Abstract
In this study, the biomass and exopolysaccharides (EPS) production in co-cultures of microalgae/cyanobacteria and macromycetes was evaluated as a technology for producing new polysaccharides for medical and/or industrial application. Based on biomass and EPS productivity of monocultures, two algae and two fungi were selected and cultured in different co-culture arrangements. The hydrosoluble EPS fractions from mono- and co-cultures were characterized by ¹³C NMR spectroscopy and gas chromatography coupled to mass spectrometry and compared. It was found that co-cultures resulted in the production of an EPS different from those produced by monocultures, showing fungal predominance with microalgal/cyanobacterial traces. Co-cultures conditions were screened (temperature, agitation speed, fungal and microalgae inoculation rate, initial pH, illumination rate, and glucose concentration) in order to achieve maximum biomass and EPS production, resulting in an increase of 33 and 61% in exopolysaccharides and biomass productions, respectively (patent pending).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fungi and algae are microorganisms that may have innumerous industrial application. They are mostly used in food or fed in form of protein supplement or lipids source and also for the extraction of flavors and other metabolites, such as enzymes and polysaccharides. Fungal polysaccharides are already used in food industry, for example, while those from microalgae and cyanobacteria are not yet completely explored. The biopolymers produced may also have potential industrial applications, as well in human health, as several fungal polysaccharides are intensively researched as antitumor agents.
Some exopolysaccharides (EPS) from microalgae and mushrooms are able to stimulate the nonspecific immune system and to exert antitumor activity through the stimulation of the host’s defense mechanism [1–5]. Molecular weight, degree of branching, numbers of substituent, as well its ultrastructure, including the presence of single and triple helices, significantly affect the EPS biological activity [6]. The high water solubility, with low level of branching and high molecular weight seems to be correlated to higher antitumor activity [7] and varies according to culture conditions.
Macromycetes have been studied extensively for their degradation capacity, specially the so-called white rot fungi, which have the capacity to degrade lignin [8] and xenobiotics [9]. There are many evidences that the extracellular polysaccharides (EPS) produced by these lignocellulolitic fungi play an important role in wood decay [8, 10]. According to [11], the gel formed by these biopolymers prevents the hyphal dehydration, allows cell adherence to others cells or surfaces, and can also immobilize exocellular enzymes.
The ability of microalgae to produce extracellular polysaccharides has been studied since the beginning of the 1950s. In stress conditions, these microorganisms produce a large amount of EPS, which is possibly a metabolic strategy for their growth and development in unfavorable conditions [12].
Co-culture systems are composed of at least two kinds of microorganisms. This interaction results in stress conditions, to which the microorganisms may respond producing large amounts of EPS, for example, as a metabolic strategy to grow in these unfavorable conditions.
The aim of this work was to evaluate the competition or synergy effect of co-cultured macromycetes and algae as a stress condition for favoring EPS production. Considering the innumerous applications of fungal, microalgal, and cyanobacterial EPS, the stressful effects of co-cultures in EPS composition were also evaluated.
Materials and Methods
Screening
Microorganisms
Eight strains of Basidiomycetes and four strains of microalgae were screened. The pure cultures came from the Bioprocess Engineering and Biotechnology Department, Federal University of Paraná, Brazil.
Fungi Cultivation
The medium was composed by (in grams per liter): glucose, 20; yeast extract, 3; K2HPO4, 0.6, and MgSO4, 0.3; pH 6.0. Erlenmeyer flasks containing 250 mL of sterilized culture medium were inoculated with a suspension in sterile water of fungal mycelium grown on potato dextrose agar slants (the so-called pre-inoculum). Incubation was done at 29 °C on a shaker at 250 rpm. A scale-up was performed, transferring the pre-inoculum to 600 mL of medium culture, prepared with same components, and incubated at the same conditions. Dry weight of mycelium was measured after repeated washing of the mycelial pellet with distilled water and drying overnight at 70 °C until constant weight.
Microalgae Cultivation
The Spirulina platensis LPBPA culture was performed in Zarrouk medium [13] and the Chlorella vulgaris LPB06 in modified Bristol’s medium [14]. Incubation was done in 250-mL Erlenmeyer flasks at 29 °C under illumination of white fluorescent light (24 h light, 3,000 lx). A scale-up was made by transferring the pre-inoculum to 3,000 mL of medium culture, prepared with the same components, and incubated at the same conditions.
Screening
Eight strains of Basidiomycetes and four strains of microalgae were separately tested for EPS and biomass production. The species are listed in Table 1. For the screening, the incubation period was 10 days for Basidiomycetes and 20 days for microalgae. The final broth was filtrated to separate the biomass, which was washed twice with distilled water and quantified by dry weight (105 °C to constant weight). Ethanol was added to the culture filtrate (4:1 v/v) and after 24 h at 4 °C the precipitated biopolymer was separated by centrifugation (10,000 rpm for 10 min) and also quantified by drying and weighting.
Co-culture
Following the screening, microorganisms that showed higher biomass and exopolysaccharides production were arranged according to the Table 2. Each combination received a correspondent code, as informed in Table 2. Co-cultures were performed using a 1:1 mixture of fungi and algae medium. The co-culture was followed daily by microscopy, certifying that both microorganisms were growing. Thus, co-cultures biomass quantifications intended to quantify algal and fungal biomass together. Biomass and EPS productions in co-cultures are an average of five trials.
EPS Characterization
EPS Extraction
The broth was vacuum filtered (paper filter Whatman no. 1), and the filtrate was concentrated to one fourth of the original volume through rotary evaporation at 50 °C. The filtrated was then dialyzed in a membrane (12–14 kDa), mixed with four times its volume of absolute ethanol, stirred vigorously, and left overnight at 4 °C and centrifuged at 10,000 rpm for 10 min. The supernatant was discarded and the purified EPS lyophilized. EPS characterization results are an average of five trials.
Sugar Content
The carbohydrate quantification was done using phenol–sulfuric method [15], and the quantification of residual sugar (glucose) was carried out according to Somogy and Nelson [16]. The absorbance reading for both methodologies was performed in spectrophotometer (Power Wave XS, BioTek, USA).
Protein Content
The quantification of protein was performed according to Lowry [17]. The determination of absorbance was also carried out in a spectrophotometer (Power Wave XS, BioTek, USA).
Monosaccharide Composition
Monosaccharide composition analysis was performed according to [18]. Approximately 1 mg of EPS was treated with 0.5 mL of 2 M trifluoracetic acid during 1 h at 121 °C. The acid was eliminated by evaporation, followed by NaBH4 reduction, acetylation, and analysis by gas chromatography (GC) and GC coupled to mass spectrometry (GC-MS). The alditol acetates produced from the EPS samples were analyzed by GC-MS using a Varian 3.300 gas chromatograph equipped with DB-225 (30 m × 0.25 mm) fused silica capillary columns interfaced to a Finnigan Mat ITD 800 mass spectrometer. The injector and FID temperatures were 250 °C. Helium was used as carrier gas (1.0 mL min−1).
¹³C NMR Spectroscopy
The EPS structures were determined and compared using ¹³C NMR spectroscopy. Resolution-enhanced 1D/2D 100.16 MHz NMR spectra were recorded in D20 on a Bruker spectrometer, 30,000 pulses with a pulse repetition time of 0.1 s, at a probe temperature of 50 to 70 °C for polysaccharides. Prior to analysis, samples were dissolved to a polysaccharide concentration of 0.6 % (w/v) in D2O/H2O. Chemical shifts are expressed in parts per million by reference to the α-anomeric signal of external [1-13C] glucose (dC-1 92.9) for ¹³C. All NMR data were processed using TopSpin® software.
Preliminary Optimization Steps
In order to evaluate the most significant variables to the process of biomass and EPS production by co-culture, a randomized Plackett–Burman experimental design was used, evaluating seven variables, as shown in Table 3. The best co-culture was then analyzed through Box–Behnken design matrix (Table 4). This was carried out using the software Statistica 7.0 (StatSoft Inc., Tulsa, OK, USA). A Pareto chart displaying the magnitude of each factor estimate was generated by using the same software (Figs. 1 and 2).
Pareto chart rationalizing the effect of each variable on the production of biomass (left) and extracellular polysaccharide (right) by A. blazei and C. vulgaris co-culture. The vertical line indicates confidence level of 95 % for the effects. VAR1–VAR7 indicates culture temperature, agitation speed, microalgal inoculation density, fungal inoculation density, initial pH, illumination, rate, and glucose concentration, respectively
Contour surface plots of the EPS (a–c) and biomass (d–f) yield. In each plot, the influence of two variables is shown while the third is set at the central level. a Glucose concentration (VAR1) vs. agitation speed (VAR2) and their interactive effect with initial pH (VAR3) set at center level; b glucose concentration (VAR1) vs. initial pH (VAR3) and their interactive effect with agitation speed (VAR2) set at center level; c effects of agitation speed (VAR2) vs. initial pH (VAR3) and their interactive effect with glucose concentration (VAR1) set at center level; d effects of glucose concentration (VAR1) vs. agitation speed (VAR2) and their interactive effect with initial pH (VAR3) set at center level; e glucose concentration (VAR1) vs. initial pH (VAR3) and their interactive effect with agitation speed (VAR2) set at center level; and f agitation speed (VAR2) vs. initial pH (VAR3) and their interactive effect with glucose concentration (VAR1) set at center level
Results and Discussion
Screening
Firstly, the fungi, microalgae, and cyanobacteria strains were screened in monocultures for biomass and EPS production capacity. The results are presented in Table 5.
The yield Y S/P (conversion of glucose to polymer) varied from 0.017 to 0.283, and the best yields were those of Trametes versicolor (0.283) and Ganoderma lucidum (0.207). The best EPS production was achieved by T. versicolor (4.13 g/L, Y S/P = 0.283) and Agaricus blazei (3.44 g/L, Y S/P = 0.192) in 10 days monocultures.
Some strains, such as Lentinus edodes and G. lucidum, showed a slow growth rate in the conditions tested, while others, such as T. versicolor, produced more than 10 g/L of biomass. This great variability of EPS and biomass production and the absence of relation between them make the screening a key step. Considering the best biomass and EPS producers (Table 5), the following microorganisms were chosen for performing co-culture systems: T. versicolor, A. blazei, C. vulgaris, and S. platensis—PARACAS.
Co-Cultures
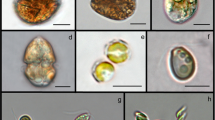
Different arrangements between the four selected strains were carried in order to evaluate EPS characterization (Table 2). Figures 3 and 4 are microscopical pictures showing the presence of both microorganisms in the all the co-culture systems tested. Co-cultures were evaluated daily through optical microscopy to ensure that both microorganisms were growing, once through dry weight it was not possible to quantify separately the fungal and microalgal/cyanobacterial biomasses.
Optical microscopical pictures of the co-cultures (A1, A2) T. versicolor and S. platensis (amplification of (A1) × 40 and (A2) × 200); (B1, B2) A. blazei and T. versicolor (amplification of (B1) × 200 and (B2) × 400); and (C1, C2) C. vulgaris and S. platensis (amplification of (C1) × 40 and (C2) × 400)
Table 6 compares the typical time courses for higher EPS production of mono- and co-cultures. Interestingly, in all cases, co-culture produced EPS much faster than monocultures.
EPS Characterization
The crude EPS obtained from co-cultures and pure cultures of C. vulgaris, S. platensis, A. blazei, and T. versicolor were analyzed for protein, neutral sugar, and total sugar content. The results (Table 7) showed that their carbohydrate portions are composed of six to eight different types of monomer units, which is quite unusual in microbial polysaccharides of industrial interest. Significant differences in EPS monosaccharide composition from mono- and co-cultures can be observed in Table 7. Mannose was the predominant monosaccharide in all EPS, except in the one produced by C. vulgaris, where glucose and fucose dominated.
Besides monosaccharide composition, the EPS structures were analyzed by ¹³C NMR. The NMR spectra (Figs. 5, 6, 7, 8, 9, and 10) of the co-cultured produced EPS showed a fungal EPS predomination but with some differences in the structure, indicating that a third type of EPS is produced, different from the ones produced by monocultures. A standard EPS 13C NMR analysis was carried out (Fig. 10) using tryptophan as a nitrogen source in the cultivation media composition in order to show no influence of the EPS present in the yeast extract used in media composition of macromycetes during the experiments.
Figure 10 shows a highly complex spectrum (4B), with a variety of signals in the anomeric region, with five main signals at 100.2, 99.5, 96.9, 102.1, and 103.4 ppm. Signs in 17.1 and 15.3 ppm corresponding to CH3 confirm the presence of 6-desoxy sugars (fucose and rhamnose) in agreement with the monosaccharide composition results. Signals around 16 ppm and around 20 ~ 25 ppm possibly indicates presence of pyruvic acid acetal group.
Co-Cultures Preliminary Optimization
Seven variables (culture temperature, agitation speed, inoculation density, initial pH, illumination intensity, and glucose concentration) were optimized for EPS and biomass production. The Plackett–Burman experimental design results for biomass and EPS production in co-cultures are shown in Table 8. A. blazei and C. vulgaris co-culture produced more biomass and EPS and was, thus, chosen for the next steps.
The Pareto chart for the co-culture of A. blazei and C. vulgaris is shown in Fig. 1. Culture temperature did not show a significant effect, while microalgal inoculation density exhibited negative influence, and agitation speed, fungal inoculation density, illumination rate, and glucose concentration showed positive effect.
Based on the Pareto chart, glucose concentration, agitation speed, and initial pH were selected as factors for further optimization. Light intensity was then fixed at 3,000 lx. Other variables with less significant effect were not included in the next step but were used in all trials at their low level (considering low cost of production).
Using the Box–Behnken method, a set of experiments with appropriate combinations of glucose concentration, agitation speed, and initial pH for the co-culture of A. blazei and C. vulgaris was conducted, as indicated in Table 4. Contour surface plots of EPS and biomass yields reflecting changes in independent variables culture temperature, agitation speed, and fungal inoculation density are presented in Fig. 2.
These surfaces show that the EPS and biomass yields increased with increasing glucose concentration until approximately 52 g/L but decreased slowly beyond that range. The agitation speed (maximum productivity at approximately 200 rpm) and the pH (maximum productivity at approximately 5.0) showed a similar behavior. Cultures carried in the optimized fermentation conditions showed an average increase of 61% in biomass and 33% in EPS production.
Conclusion
The present work presents an innovative technological process of EPS production through cyanobacteria, microalgae, and Basidiomycetes co-culture in submerged fermentation (patent pending). When cultivated in co-culture, EPS production was favored and time of fermentation was significantly reduced. Significant enhancement of EPS and biomass production were achieved using Plackett–Burman followed by Box–Behnken design matrix. The composition and structure of the EPS produced indicated the production of a new EPS composed by a mixture of the EPS produced separately by each microorganism but mainly with fungal predominance. This indicates the existence of a synergistic effect, rather than an addition effect, in EPS production when co-cultures are performed. Further studies are therefore required to better characterize the EPS and evaluate medical and/or industrial applications.
References
Chihara, G., Hamuro, J., Maeda, Y. Y., Arai, Y., & Fukuoka, F. (1970). Cancer Research, 30, 2776–2781.
Fujimiya, Y., Suzuki, Y., Oshiman, K., Kobori, H., Moriguchi, K., Nakashima, H., et al. (1999). Biochemistry and Molecular Biology International, 47, 707–714.
Wasser, S., & Weis, A. (1999). Critical Reviews in Immunology, 19, 65–96.
Lindequist, U., Niedermeyer, T., & Jülich, W. (2005). eCAM, 2, 285–299.
Fan, L., Soccol, A. T., Pandey, A., & Soccol, C. R. (2007). LWT- Food Science and Technology, 40, 30–36.
Suzuki, Y., Adachi, Y., Ohno, N., & Yadomae, T. (2001). Biological and Pharmaceutical Bulletin, 24, 811–819.
Zjawiony, J. K. (2004). Journal of Natural Products, 67, 300–310.
Sánchez, C. (2009). Biotechnology Advances, 27, 185–194.
Rabinovich, M. L., Bolobova, A. V., & Vasil’chenko. (2004). Applied Biochemistry and Microbiology, 40, 1–17.
Gutiérrez, A. (1995). PhD Thesis, Facultad de Farmacia, España: Universidad de Sevilla.
Catley, B. (1992). In D. K. Arora, R. P. Elander, K. G. Mukerji (Eds.), Handbook of applied mycology, vol 4: Fungal biotechnology (pp. 259–279). New York: Dekker.
Tribelli, P. M., & López, N. I. (2011). Extremophiles, 15, 541–547.
Zarrouk, C. (1966). Ph.D Thesis, Université Des Paris, Paris, France
Watanabe, A. (1960). Journal of General and Applied Microbiology, 6, 1–4.
Dubois, M. (1956). Analytical Chemistry, 28, 350–356.
Somogyi-Nelson. (1952). Journal of Biological Chemistry, 195, 19.
Lowry, O. (1951). Journal of Biological Chemistry, 193, 265.
Yang, X. M., Beyenal, H., Harkin, G., & Lewandowski, Z. (2000). Journal of Microbiological Methods, 39, 109–119.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Angelis, S., Novak, A.C., Sydney, E.B. et al. Co-Culture of Microalgae, Cyanobacteria, and Macromycetes for Exopolysaccharides Production: Process Preliminary Optimization and Partial Characterization. Appl Biochem Biotechnol 167, 1092–1106 (2012). https://doi.org/10.1007/s12010-012-9642-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9642-7