Abstract
Background and aims
A major concern in developing microbially-assisted phytoextraction (MAP) is that the effects of introduced microbes on indigenous soil microbial community are profound and irreversible. To date, however, the microbial properties of soils subjected to MAP remain poorly understood. Therefore, we explored the effects of inoculation with a bacterial consortium enriched from acid mine drainage on not only the cadmium (Cd) phytoextraction efficiency of Averrhoa carambola but also the microbial properties of the Cd-contaminated soil.
Methods
We conducted a field experiment and characterized the microbial community in the contaminated soil using next generation sequencing technology (Illumina MiSeq).
Results
The bacterial inoculation increased the Cd concentration in A. carambola shoot tissues by 20%–65%, leading to a relatively high Cd removal efficiency (4.63% annually). Meanwhile, there were no significant differences between the treatments in soil bacterial diversity and community composition one year after the initiation of the bacterial inoculation treatment. The most abundant genera of the introduced bacteria were found to either disappear from, or be present in similar relative abundance, in the soils of the different treatments, except Sulfobacillus.
Conclusions
Collectively, our results provide evidence that MAP could be practiced with minor effects on indigenous soil microbial community.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is ranked as one of the most toxic pollutants in the environment and is classified as a human carcinogen (WHO 1992a). The background concentration of Cd in soils worldwide is generally low, with the median ranging from 0.2 to 0.4 mg kg−1 (WHO 1992b). However, as a consequence of intensive human activities over the last century, elevated levels of Cd in soils have been widely reported in many parts of the world (WHO 1992b; Adriano 2001; Zhao et al. 2015). Cadmium in agricultural soils is readily accumulated by crops (McLaughlin et al. 1999), thereby entering the human food chain and posing significant health risks to human beings (WHO 1992a). An important approach to reduce human exposure to dietary Cd is therefore to clean up the agricultural soils contaminated by Cd.
Due to its low cost and eco-friendly nature, phytoextraction, the use of plants to remove toxic heavy metals from soils, holds promise for in situ clean-up of Cd-contaminated agricultural soils (Salt et al. 1995). Currently, however, full-scale applications of this technique are very limited (Robinson et al. 2015). In most cases, poorly available heavy metals in soils and low biomass yield of phytoextractor plants are among the main bottlenecks limiting successful phytoextraction (Krämer 2005). As a strategy to overcome these two constraints, microbially-assisted phytoextraction (MAP) has received increasing attention over the last decade (Lebeau et al. 2008; Sessitsch et al. 2013). The fundamental principle in this strategy is straightforward: introducing microbes to the roots and/or rhizosphere of phytoextractor plants for enhancing plant biomass yield and/or availability of heavy metals in the rhizosphere (Lebeau et al. 2008; Sessitsch et al. 2013).
A large number of microbes, in particular plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi (AMF), have been reported to be able to enhance plant biomass yield and/or heavy metal accumulation of phytoextractor plants (Lebeau et al. 2008; Sessitsch et al. 2013). However, most of these microbes are not indigenous to the contaminated soils and the introduction of exogenous microbes may result in profound and irreversible effects on indigenous soil microbial community, which has increasingly become a major concern in further developing MAP (Teixeira et al. 2014). Indeed, the potential environmental impact dealing with this aspect has been recognized for some time (Lebeau et al. 2008). However, most previous studies on MAP have concentrated on the effects of microbial inoculation on plant growth, plant nutritive status and uptake of heavy metals from contaminated soils, leaving the potential effects on indigenous soil microbial community largely uninvestigated (e.g. Zaidi et al. 2006; Langella et al. 2014; Phieler et al. 2015). To date, there have been a few attempts to address such an important concern (Di Gregorio et al. 2006; Braud et al. 2009; Chen et al. 2014). In a previous study, Di Gregorio et al. (2006) conducted a 10-week microcosm experiment to explore the effects of inoculation with an indigenous plant growth-promoting rhizobacterium on the soil bacterial community of a phytoextractor (Brassica juncea). Subsequently, Braud et al. (2009) examined the effects of inoculation with siderophore-producing bacteria on the phytoextraction of a heavy metal-contaminated soil by pot-grown Zea mays for 56 days. In a more recent study, Chen et al. (2014) performed a 2-month microcosm phytoextraction experiment to investigate the effects of inoculation with an endophytic bacterium on the total bacterial number and microbial biomass in a Cd-contaminated soil phytoextracted by Solanum nigrum. Together, these studies have contributed to our understanding of the potential effects of MAP on indigenous soil microbial community. However, it is obvious that they are laboratory-based studies wherein conventional microbiological approaches were employed. Therefore, the critical next step towards a better understanding of such an important issue requires researchers to take advantage of modern molecular microbiological tools (e.g. next generation high throughput sequencing; Shokralla et al. 2012) to characterize the microbial properties of contaminated soils subjected to MAP under field conditions.
More field experiments are also needed to improve the applicability of MAP, given that only a few field evaluations of this remediation approach have been made (Wang et al. 2005, 2007a; Farwell et al. 2007; Jankong et al. 2007; Phieler et al. 2015; Prapagdee and Khonsue 2015). The results of these studies provide some evidence that microbial inoculation was able to enhance phytoextraction efficiency through increasing plant biomass yield and/or shoot heavy metal concentration. It should be noted, however, that the efficiencies of MAP observed in these evaluations seemed to be low (< 1%). For example, the Cd phytoextraction efficiencies observed by Wang et al. (2007a) and Phieler et al. (2015) were estimated to be 0.85% and 0.03%, respectively.
In this study, the effectiveness of a bacterial consortium enriched from acid mine drainage (AMD) in enhancing Cd phytoextraction by Averrhoa carambola was examined under field conditions. A. carambola was employed due to its high efficiency in Cd phytoextraction (Li et al. 2009, 2012); while the bacterial consortium from AMD was chosen because it is efficient in mobilizing Cd in contaminated soils (Marhual et al. 2008; Xu 2012). Meanwhile, we characterized the microbial community in the contaminated soil using next generation sequencing technology (Illumina MiSeq). To our knowledge, this study is the first attempt to adopt next generation sequencing technology to analyze the microbial community structure of heavy metal-contaminated soils subjected to MAP.
Materials and methods
Preparation of bacterial consortium
The bacterial consortium used in this study was enriched from AMD as described by Xiang et al. (2010). Briefly, a variety of AMD samples were collected from a mine located in Shaoguan, southern China (25°02′ N, 113°39′ E) and were combined as the initial inoculum for the bacterial consortium. The modified 9 K medium (Silverman and Lundgren 1959), supplemented with 1% pyrite as the sole energy source (Rodriguez-Leiva and Tributsch 1988), was used to enrich the bacterial consortium. Cultures were incubated on a rotary shaker (150 rpm) at 25 °C. The top five dominant genera in the enriched bacterial consortium included Leptospirillum, Sulfobacillus, Acidithiobacillus, Ferrimicrobium and Acidisphaera (characterized using 16S rRNA gene-clone library analysis; Xu 2012). Their relative abundances were 50.1%, 20.7%, 12.2%, 9.76% and 1.22%, respectively. The final cell density of the enrichment culture was approximately 5 × 106 cells mL−1, which was counted by using a phase-contrast microscopy (Olympus BX50, Olympus Optical Co, Ltd., Japan) and a Hawksley bacterial counting chamber (Dopson et al. 2004).
Field experiment
The present field experiment was carried out at a paddy field located in Shaoguan, southern China (24°40′ N, 113°20′ E). In this area, the average annual temperature and rainfall are approximately 19.5 °C and 1520 mm, respectively. The field soil was contaminated by Cd (Table 1) and a high-biomass Cd accumulator (A. carambola) has been cultivated as part of a field trial for Cd phytoextraction since June 2008. The field trial comprised five blocks and each block was divided into five plots (4 m × 2 m each; planting density: 20 seedlings m−2). To determine the plant growth and shoot Cd concentration, the phytoextractor has been harvested at 20 cm above ground level twice a year (in January and July) since July 2010. Such an undestructive harvest approach (i.e. roots were not harvested) was applied because it allowed repeated harvests of shoot biomass based on one planting and was desirable for reducing cost of phytoextraction. Note also that the Cd concentrations in A. carambola roots were generally lower than those in shoots (Li et al. 2009). The harvested plant materials of each plot were divided into three fractions (stem, twig and leaf), dried and weighed. For analysis of shoot Cd concentration, three subsamples of each fraction of the shoot dry matter from each plot were collected to form a composite sample. Immediately after each harvest in July, a composite soil sample consisting of three subsamples was also collected from each plot to examine the potential changes in soil chemical properties. Taking advantage of the high regrowth ability of these phytoextractor plants, the present field experiment was initiated directly in these pre-existing plots on 1st August 2013 (the plant’s active growing season) and consisted of three treatments: A. carambola uninoculated (CK1), A. carambola inoculated with the bacterial consortium sterilized by autoclaving (CK2) and A. carambola inoculated with the bacterial consortium (Inoculated). Each treatment was replicated three times and each replicate was arranged at random in a plot of the three blocks that were selected randomly from the five blocks set up since June 2008. For the plots of CK2 and Inoculated, the soils in the plant root zone were directly sprayed with the suspensions (pH = 2) of the enriched bacterial inoculum (approximately 5 × 106 cells mL−1) either sterilized or not sterilized at a dose of 0.5 L m−2, respectively. This dose was selected because our preliminary experiment showed that a higher dose (1 L m−2) did not lead to a greater Cd availability in the soil. To exclude the potential effects associated with low pH of the suspensions, the plots of CK1 were treated with ultra-pure water (adjusted to pH 2 with HCl) in a similar manner as described above. During the duration of the present field experiment (August 2013 to July 2014), phytoextractor plants were harvested twice and soil samples were collected once as described above. To evaluate the potential effects of the bacterial inoculation on indigenous soil microbial community, additional soil samples (a composite sample consisting of three subsamples was collected in each plot) were taken from the plant root zone immediately after the plant sampling in July 2014.
Chemical analysis
Soil samples were air-dried and ground to pass a nylon sieve (0.15 mm for total Cd concentration and 1 mm for the other chemical properties) before analysis. Method 3052 recommended by the US EPA (1996) was used to determine the total Cd concentration in the soils. The diethylenetriaminepentaacetic acid (DTPA) method of Lindsay and Norvell (1978) was employed to evaluate Cd availability in these soils. Soil pH was measured in a 1:5 soil:deionized water suspension using a pH meter (pH 510, Eutech Instruments Pvt. Ltd., Singapore). Ammonium-nitrogen (NH4 +-N) and nitrate-nitrogen (NO3 −-N) were extracted with 2 M KCl at a soil:solution ratio of 1:5 and determined using an automatic chemical analyzer (Smart Chem 200, AMS, Italy). Available phosphorus (P) and potassium (K) were extracted using the Bray-1 and ammonium acetate extraction method (Lu 2000), and determined photometrically and by a flame photometer (Model 52-A, Perkin-Elmer, USA), respectively.
Microbiological analysis
Genomic DNA was extracted from each of the soil samples using the Power Soil DNA Extraction Kit (MoBio, USA) according to the manufacturer’s instructions. The universal primers of F515 and R806 were used to amplify the V4 region of bacterial and archaeal 16S rRNA genes, with the reverse primer modified to contain a sample-specific 8-bp barcode. All PCRs were conducted in a total volume of 20 μL consisting of 0.4 μL of 10 μM each primer, 2 μL of 10× Ex Taq Buffer (Mg2+ Plus), 0.4 μL of 20 mg mL−1 bovine serum albumin (Takara, Japan), 0.1 μL of 5 U Taq DNA polymerase (Takara, Japan) and 2 μL of 200 μM dNTP mix. Cycling conditions were as follows: initial denaturation at 94 °C for 5 min, followed by 30 amplification cycles consisting of denaturation at 94 °C for 30 s, annealing at 52 °C for 30 s and extension at 72 °C for 45 s, and a final extension step at 72 °C for 10 min. For each soil sample, PCR products were amplified from three subsamples, pooled and purified with a QIAquick gel extraction kit (Qiagen, USA). The purified PCR products from each sample were mixed in approximately equimolar proportions to form a composite sample that was sequenced using Illumina MiSeq platform (San Diego, CA, USA). The Illumina sequencing data have been deposited in the NCBI Sequence Read Archive database (accession number: SRR2924889).
The raw Illumina data were processed using Mothur v.1.30.2 (Schloss et al. 2009) and QIIME v.1.8.0 (Caporaso et al. 2010). Sequences were clustered into operational taxonomic units (OTUs) based on a 97% sequence similarity. Taxonomy for each OTU was assigned with the RDP classifier version 2.6 (Wang et al. 2007b) at an 80% confidence threshold. The diversity and structure of the soil microbial communities were assessed after resampling of the sequences to the same depth (6853 sequences per community, i.e. the minimum number of quality sequences per sample in this study). The Bray-Curtis coefficient-based principal coordinates analysis (PCoA) was performed at OTU level to evaluate the similarity among the soil microbial communities of different treatments.
Statistical analysis
Statistical analyses were conducted with the SPSS version 18.0 for Windows (SPSS Inc., Chicago, USA). Differences between the treatments in soil properties, plant biomass yield, Cd accumulation, soil microbial diversity and composition were analyzed using the one-way Analysis of Variance (ANOVA). The least significant difference test (LSD) was used to identify significant (P < 0.05) differences between means.
Results
Plant biomass yields
The potential effects of bacterial inoculation on the biomass yield of A. carambola were evaluated by harvesting shoot tissues from different treatments before the initiation of treatments and 0.5 and 1 year after the treatments. No significant (P > 0.05) differences in shoot biomass yield were observed between the plots for the three treatments before the initiation of the present study (Fig. 1a). The bacterial inoculation did not lead to significant differences in shoot biomass yield between treatments 0.5 and 1 year after treatment application (Fig. 1b, c).
Biomass yield (t ha−1) of A. carambola tissues harvested from different treatments before (A panel), 0.5- (B panel) and 1-y after (C panel) treatment application (i.e. in July 2013, January 2014 and July 2014, respectively). Data are presented as means ± s.e. (n = 3) and on a dry weight basis. No significant differences were found (P < 0.05, LSD) between the treatments in shoot Cd concentration at the same sampling time
Cd uptake and removal by plants
Before treatment application, there were no statistically significant (P > 0.05) differences between the treatments in the concentration of Cd in shoot tissues (Fig. 2a). In contrast, significant (P < 0.05) differences were found between the treatments in shoot Cd concentration not only 0.5 but also 1 year after treatment application (Fig. 2b, c). Specifically, the Cd concentrations in individual shoot tissues of A. carambola inoculated with the bacterial consortium were significantly (P < 0.05) higher than those of the other two control treatments at both of the two sampling time points.
Cd concentrations (mg kg−1) in A. carambola tissues harvested from different treatments before (A panel), 0.5- (B panel) and 1-y after (C panel) treatment application. Data are presented as means ± s.e. (n = 3) and on a dry weight basis. Different lowercase letters above the bars indicated a significant difference (P < 0.05, LSD) between the treatments in tissue Cd concentration at the same sampling time
The amounts of Cd removal by the shoot tissues at different sampling times were calculated (Table 2), based on the shoot biomass yields and Cd concentrations. Higher amounts of Cd removal by the individual shoot tissues were observed in the Inoculated treatment (Table 2), which could largely be attributed to the significant higher Cd concentrations in these tissues as compared to those of the other two treatments (Fig. 2b, c).
Soil chemical properties
Before treatment application, the plots for the three treatments did not show significant (P > 0.05) differences in the selected soil chemical properties, with the exception of NO3 −-N (Table 1). However, the concentration of DTPA-extractable Cd in the soil of the Inoculated treatment was significantly (P < 0.05) higher than that of CK1 1 year after treatment application, besides the significantly (P < 0.05) higher NO3 −-N concentration in the soil as compared to those of the other two treatments (Table 1). Conversely, the other selected chemical properties of the soils did not show any significant differences between the treatments 1 year after treatment application (Table 1).
Soil microbial diversity and community composition
A total of 31,459 OTUs were recorded from the 141,214 quality sequences across the soil samples. The three treatments varied in OTU number, but no significant (P > 0.05) differences were found between them on any of the two sampling dates (Fig. 3a, b). Similarly, there were no significant (P > 0.05) differences between the treatments in the other soil microbial α-diversity measures (including Chao1, ACE, Simpson, Shannon and Faith’s PD) considered in the present study (Fig. 3a, b). In addition, PCoA analysis of the community composition showed that the Inoculated treatment did not form a cluster that was different from those of the two control treatments either before or 1 year after treatment application (Fig. 4).
Microbial species richness and diversity of the soils collected from different treatments either before (A panel) or 1-y after (B panel) treatment application. Data are presented as means ± s.e. (n = 3). No significant differences were found (P < 0.05, LSD) between the treatments in microbial species richness and diversity at the same sampling time
Principal coordinate analysis (PCoA) plot with Bray-Curtis dissimilarity among soil microbial communities of different treatments either before (A panel) or 1-y after (B panel) treatment application. No obvious clustering was observed with respect to different treatments. Two replicates of CK1 were highly overlapped in both sampling time points
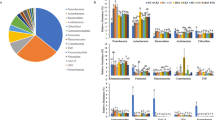
The top 10 dominant microbial phyla in the soil samples collected from different treatments before and 1 year after treatment application were Acidobacteria, Proteobacteria, Chloroflexi, Crenarchaeota, Planctomycetes, Nitrospirae, Verrucomicrobia, Actinobacteria, Bacteroidetes and Gemmatimonadetes (Fig. 5). Moreover, there were no significant (P > 0.05) differences between the treatments in relative abundance of these dominant phyla at each sampling time point (Fig. 5). Similarly, the top 10 dominant soil microbial genera (Candidatus Nitrososphaera, Rhodoplanes, Nitrospira, DA101, Steroidobacter, Pirellula, Anaeromyxobacter, Gemmata, Flavisolibacter and Pilimelia) did not show a significant (P > 0.05) difference between the treatments on either sampling date (Fig. 6).
Relative abundance (%) of the top 10 dominant microbial phyla in the soils collected from different treatments either before (A panel) or 1-y after (B panel) treatment application. Data are presented as means ± s.e. (n = 3). No significant differences were found (P < 0.05, LSD) between the treatments in relative abundance of these phyla at the same sampling time
Relative abundance (%) of the top 10 dominant microbial genera in the soils collected from different treatments either before (A panel) or 1-y after (B panel) treatment application. Data are presented as means ± s.e. (n = 3). No significant differences were found (P < 0.05, LSD) between the treatments in relative abundance of these genera at the same sampling time
When the top 5 dominant genera in the microbial consortium were considered, they showed four different patterns of occurrence and abundance in the contaminated soils. Leptospirillum was present in the soils CK1 and CK2 before treatment application, but significant differences between the treatments in this aspect were no longer observed 1 year after treatment application although enhanced relative abundances were recorded (Fig. 7). Sulfobacillus occurred in similar abundance in the soils of different treatments before treatment application, but was persistent only in the Inoculated treatment soil 1 year after treatment application (with an elevated relative abundance; Fig. 7). Acidithiobacillus was absent from the soils of different treatments before treatment application, but present in the soils in similar abundance 1 year after treatment application (Fig. 7). Ferrimicrobium and Acidisphaera were not detectable in all the soil samples examined in this study. Notably, the relative abundances of these genera in the soils were low (< 1%).
Relative abundance (%) of Leptospirillum, Sulfobacillus and Acidithiobacillus in the soils collected from different treatments either before (A panel) or 1-y after (B panel) treatment application. Data are presented as means ± s.e. (n = 3). Different lowercase letters above the bars indicated significant difference (P < 0.05, LSD) between the treatments in relative abundance of these genera at the same sampling time
Discussion
A new type of bacterial consortium was employed in this study to improve Cd phytoextraction by the high biomass Cd-accumulator A. carambola in an attempt to demonstrate that MAP can be more efficient at the field scale than previously reported (Wang et al. 2007a; Phieler et al. 2015). Unlike other soil-borne microbes tested by other workers (Lebeau et al. 2008; Sessitsch et al. 2013), this bacterial consortium was enriched from AMD. The top three dominant microbial genera (Leptospirillum, Sulfobacillus and Acidithiobacillus, with relative abundance >80%) in this consortium are thought to be directly relevant for metal sulfide oxidation and may thereby mobilize heavy metals (Marhual et al. 2008; Schippers et al. 2010). Indeed, our pilot study based on a 12-week bench-scale experiment showed that addition of the consortium to the contaminated soil increased the Cd removal efficiency of A. carambola by approximately 3-times, which was associated with an apparent decrease in soil pH (from 6.70 to 5.12) for the first 4 weeks and then a steady increase in soil pH (from 5.12 to 5.88) for the subsequent 8 weeks. Further, the existence of the acidophilic bacteria Leptospirillum, Sulfobacillus and Acidithiobacillus (cell number: 3.1, 1.8 and 1.3 × 105 cells g−1 dry soil, respectively; unpublished results) in the contaminated soil at 12 weeks after addition of the consortium was confirmed using the fluorescent in situ hybridization method as described by Kock and Schippers (2008). These results were reasonable given that many acidophilic microbes in AMD can grow over a relatively broad range of pH. More specifically, members of Leptospirillum, Sulfobacillus, Acidithiobacillus, Ferrimicrobium and Acidisphaera (i.e. the top 5 dominant genera in the consortium enriched in this study) were reported to adapt to pH values from 1.3 to 5.7, 2.0 to 6.5, 0.5 to 6.0, 1.3–5.5 and 1.5–6.0, respectively (Baker and Banfield 2003; Karavaiko et al. 2005; Liu et al. 2007). In addition, acidophilic microbes are considered to be able to create acidic microsites in soils (Chesworth 2008), which, in turn, can provide shelters for acidophilic microbes themselves. It is therefore intuitive to expect that inoculation with such a bacterial consortium can lead to elevated Cd removals by A. carambola from the near-neutral pH Cd-contaminated soil under field conditions.
In agreement with our expectation, we found that inoculation with the bacterial consortium enhanced Cd concentrations in shoot tissues of A. carambola by approximately 20%–65% (Fig. 2b, c). Such an increment might be considered normal and so could often be observed at the field scale, given that a systematic review of the literature on performance of MAP showed that inoculation with microbes was able to increase metal concentrations in plant shoots by a factor of about 2 on average (Lebeau et al. 2008). However, the data from the currently available field-based studies deviated markedly from the finding of that review, which was likely due to the fact that most studies reviewed therein were laboratory-based. Indeed, the increase of heavy metal concentration in plant shoots associated with MAP under natural field conditions seemed to be recorded only by two research groups. Wang and his colleagues found that inoculation with AMF enhanced Cu, Zn and Pb concentrations in shoot of Elsholtzia splendens by approximately 20–40% (Wang et al. 2005, 2007a), while Prapagdee and Khonsue (2015) showed that inoculation with Arthrobacter sp. led to an approximately 43% increase in Cd concentration of Ocimum gratissimum shoots. In contrast, no significant effects of microbial inoculation on shoot heavy metal concentration of phytoextractor plants were observed in other two field experiments (Farwell et al. 2007; Phieler et al. 2015). Jankong et al. (2007) reported a negative effect of inoculation with rhizofungi on As concentrations in fronds of Pityrogramma calomelanos (24–30% reduction in As concentration). These discrepancies reflect the complex nature of the extrapolation of the laboratory-based results to field conditions, although they may be partly due to the relatively small number of field-scale studies currently available.
The increased shoot Cd concentrations observed in this study could be attributed to a beneficial effect of the bacterial consortia on Cd mobilization in the contaminated soil (Marhual et al. 2008; Schippers et al. 2010). Yet, we found that concentration of DTPA-extractable Cd in the soil inoculated with the bacterial consortium was similar to that of the control soil inoculated with the autoclaved microbial consortium (i.e. CK2) 1 year after treatment application (Table 1). This result could be explained by a scenario that the introduced bacteria had functioned to mobilize soil Cd but the bacterium-induced increase in soil Cd availability was counteracted by the increased Cd uptake by A. carambola (Fig. 2a, b). Surprisingly, microbe-induced changes in soil chemical properties have rarely been investigated under field conditions, although microbes are thought to assist phytoextraction through biochemical processes occurring at the soil-root interface (Sessitsch et al. 2013). An exception was a study by Phieler et al. (2015) who examined the effects of inoculation with either R. irregularis or a mixture of mycorrhiza and streptomycetes on heavy metal availability of a field soil planted with S. bicolor. In agreement with our results, no significant changes in soil heavy metal availability were observed by these authors (Phieler et al. 2015). In a wider context, the lack of a clear connection between the capacities of introduced microbes to mobilize heavy metals and the changes in soil heavy metal availability was frequently observed even in laboratory-based studies on MAP (Sessitsch et al. 2013); this deserves further investigation.
In contrast to our results on shoot Cd concentration, no significant differences were found between treatments in shoot biomass yield of A. carambola within 1 year after treatment application (Fig. 1b, c). This finding was reasonable, although most field-scale studies showed that microbial inoculation often led to an increase in shoot biomass yield (Wang et al. 2005, 2007a; Farwell et al. 2007; Jankong et al. 2007; Phieler et al. 2015). Firstly, no characteristics desirable for improving plant growth were reported for the dominant genera of the microbial consortium used in this study (Schippers et al. 2010). Secondly, a recent meta-analysis of 73 papers on MAP revealed that only about 19% of the 738 individual cases (treatments) presented in the papers were associated with an increase in both shoot biomass yield and heavy metal concentration (Sessitsch et al. 2013). Nonetheless, the shoot biomass yields observed here were considerably high, which supported our previous results (Li et al. 2009). Note also that the Inoculated treatment was associated with a higher concentration of soil NO3 −-N (Table 1), which may increase the shoot biomass yield over a long time scale. This observation could be attributed tentatively to the fact that Nitrobacter occurred in Inoculated treatment (albeit low relative abundance of approximately 0.1%) but was absent in the other two CKs. Because it is known that almost all acid-tolerant nitrifying bacteria belong to Nitrobacter (De Boer and Kowalchuk 2001) and that the existence of acid-tolerant nitrifying bacteria can help non-acid-tolerant nitrifying bacteria (e.g. those belonging to Nitrospira, whose relative abundance was approximately 2% in this study, Fig. 6) to oxidize ammonium to form NO3 − in acidic environments (De Boer and Kowalchuk 2001).
From a practical perspective, the actual efficiency of MAP under field conditions is more important than the magnitude of an increase in either shoot biomass yield or heavy metal concentration per se, although it has always been neglected in previous studies (e.g. Wang et al. 2005; Farwell et al. 2007; Jankong et al. 2007; Prapagdee and Khonsue 2015). In this study, the shoots of A. carambola inoculated with the bacterial consortium were able to remove 623 g Cd ha−1 (Table 2) from the contaminated soil (total soil Cd approximately 4.4 mg kg−1; Table 1) within 1 year after treatment application. This figure (623 g Cd ha−1) was higher than not only that (approximately 0.5 g Cd ha−1) of the S. bicolor inoculated with mycorrhiza and Streptomyces at a low soil Cd level (total soil Cd: 0.72 mg kg−1; Phieler et al. 2015) but also that (155 g Cd ha−1) recorded for the E. splendens inoculated with AMF and Penicillium at a high soil Cd level (total soil Cd: 7 mg kg−1; Wang et al. 2007a). Assuming that Cd contamination occurred only in the 0–20 cm layer of the soil and that the soil had a bulk density of 1.3 g cm−3 (Zhang et al. 2010), the annual Cd removal by the shoots of inoculated A. carambola accounted for 4.63% of the total amount of Cd in the contaminated soil. Such a Cd removal efficiency was about 4–150 times higher than those reported in comparable field-scale studies (Wang et al. 2007a; Phieler et al. 2015). Moreover, if the Cd removal efficiency observed here does not change over time, a 50% reduction in total soil Cd can be achieved within 15 years (a reasonable time span; Huang et al. 1997). During this period, repeated inoculation is needed because the most abundant genera of the introduced bacteria were found to either disappear from, or be present in similar relative abundance, in the soils of the different treatments (as discussed below). It should be noted that the potential environmental risk associated with Cd accumulation in fruits of A. carambola can be avoided, since our observation in the past decade showed that the plant individuals did not bear fruit when their shoots were harvested annually. Despite this, further study is required to examine whether the bacterial consortium increases migration of Cd into deeper soil layers and groundwater.
The Illumina next generation sequencing technology employed in this study enabled us to capture more of the soil microbial diversity. For example, the average number of microbial species (OTUs, Fig. 3) observed per soil sample here was higher than that detected using MiSeq in another Cd-contaminated soil phytoextracted by bioenergy crops (Ding et al. 2016). Note, however, that the identities of dominant microbial phyla and genera recorded here (Figs. 5 and 6) were similar to those reported for other contaminated agricultural soils (Liu et al. 2014; Sun et al. 2015; Ding et al. 2016). More importantly, our results revealed no significant differences between the treatments in microbial diversity (both alpha and beta), relative abundance of dominant phyla and that of dominant genera (Figs. 3–6), indicating that introducing the microbial consortium had minor effects on the indigenous soil microbial community of A. carambola. This finding seems to be inconsistent with that of Di Gregorio et al. (2006) who reported that inoculation with Sinorhizobium sp. Pb002 strongly altered the soil microbial community structure of pot-grown B. juncea. Such a discrepancy can be explained in several possible ways. Firstly, the next generation sequencing technology used here is more robust in characterizing the soil microbial community than PCR-DGGE analysis of that study (Shokralla et al. 2012). Secondly, soil microbial communities are able to recover from the disturbances associated with introduced microbes within a period of time, which is longer than the time-scale of the experiment (6 weeks) performed by Di Gregorio et al. (2006) but not that of ours (1 year). Thirdly, laboratory studies do not fully reflect field conditions where the effects of certain environmental variables on soil microbial community may overcome those of introduced microbes.
As to the introduced microbes per se, the top 5 genera were either absent from or present in the soils in similar abundance 1 year after treatment application, except Sulfobacillus (Fig. 7b). Moreover, their relative abundances recorded here were low (< 1%) and comparable to those observed in other contaminated agricultural soils (Lin et al. 2010; Sun et al. 2015), suggesting that they are less likely to have a more visible effect on indigenous soil microbial community. It should be noted that the quantity and metabolic activity of soil microbes cannot be unequivocally revealed by the microbiological analysis performed in this study, which is worthy of further exploration.
Conclusions
This study showed that A. carambola inoculated with the bacterial consortium enriched from AMD removed an estimated 4.6% of the total Cd in the topsoil, which was much higher than those previously reported. Meanwhile, our results provided evidence for the first time that MAP could be practiced with minor effects on the indigenous soil microbial community. Future studies should conduct more field experiments to test the extent to which our findings are applicable to other similar situations, which will help pave the way towards the full-scale implementation of MAP.
References
Adriano DC (2001) Trace elements in the terrestrial environment. Springer, New York
Baker BJ, Banfield JF (2003) Microbial communities in acid mine drainage. FEMS Microbiol Ecol 44:139–152
Braud A, Jézéquel K, Bazot S, Lebeau T (2009) Enhanced phytoextraction of an agricultural Cr- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 74:280–286
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Chen L, Luo S, Li X, Wan Y, Chen J, Liu C (2014) Interaction of Cd-hyperaccumulator Solanum nigrum L. and functional endophyte Pseudomonas sp. Lk9 on soil heavy metals uptake. Soil Biol Biochem 68:300–308
Chesworth W (2008) Encyclopedia of soil science. Springer, Dordrecht
De Boer W, Kowalchuk GA (2001) Nitrification in acid soils: micro-organisms and mechanisms. Soil Biol Biochem 33:853–866
Di Gregorio S, Barbafieri M, Lampis S, Sanangelantoni AM, Tassi E, Vallini G (2006) Combined application of triton X-100 and Sinorhizobium sp. Pb002 inoculum for the improvement of lead phytoextraction by Brassica juncea in EDTA amended soil. Chemosphere 63:293–299
Ding CY, Zheng Y, Ren XM, Chen ZJ (2016) Changes in bacterial community composition during the remediation of Cd-contaminated soils of bioenergy crops. Acta Sci Circumst 36:3009–3016
Dopson M, Baker-Austin C, Hind A, Bowman JP, Bond PL (2004) Characterization of Ferroplasma isolates and Ferroplasma acidarmanus sp. nov., extreme acidophiles from acid mine drainage and industrial bioleaching environments. Appl Environ Microbiol 70:2079–2088
Farwell AJ, Vesely S, Nero V, Rodriguez H, McCormack K, Shah S, Dixon DG, Glick BR (2007) Tolerance of transgenic canola plants (Brassica napus) amended with plant growth-promoting bacteria to flooding stress at a metal-contaminated field site. Environ Pollut 147:540–545
Huang JW, Chen JJ, Berti WR, Cunningham SD (1997) Phytoremediation of lead-contaminated soils: role of synthetic chelates in lead phytoextraction. Environ Sci Technol 31:800–805
Jankong P, Visoottiviseth P, Khokiattiwong S (2007) Enhanced phytoremediation of arsenic contaminated land. Chemosphere 68:906–1912
Karavaiko GI, Kondrat’eva TF, Tsaplina IA, Egorova MA, Krasil’nikova EN, Zakharchuk LM (2005) Reclassification of ‘Sulfobacillus thermosulfidooxidans subsp. thermotolerans’ strain K1 as Alicyclobacillus tolerans sp. nov. and Sulfobacillus disulfidooxidans Dufresne et al. 1996 as Alicyclobacillus disulfidooxidans comb. Nov., and emended description of the genus Alicyclobacillus. Int J Syst Evol Microbiol 55:941–947
Kock D, Schippers A (2008) Quantitative microbial community analysis of three different sulfidic mine tailing dumps generating acid mine drainage. Appl Environ Microbiol 74:5211–5219
Krämer U (2005) Phytoremediation: novel approaches to cleaning up polluted soils. Curr Opin Biotechnol 16:133–141
Langella F, Grawunder A, Stark R, Weist A, Merten D, Haferburg G, Büchel G, Kothe E (2014) Microbially assisted phytoremediation approaches for two multi-element contaminated sites. Environ Sci Pollut Res 21:6845–6858
Lebeau T, Braud A, Jézéquel K (2008) Performance of bioaugmentation-assisted phytoextraction applied to metal contaminated soils: a review. Environ Pollut 153:497–522
Li JT, Liao B, Dai ZY, Zhu R, Shu WS (2009) Phytoextraction of Cd-contaminated soil by carambola (Averrhoa carambola) in field trials. Chemosphere 76:1233–1239
Li JT, Baker AJM, Ye ZH, Wang HB, Shu WS (2012) Phytoextraction of Cd-contaminated soils: current status and future challenges. Crit Rev Environ Sci Technol 42:2113–2152
Lin H, Shi J, Chen X, Yang J, Chen Y, Zhao Y, Hu T (2010) Effects of lead upon the actions of sulfate-reducing bacteria in the rice rhizosphere. Soil Biol Biochem 42:1038–1044
Lindsay WL, Norvell WA (1978) Development of a DTPA test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Liu JS, Xie XH, Xiao SM, Wang XM, Zhao WJ, Tian ZL (2007) Isolation of Leptospirillum ferriphilum by single-layered solid medium. J Cent S Univ Technol 14:467–473
Liu YR, Wang JJ, Zheng YM, Zhang LM, He JZ (2014) Patterns of bacterial diversity along a long-term mercury-contaminated gradient in the paddy soils. Microb Ecol 68:575–583
Lu RK (2000) Analytical methods of soil and agricultural chemistry. China Agricultural Science and Technology Press, Beijing
Marhual NP, Pradhan N, Kar RN, Sukla LB, Mishra BK (2008) Differential bioleaching of copper by mesophilic and moderately thermophilic acidophilic consortium enriched from same copper mine water sample. Bioresour Technol 99:8331–8336
McLaughlin MJ, Parker DR, Clarke JM (1999) Metal and micronutrientsdfood safety issues. Field Crop Res 60:143–163
Phieler R, Merten D, Roth M, Büchel G, Kothe E (2015) Phytoremediation using microbially mediated metal accumulation in Sorghum bicolor. Environ Sci Pollut Res 22:19408–19416
Prapagdee B, Khonsue N (2015) Bacterial-assisted cadmium phytoremediation by Ocimum gratissimum L. in polluted agricultural soil: a field trial experiment. Int J Environ Sci Technol 12:3843–3852
Robinson BH, Anderson CWN, Dickinson NM (2015) Phytoextraction: where’s the action? J Geochem Explor 151:34–40
Rodriguez-Leiva M, Tributsch H (1988) Morphology of bacterial leaching patterns by Thiobacillus ferrooxidans on synthetic pyrite. Arch Microbiol 149:401–405
Salt DE, Blaylock M, Kumar NP, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Nat Biotechnol 13:468–474
Schippers A, Breuker A, Blazejak A, Bosecker K, Kock D, Wright TL (2010) The biogeochemistry and microbiology of sulfidic mine waste and bioleaching dumps and heaps, and novel Fe(II)-oxidizing bacteria. Hydrometallurgy 104:342–350
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Sessitsch A, Kuffner M, Kidd P, Vangronsveld J, Wenzel WW, Fallmann K, Puschenreiter M (2013) The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 60:182–194
Shokralla S, Spall JL, Gibson JF, Hajibabaei M (2012) Next-generation sequencing technologies for environmental DNA research. Mol Ecol 21:1794–1805
Silverman MP, Lundgren DG (1959) Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans: I. An improved medium and harvesting procedure for securing high cell yields. J Bacteriol 77:642–647
Sun M, Xiao T, Ning Z, Xiao E, Sun W (2015) Microbial community analysis in rice paddy soils irrigated by acid mine drainage contaminated water. Appl Microbiol Biotechnol 99:2911–2922
Teixeira C, Almeida CMR, Nunes da Silva M, Bordalo AA, Mucha AP (2014) Development of autochthonous microbial consortia for enhanced phytoremediation of salt-marsh sediments contaminated with cadmium. Sci Total Environ 493:757–765
US EPA (1996) Method 3052: microwave assisted acid digestion of siliceous and organically based matrice. US EPA, Washington, DC
Wang FY, Lin XG, Yin R (2005) Heavy metal uptake by arbuscular mycorrhizas of Elsholtzia splendens and the potential for phytoremediation of contaminated soil. Plant Soil 269:225–232
Wang FY, Lin XG, Yin R (2007a) Role of microbial inoculation and chitosan in phytoextraction of Cu, Zn, Pb and Cd by Elsholtzia splendens - a field case. Environ Pollut 147:248–255
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007b) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
WHO (1992a) Cadmium. Environmental health criteria, vol. 134. WHO, Geneva
WHO (1992b) Cadmium─environmental aspects. Environmental health criteria, vol. 135. WHO, Geneva
Xiang Y, Wu P, Zhu N, Zhang T, Liu W, Wu J, Li P (2010) Bioleaching of copper from waste printed circuit boards by bacterial consortium enriched from acid mine drainage. J Hazard Mater 184:812–818
Xu BB (2012) Bioleaching of heavy metals from contaminated soils using microbes from acid mine drainage. M.S. Dissertation, Sun Yat-sen University
Zaidi S, Usmani S, Singh BR, Musarrat J (2006) Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 64:991–997
Zhang X, Xia H, Li Z, Zhuang P, Gao B (2010) Potential of four forage grasses in remediation of Cd and Zn contaminated soils. Bioresour Technol 101:2063–2066
Zhao FJ, Ma Y, Zhu YG, Tang Z, McGrath SP (2015) Soil contamination in China: current status and mitigation strategies. Environ Sci Technol 49:750–759
Acknowledgements
We thank Prof. Alan Baker of the University of Melbourne for his insightful comments that have helped to improve the quality of the manuscript. This work was funded by the National Natural Science Foundation of China (Nos. 31100372 and 41471257), the Pearl River Nova Program of Guangzhou (No. 2014 J2200100), the Fok Ying Tong Education Foundation (No. 142025), the Guangdong Provincial Natural Science Foundation (No. 10451027501005629), and the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20110171120042).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Fangjie Zhao.
Rights and permissions
About this article
Cite this article
Li, Jt., Liang, Zw., Jia, P. et al. Effects of a bacterial consortium from acid mine drainage on cadmium phytoextraction and indigenous soil microbial community. Plant Soil 415, 347–358 (2017). https://doi.org/10.1007/s11104-016-3170-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3170-0