Abstract
The effects of elevated CO2 on metal species and mobility in the rhizosphere of hyperaccumulator are not well understood. We report an experiment designed to compare the effects of elevated CO2 on Cd/Zn speciation and mobility in the rhizosphere of hyperaccumulating ecotype (HE) and a non-hyperaccumulating ecotype (NHE) of Sedum alfredii grown under ambient (350 μl l−1) or elevated (800 μl l−1) CO2 conditions. No difference in solution pH of NHE was observed between ambient and elevated CO2 treatments. For HE, however, elevated CO2 reduced soil solution pH by 0.22 unit, as compared to ambient CO2 conditions. Elevated CO2 increased dissolved organic carbon (DOC) and organic acid levels in soil solution of both ecotypes, but the increase in HE solution was much greater than in NHE solution. After the growth of HE, the concentrations of Cd and Zn in soil solution decreased significantly regardless of CO2 level. The visual MINTEQ speciation model predicted that Cd/Zn–DOM complexes were the dominant species in soil solutions, followed by free Cd2+ and Zn2+ species for both ecotypes. However, Cd/Zn–DOM complexes fraction in soil solution of HE was increased by the elevated CO2 treatment (by 8.01 % for Cd and 8.47 % for Zn, respectively). Resin equilibration experiment results indicated that DOM derived from the rhizosphere of HE under elevated CO2 (HE-DOM-E) (90 % for Cd and 73 % for Zn, respectively) showed greater ability to form complexes with Cd and Zn than those under ambient CO2 (HE-DOM-A) (82 % for Cd and 61 % for Zn, respectively) in the undiluted sample. HE-DOM-E showed greater ability to extract Cd and Zn from soil than HE-DOM-A. It was concluded that elevated CO2 could increase the mobility of Cd and Zn due to the enhanced formation of DOM–metal complexes in the rhizosphere of HE S. alfredii.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities, particularly burning fossil fuel, have increased atmospheric carbon dioxide (CO2) and the concentration is predicted to double by 2100 (IPCC 2007). A large number of studies have shown that plants generally respond to elevated CO2 concentration with increased biomass production, water and nutrient use efficiency (de Graaff et al. 2007; Hungate et al. 1999; Kimball et al. 1995; Mishra et al. 2012), and photosynthetic rate (Cousins et al. 2003; Luttge 2004). The effects of CO2 enrichment on plant growth and physiological performance are diverse and complex, depending on the carbon fixation pathway (Körner 2001). According to the carbon fixation pathway, three functional groups can be distinguished, i.e., C3, C4 and CAM. For C3 plants, elevated CO2 concentration will reduce photorespiration, enhance photosynthetic CO2 exchange rate and increases carbon assimilates for plant growth and development, and hence increase growth and yield (Dijkstra et al. 2002). Although C4 plants represent only 1 % of the total terrestrial plant species, however, about 21 % of gross primary productivity is provided by C4 plants (Cerling et al. 1997). Thus, research on plant responses to rising atmospheric CO2 concentration and climate changes has primarily focused on C3 and C4 species, relatively few studies have been conducted on the effects of elevated atmospheric CO2 concentrations on CAM plants.
On the other hand, contamination of natural and agricultural soils with heavy metals is another serious problem throughout the world (Peris et al. 2007). Anthropogenic activities in modern society including mining, smelting, land application of sewage sludge, fertilization, and reclaimed water irrigation have accelerated soil contamination by heavy metals (Khan et al. 2000; Terzano et al. 2007). The remediation of contaminated soil is necessary not only to preserve the soil resource but also to safeguard human health (Kramer 2005; Vangronsveld et al. 2009). Elevated CO2 concentration has a profound impact on plant growth and development in metal polluted soils (Noyes et al. 2009). Thus, the responses of plants to elevated CO2 under heavy metals stress have been investigated by several researchers (Li et al. 2010; Guo et al. 2011; Rodriguez et al. 2011). In the majority of cases, elevated CO2 had a stimulatory effect on plant growth and metal uptake. For instance, Li et al. (2010) reported that elevated CO2 levels decrease Cu concentrations in six rice varieties grown in contaminated soils, but increase Cd levels in three rice varieties. Rodriguez et al. (2011) found that elevated CO2 significantly increased the total biomass production in soybean plants grown in fly ash amended soils and found significantly higher concentrations of Cu Ni, Pb and Zn in roots, stems and seeds under elevated CO2 condition. Guo et al. (2011) reported that elevated CO2 levels led to higher Cd concentrations in shoots and grain of both rice and wheat varieties grown in contaminated soil. These studies have been conducted with respect to food safety for crops, and demonstrate that elevated CO2 will greatly influence crop quality through altering the dynamics of the relationships between soil components, rhizosphere microbes and crops (Guo et al. 2011; Rodriguez et al. 2011).
Recently, some studies have been conducted from the viewpoint of phytoremediation. For instance, a variety of plant species including Indian mustard, Petridium revolutum, Sorghum, Trifolium, Pinus densiflora, Lolium mutiforum and Lolium perenne were shown to produce large biomass and/or remove significant amount of heavy metals from polluted soil under elevated CO2 level (Jia et al. 2010; Kim and Kang 2011; Tang et al. 2003; Wu et al. 2009; Zheng et al. 2008). These studies highlight that elevated CO2 have positive implications for improving phytoremediation efficiency. However, to our knowledge, most of these studies were conducted with C3 or C4 species, further research with a wider range of plant species, such as CAM plant, is required for a better understanding of the potential applications of these findings for phytoremediation practices. Moreover, understanding how hyperaccumulator species respond to future rise in atmospheric CO2 concentration and changing climate will be a challenging opportunity for plant science research.
A better understanding of the mechanisms by which elevated CO2 affect metal accumulation in plants will provide an improved knowledge on how elevated CO2 influences the plant–microbe–metal interactions in polluted soils. Recent studies have shown that increased root exudation and/or its consequence on biochemical features of rhizosphere soils caused by elevated CO2 could facilitate heavy metal accumulation by increasing nutrient cycling and availability, metal mobilization, plant growth, and/or improving soil quality (Rajkumar et al. 2013). For instance, Lieffering et al. (2004) pointed out that increased Fe, Zn and Mn bioavailability due to the increasing dissolved organic carbon (DOC) concentrations accounted for increased Fe, Zn and Mn uptake by rice plant under elevated CO2 condition. Wu et al. (2009) explained that CO2 induced Cs uptake in Sorghum and Trifolium species can be attributed to CO2 mediated decrease in the rhizosphere soil pH as a result of greater root exudation of carbonic acid. Recently, Guo et al. (2011) and Kim and Kang (2011) also found a similar effect on the uptake of Pb by Pinus densiflora and Cd by rice plants under elevated CO2. Although researches have shown that CO2 induced alteration in the rhizosphere processes such as increasing root exudation by the plant roots, lowering the rhizosphere soil pH play significant role on metal mobilization and its uptake by plants, experimental evidence supporting the above explanations is still lacking.
Sedum alfredii, a Crassulaceae species, originally grown in a Pb/Zn mined area of South China, is the first non-Brassicaceae Zn/Cd hyperaccumulator identified so far (Yang et al. 2004). It is a good candidate for phytoremediation of metal-contaminated soil, due to its rapid growth, asexual propagation, and high biomass yield (Li et al. 2012a). Previous study showed that elevated CO2 not only facilitate S. alfredii root growth, but also enhance total uptake of Cd and Zn (Li et al. 2012a). Moreover, bioavailability of Cd and Zn in the rhizosphere of S. alfredii increased significantly under elevated CO2 due to increased dissolved organic matter (DOM) (Li et al. 2013a). These results indicated that elevated CO2 induced DOM in the rhizosphere of S. alfredii plays a key role on metal mobilization and its uptake by plants. However, the mechanism involved in this process has never been characterized. The objectives of this study were (1) to investigate the effects of elevated CO2 on Cd and Zn speciation in rhizosphere of S. alfredii and (2) to investigate the effects of DOM from the rhizosphere of S. alfredii under elevated CO2 on Cd and Zn mobility. The results of this study will improve our understanding of the mechanisms by which S. alfredii mobilizes and uptake Cd and Zn from soil under elevated CO2.
Materials and methods
Plant material and soil characterization
The hyperaccumulating ecotype (HE) of S. alfredii was collected from an old Pb/Zn mine area in the city of Quzhou (29°17′N, 118°56′E), Zhejiang, China, and the non-hyperaccumulating ecotype (NHE) of S. alfredii was obtained from a tea garden in Hangzhou (30°18′N, 120°12′E), Zhejiang, China. Plants were grown in non-contaminated soil for several generations to minimize internal metal concentrations. The healthy and equal sized plant shoots were selected and grown for 3 weeks in the greenhouse using a basic nutrient solution (Li et al. 2012a). Plants were grown under glasshouse conditions with natural light, day/night temperature of 26/20 °C and day/night humidity of 70/85 %.The nutrient solution was aerated continuously and renewed every 3 days.
The paddy soil used in the pot experiment was collected from an abandoned site in Fuyang county of Hangzhou, Zhejiang, China. The site was heavily contaminated with Cd and Zn due to mining activities and not suitable for crop growth. The chemical and physical properties are shown in Table 1.
Design of the pot experiment
This was a continued study from our earlier experiments (Li et al. 2013a). The soil used in the pot experiment was collected from the surface layer (0–20 cm), air-dried, ground and sieved by 4-mm mesh. Soil samples (2.0 kg) were placed in each plastic pot (15 cm in diameter and 18 cm in height). The soil was then left to equilibrate outdoors under a waterproof tarpaulin for about 2 weeks after being moistened to 70 % field capacity. After pre-culturing for 2 weeks in hydroponic solution, four S. alfredii plants were transplanted in each pot. Control pots without plants were included. All pots were transferred into growth chambers (Conviron® E7/2) at a humidity of 70 %, with day and night temperatures of 26 °C and 20 °C, respectively. The average light intensity was maintained at 180 μmol m−2 s−1 during a 14-h light cycle. Four chambers were employed in this study, two of the chambers were maintained at ambient CO2 (350 μl l−1), the other two were maintained at elevated CO2 (800 μl l−1). Each growth chamber has two completely independent growth sectors, thus four replicates were established for each treatment, with one pot placed in each growth sector. Total CO2 treatment time was 60 days.
Soil solution extraction and analysis
Prior to harvest, each pot was saturated with Milli-Q water and allowed to equilibrate for 24 h for further extraction of field moisture. After harvest of the plants, soil solution was collected following centrifugation. The moist soil was packed into 25-ml filtration tubes. Soils were then centrifuged at 8,000 × g for 30 min with the filtration tube inside a 50-ml centrifuge tube that contained a small spacer in the bottom. Extracted solutions were then centrifuged at 12,000 × g for 30 min and filtered through a 0.45-μm membrane filter. DOC in soil solution was determined immediately after the isolation of soil solution using a total organic carbon analyzer (TOC-5050A; Shimadzu, Japan). The solution pH was measured using an Orion model 720A pH meter (Orion Research Inc., Boston, MA, USA). The concentrations of total Ca, Mg, K, Na, Cd and Zn were determined by inductively coupled plasma atomic emission spectrometry (ICP-AES, iCAP 6000 SERIES; Thermo Scientific), and the concentrations of anions (Cl−, SO4 2−, and NO3 −) were analyzed by Ion Chromatography (LC-20A; Shimadzu, Japan). In order to determine organic acid, the extracted solutions was allowed to pass first through a cation exchange column filled with 5 g Amerlite IR-120B resin (H+ form; Muromachi Chemical, Tokyo, Japan) and then through an anion exchange column filled with 1.5 g Dowex 1 × 8 resin (100–200 mesh, formate form). The organic acid anions retained in the anion exchange resin were eluted with 20 ml of 1 M HCl, and the eluent was freeze-dried (FreezZone 12; LABCONCO, USA). The residue was re-dissolved in 1 ml of mobile phase and filtered (0.2 μm) before analysis. The organic acid anions were detected by HPLC (Agilent 1100, USA) with mobile phase of 15 mM KH2PO4 (pH2.5).
Cd and Zn speciation in soil solution
The speciation of Cd and Zn in soil solution was assessed by calculation using Visual MINTEQ version 3.0, which is the Windows version of the MINTEQA2 model (Gustafsson 2011). The modeling was performed using soil solution pH, anions (Cl−, SO4 2−, and NO3 −) and total dissolved Ca, Mg, K, Na, Cd and Zn as input data (Cornu et al. 2011). The NICA-Donnan model was used to evaluate the metal binding to DOM. The modeling was made using generic NICA-Donnan parameters optimized for fulvic acid (FA) and humic acid (HA) (Milne et al. 2003). The DOM was simulated by 65 % FA and 35 % HA.
Complexation of Cd and Zn by dissolved organic matter
After harvest of the plants, DOM in the soil was extracted according to the method of Jones and Willett (Jones and Willett 2006). Field moist soil was extracted with deionized–distilled water using a solid/water ratio of 1:2.5 (w/v) on a dry weight basis and shaken at 200 rpm for 2 h at 20 °C on a reciprocal shaker. The suspension was centrifuged at 10,000 × g for 25 min, and the supernatant was filtered through a 0.45-μm membrane filter. The samples were stored in the dark at 1 °C. DOC, anions, heavy metals and major cations were determined as described above. Complexation of Cd and Zn by DOM was measured using a resin equilibrium method, and the experimental process has been described in detail by Li et al. (2012b). In brief, the complexation of Cd and Zn by DOM was assessed by equilibrating 150 mg resin with 25 ml of solution (a sample solution that containing different concentrations of DOC in its original matrix, or a reference solution that is DOC-free but is otherwise identical to the DOC-containing sample). The degree of DOC–metal complexation is defined as (1- K d,DOC/K d,R) × 100 %, where K d,DOC is the distribution of metal between the solution and the resin in the presence of DOC, K d,R is the distribution of metal between the solution and the resin in the absence of DOC. In addition, Visual MINTEQ version 3.0 was used to calculate the degree of DOC–metal complexation in sample solution that containing different concentrations of DOC in its original matrix. The solution was prepared by dilute the original DOM using deionized–distilled water. The modeling was performed using solution pH, anions and total dissolved Ca, Mg, K, Na, Zn, and Cd as input data (Cornu et al. 2011).
Metal mobilization tests
An extracting experiment was conducted to evaluate the effect of DOM from the rhizosphere of S. alfredii under elevated CO2 on metal mobility. The DOM was filtered through a 0.45-μm filter and subsequently through a sodium type of cation exchanger (Chelex-100 resin, 200–400 mesh; Bio-Rad, USA) to remove cations (e.g., Cd2+, Zn2+, Ca2+, Mg2+). The filtrates were stored at 4 °C before use. Two grams of paddy soil was placed into 50-ml polyethylene centrifuge tubes and equilibrated with 20 ml of DOM. Deionized water was added to each soil as a control. The suspensions were shaken on a reciprocal shaker at 200 rpm and 25 °C for 2 h and then centrifuged at 5,000 × g and filtered through a 0.45-μm filter. Cd and Zn concentrations in the filtrates were determined by ICP-AES (iCAP 6000 SERIES; Thermo Scientific).
Data analysis
All data were statistically analyzed using the SPSS package (Version 16.0). The data were analyzed with a two-way analysis of variance (ANOVA). Differences were considered significant at P < 0.05. Means of significant difference were separated using least significant difference (LSD, P < 0.05) or t test.
Results and discussion
Effect of elevated CO2 on physico-chemical properties of soil solutions
Solution pH is considered to be one of the most important chemical factors controlling metals speciation in soil solution. At the end of the experiment, solution pH was slightly decreased in soil planted with NHE S. alfredii under both ambient and elevated CO2, as compared to the control, but no difference was observed between ambient and elevated CO2 treatments (Table 2). For HE S. alfredii, however, soil solution pH was decreased significantly for both ambient and elevated CO2 treatments, moreover, the decrease in elevated CO2 treatment (by 0.51 units) was greater than in ambient CO2 treatment (by 0.29 units). This result is in accordance with previous studies on sunflower and Indian mustard (Tang et al. 2003), Sorghum vulgare (Wu et al. 2009), pine (Kim and Kang 2011), and rice (Li et al. 2010; Guo et al. 2011). In general, decreased soil solution pH tends to reduce the adsorption of heavy metals onto soil organic matter and clay mineral particle, resulting in more release of heavy metals into the soil solution. In this study, the soluble Cd and Zn in soil solution extracted from soil planted with HE S. alfredii increased significantly by elevated CO2 (Table 2). This result suggested that elevated CO2 can facilitate rhizosphere acidification and increase metal bioavailability, thereby increasing metal uptake by HE S. alfredii.
Lowering of rhizosphere pH has been attributed to the release of H+ and organic acids by plants causing acidification of the soils (McGrath et al. 1997). In this study, the reduction in solution pH was probably due to increased release of root exudates, which was consistent with the increased DOC derived from root exudation of HE S. alfredii (Tables 2 and 3). For both ecotypes of S. alfredii, the DOC concentration in soil solution was increased by elevated CO2, as compared with ambient CO2, but the increase in the HE S. alfredii (by 30.1 %) was much greater than in the NHE S. alfredii (by 12.2 %). This result is consist with Kim and Kang (2011) who pointed out that DOC concentrations in the rhizosphere of pine increased significantly by elevated CO2. Previous studies have shown that DOC released from plant roots can release the heavy metals from sorbents, sediments, and soil through DOC–metal complexation reaction, and then increase the mobility of heavy metals (Fitz et al. 2003; Li et al. 2012b). Thus, we propose that elevated CO2 can increase DOC in the rhizosphere of HE S. alfredii and thereby increase the bioavailability of Cd and Zn.
Guo et al. (2011) proposed that elevated CO2 increase exudation of low-molecular-weight organic compounds by the roots of rice. This result was also verified in present study (Table 3). In general, the total organic acid in soil solution of HE S. alfredii was higher than that of NHE S. alfredii. For both ecotypes of S. alfredii, the total organic acid in soil solution was increased by elevated CO2, as compared with ambient CO2, but the increase in the HE S. alfredii (by 23.8 %) was much greater than in the NHE S. alfredii (by 8.4 %). The low molecular weight organic acid (LMWOA) detected in all soils solution included oxalic acid, malic acid, citric acid and acetic acid, together with tartaric acid which were only detected in soil solution of HE S. alfredii (Table 3). LMWOA released by plant root, have the ability to form complexes with heavy metals, so they are likely to increase the solubility and phytoavailability of metals in soil (Tu et al. 2004). The present result indicated that elevated CO2 could promote the secretion of low-molecular-weight organic acids by HE S. alfredii, especially for oxalic acid, tartaric acid and citric acid, and thus increase the solubility of Cd and Zn.
The concentration of Cd, Zn, Mg, Ca, Na and K in soil solution was determined for both ecotypes of S. alfredii (Table 2). Soluble Mg, Ca, Na and K in soil solution were generally smaller after the growth of S. alfredii under both ambient and elevated CO2, as compared to control soil, but the difference was not significant for both ecotypes. For NHE S. alfredii, no significant changes in soluble Cd and Zn were observed under both ambient and elevated CO2, as compared with the control soil solution. In contrast, the concentrations of Cd and Zn in soil solution decreased significantly (P < 0.05) after growth of HE S. alfredii, regardless of CO2 level. This consistent with previous study that the labile Cd and Zn fraction (1 M NH4NO3-extractable) was depleted due to excessive Cd and Zn uptake (Li et al. 2013a). It’s important to point out that, the decreases under ambient CO2 (31.7 % for Cd and 28.9 % for Zn, respectively) was much greater than elevated CO2 (19.5 % for Cd and 18.2 % for Zn, respectively). However, the total uptake of Cd and Zn in the shoots of HE S. alfredii under elevated CO2 was much greater than ambient CO2 (Li et al. 2013a). This result indicated that elevated CO2 significantly increased soluble Cd and Zn in the rhizosphere of HE S. alfredii. Previous studies on rice (Guo et al. 2011) and pine (Kim and Kang 2011) have shown that the increased metal solubility and bioavailability in the rhizosphere of these plants under elevated CO2 was mainly attributed to the increases of DOC. Our results indicate that elevated CO2 can increase DOC in the rhizosphere of HE S. alfredii and thus increase bioavailability of Cd and Zn.
Effect of elevated CO2 on Cd and Zn speciation in soil solution
The bioavailability of trace metals to the plant can be better understood in terms of their chemical speciation. Our previous study demonstrated that Visual MINTEQ is a useful model for determination of metal species in soil solution of hyperaccumulators (Li et al. 2013b). Table 4 shows the speciation of Cd and Zn in soil solution calculated by Visual MINTEQ after the growth of S. alfredii under ambient or elevated CO2. The data indicated that Cd/Zn–DOM complexes were the dominant (50.25–89.90 %) Cd and Zn species in all soil solutions, followed by the free metal Cd2+ and Zn2+ species (9.84–48.97 %), with low amounts of Cd/Zn–inorganic complexes (0.23–1.52 %). These results are in agreement with previous work reported by (Krishnamurti et al. 2004; Sauve et al. 2000; Weng et al. 2002). However, other studies have reported speciation data showing that Cd and Zn are dominated by free metals ions (Cornu et al. 2009; Lorenz et al. 1997).
In control soil solution, the fractions of metal–DOM complex calculated by Visual MINTEQ were 75.42–75.64 % for Cd and 50.25–51.40 % for Zn, no difference was observed between two CO2 levels. After the growth of S. alfredii under elevated CO2, however, the increased DOC concentration induced by root exudates (Table 3) resulted in a higher fraction of metal–DOM complex than in control soil solutions, furthermore, the magnitude of increase for HE S. alfredii was much greater than for NHE S. alfredii, this result indicated that both plant ecotype and CO2 levels affect on metal speciation considerably. For instance, under elevated CO2, after the growth of HE S. alfredii, the increases in DOC concentrations resulted in 89.90 % of Cd and 69.22 % of Zn being complexed with DOM and only 9.84 % Cd and 30.25 % Zn remaining free in the soil solutions. However, the fraction of metal–DOM complex as a percentage of soluble Cd and Zn in soil solution of NHE S. alfredii was 81.89 % and 60.75 %, respectively (Table 4). Generally, soil solution pH and DOC concentration are considered the two main factors governing metal speciation in soil solution (Christensen and Christensen 2000). Previous studies have shown that metal speciation was governed by DOC rather than soil pH when the soil pH exceeded a certain level (Christensen and Christensen 2000; Schmitt et al. 2003; Weng et al. 2002). In this study, although decrease of soil solution pH was observed in soil solution of both ecotypes of S. alfredii grown under elevated CO2, most of the water soluble metals were DOM complex and exhibited positive correlation with DOC concentration in the solution, this result suggested that the change in metal speciation in soil solution is mainly governed by the enhanced DOC in the rhizosphere S. alfredii. The present study indicated that elevated CO2 could significantly promote the secretion of low-molecular-weight organic acids by HE S. alfredii, and thus increase the DOC concentration in soil solution, which resulted in a higher fraction of metal–DOM complex, and increase the mobility of heavy metals and their phytoavailability, resulting greater amounts of metal uptake by HE S. alfredii (Li et al. 2013b; Rajkumar et al. 2013).
Metal complexation with DOM in the rhizosphere of S. alfredii under elevated CO2
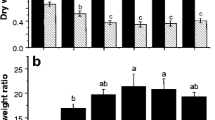
Previous studies have shown that the complexation of heavy metals with dissolved organic matter in the environment influences the solubility and mobility of these metals (Baken et al. 2011; Weng et al. 2002). In this study, the complexation of Cd and Zn by DOM derived from the rhizosphere of S. alfredii under ambient and/or elevated CO2 were measured using a resin equilibrium method as function of concentration (Fig. 1). The results confirmed that the DOM derived from the rhizosphere of S. alfredii has a significant ability to form complexes with Cd and Zn (Li et al. 2012b, 2013b). However, the degree of complexation varied with plant ecotype and CO2 level. In general, DOM derived from the rhizosphere of HE S. alfredii showed greater complexation ability than DOM derived from the rhizosphere of NHE S. alfredii. For instance, about 81–90 % of Cd and 61–73 % of Zn were bound in DOM complexes in the undiluted sample of HE S. alfredii. For NHE S. alfredii, however, the corresponding values were 74–79 % for Cd and 54–59 % for Zn, respectively. This difference may be due to the differences in ionic strength, cation composition, as well as function group of DOM (Baken et al. 2011; Christensen and Christensen 2000). This result also indicated that in the rhizosphere of S. alfredii, the complexation with DOM was more significant for Cd than for Zn. Similar results have been observed in other studies (Christensen and Christensen 2000; Nolan et al. 2003; Sauve et al. 2000).
Complexation of Cd and Zn by dissolved organic matter derived from soil planted with two ecotypes of S. alfredii grown under ambient (350 μl l−1) or elevated (800 μl l−1) CO2 as function of DOC concentration. HE-DOM-A DOM derived from soil planted with HE under ambient CO2, HE-DOM-E DOM derived from soil planted with HE under elevated CO2, NHE-DOM-A DOM derived from soil planted with NHE under ambient CO2, NHE-DOM-E DOM derived from soil planted with NHE under elevated CO2. Model predictions obtained by Visual MINTEQ computer model are also shown
Metal complexation with DOM was influenced by elevated CO2 significantly (Fig. 1). For HE S. alfredii, DOM derived from soil under elevated CO2 (HE-DOM-E) showed higher degree of complexation than DOM derived from soil under ambient CO2 (HE-DOM-A), even at relatively low DOC concentrations, e.g., about 90 % of Cd and 73 % of Zn were bound in DOM complexes in the undiluted sample of HE-DOM-E, which were 1.1 and 1.2 times of HE-DOM-A, respectively. For NHE S. alfredii, however, no significant difference (P < 0.05) was observed between the two CO2 levels. The complexation of heavy metals with DOM in the rhizosphere influences the solubility and mobility of these metals (Weng et al. 2002). Considering the increase of hydrophilic fractions of the DOM (Li et al. 2013a), as well as the organic acids (Table 3) in the rhizosphere of HE S. alfredii under elevated CO2, it is proposed that elevated CO2 can increase mobilization of Cd and Zn due to enhanced formation of DOM–metal complexes by changing DOM composition.
The complexation of Cd and Zn by dissolved organic matter derived from the rhizosphere of S. alfredii under ambient and/or elevated CO2 was also simulated using Visual MINTEQ model (Fig. 1). Generally, the Visual MINTEQ model gave excellent predictions of Cd and Zn complexation to DOM (R 2 > 0.96). Visual MINTEQ is a freeware chemical equilibrium model for the calculation of metal speciation, solubility equilibria, etc., for natural waters (Christensen and Christensen 2000; Cornu et al. 2011; Krishnamurti et al. 2004). In this study, the predictions of DOM binding of Zn and Cd showed good agreement with the data obtained by resin equilibrium method, this result further confirmed that Visual MINTEQ is an easy and useful approach to study the effect of climate change (e.g., elevated atmospheric CO2) on the species of heavy metal in the rhizosphere of hyperaccumulator plants.
Metal extractability by DOM from the rhizosphere of S. alfredii under elevated CO2
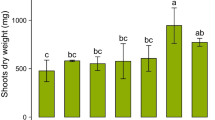
In this study, the effect of elevated CO2 on metal mobility was investigated by extracting experiment and the results are shown in Fig. 2. Under both ambient and elevated CO2 treatments, DOM in the rhizosphere of HE S. alfredii showed greater extract ability than DOM in the rhizosphere of NHE S. alfredii. For example, the metal concentrations extracted by DOM derived from the rhizosphere of HE S. alfredii were 0.23–0.32 mg l−1 for Cd and 1.78–2.20 mg l−1 for Zn, which were 1.6–2.0 and 1.6–1.9 times of NHE S. alfredii, respectively. This result is in agreement with our previous reports that DOM from the rhizosphere of HE S. alfredii significantly increased the solubility of Cd and Zn minerals (Li et al. 2012b, 2013b). Numerous studies have shown that the complexation with DOM can increase the extraction of metal from contaminated soil. Li and Shuman (1997) found that poultry litter DOM removed more Cd and Zn from contaminated soil. Antoniadis and Alloway (2002) reported that DOM significantly increased Cd, Ni, Zn extractability from soils and their uptake by ryegrass. Kim et al. (2010) found that increased metal solubility in the rhizosphere of Indian mustard was mainly attributed to the complexation of metal with DOC. Our results indicated that DOM in the rhizosphere of HE S. alfredii could significantly increased Cd and Zn extractability due to formation of Cd/Zn–DOM complexes, and thus increased Cd and Zn uptake by HE S. alfredii.
The extracting ability of dissolved organic matter derived from soil planted with two ecotypes of S. alfredii grown under ambient (350 μl l−1) or elevated (800 μl l−1) CO2. HE-DOM-A DOM derived from soil planted with HE under ambient CO2, HE-DOM-E DOM derived from soil planted with HE under elevated CO2, NHE-DOM-A DOM derived from soil planted with NHE under ambient CO2, NHE-DOM-E DOM derived from soil planted with NHE under elevated CO2. Bars sharing the same letter code among different DOM are not significantly different at P < 0.05
It is interesting to note that the extracting ability of DOM from the rhizosphere of HE S. alfredii was increased by elevated CO2 (Fig. 2). For instance, the metal concentrations extracted with HE-DOM-E were 0.32 mg l−1 for Cd and 2.20 mg l−1 for Zn, which were 1.4 and 1.2 times of HE-DOM-A, respectively. For NHE S. alfredii, although metal extracting ability of NHE-DOM-E was higher than NHE-DOM-A, but the difference was not significant (P < 0.05). The composition and characteristic of DOM will have profound impact on the complexation of metals and DOM and consequently metal mobility. Previous studies indicated that organic acids released by plants under elevated CO2 and/or heavy metal stress, play a crucial role in heavy metal acquisition by plants (Guo et al. 2011; Phillips et al. 2009; Wu et al. 2009). Our previous study showed that hydrophilic fraction, especially for hydrophilic acid fraction in DOM of HE S. alfredii was increased under elevated CO2 (Li et al. 2013a). In the present study, the total organic acid in soil solution was increased by elevated CO2 for both ecotypes of S. alfredii; however, the increase in the HE S. alfredii was much greater than in the NHE S. alfredii (Table 3). Thus, it is proposed that elevated CO2 can increase DOM, especially hydrophilic acid fraction in the rhizosphere of HE S. alfredii, and thus increase mobility of Cd and Zn in soil solutions. Further research is needed to elucidate how elevated CO2 regulate the release of hydrophilic acid fraction in the rhizosphere of HE S. alfredii, as well as to reveal the mechanisms of interaction between metal and hydrophilic acid fraction.
Our study indicates that elevated CO2 could increase DOM, especially hydrophilic acid fraction, changes the metal fractionation of rhizosphere soil, facilitates metal solubility and bioavailability due to enhanced formation of DOM–metal complexes, and thus increases uptake of metal by plants, these results suggest that use of elevated CO2 may be a useful way to improve phytoremediation efficiency of metal contaminated soil. On the other hand, this study indicates that elevated CO2 alters the speciation and distribution of contaminant elements in soil, thereby probably affecting food quality and safety (Guo et al. 2011). Thus a better understanding of the mechanisms by which CO2 and heavy metals jointly affect crop growth and uptake of metals is necessary, especially from the viewpoint of food safety.
Conclusion
This study demonstrated that elevated CO2 (800 μl l−1) could decrease soil solution pH of HE of S. alfredii by increasing DOC and low-molecular-weight organic acid in soil solution. For both ecotypes of S. alfredii, Cd/Zn–DOM complexes were the dominant species in soil solutions, followed by free Cd2+ and Zn2+ species; however, elevated CO2 resulted in a higher fraction of metal–DOM complex in soil solution of HE S. alfredii. DOM derived from the rhizosphere of HE S. alfredii under elevated CO2 showed greater ability to form complexes with Cd and Zn than DOM derived from the rhizosphere of NHE S. alfredii at the same DOC concentration. All these results suggest that elevated CO2 could increase mobility of Cd and Zn due to enhanced formation of DOM–metal complexes in the rhizosphere of HE S. alfredii and increase phytoextraction efficiency of Cd/Zn-contaminated soil by HE S. alfredii.
References
Antoniadis V, Alloway BJ (2002) The role of dissolved organic carbon in the mobility of Cd, Ni and Zn in sewage sludge-amended soils. Environ Pollut 117:515–521
Baken S, Degryse F, Verheyen L, Merckx R, Smolders E (2011) Metal complexation properties of freshwater dissolved organic matter are explained by its aromaticity and by anthropogenic ligands. Environ Sci Technol 45:2584–2590
Cerling TE, Harris JM, MacFadden BJ, Leakey MG, Quade J, Eisenmann V, Ehleringer JR (1997) Global vegetation change through the Miocene/Pliocene boundary. Nature 389:153–158
Christensen JB, Christensen TH (2000) The effect of pH on the complexation of Cd, Ni and Zn by dissolved organic carbon from leachate-polluted groundwater. Water Res 34:3743–3754
Cornu JY, Parat C, Schneider A, Authier L, Dauthieu M, Sappin-Didier V, Denaix L (2009) Cadmium speciation assessed by voltammetry, ion exchange and geochemical calculation in soil solutions collected after soil rewetting. Chemosphere 76:502–508
Cornu JY, Schneider A, Jezequel K, Denaix L (2011) Modelling the complexation of Cd in soil solution at different temperatures using the UV-absorbance of dissolved organic matter. Geoderma 162:65–70
Cousins AB, Adam NR, Wall GW, Kimball BA, Pinter PJ, Ottman MJ, Leavitt SW, Webber AN (2003) Development of C-4 photosynthesis in sorghum leaves grown under free-air CO2 enrichment (FACE). J Exp Bot 54:1969–1975
de Graaff MA, Six J, van Kessel C (2007) Elevated CO2 increases nitrogen rhizodeposition and microbial immobilization of root-derived nitrogen. New Phytol 173:778–786
Dijkstra P, Hymus G, Colavito D, Vieglais DA, Cundari CM, Johnson DP, Hungate BA, Hinkle CR, Drake BG (2002) Elevate atmospheric CO2 stimulates aboveground biomass in a fire-regenerated scrub–oak system. Glob Chang Biol 8:90–103
Fitz WJ, Wenzel WW, Zhang H, Nurmi J, Stipek K, Fischerova Z, Schweiger P, Kollensperger G, Ma LQ, Stingeder G (2003) Rhizosphere characteristics of the arsenic hyperaccumulator Pteris vittata L. and monitoring of phytoremoval efficiency. Environ Sci Technol 37:5008–5014
Guo HY, Zhu JG, Zhou H, Sun YT, Yin Y, Pei DP, Ji R, Wu JC, Wang XR (2011) Elevated CO2 levels affects the concentrations of copper and cadmium in crops grown in soil contaminated with heavy metals under fully open-air field conditions. Environ Sci Technol 45:6997–7003
Gustafsson JP (2011) Visual MINTEQ ver 3.0. http://www2.lwr.kth.se/English/OurSoftware/vminteq/
Hungate BA, Dijkstra P, Johnson DW, Hinkle CR, Drake BG (1999) Elevated CO2 increases nitrogen fixation and decreases soil nitrogen mineralization in Florida scrub oak. Glob Chang Biol 5:781–789
IPCC, Solomon S et al (2007) Climate Change 2007, The physical science basis. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge Univ. Press, Cambridge, UK, 2007
Jia Y, Tang SR, Wang RG, Ju XH, Ding YZ, Tu SX, Smith DL (2010) Effects of elevated CO2 on growth, photosynthesis, elemental composition, antioxidant level, and phytochelatin concentration in Lolium mutiforum and Lolium perenne under Cd stress. J Hazard Mater 180:384–394
Jones DL, Willett VB (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38:991–999
Khan AG, Kuek C, Chaudhry TM, Khoo CS, Hayes WJ (2000) Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 41:197–207
Kim S, Kang H (2011) Effects of elevated CO2 and Pb on phytoextraction and enzyme activity. Water Air Soil Pollut 219:365–375
Kim S, Lim H, Lee I (2010) Enhanced heavy metal phytoextraction by Echinochloa crus-galli using root exudates. J Biosci Bioeng 109:47–50
Kimball BA, Pinter PJ, Garcia RL, LaMorte RL, Wall GW, Hunsaker DJ, Wechsung G, Wechsung F, Kartschall T (1995) Productivity and water use of wheat under free-air CO2 enrichment. Global Chang Biol 1:429–442
Körner C (2001) Biosphere responses to CO2 enrichment. Ecol Appl 10:1590–1619
Kramer U (2005) Phytoremediation: novel approaches to cleaning up polluted soils. Curr Opin Biotechnol 16:133–141
Krishnamurti GS, Megharaj M, Naidu R (2004) Bioavailability of cadmium–organic complexes to soil alga—an exception to the free ion model. J Agric Food Chem 52:3894–3899
Li ZB, Shuman LM (1997) Mobility of Zn, Cd and Pb in soils as affected by poultry litter extract. 1. Leaching in soil columns. Environ Pollut 95:219–226
Li ZY, Tang SR, Deng XF, Wang RG, Song ZG (2010) Contrasting effects of elevated CO2 on Cu and Cd uptake by different rice varieties grown on contaminated soils with two levels of metals: implication for phytoextraction and food safety. J Hazard Mater 177:352–361
Li TQ, Di ZZ, Han X, Yang XE (2012a) Elevated CO2 improves root growth and cadmium accumulation in the hyperaccumulator Sedum alfredii. Plant Soil 354:325–334
Li TQ, Xu ZH, Han X, Yang XE, Sparks DL (2012b) Characterization of dissolved organic matter in the rhizosphere of hyperaccumulator Sedum alfredii and its effect on the mobility of zinc. Chemosphere 88:570–576
Li TQ, Tao Q, Han X, Yang XE (2013a) Effects of elevated CO2 on rhizosphere characteristics of Cd/Zn hyperaccumulator Sedum alfredii. Sci Total Environ 454–455:510–516
Li TQ, Liang CF, Tao Q, Yang XE (2013b) Mobilization of cadmium by dissolved organic matter in the rhizosphere of hyperaccumulator Sedum alfredii. Chemosphere 91:70–976
Lieffering M, Kim HY, Kobayashi K, Okada M (2004) The impact of elevated CO2 on the elemental concentrations of field-grown rice grains. Field Crop Res 88:279–286
Lorenz SE, Hamon RE, Holm PE, Domingues HC, Sequeira EM, Christensen TH, McGrath SP (1997) Cadmium and zinc in plants and soil solutions from contaminated soils. Plant Soil 189:21–31
Luttge U (2004) Ecophysiology of crassulacean acid metabolism (CAM). Ann Bot Lond 93:629–652
McGrath SP, Shen ZG, Zhao FJ (1997) Heavy metal uptake and chemical changes in the rhizosphere of Thlaspi caerulescens and Thlaspi ochroleucum grown in contaminated soils. Plant Soil 188:153–159
Milne CJ, Kinniburgh DG, van Riemsdijk WH, Tipping E (2003) Generic NICA-Donnan model parameters for metal-ion binding by humic substances. Environ Sci Technol 37:958–971
Mishra S, Heckathorn SA, Frantz JM (2012) Elevated CO2 affects plant responses to variation in boron availability. Plant Soil 350:117–130
Nolan AL, McLaughlin MJ, Mason SD (2003) Chemical speciation of Zn, Cd, Cu, and Pb in pore waters of agricultural and contaminated soils using Donnan dialysis. Environ Sci Technol 37:90–98
Noyes PD, McElwee MK, Miller HD, Clark BW, Van Tiem LA, Walcott KC, Erwin KN, Levin ED (2009) The toxicology of climate change: Environmental contaminants in a warming world. Environ Int 35:971–986
Peris M, Mico C, Recatala L, Sanchez R, Sanchez J (2007) Heavy metal contents in horticultural crops of a representative area of the European Mediterranean region. Sci Total Environ 378:42–48
Phillips RP, Bernhardt ES, Schlesinger WH (2009) Elevated CO2 increases root exudation from loblolly pine (Pinus taeda) seedlings as an N-mediated response. Tree Physiol 29:1513–1523
Rajkumar M, Prasad MNV, Swaminathan S, Freitas H (2013) Climate change driven plant–metal–microbe interactions. Environ Int 53:74–86
Rodriguez JH, Klumpp A, Fangmeier A, Pignata ML (2011) Effects of elevated CO2 concentrations and fly ash amended soils on trace element accumulation and translocation among roots, stems and seeds of Glycine max (L.) Merr. J Hazard Mater 187:58–66
Sauve S, Norvell WA, McBride M, Hendershot W (2000) Speciation and complexation of cadmium in extracted soil solutions. Environ Sci Technol 34:291–296
Schmitt D, Saravia F, Frimmel FH, Schuessler W (2003) NOM-facilitated transport of metal ions in aquifers: importance of complex-dissociation kinetics and colloid formation. Water Res 37:3541–3550
Tang S, Xi L, Zheng J, Li H (2003) Response to elevated CO2 of Indian mustard and sunflower growing on copper contaminated soil. Bull Environ Contam Toxicol 71:988–997
Terzano R, Spagnuolo M, Vekemans B, De Nolf W, Janssens K, Falkenberg G, Fiore S, Ruggiero P (2007) Assessing the origin and fate of Cr, Ni, Cu, Zn, Pb, and V in industrial polluted soil by combined microspectroscopic techniques and bulk extraction methods. Environ Sci Technol 41:6762–6769
Tu SX, Ma L, Luongo T (2004) Root exudates and arsenic accumulation in arsenic hyperaccumulating Pteris vittata and non-hyperaccumulating Nephrolepis exaltata. Plant Soil 258:9–19
Vangronsveld J, Herzig R, Weyens N, Boulet J, Adriaensen K, Ruttens A, Thewys T, Vassilev A, Meers E, Nehnevajova E, van der Lelie D, Mench M (2009) Phytoremediation of contaminated soils and groundwater: lessons from the field. Environ Sci Pollut Res 16:765–794
Weng LP, Temminghoff EJM, Lofts S, Tipping E, Van Riemsdijk WH (2002) Complexation with dissolved organic matter and solubility control of heavy metals in a sandy soil. Environ Sci Technol 36:4804–4810
Wu HB, Tang SR, Zhang XM, Guo JK, Song ZG, Tian SA, Smith DL (2009) Using elevated CO2 to increase the biomass of a Sorghum vulgare × Sorghum vulgare var. sudanense hybrid and Trifolium pratense L. and to trigger hyperaccumulation of cesium. J Hazard Mater 170:861–870
Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ (2004) Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259:181–189
Zheng JM, Wang HY, Li ZQ, Tang SR, Chen ZY (2008) Using elevated carbon dioxide to enhance copper accumulation in Pteridium revolutum, a copper-tolerant plant, under experimental conditions. Int J Phytoremediation 10:161–172
Acknowledgement
The study was financially supported by the National Natural Science Foundation of China (41271333), the National Key Technology R&D Program of China (2012BAC17B04), and Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Li, T., Tao, Q., Liang, C. et al. Elevated CO2 concentration increase the mobility of Cd and Zn in the rhizosphere of hyperaccumulator Sedum alfredii . Environ Sci Pollut Res 21, 5899–5908 (2014). https://doi.org/10.1007/s11356-014-2560-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2560-1