Abstract
Heavy metal (HM) contamination of the environment is a serious threat to sustainable crop production. Among the HMs, chromium (Cr) is one of the most toxic HMs that is known to negatively affect growth and metabolic activities of diverse crop plants. The present study was designed to investigate the ameliorative role of 5-aminolevulinic acid (ALA) under Cr stress in two maize (Zea mays L.) cultivars showing differential sensitivity to Cr tolerance. ALA is a biosynthesis precursor and it has a dominant regulatory effect related to physiological, respiratory, and photosynthesis processes in various plant species. Three concentrations of Cr (0, 5, and 10 mg kg−1) were tested under the graded levels of ALA application (0, 12.5, and 25 mg L−1). The results indicated that Cr stress differentially reduced plant growth attributes, gas exchange characteristics, photosynthetic pigments, and biomass in both the cultivars. Oxidative stress increased as evidenced in the form of electrolyte leakage, malondialdehyde, and hydrogen peroxide (H2O2) accumulation in plants. The anti-oxidative enzyme activities, that is, catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD) both in the leaves and roots of maize cultivars decreased due to Cr stress. The concentration of Cr increased in roots and shoots of maize under Cr levels without ALA. Under Cr stress, ALA exogenous application markedly enhanced plant growth, photosynthetic pigments, gas exchange capacity, and biomass. Furthermore, ALA application decreased the Cr-induced oxidative stress in maize cultivars by improving the activities of CAT, POD, and SOD in plants. After ALA application, the Cr concentrations and total Cr uptake by plants differently decreased in both cultivars. The 6103 cultivar of maize was found to be a tolerant cultivar against Cr stress due to its strong defensive system with a higher rate of antioxidant enzyme activities. On the other hand, the other maize cultivar (9108) was found to be a sensitive cultivar against Cr stress due to its weak defense system with higher contents of reactive oxygen species. These findings suggest that ALA can play a regulatory role in maintaining optimum plant growth and efficient photosynthetic processes under Cr-challenged habitats in maize. Thus, ALA application may be used as a sustainable remedial strategy to alleviate Cr-induced stress in maize cultivars.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals (HMs) are non-essential, carcinogenic, and frequently enter into the food web by plant uptake and are passed to end consumers and cause serious health issues in humans (Wang and Chen 2009; Farid et al. 2018; Hussain et al. 2018a). The rapid industrialization and disposal of waste in the environment is a main threat to the agricultural ecosystem as well as growth and yield of crops (Wang et al. 2013). Among HMs, chromium (Cr) is one of the most non-essential toxic metals and the level of Cr is increasing in soil and water because of its common usage in industries (Ashfaque et al. 2017; Hussain et al. 2018b). Cr toxicity is causing serious problems in the soil, particularly in underdeveloped countries including Pakistan (Daud et al. 2014; Afshan et al. 2015) due to anthropogenic activities (Ali et al. 2013a; Hernández-Madrigal et al. 2018). Chromium is regarded as the 7th most abundant element in the Earth’s crust and also classified under the top 20 hazardous substances by the Agency for Toxic Substances and Disease Registry (Oh et al. 2007). Chromium occupied the top slot as far as its carcinogenicity is concerned (Kishore et al. 2018). Chromium mainly exists in two oxidation states, that is, Cr(III) and Cr(VI) (Wu et al. 2016). Chromium(VI) is considered more toxic than Cr(III) owing to the high mobility and high oxidizing potential of Cr(III), therefore Cr(VI) causes deleterious effects on plant growth and productivity (Tripathi et al. 2012; Farid et al. 2017). The uptake of Cr in plants occurs along with uptake of water and other essential nutrients which leads to the alteration of different physiochemical systems of plants that ultimately reduce plant growth (Ranieri and Gikas 2014; Stambulska et al. 2018). Excess Cr usually induces the overproduction of reactive oxygen species (ROS), which leads to oxidative stress (Ahmad et al. 2010; Choudhary et al. 2012; Wu et al. 2018). Thus, Cr in plants caused oxidative stress, and as a result plants experience altered metabolic functions and cell death. Therefore, owing to its toxicity in the soil–plant system, this metal needs in-depth understanding in the plant soil continuum.
Different approaches have been used to alleviate the toxic impacts of various heavy metals in crops (Keller et al. 2015; Ahmad et al. 2016, 2017a, b; Rizwan et al. 2016; Qayyum et al. 2017; Farid et al. 2018). ALA exogenous application might have improved water transport efficiency of plants and recovered chloroplast injury under stressful environments (Gill et al. 2015; Herman et al. 2016). Plants can cope with various abiotic stresses with the application of plant growth regulators (PGRs). Exogenous application of PGRs to plants is one the effective means of boosting plant stress tolerance (Yiu et al. 2009; Dwivedi et al. 2018). 5-Aminolevulinic acid (ALA) is a common plant growth regulator (Bindu and Vivekanandan 1998; Akram and Ashraf 2013; Liu et al. 2016). ALA is a biosynthesis precursor, exists in bacteria, fungus, and amino acids of various plants and animals (Naeem et al. 2011; Xiong et al. 2018). It is known as a crucial plant growth regulator against the stress of various HMs (Ali et al. 2014). ALA has a dominant regulatory effect and it is related to physiological, respiratory, and photosynthesis processes in various plant species (Naeem et al. 2011; Xiong et al. 2018). ALA modulates key plant physiological processes such as seed germination (Wang et al. 2005; Han et al. 2018) rate of net photosynthesis (Wang et al. 2004; Xiaomeng et al. 2018), contributes to signaling of plastids to the nucleus (Czarnecki et al. 2012), and mediates plant abiotic stress tolerance (Akram and Ashraf 2013). Thus, these results suggest that ALA is an important signaling compound in mediating diverse plant processes under dynamic environmental pressures. Exogenous application of ALA has earlier been used to increase tolerance against cold stress in Glycine max (Balestrasse et al. 2010); Solanum lycopersicum (Liu et al. 2018), salt stress in Brassica napus (Naeem et al. 2012), and heat stress in Cucumis sativus (Zhang et al. 2012). Various activities of antioxidant enzymes can be controlled through the foliar application of ALA, so plants can be protected against a number of abiotic stresses in which salinity, low irradiance, cold and chilling are involved (Naeem et al. 2012). However, data are scarce on the effect of ALA-mediated Cr stress tolerance in maize cultivars. ALA exogenous application might have recovered cell injury due to Cr via reducing Cr mobility (Afshan et al. 2015; Ahmad et al. 2017a, b; Farid et al. 2018). Several studies reported the beneficial role of ALA under various stresses (Kosar et al. 2015; Air et al. 2018). Maize is the main concern of researchers due to its ability to survive in dynamic environmental regimes. The green fodder of maize is enriched with protein and its grains are enriched with edible oil. After wheat and rice crops, maize is a very important cereal crop in Pakistan. The metal stress had adverse effects on maize plants (Rehman et al. 2016; Rizwan et al. 2017). Chromium is known to induce a significant reduction in growth and yield of maize, and also depresses the antioxidant defense system (Anjum et al. 2017). Our hypothesis is that ALA may alleviate the Cr stress in maize plants under Cr stress conditions. The present study has been designed to highlight the impacts of exogenous ALA in alleviating Cr stress in two maize cultivars (6103 and 9108) with respect to biomass, photosynthesis, oxidative stress, antioxidants, and Cr uptake by plants.
Materials and Methods
Two maize cultivars including one tolerant (6103) and one sensitive (9108) were selected from our previous study (Habiba et al. 2018). The study was conducted in the Green House of the Government College University Faisalabad, Pakistan with a complete randomized design. The pots were filled with 7 kg of sandy clay loam soil having physicochemical properties as described (Table 1) with various Cr concentrations (0, 5, 10 mg kg−1) by using K2Cr2O7 as a source of Cr. Three concentrations of ALA (0, 12.5, and 25 mg L−1) were selected for foliar application. The trial was conducted under natural conditions. After 15 days, thinning of plants was done and only morphologically homogenous plants were selected for further experiments. The selected levels of ALA were foliarly applied after 2 and 4 weeks of sowing the seeds. The experiment was harvested after 10 weeks of treatment. The growth characteristics including dry weights, leaf area, root length, plant height, number of leaves per plant, and leaves and stems dry weights were measured.
Chlorophyll Contents and Electrolyte Leakage
After 8 weeks of treatment, the chlorophyll and carotenoid contents were measured according to methodology of Porra et al. (1989), which was also antecedently described by Pei et al. (2010). The upmost abundantly stretched leaves were cut into small pieces and placed into test tubes in which deionized water was used up to 8 mL. Then the incubation was done at 32 °C for 2 h in a water bath and the EC1 was calculated. For electrolyte discharge, samples of plants were autoclaved at 121 °C for 20 min; then cooled at room temperature and later the EC2 calculated later (Dionisio-Sese and Tobita 1998). The electrolyte leakage (EL) was then calculated by using the following formula:
Determination MDA and H2O2 Contents and Antioxidant Enzyme Activity
The MDA contents in roots and leaves were obtained by the method given by Heath and Packer (1968) through the reaction of thiobarbituric acid (TBA) and further modified by Dhindsa et al. (1981) followed by Zhang and Kirkham (1994). The samples of roots and leaves (0.25 mg) were mixed into 5 mL of 0.1% trichloroacetic acid. Hydrogen peroxide was measured by homogenizing the leaf and root tissues by use of 3 mL of phosphate buffer with a concentration of 50 mM and the pH was kept at 6.5. Afterwards, the homogenized samples were centrifuged for 25 min. The extracted solution (3 mL) was used with one milliliter of titanium sulfate of percentage 0.1% along with the H2SO4 20% (v/v) and it was centrifuged for 15 min. Then the absorbance was taken at 410 nm to calculate the supernatant intensity (Jana and Choudhuri 1981). The coefficient of extinction with 0.28 µmol−1 cm−1 was used for the calculation of H2O2 contents.
The activities of superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) in roots and leaves were measured by a spectrophotometer via the given procedure of Zhang (1992). The activity of CAT was examined via the use of the Aebi (1984) method.
Chromium Concentration
The digestion of plants (1.0 g each) was done in HNO3:HClO4 (3:1, v:v) as reported by Rehman et al. (2015). An atomic absorption spectrophotometer was used to determine the Cr concentration in maize.
Statistical Analysis
The data were evaluated by SPSS (a statistical software) by using a multivariate post hoc test and followed by the Duncan test to check the interaction between the significant values. A significant difference between the values was defined at P < 0.05 and shown by different letters.
Results
Plant Growth Traits
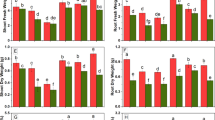
The data regarding plant growth in terms of plant height, root length, number of leaves per plant, and leaf area are shown in Fig. 1. The results indicated that maximum root and shoot length, number of leaves per plant, and leaf area were observed in control plants without Cr application. The maximum reduction in all of these attributes was observed under 10 mg kg−1 Cr concentration. As indicated in Fig. 1, with increasing concentration of Cr, the above plant parameters gradually reduced; however, this reduction was more severe in the 9108 than in the 6103 cultivar of maize. The application of ALA markedly enhanced plant height, root length, and number of leaves per plant in all concentration of Cr. The root length increased by 19 and 15% in Cr 0 and 5 mg kg−1, respectively, at ALA 25 mg L−1 level in 6103, and increased by 16 and 40% in Cr 0 and 5 mg kg−1, respectively, at ALA 25 mg L−1 level in 9108.
Impact of chromium on plant height, root length, no. of leaves, and leaf area in two different hybrids of maize, one is tolerant (6103) and other is sensitive (9108), cultivated in soil along with three different chromium levels (0, 5, and 10 mg kg−1) and three different 5-aminolevulinic acid levels (0, 12.5, 25 mg L−1) with three replicates. The significant difference between the values is at P < 0.05 which is shown by different letters
Cr toxicity significantly reduced the dry weight of roots, leaves, and stems as shown in Fig. 2. Cr stress reduced the dry weight of shoots in both cultivars and this reduction was more severe in 9108 as compared to 6103. The dry weights of leaves and roots also reduced under Cr stress. The foliar use of ALA enhanced the dry weight of roots, leaves, and stems in both stressed and control plants of both cultivars. The dry weights of stems increased by 17, 25, and 33% in Cr 0, 5, and 10 mg kg−1, respectively, at ALA 25 mg L−1 level in 6103, and increased by 18, 25, and 35% in Cr 0, 5, and 10 mg kg−1, respectively, at ALA 25 mg L−1 level in 9108.
Impact of chromium on root, stem, and leaves dry weights (c, b, a), respectively, in two different hybrids of maize, one is tolerant (6103) and other is sensitive (9108), cultivated in soil along with three different chromium levels (0, 5, and 10 mg kg−1) and three different 5-aminolevulinic acid levels (0, 12.5, 25 mg L−1) with three replicates. The significant difference between the values is at P < 0.05 which is shown by different letters
Photosynthetic Parameters
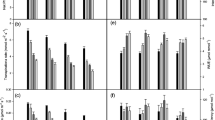
The exposure of maize cultivars to Cr stress minimized the studied photosynthetic parameters (Fig. 3). Cr stress reduced stomatal conductance, net photosynthetic rate, and transpiration rate in both cultivars in a concentration-dependent manner. The maximum reduction in these parameters was observed at 10 mg kg−1 of Cr concentration; however, this reduction was maximum in 9108 than 6103 cultivar. The application of ALA enhanced the photosynthetic parameters at all concentration of Cr. The most beneficial level of ALA was 25 mg L−1 in all Cr levels applied. The net photosynthetic rate was enhanced by 10, 15, and 14% in Cr 0, 5, and 10 mg kg−1, respectively, at ALA of 25 mg L−1 in 6103, and increased by 12, 14, and 15% in Cr 0, 5, and 10 mg kg−1, respectively, at ALA of 25 mg L−1 in 9108.
Impact of chromium on net photosynthetic rate, stomatal conductance, and transpiration rate (c, b, a), respectively, in two different hybrids of maize, one is tolerant (6103) and other is sensitive (9108), cultivated in soil along with three different chromium levels (0, 5, and 10 mg kg−1) and three different 5-aminolevulinic acid levels (0, 12.5, 25 mg L−1) with three replicates. The significant difference between the values is at P < 0.05 which is shown by different letters
Chlorophyll Contents and Carotenoids
Photosynthetic pigments and carotenoids were reduced under Cr stress (Fig. 4). The highest reduction was observed at the 10 mg kg−1 Cr concentration as compared with their relative controls. It was also noticed that 9108 was a Cr susceptive cultivar because it showed the maximum reduction in chlorophyll pigments at both (5 and 10 mg kg−1) levels of Cr. The foliar supply of ALA markedly enhanced the chlorophyll pigments in stressed and non-stressed plants. The total chlorophyll contents were enhanced by 13, 10, and 17% in Cr 0, 5, and 10 mg kg−1, respectively, at the ALA 12.5 mg L−1 level in 6103, and increased by 10, 12, and 17% in Cr 0, 5, and 10 mg kg−1, respectively, at the ALA 12.5 mg L−1 level in 6103.
Impact of chromium on Chl (a, b, total) and carotenoids (a, b, c, d), respectively, in two different hybrids of maize, one is tolerant (6103) and other is sensitive (9108), cultivated in soil along with three different chromium levels (0, 5, and 10 mg kg−1) and three different 5-aminolevulinic acid levels (0, 12.5, 25 mg L−1) with three replicates. The significant difference between the values is at P < 0.05 which is shown by different letters
Antioxidant Enzyme Activities
The leaf and root enzymatic activities are shown in Fig. 5. The Cr stress potentially disturbs the enzymatic actives in maize plants. Cr stress (5 and 10 mg kg−1) reduced the activities of SOD, POD, and CAT in both roots and shoots of maize when compared to their relative controls; however, this reduction was maximum in the 9108 cultivar compared to 6103 which demonstrated that 9108 is a susceptive cultivar. The greatest reduction was noted at the 10 mg kg−1 Cr concentration in both varieties. Application of 25 mg L−1 ALA was found to be the best for significantly increasing the activities of SOD, POD, and CAT in both roots and shoots at all concentrations of Cr. The POD activities in plant roots increased by 25, 32, and 36% in Cr 0, 5, and 10 mg kg−1, respectively, at the ALA 25 mg L−1 level in 6103 and increased by 22, 29, and 39% in Cr 0, 5, and 10 mg kg−1, respectively, at the ALA 25 mg L−1 level in 9108.
Impact of chromium on SOD leaf and root (a, b), POD leaf and root (c, d), and CAT leaf and root (e, f) in two different hybrids of maize, one is tolerant (6103) and other is sensitive (9108), cultivated in soil along with three different chromium levels (0, 5 and 10 mg kg−1) and three different 5-aminolevulinic acid levels (0, 12.5, 25 mg L−1) with three replicates. The significant difference between the values is at P < 0.05 which is shown by different letters
Electrolyte Leakage, MDA, and H2O2 Contents
The oxidative stress parameters like EL, MDA, and H2O2 contents were analyzed to determine the oxidative stress in maize cultivars under different concentrations of Cr (Fig. 6). The results demonstrated that under Cr stress EL, MDA, and H2O2 contents markedly increased; however, this effect was maximum at 10 mg kg−1 Cr concentration. It was also noticed that EL, MDA, and H2O2 contents were high in 9108, indicating that 9108 is a Cr susceptive cultivar. Application of ALA minimized EL, MDA, and H2O2 contents in both cultivars at all concentration of Cr when compared to their relative controls. The EL contents in plant leaves were decreased by 15, 19, and 18% in Cr 0, 5, and 10 mg kg−1, respectively, at the ALA 25 mg L−1 level in 6103, and decreased by 12, 17, and 19% in Cr 0, 5, and 10 mg kg−1, respectively, at the ALA 25 mg L−1 level in 9108.
Impact of chromium on leaf EL (a), root EL (b), leaf MDA (c), root MDA (d), leaf H2O2 (e), and roots H2O2 (f) in two different hybrids of maize, one is tolerant (6103) and other is sensitive (9108), cultivated in soil along with three different chromium levels (0, 5, and 10 mg kg−1) and three different 5-aminolevulinic acid levels (0, 12.5, 25 mg L−1) with three replicates. The significant difference between the values is at P < 0.05 which is shown by different letters
Cr Concentration in Different Parts of Plant
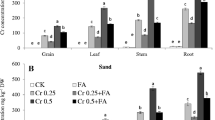
The endogenous concentration of Cr markedly increased in both parts of the maize plants with increasing exogenous Cr concentrations (Fig. 7). The values of Cr in leaves were 50.32 mg kg−1 in 6103 and in 9108 it was 54.66 mg kg−1 at the higher Cr concentration (10 mg kg−1). The Cr concentration was higher in roots as compared to the leaves in both cultivars. When foliar ALA was applied in both cultivars, it markedly decreased the Cr uptake in both parts of the plants such as, values of Cr in roots were recorded as 41.32 mg kg−1 in 6103 and in 9108 the values were noted as 48.65 mg kg−1 in comparison with the respective Cr concentrations alone.
Chromium uptake in leaves (a) and roots (b) in two different hybrids of maize, one is tolerant (6103) and other is sensitive (9108), cultivated in soil along with three different chromium levels (0, 5, and 10 mg kg−1) and three different 5-aminolevulinic acid levels (0, 12.5, 25 mg L−1) with three replicates. The significant difference between the values is at P < 0.05 which is shown by different letters
Discussion
Chromium treatments alone reduced the morpho-physiological parameters and dry weights in both cultivars (Figs. 1, 2). This reduction might be due to the inhibitory impacts of Cr on plant growth and biomass (Ahmad et al. 2017a, b; Farid et al. 2017; Hussain et al. 2018b). The reduction in plant biomass and growth has been noticed in various crops (Saleem et al. 2015; Tassi et al. 2017). However, ALA exogenous application potentially reduced the Cr toxic effect and improved plant biomass and growth in a dose-additive manner in both studied maize cultivars. Several studies reported the beneficial role of ALA under various stresses (Kosar et al. 2015; Ahmad et al. 2017a, b; Air et al. 2018). ALA exogenous application might have recovered cell injury due to Cr via reducing Cr mobility (Afshan et al. 2015; Farid et al. 2018).
Photosynthetic parameters, chlorophyll contents, and carotenoids were also reduced under Cr treatments alone as shown in Figs. 3 and 4. The reduction in photosynthetic parameters might be due to the lower efficiency to transport the water from roots to upper parts of plants due to Cr toxicity. This type of reduction in photosynthetic parameters has been described in plants under heavy metal stress (Shakoor et al. 2014; Farid et al. 2018). The reduction in chlorophyll contents and carotenoids might be due to chloroplast destruction (Mohanty et al. 1989; Balasaraswathi et al. 2017) or electron transport chain inhibition (Vassilev et al. 1995; Srivastava et al. 2018). Similar responses of Cr have been demonstrated in Brassica under Cr toxicity (Ali et al. 2015; Nafees et al. 2018). However, ALA exogenous application potentially reduced the Cr toxic effect and improved plant photosynthesis, chlorophyll contents, and carotenoids in both maize cultivars. ALA exogenous application might have improved water transport efficiency of plants and recovered chloroplast injury under stress (Gill et al. 2015; Herman et al. 2016).
Our results depicted that the activities of antioxidant enzymes were reduced, whereas reactive oxygen species were enhanced in both cultivars under Cr treatments alone (Figs. 5, 6). The reduction in the enzyme activities might be due to the overproduction of reactive oxygen species due to Cr toxicity (Tauqeer et al. 2016) and enhancement in reactive oxygen species might be due to plants poor defensive mechanism due to Cr toxicity (Farid et al. 2017). However, ALA exogenous application potentially reduced the Cr toxic effect and improved plant enzyme activities while scavenging reactive oxygen species in both maize cultivars. ALA exogenous application might have improved the plant self-defense system by promoting antioxidant enzyme activities and scavenging oxygen reactive species (Ali et al. 2015; Gill et al. 2015).
Both the uptake and translocation of Cr increased with increasing concentrations of Cr alone in a dose-additive manner both in leaves and roots of both maize cultivars (Fig. 6). Similar results have been found in various crops (Gill et al. 2015; Jabeen et al. 2016; Ahmad et al. 2017a, b) under Cr stress. However, ALA exogenous application potentially lowered the Cr uptake and translocation in both maize cultivars. This reduction in metal uptake is due to the ameliorative role of ALA towards heavy metals under stressful conditions (Gill et al. 2015; Farid et al. 2017). Moreover, ALA might have recovered plant injuries by improving their defense system. More Cr uptake was observed in 9108 as compared with 6103 which showed that 9108 is more sensitive towards Cr stress tolerance than 6103.
Conclusion
The Cr-only treatment markedly reduced plant growth, photosynthesis, antioxidant enzyme activities, and enhanced ROS contents in both cultivars. ALA exogenous application significantly reduced Cr-induced stress by reducing Cr translocation to shoots in both studied cultivars (9108–6103). ALA foliar application decreases the oxidative stress and increased the activities of antioxidant enzymes under Cr toxicity. Hence, exogenous application of ALA is beneficial to alleviate heavy metal toxicity in maize and probably in other plants. However, further studies are needed to understand the ALA detailed mechanisms under heavy metals stress in different plants species.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Afshan S, Ali S, Bharwana SA, Rizwan M, Farid M, Abbas F, Ibrahim M, Mehmood MA, Abbasi GH (2015) Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ Sci Pollut Res 22:11679–11689
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30:161–175
Ahmad P, Latef AA, Abd_Allah EF, Hashem A, Sarwat M, Anjum NA, Gucel S (2016) Calcium and potassium supplementation enhanced growth, osmolytes, secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum L.). Front Plant Sci 7:112
Ahmad P, Ahanger MA, Egamberdieva D, Alam P, Alyemeni MN, Ashraf M (2017a) Modification of osmolytes and antioxidant enzymes by 24-epibrassinolide in chickpea seedlings under mercury (Hg) toxicity. J Plant Growth Regul 37:309–322
Ahmad R, Ali S, Hannan F, Rizwan M, Iqbal M, Hassan Z, Akram NA, Maqbool S, Abbas F (2017b) Promotive role of 5-aminolevulinic acid on chromium-induced morphological, photosynthetic, and oxidative changes in cauliflower (Brassica oleracea botrytis L.). Environ Sci Pollut Res 24:8814–8824
Air T, Akram NA, Kausar S, Farid N, Ashraf M, AL-Qurainy FA (2018) 5-Aminolevulinic acid induces regulation in growth, yield and physio-biochemical characteristics of wheat under water stress. Sains Malays 47:661–670
Akram NA, Ashraf M (2013) Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J Plant Growth Regul 32:663–679
Ali B, Huang CR, Qi ZY, Ali S, Daud MK, Geng XX, Liu HB, Zhou WJ (2013a) 5-Aminolevulinic acid ameliorates cadmium-induced morphological, biochemical, and ultrastructural changes in seedlings of oilseed rape. Environ Sci Pollut Res 20:7256–7267
Ali B, Wang B, Ali S, Ghani MA, Hayat MT, Yang C, Xu L, Zhou WJ (2013b) 5-Aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity, and ultrastructural changes under cadmium stress in Brassica napus L. J Plant Growth Regul 32:604–614
Ali B, Xu X, Gill RA, Yang S, Ali S, Tahir M, Zhou W (2014) Promotive role of 5-aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Ind Crops Prod 52:617–626
Ali S, Chaudhary A, Rizwan M, Anwar HT, Adrees M, Farid M, Irshad MK, Hayat T, Anjum SA (2015) Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ Sci Pollut Res 22:10669–10678
Anjum SA, Ashraf U, Imran KH, Tanveer M, Shahid M, Shakoor A, Longchang WA (2017) Phyto-toxicity of chromium in maize: oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 27:262–273
Ashfaque F, Inam A, Iqbal S, Sahay S (2017) Response of silicon on metal accumulation, photosynthetic inhibition and oxidative stress in chromium-induced mustard (Brassica juncea L.). S Afr J Bot 111:153–160
Balasaraswathi K, Jayaveni S, Sridevi J, Sujatha D, Aaron KP, Rose C (2017) Cr-induced cellular injury and necrosis in Glycine max L.: biochemical mechanism of oxidative damage in chloroplast. Plant Physiol Biochem 118:653–666
Balestrasse KB, Tomaro ML, Batlle A, Noriega GO (2010) The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry 71:2038–2045
Bindu RC, Vivekanandan M (1998) Hormonal activities of 5-aminolevulinic acid in callus induction and micropropagation. Plant Growth Regul 26:15–18
Choudhary SP, Kanwar M, Bhardwaj R, Yu JQ, Tran LS (2012) Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS ONE 7:1–11
Czarnecki O, Gläßer C, Chen JG, Mayer KF, Grimm B (2012) Evidence for a contribution of ALA synthesis to plastid-to-nucleus signaling. Front Plant Sci 3:1–19
Daud MK, Mei L, Variath MT, Ali S, Li C, Rafiq MT, Zhu SJ (2014) Chromium(VI) uptake and tolerance potential in cotton cultivars: effect on their root physiology, ultramorphology, and oxidative metabolism. BioMed Res Int 2014:1–12
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Dwivedi SK, Arora A, Singh VP, Singh GP (2018) Induction of water deficit tolerance in wheat due to exogenous application of plant growth regulators: membrane stability, water relations and photosynthesis. Photosynthetica 56:478–486
Farid M, Ali S, Rizwan M, Ali Q, Abbas F, Bukhari SA, Saeed R, Wu L (2017) Citric acid assisted phytoextraction of chromium by sunflower; morpho-physiological and biochemical alterations in plants. Ecotoxicol Environ Saf 145:90–102
Farid M, Ali S, Rizwan M, Ali Q, Saeed R, Nasir T, Abbasi GH, Rehmani MI, Ata-Ul-Karim ST, Bukhari SA, Ahmad T (2018) Phyto-management of chromium contaminated soils through sunflower under exogenously applied 5-aminolevulinic acid. Ecotoxicol Environ Saf 151:255–265
Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Ali S, Zhou W (2015a) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164
Gill RA, Ali B, Islam F, Farooq MA, Gill MB, Mwamba TM, Zhou W (2015b) Physiological and molecular analyses of black and yellow seeded Brassica napus regulated by 5-aminolivulinic acid under chromium stress. Plant Physiol Biochem 94:130–143
Habiba U, Ali S, Hafeez F, Rizwan M, Rehman MZ, Hussain A, Asad SA (2018) Morpho-physiological responses of maize cultivars exposed to chromium stress. Int J Agric Biol. https://doi.org/10.17957/IJAB/15.0874
Han R, Gao G, Li Z, Dong Z, Guo Z (2018) Effects of exogenous 5-aminolevulinic acid on seed germination of alfalfa (Medicago varia Martyn.) under drought stress. Grassland Sci 64:100–107
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys 125:189–198
Herman S, Marco G, Cecilia B, Alfonso V, Luis M, Cristián V, Sebastián P, Sebastián A (2016) Effect of water availability on growth, water use efficiency and omega 3 (ALA) content in two phenotypes of chia (Salvia hispanica L.) established in the arid Mediterranean zone of Chile. Agric Water Manag 173:67–75
Hernández-Madrigal F, Ortiz-Castro R, Ruiz-Herrera LF, Cervantes C, López-Bucio J, Martínez-Trujillo M (2018) Sucrose protects arabidopsis roots from chromium toxicity influencing the auxin–plethora signaling pathway and improving meristematic cell activity. J Plant Growth Regul 37:530–538
Hussain A, Ali S, Rizwan M, Rehman MZ, Javed MR, Imran M, Chatha SA, Nazir R (2018a) Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ Pollut 242:1518–1526
Hussain A, Ali S, Rizwan M, Rehman MZ, Hameed A, Hafeez F, Alamri SA, Alyemeni MN, Wijaya L (2018b) Role of zinc–lysine on growth and chromium uptake in rice plants under Cr stress. J Plant Growth Regul 2018:1–10
Jabeen N, Abbas Z, Iqbal M, Rizwan M, Jabbar A, Farid M, Ali S, Ibrahim M, Abbas F (2016) Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch Agron Soil Sci 62:648–662
Jana S, Choudhuri MA (1981) Glycolate metabolism of three submersed aquatic angiosperms: effect of heavy metals. Aquat Bot 11:67–77
Keller C, Rizwan M, Davidian JC, Pokrovsky OS, Bovet N, Chaurand P, Meunier JD (2015) Effect of Silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta 241:847–860
Kishore PV, Madhuvarasu SS, Moru S (2018) Stimulus responsive hydrogel-coated etched fiber Bragg grating for carcinogenic chromium(VI) sensing. Opt Eng 57:017101
Kosar F, Akram NA, Ashraf M (2015) Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. S Afr J Bot 96:71–77
Liu T, Hu X, Zhang J, Zhang J, Du Q, Li J (2018) H2O2 mediates ALA-induced glutathione and ascorbate accumulation in the perception and resistance to oxidative stress in Solanum lycopersicum at low temperatures. BMC Plant Biol 18:34
Liu M, Li J, Niu J, Wang R, Song J, Lv J, Zong X, Wang S (2016) Interaction of drought and 5-aminolevulinic acid on growth and drought resistance of Leymus chinensis seedlings. Acta Ecol Sin 36:180–188
Mohanty N, Vass I, Demeter S (1989) Impairment of photosystem 2 activity at the level of secondary quinone electron acceptor in chloroplasts treated with cobalt, nickel and zinc ions. Physiol Plant 76:386–390
Naeem MS, Rasheed M, Liu D, Jin ZL, Ming DF, Yoneyama K, Takeuchi Y, Zhou WJ (2011) 5-Aminolevulinic acid ameliorates salinity-induced metabolic, water-related and biochemical changes in Brassica napus L. Acta Physiol Plant 33:517–528
Naeem MS, Warusawitharana H, Liu H, Liu D, Ahmad R, Waraich EA, Xu L, Zhou W (2012) 5-Aminolevulinic acid alleviates the salinity-induced changes in Brassica napus as revealed by the ultrastructural study of chloroplast. Plant Physiol Biochem 57:84–92
Nafees M, Ali S, Naveed M, Rizwan M (2018) Efficiency of biogas slurry and Burkholderia phytofirmans PsJN to improve growth, physiology, and antioxidant activity of Brassica napus L. in chromium-contaminated soil. Environ Sci Pollut Res 25:6387–6397
Oh YJ, Song H, Shin WS, Choi SJ, Kim YH (2007) Effect of amorphous silica and silica sand on removal of chromium(VI) by zero-valent iron. Chemosphere 66:858–865
Pei ZF, Ming DF, Liu D, Wan GL, Geng XX, Gong HJ, Zhou WJ (2010) Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J Plant Growth Regul 29:106–115
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Qayyum MF, Rehman MZ, Ali S, Rizwan M, Naeem A, Maqsood MA, Khalid H, Rinklebe J, Ok YS (2017) Residual effects of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere 174:515–523
Ranieri E, Gikas P (2014) Effects of plants for reduction and removal of hexavalent chromium from a contaminated soil. Water Air Soil Pollut 225:1981
Rehman MZ, Rizwan M, Ghafoor A, Naeem A, Ali S, Sabir M, Qayyum MF (2015) Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ Sci Pollut Res 22:16897–16906
Rehman MZ, Rizwan M, Ali S, Fatima N, Yousaf B, Naeem A, Sabir M, Ahmad HR, Ok YS (2016) Contrasting effects of biochar, compost and farm manure on alleviation of nickel toxicity in maize (Zea mays L.) in relation to plant growth, photosynthesis and metal uptake. Ecotoxicol Environ Saf 133:218–225
Rizwan M, Meunier JD, Davidian JC, Pokrovsky OS, Bovet N, Keller C (2016) Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ Sci Pollut Res 23:1414–1427
Rizwan M, Ali S, Qayyum MF, Ok YS, Rehman MZ, Abbas Z, Hannan F (2017) Use of maize (Zea mays L.) for phytomanagement of Cd-contaminated soils: a critical review. Environ Geochem Health 39:259–277
Saleem M, Asghar HN, Khan MY, Zahir ZA (2015) Gibberellic acid in combination with pressmud enhances the growth of sunflower and stabilizes chromium(VI)-contaminated soil. Environ Sci Pollut Res 22:10610–10617
Shakoor MB, Ali S, Hameed A, Farid M, Hussain S, Yasmeen T, Najeeb U, Bharwana SA, Abbasi GH (2014) Citric acid improves lead (Pb) phytoextraction in Brassica napus L. by mitigating Pb-induced morphological and biochemical damages. Ecotoxicol Environ Saf 109:38–47
Srivastava RK, Rajpoot R, Pandey P, Rani A, Dubey RS (2018) Cadmium alters mitochondrial membrane potential, inhibits electron transport chain activity and induces callose deposition in rice seedlings. J Plant Growth Regul 37:335–344
Stambulska UY, Bayliak MM, Lushchak VI (2018) Chromium(VI) toxicity in legume plants: modulation effects of rhizobial symbiosis. BioMed Res Int 2018:1–10
Tassi E, Giorgetti L, Morelli E, Peralta-Videa JR, Gardea-Torresdey JL, Barbafieri M (2017) Physiological and biochemical responses of sunflower (Helianthus annuus L.) exposed to nano-CeO2 and excess boron: modulation of boron phytotoxicity. Plant Physiol Biochem 110:50–58
Tauqeer HM, Ali S, Rizwan M, Ali Q, Saeed R, Iftikhar U, Ahmad R, Farid M, Abbasi GH (2016) Phytoremediation of heavy metals by Alternanthera bettzickiana: growth and physiological response. Ecotoxicol Environ Saf 126:138–146
Tripathi DK, Singh VP, Kumar D, Chauhan DK (2012) Impact of exogenous silicon addition on chromium uptake, growth, mineral elements, oxidative stress, antioxidant capacity, and leaf and root structures in rice seedlings exposed to hexavalent chromium. Acta Physiol Plant 34:279–289
Vassilev A, Iordanov I, Chakalova E, Kerin V (1995) Effect of cadmium stress on growth and photosynthesis of young barley (H. vulgare L.) plants and structural and functional changes in the photosynthetic apparatus. Bulg J Plant Physiol 21:2–21
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Wang LJ, Jiang WB, Huang BJ (2004) Promotion of 5-aminolevulinic acid on photosynthesis of melon (Cucumis melo) seedlings under low light and chilling stress conditions. Physiol Plant 121:258–264
Wang LJ, Jiang WB, Liu H, Liu WQ, Kang L, Hou XL (2005) Promotion by 5-aminolevulinic acid of germination of pakchoi (Brassica campestris ssp. chinensis var. communis Tsen et Lee) seeds under salt stress. J Integ Plant Biol 47:1084–1091
Wang Q, Liang X, Dong Y, Xu L, Zhang X, Hou J, Fan Z (2013) Effects of exogenous nitric oxide on cadmium toxicity, element contents and antioxidative system in perennial ryegrass. Plant Growth Regul 69:11–20
Wu LE, Levina A, Harris HH, Cai Z, Lai B, Vogt S, James DE, Lay PA (2016) Carcinogenic chromium(VI) compounds formed by intracellular oxidation of chromium(III) dietary supplements by adipocytes. Angew Chem Int Ed 55:1742–5174
Wu S, Hu Y, Zhang X, Sun Y, Wu Z, Li T, Lv J, Li J, Zhang J, Zheng L, Huang L (2018) Chromium detoxification in arbuscular mycorrhizal symbiosis mediated by sulfur uptake and metabolism. Environ Exp Bot 147:43–52
Xiaomeng LI, Li ZH, Qiling SO, Chang J, Jiabao YE, Zhang W, Yongling LI, Feng XU (2018) Effects of 5-aminolevulinic acid on the photosynthesis, antioxidant system, and α-bisabolol content of Matricaria recutita. Notulae Bot Hortic Agrobotanici Cluj-Napoca 46:418–425
Xiong JL, Wang HC, Tan XY, Zhang CL, Naeem MS (2018) 5-Aminolevulinic acid improves salt tolerance mediated by regulation of tetrapyrrole and proline metabolism in Brassica napus L. seedlings under NaCl stress. Plant Physiol Biochem 124:88–99
Yiu JC, Juang LD, Fang DY, Liu CW, Wu SJ (2009) Exogenous putrescine reduces flooding-induced oxidative damage by increasing the antioxidant properties of Welsh onion. Sci Hortic 120:306–314
Zhang XZ (1992) The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In: Research methodology of crop physiology. Agriculture Press, Beijing, pp 208–211
Zhang J, Li D-M, Gao Y, Yu B, Xia C-X, Bai J-G (2012) Pretreatment with 5-aminolevulinic acid mitigates heat stress of cucumber leaves. Biol Plantarum 56:780–784
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35:785–791
Acknowledgements
This study was funded by Higher Education Commission (HEC) Islamabad, Pakistan (IPFP/HRD/HEC/2014/1035) and Government College University Faisalabad, Pakistan. The authors would also like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding to the Research Group number (RG-1435-014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors do not have any conflicts of interest.
Rights and permissions
About this article
Cite this article
Habiba, U., Ali, S., Rizwan, M. et al. The Ameliorative Role of 5-Aminolevulinic Acid (ALA) Under Cr Stress in Two Maize Cultivars Showing Differential Sensitivity to Cr Stress Tolerance. J Plant Growth Regul 38, 788–798 (2019). https://doi.org/10.1007/s00344-018-9890-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9890-z