cycles at distinct growth phases significantly alter the algal lipid composition (Table 4). Specific components (wavelengths) of light influence the cellular processes such as chlorophyll synthesis and cell division. The algal growth rate and composition of biochemical contents are also influenced by the wavelength of illuminating light. Specifically, the effect of blue light (400–480 nm), red light (620–750 nm), and UV radiations on the microalgae growth and lipid content has been reported (Table 4).
Algae have the capability to survive and grow under varied temperature (15 to 40 °C), and it is one of the crucial environmental factors that affect the growth rate and composition of biochemical contents in algae. Temperature significantly influences the fatty acid composition of algae with an alteration of fatty acid saturation (membrane lipids) to adapt against the changing environment due to a temperature shift (Table 4). In most microalgae species, fatty acid unsaturation increases with decreasing temperature, while fatty acid saturation increases with increasing temperature.
4.3 Salinity-, pH-, and Metal-Induced Stress
Salinity (salt concentration) is another important parameter that influences algal growth. Algae show different growth rate and biochemical composition under the presence of salt concentration other than their natural/adapted concentration in the growth medium (Table 5). Gradual rise in initial NaCl concentration from 0.5 M to 2.0 M during cultivation of Dunaliella tertiolecta enhanced the lipid content (intracellular) and level of TAG (Takagi 2006). In another study, increase in saturated and monounsaturated fatty acids of Dunaliella was observed in response to increase in NaCl concentration from 0.4 to 4 M (Xu and Beardall 1997). The growth rate and lipid content of freshwater alga Botryococcus braunii was increased with increasing NaCl levels in the culture medium (Ben-Amotz et al. 1985).
Medium pH significantly influences algal growth because it regulates the solubility and availability of nutrients and CO2 (Juneja et al. 2013). Alteration in lipid composition of microalgae has been reported with fluctuations of the medium pH (Table 5). Incrementally adjusted pH during the growth promoted accumulation of lipids compared to constant pH in five species of Chlorellaceae (Skrupski et al. 2014). Chlorella cultivated under alkaline pH stress conditions showed an increased TAG level with a decrease of membrane lipids (Guckert and Cooksey 1990). In another study, the TAG content was enhanced to 63% in Chlorella at initial pH of 5.0 (Zhang et al. 2014).
Metal ions also enhance the lipid content in several microalgae species (Table 5). Exposure of Euglena gracilis to low chromium (Cr6+) concentration increased the lipid content under photoautotrophic or mixotrophic growth conditions (Rocchetta et al. 2006). Zerovalent iron nanoparticles increased the lipid productivity of Arthrospira maxima and Parachlorella kessleri by 40 and 66%, respectively (Padrova et al. 2015). Fatty acid saturation was increased in Dunaliella salina and Nannochloropsis salina cells by nickel (Mohammady and Fathy 2007). Arsenic [As(III)] exposure enhanced the cell lipid content in Nannochloropsis sp. with a decreased fraction of polyunsaturated fatty acids and increased fractions of short-chain saturated (C16:0, C18:0) and monounsaturated (C16:1, C18:1) fatty acids (Sun et al. 2015). Thus, metal stress can be used to modulate the fatty acid profile of microalgae and obtain biodiesel with desired properties (Miazek et al. 2015).
4.4 Supplementation of CO2 and Phytohormones
Carbon constitutes around 50% of microalgae biomass on a dry weight basis, which majorly comes from the photosynthetically fixed carbon dioxide. Photosynthesis includes light and dark reactions, with dark reaction as one of the rate-limiting steps because of the insufficient availability of CO2. Carbon fixation in microalgae is initiated by sequestration of CO2 into Calvin cycle, and low concentrations of CO2 in air become a key limiting factor. Thus, external supply of CO2 can overcome substrate limitation and enhance the photosynthetic efficiency, subsequently improving the biomass and constitutes including carbohydrates and lipids (Sun et al. 2016). Supplementation of 15% CO2 increased the biomass concentration and total lipid content of Nannochloropsis sp. from 0.71 to 2.23 g L−1 and 33.8–59.9%, respectively (Jiang et al. 2011). The highest specific lipid productivity of 0.164 g-lipids g-cell−1 day−1and oleic acid content of 44% was obtained in C. vulgaris with 15% CO2 after 7 days of cultivation (Ji et al. 2013). The oleic and linoleic fatty acid levels were increased in Scenedesmus sp. and Chlorococcum sp. on supplementation of 5% CO2 (Prabakaran and Ravindran 2013).
Plant hormones (phytohormones) increase the microalgae growth by modulating the intrinsic biochemical pathways (Hunt et al. 2011). Phytohormones are chemical messengers which regulate the plant growth and developmental processes. Phytohormones, including auxins, brassinosteroids, cytokinins, jasmonides, gibberellins, ethylene, abscisic acid, polyamines, , salicylates, and signal peptides, have been identified in various algae species (Tarakhovskaya et al. 2007; Raposo and Morais 2013). Supplementation of phytohormones for enhanced microalgae biomass and metabolite production has been extensively studied (Hunt et al. 2011; Bajguz and Piotrowska-Niczyporuk 2013; Tate et al. 2013; Raposo and Morais 2013; Czerpak and Bajguz 1997). A newly discovered phytohormones diethyl aminoethyl hexanoate enhanced the growth by 2.5-fold and the total fatty acid content up to 100 mg g−1 DCW with Scenedesmus obliquus (Salama et al. 2014b). The cell number of Chlorella vulgaris was increased with supplementation of indole-3-acetic acid (IAA) at 0.1 μM by 53%, indole-3-n-butyric (IBA) at 0.1 μM by 46%, phenylacetic acid (PAA) at 1 μM by 34%, and naphthyl-3-acetic acid (NAA) at 1 μM by 24% compared to control after 48 h of cultivation (Piotrowska-Niczyporuk and Bajguz 2014). The levels of photosynthetic pigments, soluble proteins, and monosaccharides were also enhanced at the respective phytohormones concentrations. The biomass production of Chlamydomonas reinhardtii was enhanced between 61 and 69% with supplementation of IAA, gibberellic acid (GA3), and kinetin (KIN) (Park et al. 2013).
Strategies involving nutrient deprivation, salinity, light, temperature, CO2, and phytohormones have been extensively utilized for enhancing the lipid content of microalgae, but such approaches have limitations to increase the feasibility of the overall process. However, the knowledge of the biochemical mechanisms and the molecular insights for lipid accumulation influenced by such stress environments in microalgae cells could be useful in inventing new strains, improving known strains and methods for greater lipid productivities. Thus, metabolic engineering approach has been recently initiated to develop highly efficient and potent strains to enable the algae-based biodiesel production feasible.
5 Molecular and Genetic Engineering Tools for the Improvement in Microalgal Lipids
A significant improvement in the strategies to improve the microalga biomass is needed in order to achieve a good quality biodiesel. The use of genetic and metabolic engineering approach to develop microalgal strains with high lipid-accumulating capability is a good approach for strain improvement (Larkum et al. 2012; Singh et al. 2016). The key genes coded for lipid synthesis pathways have been recently identified, and full genome of several microalgal strains have been deciphered (Tabatabaei et al. 2011). Environmental risk assessment and long-term viability of genetically engineered microalgal strains in open ponds are the major challenges.
5.1 Strain Improvement Using Mutagenesis Approach
Specific algal strains are utilized for a particular purpose. For example, fast-growing strains are used for biomass production, whereas other strains are utilized for biomass production of astaxanthin, eicosapentaenoic acid (EPA), and oils (Pulz and Gross 2004; Trentacoste et al. 2013). UV irradiation, reactive oxygen species, and changes in genetic material result in transformation of wild-type strain into mutants, which causes genetic variability with potential for evolution (Hlavova et al. 2015; Eyre-Walker and Keightley 2007). Different types of mutagens can be used to generate the mutants. The mutation frequency depends upon the intensity of mutagenic compounds used for mutations. Mutagenic effect is very specific; hence, to maximize and cover the mutation in an entire genome, several thousands of independent mutants should be produced. A desired phenotype can be selected from this mutant population. Although mutant population generation is a simple task, the selection of mutants with desired phenotypes through mutational screening is extremely challenging, creating a major hurdle of any mutagenic screen (Hlavova et al. 2015). Specific screening protocols to screen the desired phenotypes based on the mutant properties such as improved growth, increased cell size, improved productivity of a biochemical content, or resistance to different compounds.
5.1.1 Available Chemical and Physical Treatment Methods for Mutagenesis
Chemical and physical treatments to generate mutations are most favorite options among the researchers due to simplicity in their application, and their mutagenic capabilities are well described (Table 6). Alkylating agents, for example, methylnitronitrosoguanidine (MNNG) and ethyl methane sulfonate (EMS), are most extensively used chemical mutagens for algal cells. For the first time, Chaturvedi and Fujita (2006) utilized these agents in mutagenic screenings in order to increase EPA production in Nannochloropsis oculata and to enhance growth of Chlorella species (Ong et al. 2010). Irradiations (UV, gamma rays, and heavy ion beams) are used as typical physical mutagens. Mutagenesis by UV is very easy to perform and do not require specialized equipment or chemicals. The mutagenic potential and mode of action of each type of radiation on cells depend on its energy. The simplicity and potential makes this method very popular, both in basic research with certain specifications and in applied science to generate engineered strains, which can synthesize higher amount of oils (Neupert et al. 2009; de Jaeger et al. 2014; Vigeolas et al. 2012). Improved production of astaxanthin using gamma irradiation is also evident (Najafi et al. 2011). Despite their tremendous capability, irradiation techniques are not very commonly used because it requires specialized equipment making these procedures highly expensive.
Point mutations can be used to isolation of essential gene mutants through alter the activity of gene product without its inactivation. Extra precautions are needed to ensure the survival of desired gene mutants. Conditional mutants show the phenotype under specific and restrictive conditions, whereas under permissive conditions, they behave as wild type. Temperature-sensitive mutants are the most commonly used type of mutants. Temperature-sensitive mutants have mutations in cell cycle regulators.
These mutants grow and divide normally at a permissive (usually lower) temperature, whereas their growth and cell division is completed inhibited at restrictive (higher) temperatures (Harper et al. 1995; Hartwell et al. 1974; Nurse et al. 1976; Thuriaux et al. 1978). These types of mutants were verified for lipid production at a restrictive temperature in Chlamydomonas reinhardtii and Chlorella vulgaris (Yao et al. 2012), which assisted the production of neutral lipids by 20%. Some of the mutants showed differences in lipid composition with temperature shift. As the main consumer of the cell’s energy reserves is blocked, the mutants can show variable amounts of starch along with lipids. The temperature-sensitive mutants could possibly produce lipid or starch with temperature increase serving as a convenient switch. However, such a temperature switch can be very costly in real-scale algal bioreactors, where temperature controller adds additional costs. Physical and chemical mutagens yield strains having enhanced properties, but these are not considered as GMOs. The spectrum of products obtained by this approach is limited by the natural properties of algal species (Hlavova et al. 2015).
5.2 Genetic Engineering of Microalgae and Its Technical Progress
Even though our Mother Nature is very diverse in terms of various algal species (approximately 10,000 species), only a few thousand are collected, several hundred are explored for biochemical characteristics, and just few are cultivated for industrial application (Spolaore et al. 2006; Parmar et al. 2011). Although lot of research was dedicated to the commercial cultivation of some limited algal species, metabolic engineering of algae is equally important to gain enhanced yield of biomass as well as their biochemical content and to optimize their growth and harvesting. GM strains are usually associated with accidental consequences to environment and public health. These problems need to be taken into consideration for designing a high-scale reactor for mass cultivation of genetically modified strains. The large-scale cultivation deals with serious risk of escape of genetically modified strains and contamination of the natural strains (Parmar et al. 2011). Modified strains have a great chance of release in air and transported over far distances and persist in diverse harsh environmental conditions. Despite these consequences, researchers are continuously developing transgenic algal strains to boost up recombinant protein expression, enhanced metabolism, and enhanced photosynthetic activities, which helps to boost the future of engineered microalgae (Rosenberg et al. 2008).
The idea of increasing valuable compounds in microalgae using genetic engineering approach is very attractive. The most impressive strategies in implementation of molecular tools for the enhancement of microalgal lipids are summarized in Fig. 5. The absence of cell differentiation and allelic genes due to their haploid nature of most vegetative stages of microalgae makes their genetic manipulations much simpler than higher plants (Pulz and Gross 2004). In the last decade, there is a significant advancement in the development for microalgal transformation methods. Genetic modifications in a variety of more than 30 algal species such as Chlorophyta, Rhodophyta, Phaeophyta, diatoms, euglenoids, and dinoflagellates have been successfully conducted to date using molecular tools (Radakovits et al. 2010). Most of the researchers studied the genetic modification of Chlamydomonas genome, because stable genetic transformation is reported for these species (Boynton et al. 1988; Fernandez et al. 1989; Merchant et al. 2012; O’Neill et al. 2012; Singh et al. 2016). Nevertheless, in recent past, whole genome sequencing for many lipid-containing microalgal strains was conducted which includes Chlorella vulgaris, Phaeodactylum tricornutum, Nannochloropsis, Coccomyxa sp., Micromonas, Ostreococcus tauri, Ostreococcus lucimarinus, Volvox carteri, and Thalassiosira pseudonana (O’Neill et al. 2012; Merchant et al. 2012; Singh et al. 2016).
Molecular schemes for enhancing accumulation of lipids in microalgae (Adapted from Singh et al. 2016)
5.3 Tools and Techniques of Genetic Transformations in Microalgae
Varieties of transformation methods are available to transfer particular DNA into microalgal cells such as agitation in the presence of DNA, particle bombardment and silicon carbide whiskers, agitation of a cell suspension along with DNA and glass beads, electroporation, artificial transposons, Agrobacterium infection, viruses, and Agrobacterium-mediated transformation. The most important steps for the successful transformation are insertion of foreign DNA molecules into the host cell and maintaining its viability for long term. The first successful genetic transformation was achieved in Chlamydomonas reinhardtii by agitating its cell suspension in the presence of DNA, polyethylene glycol (PEG), and glass beads (Kindle 1990). A few years later, this method was successfully applied for gene transformation in some other microalgae such as Amphidium and Sybiodium (Wijffels et al. 2013). Nevertheless, the major weakness of this method is the requirement of cell wall-deficient host strain. Therefore, this method cannot be used for microalgal strains having thick and complex cell wall structures, viz., Scenedesmus and Chlorella (Misra et al. 2014; Voigt et al. 2014).
The more advanced method such as electroporation is more suitable in these circumstances, as this technique can easily disrupt the lipid bilayers of the cell wall creating a channel for the efficient transport of genetic material through the plasma membrane by means of electric current. This method was used for transformation in Chlamydomonas reinhardtii, Chlorella vulgaris, Chlorella eliopdodeia, Chlorella sp., Phaeodactylum, Dunaliella salina, and Nannochloropsis oculata (Singh et al. 2016). Diacylglycerol acyltransferase (BnDGAT2) gene from Brassica was successfully transformed in Chlamydomonas reinhardtii to improve its lipid accumulation using electroporation method (Ahmad et al. 2015). However, its efficiency depends on several factors such as pulse length, temperature, field strength, membrane characteristics, and medium composition and concentration of DNA (Kumar et al. 2004). Particle bombardment is the widely used method for chloroplast and nuclear genome transformation to manipulate metabolic pathways such as fatty acid biosynthesis and TAG synthesis. The multiple copies of recombinant DNA can be delivered through cellular as well as chloroplast membranes using this method, resulting in increased chances of successful mixing regime (Leon-Banares et al. 2004). This method has been successfully applied for stable chloroplast and nuclear transformation of Chlamydomonas reinhardtii, Chlorella ellipsoidea, Chlorella kessleri, Chlorella sorokiniana, and diatom Phaeodactylum tricornutum (Niu et al. 2012; Singh et al. 2016). Several researchers have demonstrated the high lipid content of these microalgal strains, which can be further enhanced by this transformation method. Electroporation and particle bombardment methods usually used for eukaryotic microalgae provided highest transformation rate and enables lipid enhancement (Tabatabaei et al. 2011).
Transformation conducted using Agrobacterium tumefaciens is the most widely used technique for plant cells (Kumar et al. 2004) due to its natural ability to transfer inter-kingdom DNA transfer. Microalgal lipids content can be improved through Agrobacterium tumefaciens-mediated transformation by expressing exogenous genes coded for lipid metabolism. Cheng et al. (2012) transformed (gfp gene) Schizochytrium using this method. The challenge in transformation of algae is the application, and efficiency of transformation method is not uniform for all the algal strains; therefore, a lot of efforts have been dedicated to develop the transformation methods (Rosenberg et al. 2008; Singh et al. 2016). A different molecular approaches overview is presented in Table 7.
5.4 Genetic Engineering in Selective Organelles of Microalgae
5.4.1 Chloroplast and Nuclear Engineering
Chloroplast is an attractive choice for genetic manipulation that results in high-level expression of foreign genes (O’Neill et al. 2012; Singh et al. 2016). Therefore, chloroplast is the best choice for the manipulation to enhance biomass, lipid, and pigment production (Napier et al. 2014). Such approach was successfully implemented previously in microalgal strains such as Haematococcus pluvialis, Chlamydomonas reinhardtii, Dunaliella sp., and Scenedesmus sp., which are reported for their high lipid-producing capacities (Guo et al. 2013; Gutierrez et al. 2012; Potvin and Zhang 2010). Photosynthetic activity in Chlamydomonas reinhardtii was improved after modifications in light-harvesting complexes (LHC) using RNAi technology (Wobbe and Remacle 2015). The genetically engineered Chlamydomonas reinhardtii showed reduction in photo inhibition, thus improving the biomass yields. Chloroplast engineering can enhance lipid accumulation and biomass production simultaneously leading to increase in the overall volumetric lipid productivity in order to make biodiesel production an economical process.
Alteration in microalgal nucleus may provide a great chance to enhance the lipid accumulation as well as the quality of microalgal biodiesel. The presence of lipid biosynthesis genes in different cell organelles has already been revealed by whole genome analyses of microalgal strains (Wang et al. 2014; Misra et al. 2012). The nuclear genome of microalgae contains approximately 6% of total genes responsible for lipid biosynthesis (Misra et al. 2012). Most of these genes are coded for membrane lipid synthesis, TAG synthesis (DGAT), and fatty acid chain termination; therefore, manipulation in these genes can improve quality as well as quantity of the microalgal lipids in genetically engineered strains. Microalgal strains such as Phaeodactylum tricornutum, Nannochloropsis oceanica CCMP1779, and Fistulifera sp. have been successfully used for nuclear transformations (Muto et al. 2013; Singh et al. 2016; Vieler et al. 2012). Most of the studies reported single gene insertion for microalgal nuclear transformations. Noor-Mohammadi et al. (2014) developed a novel technique involving multigene expression, in which multigene pathway in yeast was constructed, integrated it in nuclear genome of Chlamydomonas reinhardtii for the co-expression of three reporter proteins (Ble, AphVIII, and GFP). The multigene expression technique can also be used to express functional genes of biomass generation and lipid synthesis pathway to improve lipid productivity. The multigene expression studies have a great potential, which can be suitably exploited to improve both quality of fatty acid synthesis and quantity of TAG synthesis.
5.5 Expression Analysis of Genes Involved in Lipid Biosynthesis
The microalgal lipid biosynthesis pathway has been intensively studied and well understood now (Cao et al. 2014; Radakovits et al. 2011; Purton et al. 2013) (Fig. 2). Lipid biosynthesis is a multistep reaction, catalyzed by fatty acid synthase (an acyl-carrier protein) (Harwood and Guschina 2009). Acyltransferases of the Kennedy pathway such as acyl-CoA:glycerol-3-phosphate acyltransferase (GPAT), acyl-CoA:diacylglycerol acyltransferase (DGAT), and acyl-CoA:lysophosphatidic acyltransferase (LPAAT) are the key enzymes in the formation of fatty acid patterns of TAGs (De Bhowmick et al. 2015). High microalgal growth rates and biomass production under favorable conditions (optimum nutrients and cultivation parameters) are the outcome of increased translation and transcription processes (Merchant et al. 2012; Singh et al. 2016). Fan et al. (2014) examined the consequence of nutrient stress (phosphorus, nitrogen, and iron) on Chlorella pyrenoidosa, whereas the upregulation in expression of accD and rbcl genes was observed at higher concentrations of iron leading to high lipid productivity in Chlorella sorokiniana (Wan et al. 2014). Such studies provide the in-depth mechanism of lipid accumulation in the microalgae due to changes in cultivation conditions (Jusoh et al. 2015; Fan et al. 2014). The recent, most advanced molecular methods including microarray analysis, transcriptome analysis, and full-length overexpressed sequence tag (EST) transcript sequencing can reveal the mechanism of lipid biosynthesis under different stress conditions and give deep understanding of the key genes involved in triggering lipid accumulation (Trentacoste et al. 2013; Shin et al. 2015). Gene expression analysis can disclose the major functional genes involved in lipid biosynthesis, and therefore existing stress strategies can be improved further to achieve better lipid yields in microalgae.
5.6 Overexpression of Lipid Biosynthesis Enzymes
5.6.1 Acetyl-CoA Carboxylase (ACC)
Lipid metabolism is a complex process, which involves a number of chemical conversion processes catalyzed by different enzymes. Acetyl-CoA carboxylase (ACC) strongly control the metabolic flux of fatty acid synthesis in plants, and hence, its overexpression is studied in several species in order to enhance the generation of lipids. Overexpression of ACCase could be one of the most successfully implemented approaches for the improvement in fatty acid synthesis in microalgae. It has been well established that overexpression of ACCase can enhance accessibility of malonyl-CoA in chloroplast which subsequently trigger increase in fatty acid biosynthesis (Blatti et al. 2013; Liang and Jiang 2013; Singh et al. 2016). A one- to twofold rise in activity of plastid ACC along with 6% increase in fatty acid content was observed when the cytosolic ACC from Arabidopsis was overexpressed in Brassica napus plastid (Roesler et al. 1997). Four ACC genes of E. coli BL21 were cloned and overexpressed in the same strain by Davis et al. (2000). It showed an enhanced ACC enzymatic activity which subsequently increased the intracellular malonyl-CoA pool. A sixfold increase in the rate of fatty acid synthesis was observed after co-expressing thioesterase I (encoded by the tesA gene) and ACCase (encoded by accA, accB, accC, accD). It confirmed that the committing step catalyzed by ACC was certainly the rate-limiting step for fatty acid biosynthesis in this strain. Nevertheless, enhancement in lipid production was not highly significant, suggesting that effective transformation of fatty acids to lipids was prevented by a secondary rate-limiting step after fatty acid formation in E. coli. ACC isolated from microalgae was also reported to be overexpressed in diatoms (N. saprophila and C. cryptica) (Roessler 1990). Similar to E. coli, the transgenic diatoms also resulted in insignificant increase of lipid accumulation (Dunahay et al. 1995, 1996). The expression of ACCase leads to an increase in microalgae under certain nutrient-limited cultivation conditions, however, not necessarily associated with higher lipid yields (Fan et al. 2014). Sheehan et al. (1998) concluded that enhancement in the whole lipid biosynthesis pathway in diatoms may not be solely dependent on the overexpression of ACC enzyme alone. In support to this conclusion, there are very rare reports mentioning the increase in the relevant enzymes with subsequent enhancement in lipid accumulation. It can be stated on a conclusive note that ACC does not catalyze the rate-limiting step alone, and a secondary rate-limiting step emerged when ACC was overexpressed in a particular species.
5.6.2 Fatty Acid Synthetase (FAS)
KAS subunit of FAS in E. coli was overexpressed by Subrahmanyam and Cronan (1998) to facilitate the C2 concatenation which was a failed trial due to extreme toxicity for the cell. In another study, overexpressed E. coli KAS III showed major alterations in the fatty acid composition of rapeseed with the significant changes in 18:1 fatty acids and short-chain fatty acids (14:0) (Verwoert et al. 1995). Likewise, KAS III from spinach Spinacia oleracea was overexpressed in cress Arabidopsis, tobacco Nicotiana tabacum, and rapeseed, which led to increase of fatty acids (16:0) along with the decline of the lipid synthesis rate (Dehesh et al. 2001). Targeting subunits of FAS for manipulation to enhance metabolism of fatty acid are challenging because the differences in multipoint controls among different species create critical complications in heterologous expression of multienzymatic complexes (Courchesne et al. 2009).
5.6.3 Acyl-CoA:Diacylglycerol Acyltransferase (DGAT)
DGAT is associated with last stage of TAG formation for the formation of triacylglycerol from fatty acyl-CoA and diacylglycerol. The insertion of Arabidopsis DGAT in yeast and tobacco showed increase of DGAT activity by 200–600-fold, and TAGs accumulation increased by three- to ninefold in the transformed yeast, whereas in the transformed tobacco, TAG content amplified to sevenfold (Bouvier-Nave et al. 2000). The overexpression of DGAT gene in plant Arabidopsis has also enhanced the oil content by 10–70% due to positive influence of DGAT activity (Jako et al. 2001). Overexpression of DGAT would force the conversion of diacylglycerol to TAG instead of phospholipid formation. Another study conducted by Thelen and Ohlrogge (2002) reported that formation of fatty acid can be stimulated by enhancing TAG synthesis rate in plants through overexpression of DGAT. These results suggest DGAT is definitely engaged in rate-limiting step of lipid biosynthesis. However, overexpression of DGAT in microalgae is hardly reported until today.
5.6.4 Lysophosphatidate Acyltransferase (LPAT)
Lysophosphatidate acyltransferase (LPAT) is one of the enzyme engaged in TAG formation, and its overexpression can enhance lipid accumulation. Zou et al. (1997) for the first time attempted the conversion of rapeseed with a putative sn-2 acyltransferase gene from the Saccharomyces cerevisiae. They overexpressed lysophosphatidate acyltransferase (LPAT) activity in rapeseed and observed 8–48% increase in oil content. However, the increasing activity of LPAT in developing seeds may disturb the steady-state level of diacylglycerol. Some of the enzymes including acetyl-CoA synthase (ACS), ATP:citrate lyase (ACL), and malic enzyme (ME), which are not related to lipid metabolism can also increase the pool of essential metabolites for lipid biosynthesis via influencing the rate of lipid accumulation.
5.6.5 Acetyl-CoA Synthase (ACS)
ACS is known to be involved in the formation of acetyl-CoA using acetate as substrate. In the presence of acetate, bacterial strains overexpress ACS with subsequent enhancement in fatty acid synthesis rate (Lin et al. 2006). For instance, overexpression of ACS gene in E. coli led to a ninefold increase in ACS activity, subsequently increasing the utilization of acetate from the medium, which can contribute to lipid biosynthesis. Brown et al. (1977) reported similar observations of enhanced lipid biosynthesis.
5.6.6 Malic Enzyme (ME)
Malic enzyme ME can convert malate into pyruvate along with reduction of a NADP+ into NADPH (Wynn et al. 1999). It was reported that ME with its increased activity can enhance the pool of cytosolic NADPH, providing additional reducing energy to lipogenic enzymes including ACL, ACC, and FAS. A metabolon could be formed between ME and FAS to create a channeling of NADPH. These are formed by ME toward the FAS active sites. Zhang et al. (2007) investigated overexpression of ME in Mucor circinelloides to process lipogenesis without energy restriction to achieve high lipid accumulation. The genes encoding ME from Mortierella alpine (malEMc) and M. circinelloides (malEMt) were overexpressed in M. circinelloides which led to three- and twofold increase of ME activity for the transgenic malEMc and malEMt strains, respectively. A faster lipid accumulation for the transgenic malEMt and malEMc strains (2.5- and 2.4-fold higher, respectively) was predicted because of the ME activity increase in both cases.
5.6.7 ATP:Citrate Lyase (ACL)
ACL provides source of acetyl-CoA for fatty acid biosynthesis by catalyzing the conversion of citrate into oxaloacetate and acetyl-CoA. ACL is one of the major enzymes in lipid accumulation regulation in mammals, oleaginous yeast, and fungi. Rangasamy and Ratledge (2000) constructed a gene that encoded for a fusion protein of the rat liver ACL. These are with the leader peptide for the small subunit of ribulose bisphosphate carboxylase and inserted into the genome of tobacco. Overexpression of this gene enhanced the total ACL activity by fourfold, subsequently increasing the quantity of fatty acids by 16%, however, without any major changes in fatty acid profile.
5.7 Inhibiting the Competitive Pathways
Blocking the pathways (e.g., carbohydrate and lipid catabolism), which are considered competitive for the desired product, is an effective strategy for improving microalgal lipid accumulation (Blatti et al. 2012; Liu and Benning 2013; Radakovits et al. 2010). Carbohydrate metabolic pathways are essential for accumulation and storage of carbon in the form of starch (Gonzalez-Fernandez and Ballesteros 2012). Therefore, suppressing the carbohydrate metabolism can divert the carbon flow toward lipids biosynthesis. A mutant of Scenedesmus obliquus showed up to 51% increase in TAG accumulation (0.217 g mol−1) over the wild type (0.144 g mol−1) under similar conditions (Breuer et al. 2014). Moreover, there was no alteration in photosynthetic behavior of both the wild type and mutants. This genetic manipulation only affected the carbohydrate metabolism and not photosynthetic performance. In another study, TAG accumulation was increased by ten times in mutant strain of Chlamydomonas. It was believed that deactivation of ADP-glucose pyrophosphorylase catalyzed the committing step in metabolism of starch (Li et al. 2010). These breakthrough investigations provided a future direction for lipid enrichment by redirecting “C” pool from synthesis of starch toward accumulation of lipids by knocking down the key genes involved in carbohydrate synthesis. However, it should be noted that interruption in synthesis of starch might result in reduced microalgal growth, which would ultimately have worse effect on the final productivity of lipids. Instead, suppression of lipid catabolism is one of the effective tactics employed to enhance the microalgae lipid accumulation. Such trials have been conducted in a mutant strain of Thalassiosira pseudonana and shown 3.5-fold increase in lipid accumulation after knocking down the regulation of multifunctional enzymes lipase/phospholipase/acyltransferase in the lipid catabolism (Trentacoste et al. 2013) (Table 5). These strategies can be employed for microalgae to enhance lipid accumulation without compromising microalgal growth.
5.8 Modification in Fatty Acid Chain Length for the Improvements in Lipid Quality
The properties of produced biodiesel depend upon the microalgal lipid’s composition. Therefore, enhancing the lipid accumulation in microalgae is not enough, and development of approaches for the advancement of lipids quality in microalgae is crucial to meet the standard specifications for biodiesel (Parsaeimehr et al. 2015). The most desirable fatty acids for biodiesel production are monounsaturated and saturated fatty acids (12:0, 14:0, 16:0, 16:1, 18:0, and 18:1). Acyl-ACP thioesterase releases the fatty acid polymer from fatty acid synthase and thereby controls the chain length of fatty acid. These enzymes can enhance the composition of the fatty acids which is useful to achieve anticipated fuel properties. The transformation of two shorter chain length fatty acid acyl-ACP thioesterases from Umbellularia californica and Cinnamomum camphora into Phaeodactylum tricornutum significantly improved the percent composition of myristic (C14:0) and lauric (C12:0) acids in overall fatty acid profile (Radakovits et al. 2011). The strategies involving the alteration of fatty acid chain length using molecular approaches to improve the microalgal lipid quality with desired compositions have significant potential for biodiesel generation in the near future.
6 Conclusion and Future Outlooks
Economical production of biodiesel from microalgae is a bottleneck in biorefinery industries. One of the most possible approaches to achieve this goal is by assuring high lipid accumulation in microalgal cells. This chapter describes the recent advancements in lipid enhancement approaches. Several novel approaches conducted to enhance biolipids in the recent past have been discussed. The strategies such as altering the light intensity and nutrient composition of the medium; inducing stress conditions such as salinity and temperature; and adding certain chemicals and phytohormones can be successfully combined with the application of wastewater as nutrients in order to make the biomass generation a cost-effective process. These innovative strategies have ensured bright future in microalgal biotechnology for successful enhancement in biomass and lipid productivity. Despite of their great potential, the traditional biochemical approaches still need a lot of significant improvements for enhanced lipid accumulation so as to fulfill the need for biodiesel commercialization. Therefore, strain improvements through mutations metabolic engineering approaches and synthetic biology strategies can possibly provide us the necessary developments in microalgal biotechnology so that microalgae can be used as a feedstock for commercial lipid production. Particularly in biocatalyst engineering, attention should be given on collection of novel genetic properties of microalgae, including genome sequencing to explore the accessibility of appropriate hosts and gene libraries, development of novel methods of nuclear transformation and controlled overexpression of lipid metabolites, and blocking competitive pathways. A rise in both technological applicability and fundamental knowledge is required to report the current bottlenecks in the developments of microalgal biodiesel and to make microalgal biodiesel production “a fully competitive process.” Employment of genetic engineering including overexpression of enzymes, inducible promoters, and redirection flux transcription factor regulation of key metabolites involved in lipid biosynthesis pathway can enhance lipid accumulation. These approaches will provide a potential breakthrough in increasing lipid accumulation as well as it can achieve the desired quality for standard biodiesel production. These advancements and innovative strategies are certainly moving toward the economical and sustainable biodiesel production.
References
Abdel-Raouf N, Al-Homaidan AA, Ibraheem IBM (2012) Microalgae and wastewater treatment. Saudi J Biol Sci 19:257–275
Ahmad AL, Mat Yasin NH, Derek CJC, Lim JK (2011) Microalgae as a sustainable energy source for biodiesel production. A review. Renew Sust Energ Rev 15:584–593
Ahmad I, Sharma AK, Daniell H, Kumar S (2015) Altered lipid composition and enhanced lipid production in green microalga by introduction of brassica diacylglycerol acyltransferase 2. Plant Biotechnol J 13(4):540–550
Alonso DL, Belarbi EH, Fernández-Sevilla JM, Rodríguez-Ruiz J, Grima EM (2000) Acyl lipid composition variation related to culture age and nitrogen concentration in continuous culture of the microalga Phaeodactylum tricornutum. Phytochemistry 54:461–471
Ansari FA, Shriwastav A, Gupta SK, Rawat I, Guldhe A, Bux F (2015) Lipid extracted algae as a source for protein and reduced sugar: a step closer to the biorefinery. Bioresour Technol 179:559–564
Baba M, Kikuta F, Suzuki I, Watanabe MM, Shiraiwa Y (2012) Wavelength specificity of growth, photosynthesis, and hydrocarbon production in the oil-producing green alga Botryococcus braunii. Bioresour Technol 109:266–270
Bajguz A, Piotrowska-Niczyporuk A (2013) Synergistic effect of auxins and brassinosteroids on the growth and regulation of metabolite content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol Biochem 71:290–297
Bandarra NM, Pereira PA, Batista I, Vilela MH (2003) Fatty acids, sterols and α-tocopherol in Isochrysis galbana. J Food Lipids 10:25–34
Bartley ML, Boeing WJ, Corcoran AA, Holguin FO, Schaub T (2013) Effects of salinity on growth and lipid accumulation of biofuel microalga Nannochloropsis salina and invading organisms. Biomass Bioenergy 54:83–88
Bellou S, Baeshen MN, Elazzazy AM, Aggeli D, Sayegh F, Aggelis G (2014) Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol Adv 32(8):1476–1493
Bellou S, Moustogianni A, Makri A, Aggelis G (2012) Lipids containing polyunsaturated fatty acids synthesized by Zygomycetes grown on glycerol. Appl Biochem Biotechnol 166:146–158
Ben-Amotz A, Tornabene TG, Thomas WH (1985) Chemical profile of selected species of microalgae with emphasis on lipids. J Phycol 21:72–81
Biller P, Ross A (2014) Pyrolysis GC-MS as a novel analysis technique to determine the biochemical composition of microalgae. Algal Res 6:91–97
Blatti JL, Beld J, Behnke CA, Mendez M, Mayfield SP, Burkart MD (2012) Manipulating fatty acid biosynthesis in microalgae for biofuel through protein-protein interactions. PLoS One 7(9):e42949
Blatti JL, Michaud J, Burkart MD (2013) Engineering fatty acid biosynthesis in microalgae for sustainable biodiesel. Curr Opin Chem Biol 17(3):496–505
Bono MS Jr, Ahner BA, Kirby BJ (2013) Detection of algal lipid accumulation due to nitrogen limitation via dielectric spectroscopy of Chlamydomonas reinhardtii suspensions in a coaxial transmission line sample cell. Bioresour Technol 143:623–631
Borowitzka MA, Moheimani NR (2013) Sustainable biofuels from algae. Mitig adapt strategies glob chang 18:13–25
Bouvier-Nave P, Benveniste P, Oelkers P, Sturley SL, Schaller H (2000) Expression in yeast and tobacco of plant cDNAs encoding acyl CoA: diacylglycerol acyltransferase. Eur J Biochem 267(1):85–96
Boynton JE, Gillham NW, Harris EH, Hosler JP, Johnson AM, Jones AR, Randolphanderson BL, Robertson D, Klein TM, Shark KB, Sanford JC (1988) Chloroplast Transformation in Chlamydomonas with high-velocity microprojectiles. Science 240(4858):1534–1538
Breuer G, de Jaeger L, Artus VPG, Martens DE, Springer J, Draaisma RB, Eggink G, Wijffels RH, Lamers PP (2014) Superior triacylglycerol (TAG) accumulation in starchless mutants of Scenedesmus obliquus: (II) evaluation of TAG yield and productivity in controlled photobioreactors. Biotechnol Biofuels 7:70
Brown TD, Jones-Mortimer MC, Kornberg HL (1977) The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J Gen Microbiol 102(2):327–336
Cao J, Yuan H, Li B, Yang J (2014) Significance evaluation of the effects of environmental factors on the lipid accumulation of Chlorella minutissima UTEX 2341 under low-nutrition heterotrophic condition. Bioresour Technol 152:177–184
Carvalho AP, Malcata FX (2005) Optimization of ω-3 fatty acid production by microalgae: crossover effects of CO2 and light intensity under batch and continuous cultivation modes. Mar Biotechnol 7:381–388
Chaturvedi R, Fujita Y (2006) Isolation of enhanced eicosapentaenoic acid producing mutants of Nannochloropsis oculata ST-6 using ethyl methane sulfonate induced mutagenesis techniques and their characterization at mRNA transcript level. Phycol Res 54(3):208–219
Chen Y, Vaidyanathan S (2013) Simultaneous assay of pigments, carbohydrates, proteins and lipids in microalgae. Anal Chim Acta 776:31–40
Chen YF (2011) Production of biodiesel from algal biomass: current perspectives and future. Academic Press, Waltham, p. 399
Cheng RB, Ma RJ, Li K, Rong H, Lin XZ, Wang ZK, Yang SJ, Ma Y (2012) Agrobacterium tumefaciens mediated transformation of marine microalgae Schizochytrium. Microbiol Res 167(3):179–186
Chia MA, Lombardi AT, Melao MDGG (2013) Growth and biochemical composition of Chlorella vulgaris in different growth media. An Acad Bras Cienc 85:1427–1438
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26:126–131
Chisti Y (2013) Constraints to commercialization of algal fuels. J Biotechnol 167:201–214
Choi WJ, Hartono MR, Chan WH, Yeo SS (2011a) Ethanol production from biodiesel-derived crude glycerol by newly isolated Kluyvera cryocrescens. Appl Microbiol Biotechnol 89:1255–1264
Choi JA, Hwang JH, Dempsey BA, Abou-Shanab RAI, Min B, Song H, Lee DS, Kim JR, Cho Y, Hong S, Jeon BH (2011b) Enhancement of fermentative bioenergy (ethanol/hydrogen) production using ultrasonication of Scenedesmus obliquus YSW15 cultivated in swine wastewater effluent. Energ Environ Sci 4:3513–3520
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151
Costa JAV, Cozza KL, Oliveira L, Magagnin G (2001) Different nitrogen sources and growth responses of Spirulina platensis in microenvironments. World J Microbiol Biotechnol 17:439–442
Courchesne NMD, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141(1–2):31–41
Cuellar-Bermudez SP, Aguilar-Hernandez I, Cardenas-Chavez DL, Ornelas-Soto N, Romero-Ogawa MA, Parra-Saldivar R (2014) Extraction and purification of high-value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins. Microb Biotechnol 8:190–209
Czerpak R, Bajguz A (1997) Stimulatory effect of auxins and cytokinins on carotenes, with differential effects on xanthophylls in the green alga Chlorella pyrenoidosa Chick. Acta Soc Bot Pol 66:41–46
Davis MS, Solbiati J, Cronan JE (2000) Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J Biol Chem 275(37):28593–28598
De Bhowmick G, Koduru L, Sen R (2015) Metabolic pathway engineering towards enhancing microalgal lipid biosynthesis for biofuel application-A review. Renew Sust Energ Rev 50:1239–1253
de Jaeger L, Verbeek RE, Draaisma RB, Martens DE, Springer J, Eggink G, Wijffels RH (2014) Superior triacylglycerol (TAG) accumulation in starchless mutants of Scenedesmus obliquus: (I) mutant generation and characterization. Biotechnol Biofuels 7:69
Dean AP, Sigee DC, Estrada B, Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour Technol 101:4499–4507
Dehesh K, Tai H, Edwards P, Byrne J, Jaworski JG (2001) Overexpression of 3-ketoacyl-acyl-carrier protein synthase IIIs in plants reduces the rate of lipid synthesis. Plant Physiol 125(2):1103–1114
Demirbas A, Demirbas MF (2011) Importance of algae oil as a source of biodiesel. Energy Convers Manag 52:163–170
Deng XD, Fei XW, Li YJ (2011) The effects of nutritional restriction on neutral lipid accumulation in Chlamydomonas and Chlorella. Afr J Microbiol Res 5:260–270
Dunahay TG, Jarvis EE, Dais SS, Roessler PG (1996) Manipulation of microalgal lipid production using genetic engineering. Appl Biochem Biotechnol 57–58:223–231
Dunahay TG, Jarvis EE, Roessler PG (1995) Genetic transformation of the diatoms Cyclotella cryptica and Navicula saprophila. J Phycol 31(6):1004–1012
Eldalatony MM, Kabra AN, Hwang JH, Govindwar SP, Kim KH, Kim H, Jeon BH (2016) Pretreatment of microalgal biomass for enhanced recovery/extraction of reducing sugars and proteins. Bioprocess Biosyst Eng 39(1):95–103
Eyre-Walker A, Keightley PD (2007) The distribution of fitness effects of new mutations. Nat Rev Genet 8(8):610–618
Fakhry EM, Maghraby DM (2015) Lipid accumulation in response to nitrogen limitation and variation of temperature in Nannochloropsis salina. Bot Stud 56:6
Fan JH, Cui YB, Wan MX, Wang WL, Li YG (2014) Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnol Biofuels 7:17
Feng P, Zhongyang D, Zhengyu H, Fan L (2011) Lipid accumulation and growth of Chlorella zofingiensis in flat plate photobioreactors outdoors. Bioresour Technol 102:10577–10584
Fernandez E, Schnell R, Ranum LPW, Hussey SC, Silflow CD, Lefebvre PA (1989) Isolation and Characterization of the Nitrate Reductase Structural Gene of Chlamydomonas-Reinhardtii. Proc Natl Acad Sci U S A 86(17):6449–6453
Gatenby CM, Parker BC, Neves RJ (1997) Growth and survival of juvenile rainbow mussels, Villosa iris (Lea, 1829) (Bivalvia: Unionidae), reared on algal diets and sediment. Am Malacol Bull 14:57–66
Gonzalez-Fernandez C, Ballesteros M (2012) Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnol Adv 30(6):1655–1661
Gordillo FJL, Goutx M, Figueroa FL, Niell FX (1998) Effects of light intensity, CO2 and nitrogen supply on lipid class composition of Dunaliella viridis. J Appl Phycol 10:135–144
Granados MR, Acien FG, Gomez C, Fernandez-Sevilla JM, Molina Grima E (2012) Evaluation of flocculants for the recovery of freshwater microalgae. Bioresour Technol 118:102–110
Guckert JB, Cooksey KE (1990) Triglyceride accumulation and fatty acid profile changes in Chlorella (chlorophyta) during high ph-induced cell cycle inhibition. J Phycol 26:72–79
Guldhe A, Singh B, Rawat I, Bux F (2014) Synthesis of biodiesel from Scenedesmus sp. by microwave and ultrasound assisted in situ transesterification using tungstated zirconia as a solid acid catalyst. Chem Eng Res Des 92(8):1503–1511
Guo SL, Zhao XQ, Tang Y, Wan C, Alam MA, Ho SH, Bai FW, Chang JS (2013) Establishment of an efficient genetic transformation system in Scenedesmus obliquus. J Biotechnol 163(1):61–68
Gupta SK, Kumar M, Guldhe A, Ansari FA, Rawat I, Kanney K, Bux F (2014) Design and development of polyamine polymer for harvesting microalgae for biofuels production. Energy Convers Manag 85:537–544
Gutierrez CL, Gimpel J, Escobar C, Marshall SH, Henriquez V (2012) Chloroplast genetic tool for the green microalgae haematococcus pluvialis (chlorophyceae, volvocales). J Phycol 48(4):976–983
Hannon M, Gimpel J, Tran M, Rasala B, Mayfield S (2010) Biofuels from algae: challenges and potential. Biofuels 1:763–784
Harper JDI, Wu L, Sakuanrungsirikul S, John PCL (1995) Isolation and partial characterization of conditional cell-division cycle mutants in Chlamydomonas. Protoplasma 186(3–4):149–162
Hartwell LH, Culotti J, Pringle JR, Reid BJ (1974) Genetic control of the cell division cycle in yeast. Science 183(4120):46–51
Harwood JL, Guschina IA (2009) The versatility of algae and their lipid metabolism. Biochimie 91(6):679–684
He Q, Yang H, Wu L, Hu C (2015) Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour Technol 191:219–228
Hlavova M, Turoczy Z, Bisova K (2015) Improving microalgae for biotechnology - From genetics to synthetic biology. Biotechnol Adv 33(6):1194–1203
Ho SH, Chen CY, Lee DJ, Chang JS (2011) Perspectives on microalgal CO2-emission mitigation systems—a review. Biotechnol Adv 29:189–198
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Hunt RW, Chinnasamy S, Das KC (2011) The effect of naphthalene acetic acid on biomass productivity and chlorophyll content of green algae, coccolithophore, diatom, and cyanobacterium cultures. Appl Biochem Biotechnol 164:1350–1365
Jako C, Kumar A, Wei YD, Zou JT, Barton DL, Giblin EM, Covello PS, Taylor DC (2001) Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol 126(2):861–874
Jeon BH, Choi JA, Kim HC, Hwang JH, Abou-Shanab R, Dempsey B, Regan J, Kim J (2013) Ultrasonic disintegration of microalgal biomass and consequent improvement of bioaccessibility/bioavailability in microbial fermentation. Biotechnol Biofuels 6:37
Ji MK, Abou-Shanab RAI, Kim SH, Salama ES, Lee SH, Kabra AN, Lee YS, Hong S, Jeon BH (2013) Cultivation of microalgae species in tertiary municipal wastewater supplemented with CO2 for nutrient removal and biomass production. Ecol Eng 58:142–148
Jiang L, Luo S, Fan X, Yang Z, Guo R (2011) Biomass and lipid production of marine microalgae using municipal wastewater and high concentration of CO2. Appl Energy 10:3336–3341
Jiang Y, Chen F (1999) Effects of salinity on cell growth and docosahexaenoic acid content of the heterotrophic marine microalga Crypthecodinium cohnii. J Ind Microbiol Biotechnol 23:508–513
Joh T, Yoshida T, Yoshimoto M, Miyamoto T, Hatano S (1993) Composition and positional distribution of fatty acids in polar lipids from Chlorella ellipsoidea differing in chilling susceptibility and frost hardiness. Physiol Plant 89:285–290
Juneja A, Ceballos RM, Murthy GS (2013) Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies 6:4607–4638
Jusoh M, Loh SH, Chuah TS, Aziz A, Cha TS (2015) Indole-3-acetic acid (IAA) induced changes in oil content, fatty acid profiles and expression of four fatty acid biosynthetic genes in Chlorella vulgaris at early stationary growth phase. Phytochemistry 111:65–71
Kaewkannetra P, Enmak P, Chiu TY (2012) The effect of CO2 and salinity on the cultivation of Scenedesmus obliquus for biodiesel production. Biotechnol Bioprocess Eng 17:591–597
Kang NK, Lee B, Choi GG, Moon M, Park MS, Lim JK, Yang JW (2014) Enhancing lipid productivity of Chlorella vulgaris using oxidative stress by TiO2 nanoparticles. Korean J Chem Eng 31:861–867
Khotimchenko SV, Yakovleva IM (2005) Lipid composition of the red alga Tichocarpus crinitus exposed to different levels of photon irradiance. Phytochemistry 66:73–79
Khozin-Goldberg I, Cohen Z (2006) The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 67:696–701
Kindle KL (1990) High-frequency nuclear transformation of chlamydomonas-reinhardtii. Proc Natl Acad Sci U S A 87(3):1228–1232
Kumar MS, Hwang JH, Abou-Shanab RAI, Kabra AN, Ji MK, Jeon BH (2014) Influence of CO2 and light spectra on the enhancement of microalgal growth and lipid content. J Renew Sustain Ener 6:063107
Kumar P, Kumar P, Sharma PK, Sharma PK, Sharma D (2015) Micro-algal Lipids: a Potential Source of Biodiesel. JIPBS 2(2):135–143
Kumar SV, Misquitta RW, Reddy VS, Rao BJ, Rajam MV (2004) Genetic transformation of the green alga—chlamydomonas reinhardtii by agrobacterium tumefaciens. Plant Sci 166(3):731–738
Lacerda LMCF, Queiroz MI, Furlan LT, Lauro MJ, Modenesi K, Jacob-Lopes E, Franco TT (2011) Improving refinery wastewater for microalgal biomass production and CO2 biofixation: predictive modeling and simulation. J Pet Sci Eng 78:679–686
Lan EI, Liao JC (2013) Microbial synthesis of n-butanol, isobutanol, and other higher alcohols from diverse resources. Bioresour Technol 135:339–349
Larkum AWD, Ross IL, Kruse O, Hankamer B (2012) Selection, breeding and engineering of microalgae for bioenergy and biofuel production. Trends Biotechnol 30(4):198–205
Leon-Banares R, Gonzalez-Ballester D, Galvan A, Fernandez E (2004) Transgenic microalgae as green cell-factories. Trends Biotechnol 22(1):45–52
Li YT, Han DX, Hu GR, Sommerfeld M, Hu QA (2010) Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnol Bioeng 107(2):258–268
Liang KH, Zhang QH, Gu M, Cong W (2013) Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J Appl Phycol 25:311–318
Liang MH, Jiang JG (2013) Advancing oleaginous microorganisms to produce lipid via metabolic engineering technology. Prog Lipid Res 52(4):395–408
Liang Y, Beardall J, Heraud P (2006) Effects of nitrogen source and uv radiation on the growth, chlorophyll fluorescence and fatty acid composition of phaeodactylum tricornutum and chaetoceros muelleri (bacillariophyceae). J Photochem Photobiol B Biol 82:161–172
Lim JK, Chieh DCJ, Jalak SA, Toh PY, Yasin NHM, Ng BWV, Ahmad AL (2012) Rapid magnetophoretic separation of microalgae. Small 8:1683–1692
Lin H, Castro NM, Bennett GN, San KY (2006) Acetyl-CoA synthetase overexpression in Escherichia coli demonstrates more efficient acetate assimilation and lower acetate accumulation: a potential tool in metabolic engineering. Appl Microbiol Biotechnol 71(6):870–874
Liu BS, Benning C (2013) Lipid metabolism in microalgae distinguishes itself. Curr Opin Biotechnol 24(2):300–309
Liu J, Yuan C, Hu G, Li F (2012) Effects of light Intensity on the growth and lipid accumulation of microalga Scenedesmus sp. 11-1 under nitrogen limitation. Appl Biochem Biotechnol 166:2127–2137
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in chlorella vulgaris. Bioresour Technol 99:4717–4722
Malcata FX (2011) Microalgae and biofuels: a promising partnership? Trends Biotechnol 29(11):542–549
Martin S, Parton RG (2006) Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol 7:373–378
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Matthew T, Zhou W, Rupprecht J, Lim L, Thomas-Hall SR, Doebbe A, Kruse O, Hankamer B, Marx UC, Smith SM (2009) The metabolome of Chlamydomonas reinhardtii following induction of anaerobic H2 production by sulfur depletion. J Biol Chem 284:23415–23425
McLarnon-Riches CJ, Rolph CE, Greenway DLA, Robinson PK (1998) Effects of environmental factors and metals on Selenastrum capricornutum lipids. Phytochemistry 49:1241–1247
Merchant SS, Kropat J, Liu BS, Shaw J, Warakanont J (2012) TAG, You’re it! Chlamydomonas as a reference organism for understanding algal triacylglycerol accumulation. Curr Opin Biotechnol 23(3):352–363
Miazek K, Iwanek W, Remacle C, Richel A, Goffin D (2015) Effect of metals, metalloids and metallic nanoparticles on microalgae growth and industrial product biosynthesis: a review. Int J Mol Sci 16:23929–23969
Millan-Oropeza A, Torres-Bustillos LG, Fernandez-Linares L (2015) Simultaneous effect of nitrate (NO3−) concentration, carbon dioxide (CO2) supply and nitrogen limitation on biomass, lipids, carbohydrates and proteins accumulation in Nannochloropsis oculata. Biofuel Res J-Brj 2(1):215–U85
Misra N, Panda PK, Parida BK, Mishra BK (2012) Phylogenomic study of lipid genes involved in microalgal biofuel production-candidate gene mining and metabolic pathway analyses. Evol Bioinforma 8:545–564
Misra R, Guldhe A, Singh P, Rawat I, Bux F (2014) Electrochemical harvesting process for microalgae by using nonsacrificial carbon electrode: a sustainable approach for biodiesel production. Chem Eng J 255:327–333
Miyachi S, Kamiya A (1978) Wavelength effects on photosynthetic carbon metabolism in Chlorella. Plant Cell Physiol 19:277–288
Mizuno Y, Sato A, Watanabe K, Hirata A, Takeshita T, Ota S, Sato N, Zachleder V, Tsuzuki M, Kawano S (2013) Sequential accumulation of starch and lipid induced by sulfur deficiency in Chlorella and Parachlorella species. Bioresour Technol 129:150–155
Mohammady NG, Fathy AA (2007) Humic acid mitigates viability reduction, lipids and fatty acids of Dunaliella salina and Nannochloropsis salina grown under nickel stress. Int J Bot 3:64–70
Mohr A, Raman S (2013) Lessons from first generation biofuels and implications for the sustainability appraisal of second generation biofuels. Energy Policy 63:114–122
Mühlroth A, Li K, Røkke G, Winge P, Olsen Y, Hohmann-Marriott MF (2013) Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of Chromista. Mar Drugs 11:4662–4697
Muto M, Fukuda Y, Nemoto M, Yoshino T, Matsunaga T, Tanaka T (2013) Establishment of a genetic transformation system for the marine pennate diatom Fistulifera sp Strain JPCC DA0580-A high triglyceride producer. Mar Biotechnol 15(1):48–55
Najafi N, Ahmadi AR, Hosseini R, Golkhoo S (2011) Gamma irradiation as a useful tool for the isolation of astaxanthin-overproducing mutant strains of Phaffia rhodozyma. Can J Microbiol 57(9):730–734
Napier JA, Haslam RP, Beaudoin F, Cahoon EB (2014) Understanding and manipulating plant lipid composition: metabolic engineering leads the way. Curr Opin Plant Biol 19:68–75
Neupert J, Karcher D, Bock R (2009) Generation of Chlamydomonas strains that efficiently express nuclear transgenes. Plant J 57(6):1140–1150
Niu YF, Yang ZK, Zhang MH, Zhu CC, Yang WD, Liu JS, Li HY (2012) Transformation of diatom Phaeodactylum tricornutum by electroporation and establishment of inducible selection marker. Biotechniques 52(6):1–3
Noor-Mohammadi S, Pourmir A, Johannes TW (2014) Method for assembling and expressing multiple genes in the nucleus of microalgae. Biotechnol Lett 36(3):561–566
Nurse P, Thuriaux P, Nasmyth K (1976) Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet 146(2):167–178
O’Neill BM, Mikkelson KL, Gutierrez NM, Cunningham JL, Wolff KL, Szyjka SJ, Yohn CB, Redding KE, Mendez MJ (2012) An exogenous chloroplast genome for complex sequence manipulation in algae. Nucleic Acids Res 40(6):2782–2792
Ong SC, Kao CY, Chiu SY, Tsai MT, Lin CS (2010) Characterization of the thermal-tolerant mutants of Chlorella sp. with high growth rate and application in outdoor photobioreactor cultivation. Bioresour Technol 101(8):2880–2883
Padrova K, Lukavsky J, Nedbalova L, Cejkova A, Cajthaml T, Sigler K, Vitova M, Rezanka T (2015) Trace concentrations of iron nanoparticles cause overproduction of biomass and lipids during cultivation of cyanobacteria and microalgae. J Appl Phycol 27:1443–1451
Parmar A, Singh NK, Pandey A, Gnansounou E, Madamwar D (2011) Cyanobacteria and microalgae: a positive prospect for biofuels. Bioresour Technol 102(22):10163–10172
Park WK, Yoo G, Moon M, Kim CW, Choi YE, Yang JW (2013) Phytohormone supplementation significantly increases growth of Chlamydomonas reinhardtii cultivated for biodiesel production. Appl Biochem Biotechnol 171:1128–1142
Parker MS, Mock T, Armbrust EV (2008) Genomic insights into marine microalgae. Annu Rev Genet 42:619–645
Parsaeimehr A, Sun Z, Dou X, Chen YF (2015) Simultaneous improvement in production of microalgal biodiesel and high-value alpha-linolenic acid by a single regulator acetylcholine. Biotechnol Biofuels 8:11
Piotrowska-Niczyporuk A, Bajguz A (2014) The effect of natural and synthetic auxins on the growth, metabolite content and antioxidant response of green alga Chlorella vulgaris (Trebouxiophyceae). Plant Growth Regul 73:57–66
Potvin G, Zhang Z (2010) Strategies for high-level recombinant protein expression in transgenic microalgae: a review. Biotechnol Adv 28(6):910–918
Prabakaran P, Ravindran D (2013) Selection of microalgae for accumulation of lipid production under different growth conditions. Carib J Sci Tech 1:131–137
Priyadarshani I, Rath B (2012) Commercial and industrial applications of micro algae–a review. J Algal Biomass Utln 3:89–100
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65(6):635–648
Purton S, Szaub JB, Wannathong T, Young R, Economou CK (2013) Genetic engineering of algal chloroplasts: progress and prospects. Russ J Plant Physiol 60(4):491–499
Radakovits R, Eduafo PM, Posewitz MC (2011) Genetic engineering of fatty acid chain length in phaeodactylum tricornutum. Metab Eng 13(1):89–95
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9(4):486–501
Rangasamy D, Ratledge C (2000) Genetic enhancement of fatty acid synthesis by targeting rat liver ATP: citrate lyase into plastids of tobacco. Plant Physiol 122(4):1231–1238
Raposo MFJ, Morais RMSC (2013) Influence of the growth regulators kinetin and 2, 4-D on the growth of two chlorophyte microalgae, Haematococcus pluvialis and dunaliella salina. J Basic Appl Sci 9:302–308
Reitan KI, Rainuzzo JR, Olsen Y (1994) Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J Phycol 30:972–979
Rocchetta I, Mazzuca M, Conforti V, Ruiz L, Balzaretti V, Molina MCR (2006) Effect of chromium on the fatty acid composition of two strains of euglena gracilis. Environ Pollut 141:353–358
Roesler K, Shintani D, Savage L, Boddupalli S, Ohlrogge J (1997) Targeting of the arabidopsis homomeric acetyl-coenzyme a carboxylase to plastids of rapeseeds. Plant Physiol 113(1):75–81
Roessler PG (1988) Effects of silicon deficiency on lipid composition and metabolism in the diatom Cyclotella cryptica. J Phycol 24:394–400
Roessler PG (1990) Purification and Characterization of Acetyl-Coa Carboxylase from the Diatom Cyclotella-Cryptica. Plant Physiol 92(1):73–78
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ (2008) A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19(5):430–436
Salama E-S, Abou-Shanab RAI, Kim JR, Lee S, Kim SH, Oh SE, Kim HC, Roh HS, Jeon BH (2014a) The effects of salinity on the growth and biochemical properties of Chlamydomonas mexicana GU732420 cultivated in municipal wastewater. Environ Technol 35:1491–1498
Salama E-S, Kabra AN, Ji MK, Kim JR, Min B, Jeon BH (2014b) Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresour Technol 172:97–0103
Schoerken U, Kempers P (2009) Lipid biotechnology: Industrial relevant production processes. Eur J Lipid Sci Technol 111:627–645
Sharma KK, Schuhmann H, Schenk PM (2012) high lipid induction in microalgae for biodiesel production. Energies 5(5):1532–1553
Sheehan TDJ, Benemann J, Roessler P (1998) A look back at the U.S. Department of energy’s aquatic species program-biodiesel from algae. NREL/TP-580-24190.
Shin H, Hong SJ, Kim H, Yoo C, Lee H, Choi HK, Lee CG, Cho BK (2015) Elucidation of the growth delimitation of Dunaliella tertiolecta under nitrogen stress by integrating transcriptome and peptidome analysis. Bioresour Technol 194:57–66
Singh P, Kumari S, Guldhe A, Misra R, Rawat I, Bux F (2016) Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew Sust Energ Rev 55:1–16
Skrupski B, Wilson KE, Goff KL, Zou J (2014) Effect of pH on neutral lipid and biomass accumulation in microalgal strains native to the Canadian prairies and the Athabasca oil sands. J Appl Phycol 25:937–949
Sorokin C, Krauss RW (1958) The Effects of light intensity on the growth rates of green algae. Plant Physiol 33:109–113
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Stockenreiter M, Haupt F, Graber AK, Seppälä J, Spilling K, Tamminen T, Stibor H (2013) Functional group richness: Implications of biodiversity for light use and lipid yield in microalgae. J Phycol 49:838–847
Subrahmanyam S, Cronan JE Jr (1998) Overproduction of a functional fatty acid biosynthetic enzyme blocks fatty acid synthesis in Escherichia coli. J Bacteriol 180(17):4596–4602
Sun Z, Dou X, Wu J, He B, Wang Y, Chen Y (2016) Enhanced lipid accumulation of photoautotrophic microalgae by high-dose CO2 mimics a heterotrophic characterization. World J Microbiol Biotechnol 32:9
Sun J, Cheng J, Yang Z, Li K, Zhou J, Cen K (2015) Microstructures and functional groups of Nannochloropsis sp. cells with arsenic adsorption and lipid accumulation. Bioresour Technol 194:305–311
Sushchik NN, Kalacheva GS, Zhila NO, Gladyshev MI, Volova TG (2003) A temperature dependence of the intra- and extracellular fatty-acid composition of green algae and cyanobacterium. Russ J Plant Physiol 50:374–380
Tabatabaei M, Tohidfar M, Jouzani GS, Safarnejad M, Pazouki M (2011) Biodiesel production from genetically engineered microalgae: Future of bioenergy in Iran. Renew Sust Energ Rev 15(4):1918–1927
Takagi M, Karseno, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101: 223–226.
Tarakhovskaya ER, Maslov YI, Shishova MF (2007) Phytohormones in algae. Russ J Plant Physiol 54:163–170
Tate JJ, Gutierrez-Wing MT, Rusch KA, Benton MG (2013) The Effects of plant growth substances and mixed cultures on growth and metabolite production of green algae Chlorella sp.: a review. J Plant Growth Regul 32:417–428
Tatsuzawa H, Takizawa E, Wada M, Yamamoto Y (1996) Fatty acid and lipid composition of the acidophilic green alga Chlamydomonas sp. J Phycol 32:598–601
Thelen JJ, Ohlrogge JB (2002) Metabolic engineering of fatty acid biosynthesis in plants. Metab Eng 4(1):12–21
Thompson GA (1996) Lipids and membrane function in green algae. Biochim Biophys Acta 1302:17–45
Thuriaux P, Nurse P, Carter B (1978) Mutants altered in the control co-ordinating cell division with cell growth in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet 161(2):215–220
Trentacoste EM, Shrestha RP, Smith SR, Gle C, Hartmann AC, Hildebrand M, Gerwick WH (2013) Metabolic engineering of lipid catabolism increases microalgal lipid accumulation without compromising growth. Proc Natl Acad Sci U S A 110(49):19748–19753
Ueda R, Hirayama S, Sugata K, Nakayama H (1996) Process for the production of ethanol from microalgae. US Patent 5, 578, 472
Verwoert II, van der Linden KH, Walsh MC, Nijkamp HJ, Stuitje AR (1995) Modification of Brassica napus seed oil by expression of the Escherichia coli fabH gene, encoding 3-ketoacyl-acyl carrier protein synthase III. Plant Mol Biol 27(5):875–886
Vieler A, Wu G, Tsai CH, Bullard B, Cornish AJ, Harvey C, Reca IB, Thornburg C, Achawanantakun R, Buehl CJ, Campbell MS, Cavalier D, Childs KL, Clark TJ, Deshpande R, Erickson E, Armenia Ferguson A, Handee W, Kong Q, Li X, Liu B, Lundback S, Peng C, Roston RL, Sanjaya, Simpson JP, Terbush A, Warakanont J, Zauner S, Farre EM, Hegg EL, Jiang N, Kuo MH, Lu Y, Niyogi KK, Ohlrogge J, Osteryoung KW, Shachar-Hill Y, Sears BB, Sun Y, Takahashi H, Yandell M, Shiu SH, Benning C (2012) Genome, functional gene annotation, and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLoS Genet 8(11): e1003064
Vigeolas H, Duby F, Kaymak E, Niessen G, Motte P, Franck F, Remacle C (2012) Isolation and partial characterization of mutants with elevated lipid content in Chlorella sorokiniana and Scenedesmus obliquus. J Biotechnol 162(1):3–12
Voigt J, Stolarczyk A, Zych M, Malec P, Burczyk J (2014) The cell-wall glycoproteins of the green alga Scenedesmus obliquus. The predominant cell-wall polypeptide of Scenedesmus obliquus is related to the cell-wall glycoprotein gp3 of Chlamydomonas reinhardtii. Plant Sci 215–216:39–47
Waltz E (2009) Biotech’s green gold? Nat Biotechnol 27:15–18
Wan M, Jin X, Xia J, Rosenberg JN, Yu G, Nie Z, Oyler GA, Betenbaugh MJ (2014) The effect of iron on growth, lipid accumulation, and gene expression profile of the freshwater microalga Chlorella sorokiniana. Appl Microbiol Biotechnol 98(22):9473–9481
Wang DM, Ning K, Li J, Hu JQ, Han DX, Wang H, Zeng XW, Jing XY, Zhou Q, Su XQ, Chang XZ, Wang AH, Wang W, Jia J, Wei L, Xin Y, Qiao YH, Huang RR, Chen J, Han B, Yoon K, Hill RT, Zohar Y, Chen F, Hu Q, Xu J (2014) Nannochloropsis genomes reveal evolution of microalgal oleaginous traits. PLoS Genet 10(1):094
Wang GY, Wang X, Liu XH (2011) Two-stage hydrolysis of invasive algal feedstock for ethanol fermentation. J Integr Plant Biol 53:246–252
Widjaja A, Chien CC, Ju YH (2009) Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J Taiwan Inst Chem Eng 40:13–20
Wijffels RH, Kruse O, Hellingwerf KJ (2013) Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr Opin Biotechnol 24(3):405–413
Wobbe L, Remacle C (2015) Improving the sunlight-to-biomass conversion efficiency in microalgal biofactories. J Biotechnol 201:28–42
Wu L, Chen PC, Lee CM (2013) The effects of nitrogen sources and temperature on cell growth and lipid accumulation of microalgae. Int Biodeter Biodegr 85:506–510
Wynn JP, Hamid ABA, Ratledge C (1999) The role of malic enzyme in the regulation of lipid accumulation in filamentous fungi. Microbiology 145(Pt 8):1911–1917
Xin L, Hu HY, Gan K, Sun YX (2010) Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour Technol 101:5494–5500
Xu XQ, Beardall J (1997) Effect of salinity on fatty acid composition of a green microalga from an antarctic hypersaline lake. Phytochemistry 45:655–658
Yang JS, Cao J, Xing GL, Yuan HL (2015) Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour Technol 175:537–544
Yao S, Brandt A, Egsgaard H, Gjermansen C (2012) Neutral lipid accumulation at elevated temperature in conditional mutants of two microalgae species. Plant Physiol Biochem 61:71–79
Yu WL, Ansari W, Schoepp NG, Hannon MJ, Mayfield SP, Burkart MD (2011) Modifications of the metabolic pathways of lipid and triacylglycerol production in microalgae. Microb Cell Factories 10:91
Zhang Q, Wang T, Hong Y (2014) Investigation of initial pH effects on growth of an oleaginous microalgae Chlorella sp. HQ for lipid production and nutrient uptake. Water Sci Technol 70:712–719
Zhang Y, Adams IP, Ratledge C (2007) Malic enzyme: the controlling activity for lipid production? overexpression of malic enzyme in Mucor circinelloides leads to a 2.5-fold increase in lipid accumulation. Microbiology 153(Pt 7):2013–2025
Zou J, Katavic V, Giblin EM, Barton DL, MacKenzie SL, Keller WA, Hu X, Taylor DC (1997) Modification of seed oil content and acyl composition in the brassicaceae by expression of a yeast sn-2 acyltransferase gene. Plant Cell 9(6):909–923
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Ravindran, B., Kurade, M.B., Kabra, A.N., Jeon, BH., Gupta, S.K. (2017). Recent Advances and Future Prospects of Microalgal Lipid Biotechnology. In: Gupta, S., Malik, A., Bux, F. (eds) Algal Biofuels. Springer, Cham. https://doi.org/10.1007/978-3-319-51010-1_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-51010-1_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51009-5
Online ISBN: 978-3-319-51010-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)