Abstract
Using hyperaccumulator plants is an important method to remove heavy metals from contaminated land. Carpobrotus rossii, a newly found Cd hyperaccumulator, has shown potential to remediate Cd-contaminated soils. This study examined the effect of nitrogen forms on Cd phytoextraction by C. rossii. The plants were grown for 78 days in an acid soil spiked with 20 mg Cd kg−1 and supplied with (NH4)2SO4, Ca(NO3)2, urea, and chicken manure as nitrogen (N) fertilizers. Nitrification inhibitor dicyandiamide (DCD) was applied to maintain the ammonium (NH4 +) form. Nitrogen fertilization increased shoot biomass but decreased root biomass with the highest shoot biomass occurring in the manure treatment. Compared to the no-N control, urea application did not affect shoot Cd concentration, but increased Cd content by 17 % due to shoot biomass increase. Chicken manure significantly decreased CaCl2-extractable Cd in soil, and the Cd concentration and total Cd uptake in the plant. Rhizosphere pH was the highest in the manure treatment and the lowest in the NH4 + treatments. The manure and nitrate (NO3 −) treatments tended to have higher rhizosphere pH than their respective bulk soil pH, whereas the opposite was observed for urea and NH4 + treatments. Furthermore, the concentrations of extractable Cd in soil and Cd in the plant correlated negatively with rhizosphere pH. The study concludes that urea significantly enhanced the Cd phytoaccumulation by C. rossii while chicken manure decreased Cd availability in soil and thus the phytoextraction efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil contamination by heavy metals has accelerated during the last few decades. Cadmium is considered as one of the most toxic heavy metals. The high concentrations of Cd observed in many agricultural soils are due to long-term use of phosphate fertilizer and sewage sludge (Stingu et al. 2012). As a non-essential element for living organisms, Cd is highly mobile in soil-plant systems and can adversely affect both human health and ecosystem functioning (Dwivedi et al. 2014). Therefore, cost-effective procedures are required to remediate Cd-contaminated soils.
Compared with classical remediation techniques, phytoremediation is a cheap and environmentally friendly technique and has attracted attention in recent years. Especially for agricultural soils, concentrations of heavy metals are generally low, and heavy metals are distributed on the surface and can be easily extracted by plant root systems. The task for effective phytoremediation is to identify hyperaccumulator plants with high biomass production. There are numerous reports on the use of hyperaccumulators to remove heavy metals from the soil (Bayramoglu et al. 2012; Li et al. 2014). However, the slow growth and low shoot biomass limit the use of many hyperaccumulator plants for a large-scale remediation. Apart from the use of hyperaccumulators with larger biomass, the application of fertilizers or soil amendments to improve soil physicochemical properties and increase plant biomass production is also important for phytoremediation. For example, Wei et al. (2010) found that adding chicken manure significantly increased total Cd extraction by Solanum nigrum although it decreased Cd concentration in plant tissues.
Nitrogen (N) is an essential macronutrient and is involved in many metabolic processes including the biosynthesis of amino acids and proteins in plants. The application of N fertilizers is important for biomass production and yield. Nitrate (NO3 −) and ammonium (NH4 +) are the main inorganic N sources for plants in most agricultural systems. It has been reported how the supply of NH4 + enhances Zn and Cd uptake by non-hyperaccumulators sunflower and tobacco, which has been related to rhizosphere acidification (Loosemore et al. 2004; Zaccheo et al. 2006). Zaccheo et al. (2006) suggested that manipulation of rhizosphere pH via the combined use of NH4 +–N and nitrification inhibitors was an effective way to enhance phytoextraction of Cd and Zn from contaminated soils. However, in hydroponic experiments, supplying NO3 − doubled Cd concentration in the shoots of Noccaea caerulescens, compared with NH4 + supply (Xie et al. 2009). Similar results were found for Sedum plumbizincicola in solution culture; NO3 − stimulated Cd uptake by roots, xylem loading, shoot-to-root translocation, and uploading to the leaves and thus enhanced Cd phytoextraction (Hu et al. 2013). Monsant et al. (2008) showed that NO3 − nutrition improved Zn phytoextraction in solution culture by increasing the biomass and Zn concentration of shoots of N. caerulescens.
This soil-based pot experiment compared the effects of manure-N and different forms of chemical N supply (NO3 −–N, NH4 +–N, and urea-N) on Cd uptake by Carpobrotus rossii, a newly found Australian Cd hyperaccumulator (Zhang et al. 2014). A nitrification inhibitor was used to maintain the NH4 + form for plant uptake. We hypothesized that the NO3 −–N fertilizer form would increase Cd phytoextraction by C. rossii above that of NH4 +–N forms, in line with earlier research.
Materials and methods
Cd-contaminated soil
The soil was collected from the surface layer (0–15 cm) in the farm of La Trobe University (37° 43′ 18″ S, 145° 02′ 52″ E). Soil samples were air-dried and ground to pass through a 4-mm sieve. The soil had pH 4.99 (1:5, soil:water), cation exchange capacity 16 cmol kg−1, and the following properties (mg kg−1): hydrolyzable N 158, available P 11.5, available K 132, and background Cd 0.5. The soil was spiked with 20 mg Cd kg−1 by mixing CdCl2 solution thoroughly into the soil. The water content was adjusted to 60 % field capacity. The spiked soil was placed in constant room temperature (25 °C) for 2 weeks to allow added Cd to equilibrate.
Experimental design and growing conditions
The experiment consisted of six treatments with three replicates. The treatments were no-N control, chicken manure, Ca(NO3)2, urea [CO(NH2)2], (NH4)2SO4, and (NH4)2SO4 plus NH2C(NH)2CN (dicyandiamide, DCD). The N and DCD were applied to the soil in 100 mL of water to bring the soil water content to 80 % field capacity. Nitrogen was applied at 20 mg N kg−1 weekly (total = 200 mg N kg−1). In the NH4 + + DCD treatment, DCD was applied at 8 mg kg−1. This application rate of DCD had no significant effect on plant growth and was effective in minimizing nitrification (Monsant et al. 2008). Chicken manure had the following properties: pH 7.68, total N 20.1 g kg−1, total Cd 0.55 mg kg−1, available N 3430 mg kg−1, available P 11.5 mg kg−1, and available K 150 mg kg−1. The manure was added to the soil at a rate of 1 % w/w (about 200 mg N kg−1) before planting.
A pot experiment was conducted in a glasshouse (20 ± 5 °C) with natural light. Plastic pots were lined with plastic bags and filled with 1.5 kg air-dried soil. Basal nutrients were applied at the following concentration expressed in milligrams per kilogram of soil: 150 KNO3, 21 MgSO4·H2O, 150 KH2PO4, 236 CaCl2·2H2O, 18 MnCl2·4H2O, 0.67 H3BO3, 10.33 ZnSO4·7H2O, 1.42 CuCl2·5H2O, and 0.15 Na2MoO4·2H2O. The soil water content was maintained at around 80 % field capacity by watering daily for the experiment duration.
Pre-culture of stem cuttings of C. rossii (family Aizoaceae) were grown in plastic nursery cells for 3 weeks (Zhang et al. 2014), and then three uniform rooted stem cuttings were transplanted into each pot. The plants were harvested on day 78.
Plant and soil analyses
At harvest, shoots were cut at their base, and roots were separated from the soil. Rhizosphere soils were collected by gently shaking off the soil adhering to the roots. The roots were first rinsed with running tap water. The root morphological parameters were determined with a root scanner at 600 dpi (Epson Perfection 4990 Scanner, model J131B, Epson Inc.). Both shoots and roots were then rinsed in distilled water, immersed in 0.1 mM HCl for approximate 5 s to remove external metal from the tissue surface, and finally rinsed with distilled water twice. These samples were dried in an oven at 105 °C for 15 min and then at 70 °C until completely dry, weighed immediately upon removal from a desiccator, and then ground. Plant materials (0.5 g) were digested with 7 mL mixture of concentrated HNO3 and HClO4 (4:1 by volume).
The soil samples taken at the harvest were air-dried and ground to pass through 2 mm. For extractable Cd, air-dried soil was extracted in 0.01 M CaCl2 solution (1:10 w/v) by shaking end-over-end for 16 h at 25 °C. The suspensions were then centrifuged at 3000 rpm for 10 min, followed by filtering the supernatant through Whatman No. 1 filter paper. The supernatant was used for Cd determination.
The concentrations of Cd in shoot and root digests, and soil extracts were determined using ICP-OES (Varian Vista AX CCD, Australia Pty Ltd.). All plant tissue concentrations were expressed on a dry weight basis. The pH of the bulk soil and rhizosphere soil was determined in the supernatant using a pH meter (Thermo Orion 720, USA) after shaking 5 g soil in 25 mL 0.01 M CaCl2 solution, end-over-end for 12 h, and then centrifuging at 2000 rpm for 10 min.
Statistical analysis
The data were statistically analyzed using Excel and SPSS 16.0 software, and expressed as means ± standard deviation. The means of the treatments were compared using the Duncan’s multiple range tests at p = 0.05.
Results
Plant growth
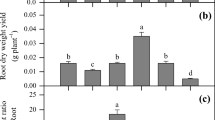
All plants appeared healthy during the experiment, with differences in shoot growth between the treatments becoming noticeable prior to the harvest at 78 days. The average shoot dry weights ranged from 6.59 to 8.77 g pot−1. Relative to the no-N control, N fertilization increased shoot weight by 13–33 %. Shoot biomass was the highest in the manure treatment while DCD did not affect the shoot dry weight in the NH4 + treatments (Fig. 1a). In contrast, the application of manure and chemical N decreased the root dry weight by 38–47 % with manure having less effect (Fig. 1a), and hence, there was an increase in the shoot-to-root dry weight ratio. The shoot-to-root dry weight ratio was the highest for the urea and NH4 + treatments and the lowest for the no-N control with no significant differences between the four chemical N treatments (Fig. 1b).
Effects of N forms and nitrification inhibitor (DCD) on dry weights of (a) shoots and roots, and (b) shoot-to-root ratio of C. rossii grown in a Cd-contaminated soil for 78 days. The control had no added nitrogen. Treatments assigned with different letters are statistically different according to the Duncan’s multiple range tests at the 5 % level. Error bars ±standard deviation (n = 3)
Root morphology
Quantitative data of the root morphology are given in Table 1. The decrease in root biomass with the chemical N treatments coincided with smaller surface areas and shorter cumulative lengths of roots than the control plants. Furthermore, root length and root surface area were greater in the manure than in the chemical N treatments. Thicker roots with larger diameters occurred with the NO3 −, NH4 +, and urea treatments.
Rhizosphere and bulk soil pH
The rhizosphere pH ranged from 4.57 to 5.65, while the bulk soil pH varied between 4.62 and 5.31 (Fig. 2). The bulk soil pH was highly correlated with the rhizosphere pH (p < 0.01), indicating that the bulk soil had been affected by the root activity. Rhizosphere pH was the highest in the manure treatment and the lowest in the NH4 + treatments while the manure and NO3 − treatments tended to have higher rhizosphere pH and the urea and NH4 + treatments lower rhizosphere pH than their respective bulk soil pH readings.
Effects of N forms and manure addition on rhizosphere and bulk soil pH of C. rossii grown in a Cd-contaminated soil for 78 days. Means for the same type of soil with different letters are statistically different according to the Duncan’s multiple range tests at the 5 % level. Error bars ±standard deviation (n = 3)
Cadmium in plants
The concentration of Cd in shoots varied from 97 mg kg−1 for the manure treatment to 172 mg kg−1 for the urea treatment (Fig. 3a). Compared to the no-N control, the manure and NO3 − treatments had lower while the urea and NH4 + + DCD treatments had similar concentrations of Cd in shoots.
Effects of N form and manure addition on (a) Cd concentrations in shoots and roots, (b) shoot-to-root Cd concentration ratio, (c) the amount of Cd in shoots and roots, and (d) shoot-to-root Cd content ratio of C. rossii grown in a Cd-contaminated soil for 78 days. Treatment means with different letters within a tissue type are statistically different according to the Duncan’s multiple range tests at the 5 % level. Error bars ±standard deviation (n = 3)
The total amount of Cd in the shoots was significantly enhanced by supply of urea and NH4 + + DCD, but decreased by manure and NO3 − treatments, compared to the control (Fig. 3c). Thus, the Cd amount was the highest in the urea and NH4 + + DCD treatments, followed by the NH4 + treatment (similar to control) and then the manure and NO3 − treatments (lower than control). The amounts of Cd in the shoots in the urea and NH4 + + DCD treatments were 15 and 14 % greater than that in the control, respectively, and were greatest among the N treatments.
The concentrations of Cd in the roots were 4–6-fold higher than those in the shoots, ranging from 540 for the manure treatment to 793 mg kg−1 for the urea treatment (Fig. 3a). DCD did not significantly affect the Cd concentration in roots although NH4 + + DCD tended to have higher Cd than the NH4 +-alone treatment (Fig. 3a, b). Unlike the shoots, the amount of Cd in the root did not vary between the N treatments, with Cd amount being lower in all N treatments compared with the control.
The shoot-to-root ratios of Cd concentration and content for the NH4 +, NH4 + + DCD and urea treatments were significantly higher than those for the manure and NO3 − treatments, indicating that NH4 + and urea enhanced Cd translocation from the roots to shoots compared with that of NO3 − and manure (Fig. 3c, d). The highest shoot-to-root ratio of Cd concentration occurred in the control treatment.
Given the treatment effects on rhizosphere pH (Fig. 2) and Cd concentrations in shoots (Fig. 3a), it was thus not surprising that the Cd concentration in shoots correlated negatively with rhizosphere pH across treatments (p < 0.01) (Fig. 4).
Extractable Cd in bulk soil
Compared to the control, most of N fertilizers tended to increase the concentration of CaCl2-extractable Cd but the only significant difference was recorded with the NH4 + + DCD treatment (Fig. 5). By contrast, manure amendment strongly decreased the concentration of CaCl2-extractable Cd by a factor of 2 compared to the control.
Discussion
This study demonstrated that C. rossii could accumulate Cd to a concentration of over 170 mg kg−1 in the shoots, and that ammonium enhanced while nitrate decreased the Cd phytoextraction by this species when grown in the acid soil. C. rossii is a succulent glabrous halophytic perennial commonly found in the coastal fore-dunes of southern Australia (Venning 1988). It was shown to hyperaccumulate Cd and to be more tolerant to Cd than most species (Zhang et al. 2014). Other Cd hyperaccumulators have been identified, notably N. caerulescens (Robinson et al. 1998), Arabidopsis halleri (Bert et al. 2002), Rorippa globosa (Sun et al. 2007), S. nigrum (Wei et al. 2006), Sedum alfredii (Yang et al. 2004), Viola baoshanensis (Liu et al. 2004), and S. plumbizincicola (Li et al. 2014), but none of these plants are native to Australia (van der Ent et al. 2013). In combination with its easy growing, and salt- and drought-tolerant traits, this native species, C. rossii, could be a promising candidate for phytoremediation of Cd-contaminated soils, especially in drought-prone areas and soils with high salinity (Zhang et al. 2014).
Nitrogen fertilization is believed helpful for phytoremediation of heavy metals because it enhances plant growth, influences the cellular charge balance (Marschner and Römheld 1994) and helps to produce proteins which form complexes with metals to affect translocation and/or storage or are antioxidative enzymes to reduce the level of oxidation caused by metals (Sarwar et al. 2010). Nitrogen fertilizers, usually in the form of NH4 +, NO3 −, urea, or organic nitrogen (manure), are used to enhance plant biomass and the phytoextraction efficiency of contaminants from soils (Giansoldati et al. 2012). This study showed that all forms of nitrogen could efficiently increase plant biomass to a similar extent. Although the highest Cd concentrations in shoots were observed in the control, urea, and NH4 + + DCD treatments, the highest amounts of Cd were obtained in the urea and NH4 + + DCD treatments due to higher biomass production in these treatments compared to the control (Fig. 3).
Nitrate and NH4 + are the two main forms of inorganic N that are taken up by plants. Since N accounts for 80 % of the total nutrients taken up by roots, the charge of these N ions has a strong effect on charge balance in root epidermal cells (Marschner and Römheld 1994). Physiologically, the uptake of NO3 − and NH4 + induces the release of OH−/HCO3 − and H+ ions, respectively. This was shown in the present study where the rhizosphere pH was lower in the NH4 + than in the NO3 − treatment (Fig. 2). Ammonium nutrition causes rhizosphere acidification which in turn increases metal bioavailability in soil and hence plant uptake (Zaccheo et al. 2006). Thus, rhizosphere acidification might explain the higher concentration and phytoextraction of Cd by C. rossii grown in the NH4 + than the NO3 − treatment, with much lower rhizosphere pH in the NH4 + treatments. This is also supported by the strong negative relationship between Cd concentration in shoots and rhizosphere pH (Fig. 4), and the higher Cd phytoextraction with the NH4 + + DCD than the NH4 +-alone treatment (Fig. 3) because DCD is able to inhibit nitrification and thereby decrease NO3 − concentration in soil (Arnamwong et al. 2015). Our results are consistent with the study of Zaccheo et al (2006) who showed that the application of NH4 + together with nitrification inhibitor DMPP resulted in higher Cd uptake compared to NO3 −, in the shoot of non-hyperaccumulator sunflower grown in contaminated soils.
The present study showed that manure addition increased the production of shoot biomass but decreased the concentration of extractable Cd in soil and Cd concentration in plants. A number of studies have also shown that the application of organic materials (e.g., farmyard manure, compost, peat soil) resulted in a decrease in Cd concentration in plants (e.g., corn, wheat, radish, alfalfa, and Indian mustard) (Wei et al. 2010). In general, organic amendments such as animal manures contain a high proportion of humidified organic matter, which forms strong complexes with Cd, resulting in Cd immobilization and decreased Cd bioavailability in the soil (Putwattana et al. 2010). Additionally, manures often contain high contents of dissolved organic matter which forms soluble organic-metal complexes (Bolan et al. 2003a; Vaca-Paulin et al. 2006) that reduce metal availability to plants (Bolan et al. 2003b). Soluble organic-Cd complexes are also likely to have contributed to the lower phytoextraction by the manure treatment.
All N fertilizers reduced root biomass (Fig. 1a), and this is likely to be in response to the alleviation of N deficiency in the soil. The chemical N treatments resulted generally in shorter, thicker roots with less surface area compared with the control and manure treatments (Table 1). An increased biomass allocation to the roots is a common phenomenon in many plant species in response to N deficiency. It is suggested that N deficiency initiates transcriptional changes that lead to the accumulation of carbohydrates in the shoots and increase the translocation of sucrose to the roots (Hermans et al. 2006). It could be argued that small root systems in the N-amended treatments would not favor the uptake of heavy metals in soil. For example, Lombi et al. (2000) reported the greater root system in the French ecotype of N. caerulescens (root:shoot = 0.21) than the Prayon ecotype (root:shoot = 0.12) could be partly responsible for the higher Cd uptake in roots and accumulation in shoots of the French ecotype. However, in this study, the NH4 + + DCD and urea treatments resulted in higher Cd uptake by shoots compared to the control and yet had significantly smaller root systems. Thus, soil factors such as rhizosphere pH and Cd extractability were more important in determining Cd extraction from the soil than root morphology.
Nitrogen form also appeared to affect Cd translocation from roots to shoots of C. rossii. It is evident that the shoot-to-root ratio of Cd concentration and content was significantly higher in the urea and NH4 + treatments than the NO3 − treatment (Fig. 3c, d), indicating that urea and NH4 + nutrition stimulates the translocation of Cd from roots to shoots to a higher extent compared with NO3 − nutrition. Wang et al. (2010) also found the shoot-to-root Cd concentration in mustard grown in pots was higher with NH4 + than with NO3 − treatment. The mechanism by which N form affects the Cd translocation is unclear. In N. caerulescens, NO3 − nutrition enhanced the formation of organic acids while NH4 + nutrition increased the formation of amino acids (White-Monsant and Tang 2013). The form of N in this study might have also affected the concentrations of different metabolites in the xylem of C. rossii and thereby affected the Cd transport from the roots to the shoots. However, N form did not affect Zn speciation in shoots, roots, or xylem even though Zn accumulation in the shoots was enhanced (Monsant et al. 2008, 2011).
The results from this study, where NH4 +–N forms resulted in greater Cd uptake by shoots than NO3 − –N, mean that our hypothesis is rejected. The results are inconsistent with those of Xie et al. (2009) and Hu et al. (2013). By using a positron-emitting 107Cd tracer, Hu et al. (2013) showed that Cd transport was 1.5-fold and 3.7-fold faster in the roots and shoots, respectively, in the Cd hyperaccumulator S. plumbizincicola supplied with NO3 − than with NH4 +. The concentration of Cd was 2.63 times higher in shoots of the NO3 −- than NH4 +-fed plants in nutrient solution. Similarly, Xie et al. (2009) showed in hydroponic experiments that compared with NH4 + nutrition, NO3 − nutrition doubled shoot Cd concentrations and increased shoot Cd accumulation by 2.6–8-fold in N. caerulescens. The reasons for the discrepancy in these studies can be attributed to the rhizosphere effect in this soil-based study. This effect, where the rhizosphere pH differs with N form (Fig. 2), is dissipated in solution culture, as ions can freely diffuse away from the root surface in the culture solution, which cannot happen in the soil. Thus, the study highlights the importance of soil-based experiments, compared to those using solution culture, when investigating Cd phytoextraction from Cd-contaminated soils.
Conclusions
This study aimed at testing chemical N fertilizers and manure to evaluate the influence of N form on Cd phytoextraction using Australian native species C. rossii, a Cd hyperaccumulator. The addition of N fertilizers significantly enhanced shoot biomass production, particularly manure application resulted in the highest shoot biomass. However, manure application significantly decreased Cd availability in soil and hence plant Cd phytoextraction efficiency. Although the four chemical N treatments resulted in similar shoot and root biomass production, the highest efficiency of Cd phytoextraction by C. rossii was in the urea- and NH4 + + DCD-amended soils. Thus, this study suggests that urea would be a good N source to enhance Cd phytoextraction using C. rossii. Future work is required to compare the effect of N form on metal extraction with other plant species and to validate the efficiency of C. rossii under field conditions.
References
Arnamwong S, Wu L, Hu P, Yuan C, Thiravetyan P, Luo Y, Christie P (2015) Phytoextraction of cadmium and zinc by Sedum plumbizincicola using different nitrogen fertilizers, a nitrification inhibitor and a urease inhibitor. Int J Phytoremediation 17:382–390
Bayramoglu G, Arica MY, Adiguzel N (2012) Removal of Ni (II) and Cu (II) ions using native and acid treated Ni-hyperaccumulator plant Alyssum discolor from Turkish serpentine soil. Chemosphere 89:302–309
Bert V, Bonnin I, Saumitou‐Laprade P, De Laguérie P, Petit D (2002) Do Arabidopsis halleri from nonmetallicolous populations accumulate zinc and cadmium more effectively than those from metallicolous populations? New Phytol 155:47–57
Bolan N, Adriano D, Mani S, Khan A (2003a) Adsorption, complexation, and phytoavailability of copper as influenced by organic manure. Environ Toxicol Chem 22:450–456
Bolan NS, Adriano DC, Natesa R, Koo BJ (2003b) Effects of organic amendments on the reduction and phytoavailability of chromate in mineral soil. J Environ Qual 32:120–128
Dwivedi G, Upadhyay S, Mishra A, Kumar Singh A (2014) Hyper accumulation of cadmium in Solanum nigrum L. and their effects on phyto-chemicals and antioxidant enzymatic activities. Int J Pharm Sci Res 5:1424–1430
Giansoldati V, Tassi E, Morelli E, Gabellieri E, Pedron F, Barbafieri M (2012) Nitrogen fertilizer improves boron phytoextraction by Brassica juncea grown in contaminated sediments and alleviates plant stress. Chemosphere 87:1119–1125
Hermans C, Hammond JP, White PJ, Verbruggen N (2006) How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci 11:610–617
Hu P, Yin YG, Ishikawa S, Suzui N, Kawachi N, Fujimaki S, Igura M, Yuan C, Huang J, Li Z (2013) Nitrate facilitates cadmium uptake, transport and accumulation in the hyperaccumulator Sedum plumbizincicola. Environ Sci Pollut Res 20:6306–6316
Li Z, Wu L, Hu P, Luo Y, Zhang H, Christie P (2014) Repeated phytoextraction of four metal-contaminated soils using the cadmium/zinc hyperaccumulator Sedum plumbizincicola. Environ Pollut 189:176–183
Liu W, Shu W, Lan C (2004) Viola baoshanensis, a plant that hyperaccumulates cadmium. Chin Sci Bull 49:29–32
Lombi E, Zhao FJ, Dunham SJ, McGrath SP (2000) Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytol 145:11–20
Loosemore AN, Straczek A, Hinsinger P, Jaillard B (2004) Zinc mobilisation from a contaminated soil by three genotypes of tobacco as affected by soil and rhizosphere pH. Plant Soil 260:19–32
Marschner H, Römheld V (1994) Strategies of plants for acquisition of iron. Plant Soil 165:261–274
Monsant AC, Tang C, Baker A (2008) The effect of nitrogen form on rhizosphere soil pH and zinc phytoextraction by Thlaspi caerulescens. Chemosphere 73:635–642
Monsant AC, Kappen P, Wang Y, Pigram PJ, Baker AJ, Tang C (2011) In vivo speciation of zinc in Noccaea caerulescens in response to nitrogen form and zinc exposure. Plant Soil 348:167–183
Putwattana N, Kruatrachue M, Pokethitiyook P, Chaiyarat R (2010) Immobilization of cadmium in soil by cow manure and silicate fertilizer, and reduced accumulation of cadmium in sweet basil (Ocimum basilicum). Sci Asia 36:349–354
Robinson BH, Leblanc M, Petit D, Brooks RR, Kirkman JH, Gregg PE (1998) The potential of Thlaspi caerulescens for phytoremediation of contaminated soils. Plant Soil 203:47–56
Sarwar N, Saifullah, Malhi SS, Zia MH, Naeem A, Bibi S, Farid G (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric 90:925–937
Stingu A, Volf I, Popa VI, Gostin I (2012) New approaches concerning the utilization of natural amendments in cadmium phytoremediation. Ind Crops Prod 35:53–60
Sun Y, Zhou Q, Wei S, Ren L (2007) Growth responses of the newly-discovered Cd-hyperaccumulator Rorippa globosa and its accumulation characteristics of Cd and As under joint stress of Cd and As. Front Environ Sci Eng China 1:107–113
Vaca-Paulin R, Esteller-Alberich MV, Lugo de la Fuente J, Zavaleta-Mancera HA (2006) Effect of sewage sludge or compost on the sorption and distribution of copper and cadmium in soil. Waste Manag 26:71–81
van der Ent A, Baker AJ, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334
Venning J (1988) Flora of Australia Vol. 4: Phytolaccaceae to Chenopodiaceae. Australian Government Publishing Service
Wang Y, Ai S, Li M, Yang S, Yao J, Tang M, Zeng Z (2010) Effect of nitrogen fertilization on cadmium translocation in soil-mustard system. Chinese J Eco-Agric 18:649–653 (in Chinese)
Wei S, Li Y, Zhou Q, Srivastava M, Chiu S, Zhan J, Wu Z, Sun T (2010) Effect of fertilizer amendments on phytoremediation of Cd-contaminated soil by a newly discovered hyperaccumulator Solanum nigrum L. J Hazard Mater 176:269–273
Wei S, Zhou Q, Koval PV (2006) Flowering stage characteristics of cadmium hyperaccumulator Solanum nigrum L. and their significance to phytoremediation. Sci Total Environ 369:441–446
White-Monsant A, Tang C (2013) Organic acids are not specifically involved in the nitrate-enhanced Zn hyperaccumulation mechanism in Noccaea caerulescens. Environ Exp Bot 91:12–21
Xie H, Jiang R, Zhang F, McGrath S, Zhao F (2009) Effect of nitrogen form on the rhizosphere dynamics and uptake of cadmium and zinc by the hyperaccumulator Thlaspi caerulescens. Plant Soil 318:205–215
Yang X, Long X, Ye H, He Z, Calvert D, Stoffella P (2004) Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259:181–189
Zaccheo P, Crippa L, Pasta VDM (2006) Ammonium nutrition as a strategy for cadmium mobilisation in the rhizosphere of sunflower. Plant Soil 283:43–56
Zhang C, Sale PW, Doronila AI, Clark GJ, Livesay C, Tang C (2014) Australian native plant species Carpobrotus rossii (Haw.) Schwantes shows the potential of cadmium phytoremediation. Environ Sci Pollut Res 21:9843–9851
Acknowledgments
We thank the Key Projects in the National Science & Technology Pillar Program (2015BAD05B04), National High Technology Research and Development Program of China (863 Program) (2012AA06A204), Jiangsu Provincial Natural Science Foundation of China (BK2012891), and Australian Research Council Linkage Project (LP100100800) for the financial support. We also thank anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Liu, W., Zhang, C., Hu, P. et al. Influence of nitrogen form on the phytoextraction of cadmium by a newly discovered hyperaccumulator Carpobrotus rossii . Environ Sci Pollut Res 23, 1246–1253 (2016). https://doi.org/10.1007/s11356-015-5231-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5231-y