Abstract
A pot experiment was performed to examine the role of foliar applied mannitol (M) in chromium (Cr) stress alleviation in different maize cultivars. Two maize cultivars, one tolerant (6103) and one sensitive (9108) to chromium stress, were grown in soil treated with three concentrations of Cr (0, 5, and 10 mg kg−1) and three levels of mannitol (0, 50, and 100 mg L−1). Chromium stress decreased the overall growth of plants by reducing the plant height, root/shoot dry weight, chlorophyll contents, and enzymatic activities, while exacerbated the severity of reactive oxygen species in both maize cultivars. Chromium-induced reduction in growth attributes of maize plants was relatively higher in sensitive cultivar than that of tolerant one. Uptake of Cr by the plants and its translocation from roots to shoots increased with increasing concentration in the soil. However, foliar application of mannitol significantly alleviated the Cr stress and improved growth, biomass, and photosynthetic pigments of maize plants. Mannitol also considerably reduced Cr contents in leaves and roots of both cultivars. Hence, it is concluded that mannitol can be helpful for crops grown on heavy metal, especially Cr, contaminated soils for remediation purpose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During past few decades, heavy metals have played a key role in deteriorating the quality of environment because of rapid urbanization, population growth, and industrialization (Farid et al. 2015; Rizwan et al. 2017a). In developing countries, the industrial effluent is often used to irrigate the agricultural soils due to shortage of freshwater. This industrial effluent with heavy metal contamination poses highly poisonous impacts on fauna and flora (Júnior et al. 2015; Sood et al. 2012). The accumulation of heavy metals in edible portions of plants, especially cereals, poses a major threat to human life (Anjum et al. 2016; Wang et al. 2017). These heavy metals have deleterious impacts on growth, photosynthesis, and antioxidant enzyme activities in plants (Anjum et al. 2017; Singh et al. 2017). Chromium is a nonessential and potentially deleterious heavy metal having no metabolic function in plants (Kamran et al. 2016). Chromium has 7th rank in the list of most abundant element and 21st in most abundant heavy metal in Earth’s crust with specific density of 7.19 g/cm3 (Economou-Eliopoulos et al. 2013). According to the International Agency for Research on Cancer, Cr is considered as no. 1 carcinogen (IARC 1987). Ashraf et al. (2016) reported that Cr has different oxidation states ranging from − 2 to + 6, but the most stable and common states present in the environment are trivalent [Cr(III)] and hexavalent chromate [Cr(VI)]. Both states show different behavior with respect to their bioavailability and sorption in soil, translocation and absorption in aboveground biomass, and toxicity inside the plant (Choppala et al. 2016). Several studies have illustrated the toxic effects of Cr on morphophysiological and biochemical processes of plants (Jabeen et al. 2016; Kamran et al. 2016). Recent studies reported that Cr toxicity severely affected the agronomic traits, photosynthetic pigments, gas exchange characteristics, ultrastructure of cell chloroplast and membrane, as well as proteomic and miRNA expression (Ali et al. 2015; Bukhari et al. 2016a, b). Several plant- and soil-related factors define the transfer of Cr from soil to plant, such as plant species and genotype, gas exchange attributes, root surface area, soil pH, texture, and electrical conductivity (Islam et al. 2016).
Maize (Zea mays L.), an important cereal crop, is one of the main staple foods and is widely cultivated under varying soil and climatic conditions all over the world. It is an important constituent of animal as well as human nutrition (Rosas-Castor et al. 2014). Maize is an important industrial crop which is used for ethanol manufacturing (Shahzad et al. 2016). China and USA are two major candidates for maize consumption (Gale et al. 2016). Maize is capable of bioaccumulation of metals from growth medium, with greater phytoextraction potential and higher transfer rates (Wuana and Okieimen 2010). According to FAOSTAT (2013), the existing production of maize is about 0.250 billion tons per annum. The demand for maize, only in East and Southeast Asia, is anticipated to reach around 0.291 billion tons in 2020 (Rosegrant et al. 2001).
Plants can fight against various biotic and abiotic stresses with the help of different organic solutes including polyols, oligosaccharides, and proline. Mannitol, a common natural polyol, is a key osmolyte synthesized by many plants which plays a pivotal role in carbon and energy storage and regulation and osmoregulation of coenzymes (Mitoi et al. 2009; Pharr et al. 1995). Because of the ability to scavenge the reactive oxygen species (ROS) and free radicals, mannitol is considered as an important antioxidant (Tandon et al. 2003; Khare et al. 2010). Mannitol is reported to have significant role in reducing salinity and osmotic stress in various plants (Bhauso et al. 2014) and maintaining the cell turgor pressure (Siringam et al. 2011). Mannitol has been reported to play an important role in stabilizing the membrane and protein structure, scavenging the ROS, maintaining the photosynthetic apparatus, and osmoprotection in various species under abiotic stress (Chan et al. 2011). Mannitol plays a vital role in salt stress tolerance (Sickler et al. 2007) and carbon utilization efficiency (Keunen et al. 2013).
The present work was designed to examine the ameliorative impact of mannitol on maize cultivars, grown under Cr stress, in terms of growth, photosynthetic pigments, and antioxidant enzymatic activities.
Materials and methods
Soil sampling and analysis
The soil samples, collected from agricultural field, were properly mixed and sieved by 2-mm sieve. Standard methods were employed to analyze the soil pH and EC (Soltanpour 1985), soil organic matter (Jackson 1962), and soil texture (Abbas et al. 2017). The physicochemical properties of soil are given in Table 1.
Pot experiment
A pot experiment was performed in the Botanical Garden of GCUF on two maize cultivars, one tolerant (6103) and the other sensitive (9108) to chromium stress (data not shown). The trial was run using completely randomized design (CRD) having six replicates. Stress was induced by using three levels of chromium (Cr VI) (0, 5, and 10 mg kg−1soil) as K2Cr2O7. Three concentrations of M (0, 50, 100 mg L−1) were selected for foliar application. Every pot was filled with 6 kg soil. The pots were irrigated until they became saturated. When the soil was fully moisturized, the seeds of maize were sown uniformly by hand. After 15 days of germination, thinning was done to maintain two plants per pot. Mannitol was sprayed four times during the experiment. First spray was done after 1 week of germination; 2nd, 3rd, and 4th spray was applied after 2, 4, and 6 weeks of sowing, respectively; and Tween 80 was used as sticking agent. The pots were covered to avoid soil contamination during spray and rotated randomly throughout the growth period. After 2 weeks of germination, the N, K, and P fertilizers were applied at the rate of 120, 25, and 50 kg ha−1, respectively. Urea, potassium sulfate, and diammonium phosphate fertilizers were used as sources of N, K, and P, respectively.
Growth traits
Plants were harvested after 10 weeks of treatment. The growth characteristics, such as leaf area, number of leaves per plant, root length, and plant height, were measured on fresh plant samples. The roots and shoots were separated, followed by oven drying at 70 °C to measure their dry biomass.
Chlorophyll and carotenoid contents
After 8 weeks of treatment, fresh leaf samples were harvested to measure chlorophyll (a, b, total chlorophylls) and carotenoid contents according to the methodology given by Lichtenthaler (1987).
Electrolyte leakage, H2O2, MDA, and antioxidant enzymes
After 8 weeks of treatment, the electrolyte leakage (EL), H2O2, MDA, and antioxidant enzymatic activities were measured on plant leaves. The samples were autoclaved at 32 °C for 2 h, and EC1 was noted. The same samples were again autoclaved at 121 °C for 20 min to measure EC2 thereafter. Then, EL was calculated as described by Dionisio-Sese and Tobita (1998) using the following equation:
The H2O2 contents were measured by homogenizing the samples with 50 mM phosphate buffer having pH 6.5. The samples were centrifuged for 25 min, followed by addition of 20% H2SO4 (v/v). The specimens were again centrifuged for 15 min. The H2O2 contents were measured by running the samples at 410 nm absorbance (Jana and Choudhuri 1981). The MDA contents were measured by using the technique described by Zhang and Kirham (1994).
The activities of SOD, POD, CAT, and APX were estimated spectrophotometrically. The leaf samples were mixed with 0.05 M phosphate buffer (pH 7.8) and centrifuged for 10 min. POD and SOD contents were obtained as described by Zhang (1992), while APX and CAT contents were measured by following Nakano and Asada (1981) and Aebi (1984), respectively.
Measurement of Cr contents
The samples were rinsed with dilute HCl, dried at 70 °C, and grinded to fine powder. The plant samples (1 g each) were burnt to ashes at 450 °C for 12 h and digested with10 mL of HNO3-HClO4 (3:1 v/v) thereafter. The specimens were kept overnight and further digested by adding 5 mL of the same solution (Rehman et al. 2015). The Cr contents were estimated by atomic absorption spectrophotometer.
Statistical analysis
Data was presented with means of the three replicates. Analysis of variance (ANOVA) was done using the statistical package (SPSS, version 16.0), followed by the Tukey’s post hoc test among means of different treatments to estimate significant difference.
Results
Growth and physiological traits
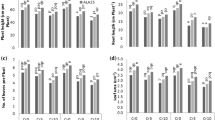
Growth characteristics of maize plants such as height, leaf area, root length, and number of leaves plant−1 were considerably decreased due to Cr stress at 10 mg kg−1. With increasing Cr concentration in growth medium, there was a regular reduction in all growth parameters of both varieties (Tolerant 6103, Sensitive 9108) (Fig. 1.). Foliar application of M (50, 100 mg L−1) on stressed plants improved root length, leaf area, plant height, and number of leaves plant−1 as compared to Cr treatment alone.
Impact of chromium on plant height (a), root length (b), number of leaves (c), and leaf area (d) in two different hybrids of maize, one is tolerant (6103) and other is sensitive (9108), cultivated in soil along with three different chromium levels (0, 5, and 10 mg kg−1) and three different mannitol levels (0, 50, and 100 mg L−1) with three replicates. The significant difference between the values is of P < 0.05 which is shown by different letters
Agronomic traits
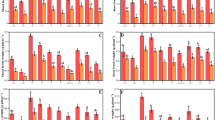
Agronomic characteristics of maize plants such as dry weight of leaves, roots, and stems were depressed with increasing Cr concentration in both varieties (Tolerant 6103, Sensitive 9108). Maximum reduction in biomass was noticed at 10 mg kg−1 Cr treatment in both varieties (Fig. 2.) However, foliar application of mannitol enhanced the dry weight of all parts of plants with dose-additive manner in both maize varieties.
Impact of chromium on leaf (a), stem (b), and root (c) dry weights in two different hybrids of maize, one is tolerant (6103) and other is sensitive (9108), cultivated in soil along with three different chromium levels (0, 5, and 10 mg kg−1) and three different mannitol levels (0, 50, and 100 mg L−1) with three replicates. The significant difference between the values is of P < 0.05 which is shown by different letters
Oxidative stress
Increasing Cr concentration imposed the oxidative stress on plants by increasing the EL, H2O2, and MDA contents in root and leaves of both cultivars (Fig. 3). Foliar application of mannitol, at both concentrations (50, 100 mg L−1), considerably ameliorated the oxidative stress in roots and leaves of plants. However, the severity of oxidative stress was more pronounced in Cr-sensitive cultivar (9108) than the tolerant one (6103) as shown by higher concentration of EL, H2O2, and MDA.
Impact of chromium on leaf MDA (a), root MDA (b), leaf H2O2 (c), root H2O2 (d), EL in leaves (e), and EL in roots (f) in two different hybrids of maize, one is tolerant (6103) and other is sensitive (9108), cultivated in soil along with three different chromium levels (0, 5, and 10 mg kg−1) and three different mannitol levels (0, 50, and 100 mg L−1) with three replicates. The significant difference between the values is of P < 0.05 which is shown by different letters
Antioxidant enzymatic activities
Under Cr stress, a significant reduction in the activities of antioxidant enzymes was observed both in leaves and root of maize plants. But foliar application of mannitol, at both concentrations (50, 100 mg L−1), on Cr-stressed plants considerably improved the activities of antioxidant enzymes (SOD, POD, CAT, and APX) in both cultivars, as compared to those treated with same Cr concentration without mannitol. The highest increase in antioxidant enzymatic activities was observed at higher mannitol concentration (100 mg L−1) (Fig. 4).
Impact of chromium on SOD leaf and root (a, b), POD leaf and root (c, d), CAT leaf and root (e, f), and APX leaf and root (g, h) in two different hybrids of maize, one is tolerant (6103) and other is sensitive (9108), cultivated in soil along with three different chromium levels (0, 5, and 10 mg kg−1) and three different mannitol levels (0, 50, and 100 mg L−1) with three replicates. The significant difference between the values is of P < 0.05 which is shown by different letters
Photosynthetic characteristics
Photosynthetic traits including chlorophyll a, b, total chlorophyll, and carotenoids were considerably reduced in leaves of plants grown under Cr stress at both levels (5 and 10 mg kg−1) in comparison with the control (Fig. 5) in both verities (Tolerant 6103, Sensitive 9108). But, foliar applied mannitol on Cr-stressed plants significantly enhanced chlorophyll pigments and carotenoids, as compared to those facing Cr stress without mannitol. However, mannitol was more effective in enhancing chlorophyll contents at lower Cr concentration (5 mg kg−1), as compared to the higher one (10 mg kg−1) in both varieties.
Impact of chromium on Chl a (a), Chl b (b), total Chl (c), and carotenoids (d) in two different hybrids of maize, one is tolerant (6103) and other is sensitive (9108), cultivated in soil along with three different chromium levels (0, 5, and 10 mg kg−1) and three different mannitol levels (0, 50, and 100 mg L−1) with three replicates. The significant difference between the values is of P < 0.05 which is shown by different letters
Chromium contents
Chromium contents in maize plants increased with increasing Cr concentration in the growth medium in a dose-additive way in roots and leaves of both maize cultivars (Fig. 6). Highest Cr contents were obtained at maximum applied Cr level (10 mg kg−1). There were higher Cr contents in roots than that in leaves. But the exogenous application of mannitol significantly reduced the Cr contents in roots and leaves of both maize varieties. The highest reduction in Cr contents was observed at maximum mannitol level (100 mg L−1).
Chromium uptake in leaves (a) and roots (b) in two different hybrids of maize, one is tolerant (6103) and other is sensitive (9108), cultivated in soil along with three different chromium levels (0, 5, and 10 mg kg−1) and three different mannitol levels (0, 50, and 100 mg L−1) with three replicates. The significant difference between the values is of P < 0.05 which is shown by different letters
Discussion
In present study, the growth and biomass of maize were severely suppressed by Cr toxicity as compared with control (Figs. 1 and 2). This growth and biomass reduction might be due to ultrastructural changes in mesophyll cells of leaves (Gill et al. 2015; Antoniadis et al. 2017). Also, the reduction in nutrient uptake by plants due to Cr stress might have resulted in plants’ biomass reduction (Tauqeer et al. 2016). Foliar application of mannitol significantly alleviated the Cr-induced deterioration in growth and biomass of maize plants. This increase in biomass and growth, because of foliar applied mannitol, has also been noticed in various plant species under drought (Ullah et al. 2014) and salinity stress (Kaya et al. 2013). The improvement in growth and biomass of plants with mannitol might be ascribed to its utilization in plant leaves, where it might have served as a source of nitrogen and carbon (Mitoi et al. 2009). Chlorophyll contents and carotenoids were reduced by Cr toxicity both in leaves and root of maize (Fig. 5). Similar results have been found in many other plants like sunflower (Singh et al. 2013), wheat, and mung bean under Cr stress (Jabeen et al. 2016). Many other heavy metals like Cd, Ni, and Cu also decreased the chlorophyll and carotenoid contents in various plants species (Farooq et al. 2016). The reduction in photosynthetic pigments might be associated with ultrastructural changes in chloroplasts (Bukhari et al. 2016a; Najeeb et al. 2011). However, the foliar applied mannitol significantly alleviated the Cr-induced stress and improved photosynthetic pigments of maize plants. It has been reported previously that the mannitol enhanced the photosynthetic pigments under Cr stress in maize (Kaya et al. 2013), which might be due to higher photosynthetic rate.
Under Cr stress, maize plants faced oxidative stress induced by overproduction of ROS (Fig. 3). Same results were observed in various plant species under stressful condition (Habiba et al. 2015; Ahmad et al. 2017). Under heavy metal stress, many plant species revealed the higher production of ROS like sunflower (Rizwan et al. 2017b), barley, and wheat (Gill et al. 2016). Like heavy metals, salinity and drought also increased the ROS production in plants (Arshad et al. 2016). In the current study, foliar application of mannitol significantly decreased the ROS production under Cr stress. This decrease in ROS production with mannitol application might be ascribed to enhanced performance of antioxidant defense system (Islam et al. 2016). It has also been reported that the application of mannitol decreased lipid peroxidation in wheat plants under salt stress (Seckin et al. 2009).
Plants develop their defense system through antioxidant enzymatic activities against various environmental stresses (Kanto et al. 2015). The Cr toxicity decreased the antioxidant enzyme activities in both leaves and roots of maize plants (Fig. 4). Similar findings have also been noticed in various plants under Cr stress (Farid et al. 2017). The decrease in antioxidant enzymatic activities might be attributed to higher Cr uptake by maize plants (Fig. 4), which might have reduced the plants’ self-defense system. However, the mannitol application significantly alleviated the Cr-induced reduction in antioxidant enzymatic activities of maize plants. It has also been reported that the mannitol enhanced the activities of antioxidant enzymes in peanut under drought and salt stress, which might be attributed to the reduction in ROS production (Bhauso et al. 2014).
In recent study, the increasing Cr concentration in soil media significantly increased the uptake and accumulation of Cr, both in leaves and root of maize plants (Fig. 6). These findings are similar to those reported in Brassica napus (Gill et al. 2015), barley (Ali et al. 2013), and tobacco (Bukhari et al. 2015). The higher Cr concentration was observed in roots than that in leaves. However, mannitol significantly reduced the Cr uptake and translocation from roots to leaves. This decline in Cr uptake might be associated with the protective role of mannitol in cell membrane stability, which might have resulted in reduced entry of Cr in cytoplasm (Bhauso et al. 2014).
Conclusion
Our study concluded that Cr application to soil media significantly reduced the physiological and morphological parameters of maize plants. But the foliar application of mannitol effectively alleviated the Cr-induced toxic effects in maize plants. The mannitol application enhanced the morphophysiological parameters, chlorophyll contents, carotenoids, and anti-oxidant enzymatic activities, while reduced the ROS production and Cr uptake and translocation both in leaves and root of plants, which suggested the protective role of mannitol in maize under heavy metals stress. However, the mechanism of Cr stress alleviation by mannitol and its uptake still bears a question mark which should be further studied.
References
Abbas T, Rizwan M, Ali S, Zia-ur-Rehman M, Qayyum MF, Abbas F, Hannan F, Rinklebe J, Ok YS (2017) Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol Environ Safe 140:37–47
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmad R, Ali S, Hannan F, Rizwan M, Iqbal M, Hassan Z, Akram NA, Maqbool S, Abbas F (2017) Promotive role of 5-aminolevulinic acid on chromium-induced morphological, photosynthetic, and oxidative changes in cauliflower (Brassica oleracea botrytis L.). Environ Sci Pollut Res 24:8814–8824
Ali B, Gill RA, Yang S, Gill MB, Farooq MA, Liu D, Daud MK, Ali S, Zhou W (2015) Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS One 10:e0123328
Ali S, Farooq MA, Jahangir MM, Abbas F, Bharwana SA, Zhang GP (2013) Effect of chromium and nitrogen form on photosynthesis and anti-oxidative system in barley. Biologiaplantarum 57:758–763
Anjum SA, Ashraf U, Khan I, Saleem MF, Wang LC (2016) Chromium toxicity induced alterations in growth, photosynthesis, gas exchange attributes and yield formation in maize. Pak J Agric Sci 53:751–757
Anjum SA, Ashraf U, Tanveer M, Khan I, Hussain S, Zohaib A, Abbas F, Saleem MF, Wang L (2017) Drought tolerance in three maize cultivars is related to differential osmolyte accumulation, antioxidant defense system, and oxidative damage. Front Plant Sci 8:69
Antoniadis V, Shaheen SM, Boersch J, Frohne T, Du Laing G, Rinklebe J (2017) Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany. J Environ Manag 186:192–200
Arshad M, Ali S, Noman A, Ali Q, Rizwan M, Farid M, Irshad MK (2016) Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants, and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Arch Agron Soil Sci 62:533–546
Ashraf A, Bibi I, Niazi NK, Ok YS, Murtaza G, Shahid M, Kunhikrishnan A, Mahmood T (2016) Chromium(VI) sorption efficiency of acid-activated banana peel over organo-montmorillonite in aqueous solutions. Int J Phytoremediation 43:121–128
Bhauso TD, Thankappan R, Kumar A, Mishra GP, Dobaria JR, VenkatRajam M (2014) Over-expression of bacterial'mtlD'gene confers enhanced tolerance to salt-stress and water-deficit stress in transgenic peanut (‘Arachishypogaea’) through accumulation of mannitol. Aus J Crop Sci 8:413–421
Bukhari SAH, Shang S, Zhang M, Zheng W, Zhang G, Wang TZ, Shamsi IH, Wu FB (2015) Genome-wide identification of chromium stress-responsive microRNAs and their target genes in tobacco (Nicotiana tabacum) roots. Environ Toxicol Chem 34:2573–2582
Bukhari SAH, Zheng W, Xie L, Zhang G, Shang S, Wu F (2016a) Cr-induced changes in leaf protein profile, ultrastructure and photosynthetic traits in the two contrasting tobacco genotypes. Plant Growth Regul 79:147–156
Bukhari SAH, Wang R, Wang W, Ahmed IM, Zheng W, Cao F (2016b) Genotype-dependent effect of exogenous 24-epibrassinolide on chromium-induced changes in ultrastructure and physicochemical traits in tobacco seedlings. Environ Sci Pollut Res 23:18229–18238
Chan Z, Grumet R, Loescher W (2011) Global gene expression analysis of transgenic, mannitol-producing, and salt-tolerant Arabidopsis thaliana indicates widespread changes in abiotic and biotic stress-related genes. J Exp Bot 62:4787–4803
Choppala G, Kunhikrishnan A, Seshadri B, Park JH, Bush R, Bolan N (2016) Comparative sorption of chromium species as influenced by pH, surface charge and organic matter content in contaminated soils. J Geochem Explor 76:110–119
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Economou-Eliopoulos M, Megremi I, Atsarou C, Theodoratou C, Vasilatos C (2013) Spatial evolution of the chromium contamination in soils from the Assopos to Thiva Basin and C. Evia (Greece) and potential source (s): anthropogenic versus natural processes. Geosciences 3:140–158
FAOSTAT D. (2013) Food and agriculture organization of the United Nations. Stat database
Farid M, Ali S, Ishaque W, Shakoor MB, Niazi NK, Bibi I, Dawood M, Gill RA, Abbas F (2015) Exogenous application of ethylenediamminetetraacetic acid enhanced phytoremediation of cadmium by Brassica napus L. Int J Environ Sci Technol 12:3981–3992
Farid M, Ali S, Rizwan M, Ali Q, Abbas F, Bukhari SA, Saeed R, Wu L (2017) Citric acid assisted phytoextraction of chromium by sunflower; morpho-physiological and biochemical alterations in plants. Ecotoxicol Environ Saf 145:90–102
Farooq MA, Detterbeck A, Clemens S, Dietz KJ (2016) Silicon-induced reversibility of cadmium toxicity in rice. J Exp Bot 67:3573–3585
Gale F, Jewison M, Hansen J (2016) Prospects for China’s corn yield growth and imports. Curr Politics Econ North West Asia 25:479–521
Gill RA, Ali B, Cui P, Shen E, Farooq MA, Islam F, Ali S, Mao B, Zhou W (2016) Comparative transcriptome profiling of two Brassica napusL. cultivars under chromium toxicity and its alleviation by reduced glutathione. BMC Genomics 17:885
Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Ali S, Zhou W (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164
Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, Abbasi GH, Hayat T, Ali B (2015) EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ Sci Pollut Res 22:1534–1544
IARC (1987) Overall evaluations of carcinogenicity: an updating of IARC monographs volumes 1 to 42. World Health Organization, International Agency for Research on Cancer
Islam F, Yasmeen T, Arif MS, Riaz M, Shahzad SM, Imran Q, Ali I (2016) Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and salicylic acid (SA) in attenuation of chromium stress in maize plants. Plant Physiol Biochem 108:456–467
Jackson ML (1962) Soil chemical analysis. Prentice-Hall, Englewood Cliffs, NJ 214–221
Jana S, Choudhuri MA (1981) Glycolate metabolism of three submersed aquatic angiosperms: effect of heavy metals. Aquat Bot 11:67–77
Jabeen N, Abbas Z, Iqbal M, Rizwan M, Jabbar A, Farid M, Ali S, Ibrahim M, Abbas F (2016) Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch Agron Soil Sci 62:648–662
Júnior CA, de Sousa Barbosa H, Galazzi RM, Koolen HH, Gozzo FC, Arruda MA (2015) Evaluation of proteome alterations induced by cadmium stress in sunflower (Helianthus annuus L.) cultures. Ecotoxicol Environ Saf 119:170–177
Kamran MA, Eqani SAMAS, Bibi S, Xu RK, Monis MFH, Katsoyiannis A, Bokhari H, Chaudhary HJ (2016) Bioaccumulation of nickel by E. sativa and role of plant growth promoting rhizobacteria (PGPRs) under nickel stress. Ecotoxicol Environ Saf 126:256–263
Kanto U, Jutamanee K, Osotsapar Y, Chai-arree W, Jattupornpong S (2015) Promotive effect of priming with 5-aminolevulinic acid on seed germination capacity, seedling growth and antioxidant enzyme activity in rice subjected to accelerated ageing treatment. Plant Prod Sci 18:443–454
Kaya C, Sonmez O, Aydemir S, Ashraf M, Dikilitas M (2013) Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L.). J Plant Interac 8:234–241
Keunen EL, Peshev D, Vangronsveld J, Van Den Ende WI, Cuypers AN (2013) Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant Cell Environ 36:1242–1255
Khare N, Goyary D, Singh NK, Shah P, Rathore M, Anandhan S, Sharma D, Arif M, Ahmed Z (2010) Transgenic tomato cv. PusaUphar expressing a bacterial mannitol-1-phosphate dehydrogenase gene confers abiotic stress tolerance. Plant Cell Tissue Organ Cult (PCTOC) 103:267–277
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Mitoi EN, Holobiuc I, Blindu R (2009) The effect of mannitol on antioxidative enzymes in vitro long term cultures of Dianthus tenuifolius and Dianthus spiculifolius. Rom J Biol Plant Biol 54:25–30
Najeeb U, Jilani G, Ali S, Sarwar M, Xu L, Zhou W (2011) Insights into cadmium induced physiological and ultra-structural disorders in Juncuseffusus L. and its remediation through exogenous citric acid. J Hazard Mater 186:565–574
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Pharr DM, Stoop JM, Feusi MS, Williamson JD, Massel MO, Conkling MA (1995) Mannitol catabolism in plant sink tissues. Curr Top Plant Physiol 13: 180–194
Rehman MZ, Rizwan M, Ghafoor A, Naeem A, Ali S, Sabir M, Qayyum MF (2015) Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ Sci Pollut Res 22:16897–16906
Rizwan M, Ali S, Qayyum MF, Ok YS, Adrees M, Ibrahim M, Zia-ur-Rehman M, Farid M, Abbas F (2017b) Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: a critical review. J Hazard Mater 322:2–16
Rizwan M, Ali S, Qayyum MF, Ok YS, Zia-ur-Rehman M, Abbas Z, Hannan F (2017a) Use of maize (Zea mays L.) for phytomanagement of Cd-contaminated soils: a critical review. Environ Geochem Health 39:259–277
Rosas-Castor JM, Guzmán-Mar JL, Hernández-Ramírez A, Garza-González MT, Hinojosa-Reyes L (2014) Arsenic accumulation in maize crop (Zea maysL.): a review. Sci Total Environ 488:176–187
Rosegrant MW, Paisner MS, Meijer S, Witcover J (2001) 2020 global food outlook: trends, alternatives, and choices (Vol. 11). Intl Food Policy Res Inst
Seckin B, Sekmen AH, Türkan I (2009) An enhancing effect of exogenous mannitol on the antioxidant enzyme activities in roots of wheat under salt stress. J Plant Growth Regul 28:12
Shahzad B, Tanveer M, Hassan W, Shah AN, Anjum SA, Cheema SA, Ali I (2016) Lithium toxicity in plants: reasons, mechanisms and remediation possibilities—a review. Plant Physiol Biochem 107:104–115
Sickler CM, Edwards GE, Kiirats O, Gao Z, Loescher W (2007) Response of mannitol-producing Arabidopsis thaliana to abiotic stress. Funct Plant Biol 34:382–391
Singh HP, Mahajan P, Kaur S, Batish DR, Kohli RK (2013) Chromium toxicity and tolerance in plants. Environ Chem Lett 11:229–254
Singh S, Tripathi DK, Singh S, Sharma S, Dubey NK, Chauhan DK, Vaculík M (2017) Toxicity of aluminium on various levels of plant cells and organism: a review. Environ Exp Bot 137:177–193
Siringam K, Juntawong N, Cha-um S, Kirdmanee C (2011) Salt stress induced ion accumulation, ion homeostasis, membrane injury and sugar contents in salt-sensitive rice (Oryza sativa L. spp. indica) roots under isoosmotic conditions. Afr J Biotechnol 10:1340–1346
Soltanpour PN (1985) Use of ammonium bicarbonate DTPA soil test to evaluate elemental availability and toxicity. Commun Soil Sci Plant Anal 16:323–338
Sood A, Uniyal PL, Prasanna R, Ahluwalia AS (2012) Phytoremediation potential of aquatic macrophyte, Azolla. Ambio 41:122–137
Tandon SK, Singh S, Prasad S, Khandekar K, Dwivedi VK, Chatterjee M, Mathur N (2003) Reversal of cadmium induced oxidative stress by chelating agent, antioxidant or their combination in rat. Toxicol Lett 145:211–217
Tauqeer HM, Ali S, Rizwan M, Ali Q, Saeed R, Iftikhar U, Ahmad R, Farid M, Abbasi GH (2016) Phytoremediation of heavy metals by Alternantherabettzickiana: growth and physiological response. Ecotoxicol Environ Saf 126:138–146
Ullah I, Akhtar N, Mehmood N, Shah IA, Noor M (2014) Effect of mannitol induced drought stress on seedling traits and protein profile of two wheat cultivars. J Animal Plant Sci 24:1246–1251
Wang D, Guo W, Zhang G, Zhou L, Wang M, Lu Y, Cai D, Wu Z (2017) Remediation of Cr (VI)-contaminated acid soil using a nanocomposite. ACS Sustain Chem Eng 5:2246–2254
Wuana RA, Okieimen FE (2010) Phytoremediation potential of maize (Zea mays L.). A review. Afr J Gen Agric 6:275–287
Zhang XZ (1992) The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. Res Methodol Crop Physiol. Agriculture Press, Beijing 208–211
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35:785–791
Acknowledgments
This study was funded by the Higher Education Commission (HEC) Islamabad, Pakistan (IPFP/HRD/HEC/2014/1035). The authors would also like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding to the Research Group number (RGP-199).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Habiba, U., Ali, S., Rizwan, M. et al. Alleviative role of exogenously applied mannitol in maize cultivars differing in chromium stress tolerance. Environ Sci Pollut Res 26, 5111–5121 (2019). https://doi.org/10.1007/s11356-018-3970-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3970-2