Abstract
The continuous growth and development of population need more fresh water for drinking, irrigation, and domestic in arid countries like Egypt. Evaluation the quality of groundwater is an essential study to ensure its suitability for different purposes. In this study, 812 groundwater samples were taken within the middle area of Upper Egypt (Sohag Governorate) to assess the quality of groundwater for drinking and irrigation purposes. Eleven water parameters were analyzed at each groundwater sample (Na+, K+, Ca2+, Mg2+, HCO3 − SO4 2−, Fe2+, Mn2+, Cl−, electrical conductivity, and pH) to exploit them in water quality evaluation. A classical statistics were applied for the raw data to examine the distribution of physicochemical parameters in the investigated area. The relationship between groundwater parameters was tested using the correlation coefficient where a strong relationship was found between several water parameters such as Ca2+ and Cl−. Water quality index (WQI) is a mathematical model used to transform many water parameters into a single indicator value which represents the water quality level. Results of WQI showed that 20% of groundwater samples are excellent, 75% are good for drinking, and 7% are very poor water while only 1% of samples are unsuitable for drinking. To test the suitability of groundwater for irrigation, three indices are used; they are sodium adsorption ration (SAR), sodium percentage (Na%), and permeability index (PI). For irrigation suitability, the study proved that most sampling sites are suitable while less than 3% are unsuitable for irrigation. The spatial distribution of the estimated values of WQI, SAR, Na%, PI, and each groundwater parameter was spatially modeled using GIS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is used for domestic, industrial water supply, and irrigation all over the world. Egypt is located within the arid zone of north Africa where the freshwater quantity is limited and mainly extracted from the river Nile (Ahmed and Ali 2011). It supplies about 97% of the annual renewable water resources in Egypt (Walaa 2014). The groundwater represents the second source for the freshwater in the study area that is originating mainly from the surface water bodies. Egypt relies heavily on surface water from the Nile river, with an annual quota of 55.5 billion m3 year−1 allocated according to the 1959 agreement between Egypt and Sudan (Hassan and Rasheedy 2007). Currently, the total annual water demands of all socio-economic sectors in Egypt are estimated to be 76 billion m3 year−1, while the total quantity of water from all sources is estimated as 65 billion m3 year−1 (Walaa 2014; MaCalister et al. 2012; Farrag 2005). Therefore, surface Nile water is insufficient to meet all the Egyptian’s demands due to increasing of the population, new cities, and new projects. Also, the Grand Ethiopian Renaissance Dam (GERD) constructed on the Blue Nile is expected to increase the water crisis in Egypt. The GERD has an active storage capacity of approximately 74 billion m3 and the energy reduction from High Aswan Dam is expected to reach 12% from the total annual energy output by the dam during the filling of GERD, while the losses of water will reach to 22% of the coming water to Egypt (Mulat and Moges 2014). So, groundwater can partially help in providing some of the new projects by the significant amount of fresh water. Urbanization, industrialization, and irrigation system in Egypt have affected the availability and quality of groundwater due to its overexploitation and improper waste disposal. Once the groundwater is contaminated, its quality cannot be restored by stopping the pollutants from the source (Ramakrishnaiah et al. 2009). Therefore, assessment of water quality is very important for decision making and water organization.
Water quality index (WQI) measures the quality of water for drinking and other purposes. It transfers several water parameters to give only a single number to assess the overall water quality at a certain location and time (Boateng et al. 2016; Akter et al. 2016). WQI is a valuable and unique rating to depict the overall water quality status in a single term that helps for selecting proper treatment technique. It was used in several studies to assess surface water quality (Ravikumar et al. 2013), groundwater (Gebrehiwot et al. 2011; Rajankar et al. 2010), and river’s bodies (Trivedi et al. 2009; Singh et al. 2008).
There are many indices used to examine the quality of water for irrigation; they are sodium adsorption ratio (SAR), sodium percentage (Na%), and permeability index (PI) (Subramani et al. 2005). SAR tests the excessive of sodium content relative to the calcium and magnesium which reduces the soil permeability and thus inhibits the supply of water needed for the crops (Aly et al. 2015). The Na% in soil is considered imperative for groundwater solubility for irrigation purpose because it reduces the soil permeability after reacting and supports little or no plant growth. PI is a significant parameter for the irrigation water suitability and affects soil permeability.
GIS is a powerful tool to assess water quality parameters, to figure water availability, to understand the natural environment, and to manage water resources (Singh and Khan 2011). Spatial analysis extension of GIS allows interpolation of the groundwater quality parameters at unknown location from known values to create a continuous surface which helps us to understand the distribution of water quality parameters of the study area (Shabbir and Ahmad 2015; Sumathi et al. 2008). There are various interpolation techniques such as inverse distance weighted (IDW), spline, trend surface analysis, and kriging available in ArcGIS 10 (Johnston et al. 2001).
Ahmed (2007) used DRASTIC GIS-based model to assess the vulnerability of contamination in the investigated area. Ahmed and Ali (2011) used geochemical modeling techniques and GIS to discuss the integrated role of geochemical processes, agriculture, and urbanization in the evaluation of groundwater composition and their impacts on groundwater quality in Sohag Governorates. Tahlawi et al. (2014) used several water quality indices to assess the suitability of groundwater for different purposes. Their study was applied on Assiut Governorate at the north of the investigated area that has similar hydrogeological and aquifer structure. Geochemical mobilization of heavy metals in water has been studied by Melegy et al. (2014) as an important reason for many diseases of Sohag Governorate. They collected 42 groundwater samples from the Quaternary aquifer and eight samples from surface water of Sohag Governorate.
The objectives of this study were to (1) assess the groundwater quality for drinking purposes using WQI, to (2) evaluate the sampling sites for irrigation purposes, to (3) model the spatial distribution of water parameters using GIS, and to (4) find the locations of the most unsuitable water.
Materials and methods
Study area
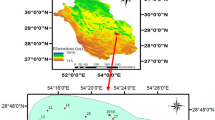
This study is applied on a district in Upper Egypt at Sohag Governorate as illustrated in Fig. 1. The area is located between 26.907 N, 31.288 E and 26.59 N, 31.653 E. It is located at 400 km south of Cairo and includes four main regions, from south to north, ElMaragha, Tahta, and Tema regions and Gehayna region at the south of Tahta. The Nile valley aquifer system is assigned to the Quaternary and Late Tertiary and occupies the Nile floodplain and desert fringes (Farrag 2005; El Arabi 2012). The Quaternary aquifer system in the study area is formed by the alluvial deposits of the Nile and consists of two layers as given in Fig. 1b. The upper layer is the clay-silt layer, which has low horizontal and vertical permeability. This layer is laterally extensive, having the greater thickness near the river channel and vanishing near the valley fringes where it is overlain by desert sands. The lower layer is the graded sand member which forms the main aquifer having high horizontal and vertical permeability. The lower boundary of the aquifer is impervious due to the presence of extensive and thick deposits of the Pliocene clays of very low permeability (Ahmed and Ali 2011; Youssef et al. 2011; Farrag 2005). The investigated subsurface lithologic well logs for the study area indicated that the area is composed of six categories which are clay, clay and sand, fine sand, coarse sand, sand and gravel, and gravel (Ahmed 2009).
According to land use, the study area can be divided to three regions, old agricultural region (located near the Nile river and water canals), reclaimed region (located at both sides of Nile valley with great extension at the west area), and desert fringes at both sides of the study area as illustrated in Fig. 1. Agriculture is the main activity of the study area but in some locations, food and cement industries may be found. Most of the population in the investigated area has no sewage system. Wastewater is disposed individually into the ground by digging a covered hole in houses. The main wastewater disposal stations are installed within the west regions of the research area.
To evaluate the groundwater quality in the study area, 812 groundwater samples were taken from the several purposes wells, most of them for irrigation and drinking. Groundwater wells have depths varying from 27 to 120 m. The groundwater samples were collected and analyzed by the sector of groundwater irrigation in Upper Egypt, belonged to Ministry of Water Resources and Irrigation.
The groundwater samples were chemically analyzed to check the quality of groundwater and sources of pollution within the study area. The measurements of physiochemical parameters pH and electrical conductivity (EC) were carried out in the field using portable instruments. Eleven water parameters were measured for each groundwater sample; they are sodium, potassium, calcium, magnesium, bicarbonates, sulfate, iron, manganese, chloride, electrical conductivity, and pH.
Water quality index
WQI is used to evaluate the quality of groundwater for drinking purposes (Rajankar et al. 2010; Kumar et al. 2007). WQI can be calculated in this study to assign groundwater quality using the 11 measured parameters at each site. The groundwater limits were assigned weights (wi) from 1 to 4 according to its impact degree of this contamination on the human health. The first step for WQI estimation is to estimate the relative weight of each parameter, as given in Eq. (1) (Shabbir and Ahmad 2015).
where w i is the weight of each parameter, W i is its relative weight, and n is the number of groundwater parameters. The next step is to estimate quality rating scale (q i ) of each parameter using Eq. (2) (Singh and Khan 2011; Srinivas et al. 2011).
where q i is the quality rating for the i water parameter, V i is the measured value for the i parameter at a given sampling site, and S i is the standard permissible value for the i parameter assigned by WHO (Vasanthavigar et al. 2010). V id is the ideal value of i parameter in pure water (i.e., 0 for all other parameters except the parameter pH is 7). The overall water quality index can be calculated by aggregating the quality rating with the unit weight using Eq. (3).
Water quality for irrigation purposes
The amount of mineral components in water affects the quality of soil and plants and the suitability of groundwater for irrigation purposes. Water quality for irrigation can be tested using SAR, Eq. (4) (Wilcox and L.V. 1955); Na%, Eq. (5) (Ayers and Westcot 1985; Shabbir and Ahmad 2015); and PI, Eq. (6) (Kumar et al. 2007), with concentrations expressed in milliequivalents of solute per liter of solvent in the following three formulas.
Results and discussion
Spatial distribution of groundwater parameters
Statistical analysis of groundwater specifications was developed to evaluate the ranges of chemical parameters and explore their deviation from WHO standards as produced in Table 1 (Organization WH 2011). The measurement unit of all water parameters is milligrams per liter except EC in microsiemens per centimeter and pH without unit. It is noticed that the maximum value for all parameters of groundwater is exceeding than the acceptable limits but the mean value of calcium, magnesium, bicarbonates, chloride, and EC is greater than the guideline values assigned by WHO.
Sodium salts are found in all food and drinking water. No firm conclusions can be drawn about the possible association between sodium in drinking water and the occurrence of hypertension. However, concentrations more than 200 mg/l as assigned by WHO may give rise to unacceptable taste. The concentration of sodium within the study area begins from 10 mg/l as the smallest value to 1254 mg/l as the greatest value. There are 200 sites of groundwater in which sodium is exceeding than the WHO limits. Most of these sites are located within the reclaimed and desert regions at the west of the investigated area. High concentration of sodium may be attributed to ion exchange and leaching of sodium salts such as halite during the movement of groundwater through sediments. Also, lack of sewage system has increased the concentration of sodium in groundwater within the old cultivated lands. Spatial distribution of sodium in the study area is developed using ArcGIS 10 and illustrated in Fig. 2a.
Potassium concentration varies from 0.2 to 53 mg/l with an average value of 4 mg/l, while the WHO limit is 12 mg/l. The application zone can be expressed as low potassium concentrations as there are only 12 sampling stations (1.5% of the total samples) increasing than 12 mg/l, while only one water sample is higher than 50 mg/l. Spatial distribution of potassium using ArcGIS is presented in Fig. 2b. Values greater than 12 mg/l are not noticed at the predicted map of K+ as its number of sampling sites is sparse.
Calcium is one of the most abundant substances in the fresh water. Dissolve Ca2+ and Mg2+ in water are the two most common minerals that make water hardness. The minimum concentration of calcium in the study area is 1.6 mg/l while the maximum value is 806 mg/l. The WHO limit is 75 mg/l; there are 46% of the sampling sites exceeding than the permissible level. The great concentration of calcium was found in the west zone of the study area and the most north of the area. The source of calcium in groundwater is the dissolution of carbonate minerals such as calcite and dolomite (Ca Mg(CO3)2) of the limestone rocks. Spatial distribution using IDW method is applied to develop the allocation of calcium in the investigated area and given in Fig. 2c.
Magnesium is directly related to hardness. Magnesium content in this study is ranging from 4.8 mg/l to 330 mg/l and average value 55 mg/l. There are only 35 measured values exceeding the WHO limit (150 mg/l). High concentration of Mg2+ is belonging to soluble from rocks such as dolomite both in the west fringe and old lands of Nile valley. Spatial distribution of Mg2+ is illustrated in Fig. 2d.
Sulfate exists in natural water in a concentration ranging from a few to several thousands of milligrams per liter. Excess sodium sulfate should not be present in drinking water as they cause cathartic action. The value of sulfate in this research was found in the range of 14 to 810 mg/l with average value 147 mg/l. Fifteen percent of water samples are exceeding than the permissible level assigned by WHO (250 mg/l). Spatial distribution of sulfate is formed using ArcGIS 10 and showed in Fig. 2f, where significant values are located in the west of the scanning zone. Higher values of sulfates in groundwater at the newly reclaimed lands may be due to the dissolution of gypsum and potassium sulfates added to the soil as fertilizers.
Iron is an essential element in human nutrition. Taste of water is not usually noticeable at iron concentrations below 0.3 mg/l; also, laundry and sanitary ware will stain at iron concentrations above 0.3 mg/l (Vasudevan et al. 2009). Iron concentrations in the study area are ranging between 0.001 and 3 mg/l. Significant concentrations are located at many batches within the subject zone as given in the spatial distribution of Fe in Fig. 3a.
Manganese is an essential element for many living organisms, including human, but adverse health effects can be caused by inadequate intake or overexposure. Concentrations below 0.05 mg/l are usually acceptable to consumers (Organization WH 2004). The minimum measured value of Mn2+ was 0.001 mg/l while maximum value was 3.5 mg/l with an average of 0.137 mg/l. Also, the spatial distribution of Mn2+ is given in Fig. 3b. The Pleistocene deposits beneath the cultivated floodplain of the Nile were containing abundant ferromagnesian minerals representing the source of Fe and Mn in the groundwater (Melegy et al. 2014).
Chloride concentration of the groundwater samples is varying from 11.9 to 2578.7 mg/l. Chloride is an important parameter in assessing groundwater quality where the higher concentration of chloride indicates a higher grade of organic pollutants (Singh and Khan 2011). Three quarters of Egyptian villages do not have sewage systems according to the latest survey made by the Central Agency for Public Mobilization and Statistics (CAPMAS), reported in September 2016 (Ahramonline 2016). The poor sewage system in Egypt contributes to groundwater contamination. Therefore, high values of Chloride may be due to contamination from sewage and fertilizer systems. Spatial distribution of Cl− is given in Fig. 3c. High concentrations of chloride are found in the newly reclaimed regions where the new wastewater disposal stations were installed.
One of the most important water parameters is pH which measures the degree of alkalinity or acidity of the groundwater. The pH scale is logarithmic and runs from 0 to 14. As the pH decreases than 3.5, water becomes more acidic. There is no value less than the lower permissible limit (3.5), so acidity of water is not encountered within the study area. In this study, pH is distributed from 5.7 least value and extending to 10.17 as the greatest measured value while mean value is 7.8. Values of pH above 8.5 are often caused by high bicarbonate (HCO3−). High carbonates cause calcium and magnesium ions to form insoluble minerals, leaving sodium as the dominant ion in solution. There are 30 locations at which pH is increasing than WHO limit. Inspection the values of carbonates at these sites proved that the minimum value of carbonates is 146 mg/l that increases the WHO limit. Spatial distribution of pH is created using ArcGIS as given in Fig. 3d. From this figure, we notice that high level of pH is located at the west of the investigated zone.
EC is a measure of water capacity to convey electric current. It signifies the amount of total dissolved salts. The EC values in the study area vary between 330 to 17,000 μS/cm with an average value of 2477 μS/cm. High EC values were observed for half of sampling sites which indicate the presence of high amount of dissolved inorganic substances in these locations.
Spatial distribution of EC was provided and given in Fig. 3e, showing that high concentrations are located in the west and south of the investigated zone.
The correlation coefficient was scanned between the groundwater parameters to investigate the degree of correlation between them that was given in Table 2. A strong correlation (r ≥ 0.8) is found between Ca+2 and Cl− while moderate correlation is noticed between several water specifications such as between Ca2+ and Mg2+, and Cl− and Mg2+. From this analysis, we can conclude that the sources of Ca, Mg, and Cl are the same. Spatial distribution of the three elements as given in Figs. 2c, d and 3c proved that the distribution of them occupied the same regions. The source of Ca and Mg in groundwater is the dissolution of carbonate minerals such as calcite and dolomite. Also, dissolution of gypsum from fertilizer system enriches the groundwater with calcium.
WQI results and evaluation
For WQI estimation, three steps were established. In the first step, each of the 11 parameters has been assigned a weight (w i ) based on their known effects on primary health as given in Table 3. Weighted values are appointed for each water parameter ranging from 2 to 4. The greatest weight (4) was selected to water parameters such as chloride and sulfate due to their major importance in water quality assessment. Bicarbonate was given the smallest weight (2) as it plays an insignificant role in the water quality assessment. In the second step, the relative weight (W i ) of each parameter is computed using Eq. (1).
A simple Matlab program was developed to estimate WQI at each sampling site and the classical statistics of the result is summarized and sorted into five classes (Sadat-Noori et al. 2014) as presented in Table 4. The first class is the excellent water with WQI less than 50, while the worst class (V) means that water is unsuitable for drinking (WQI is greater than 300). Calculations of WQI showed that 20% of the sampling sites are excellent water and healthy for drinking. Also, more than 45% of the tested water are good water for drinking, while 33% of the sampling sites are poor to very poor water. There are only nine sites of the sampling water at where groundwater is unsuitable for drinking. The spatial distribution of WQI is developed and illustrated in Fig. 3f, while the nine sites of unsuitable groundwater for drinking are presented in Fig. 4. From this figure, we can see that unsuitable water is existing within the southwest zone of the investigated area.
Assessment of groundwater for irrigation
To explore the conditions of groundwater for irrigation purposes, three quality indices were estimated for each sampling site using Eqs. (4), (5), and (6). The results of SAR, Na%, and PI are classified and tabulated in Tables 5, 6, and 7, respectively. According to the results, most of the groundwater sites are suitable and good for irrigation. There are 3.6% of the sampling data unsuitable for irrigation according to SAR, 14 samples are unsuitable according to Na%, while 28 samples are unsuitable for irrigation corresponding to PI. These sites are illustrated in Fig. 4.
From Fig. 4, we note that poor groundwater is located between two wastewater stations, Tahta and El-Dair, at the southwest of the investigated area. The raw wastewater at the two stations is accumulating on the ground surface forming large uncontrolled ponds as shown in Fig. 5 (Youssef et al. 2011). Also, no buffer zones are created around these wastewater ponds, and farmers have illegally irrigated their crops using this wastewater. Also, the region is located at the newly reclaimed lands which consist of a thick layer of sandy and gravelly sediments covered with a thin layer of recent wadi deposits (sandy gravel) (El-Haddad and El-Shater 1988; Ibrahim et al. 2010). The sediments have a high vertical permeability that facilitates the infiltration of pollutants downward and reach to the groundwater.
Wastewater ponds in El-Dair station with bio-solids (Youssef et al. 2011)
Conclusion
In the present research, an attempt was carried out to evaluate and to outline the groundwater quality in the north part of Sohag Governorate, Egypt. The predominant cation trend in the study area is Na+ > Ca+2 > Mg+2 > K+; they are 115 ± 131, 70 ± 97, 65 ± 43, and 5.3 ± 3.3 mg/l, respectively, while, the abundance of the major anions is in the following order: Cl− > HCO3 − > SO4 − with values of 239 ± 132, 169 ± 374, and 119 ± 106 mg/l, respectively.
Spatial distribution of each groundwater parameter was developed and showed that high concentrations are located in the west and southwest zones of the surveyed field. Evaluation of groundwater quality for using WQI showed that almost 65% of the sampling sites are good water while the rest of water ranges between poor to very poor water for drinking. There are only nine water samples unsuitable for drinking purposes. Assessment of groundwater for irrigation purposes enhanced the first part of this study. As the estimated values of SAR, Na%, and PI proved that the sampling water is suitable for agriculture purpose, only less than 3% of sampling sites are unsuitable for agriculture. These sites are concentrated also within the southwest zone of the investigated area.
The plateau bounding the Nile valley on the west side consists of the lower Eocene rocks (thin-bedded limestone with chert bands and flint nodules) that increases the concentration of several salts in groundwater at the west regions. Also, the four sites of wastewater disposal designated within west regions increase the contaminations in the groundwater. At the Nile valley (old agricultural lands), lack of sewage system especially at villages regions has also negative impacts on the quality of groundwater, also the bedrocks of the Quaternary aquifer enrichment the water with some salts.
We can generally say that groundwater in the study area at the time of investigation is valid for drinking and irrigation except the hot spot located in the southwest zone. At this area, groundwater is severely contaminated due to the wastewater disposal stations of Tahta and El-Dair. Also, the natural of the unconfined aquifer in this contaminated region enhanced the infiltration of pollutants to groundwater.
References
Ahmed AA, Ali MH (2011) Hydrochemical evolution and variation of groundwater and its environmental impact at Sohag. Egypt Arabian Journal of Geosciences 4:339–352. doi:10.1007/s12517-009-0055-z
Ahmed AA (2009) Using lithologic modeling techniques for aquifer characterization and groundwater flow modeling of the Sohag area. Egypt Hydrogeology Journal 17:1189–1201. doi:10.1007/s10040-009-0461-z
Ahmed AA (2007) Using contamination and pesticide DRASTIC GIS-based models for vulnerability assessment of Quaternary aquifer at Sohag, Egypt. In: The 5th Intern. Conf Geol Africa (1), pp 29–47
Akter T et al (2016) Water quality index for measuring drinking water quality in rural Bangladesh: a cross-sectional study. J Health Popul Nutr 35:4–4. doi:10.1186/s41043-016-0041-5
Aly AA, Al-Omran AM, Alharby MM (2015) The water quality index and hydrochemical characterization of groundwater resources in Hafar Albatin. Saudi Arabia Arabian Journal of Geosciences 8:4177–4190
Ahramonline J (2016) 3/4 of Egyptian villages lack sewage systems: CAPMAS. http://english.ahram.org.eg/NewsContent/1/64/177602/Egypt/Politics-/-of-Egyptian-villages-lack-sewage-systems-CAPMAS.aspx
Awad MA, Nada AA, Hamza MS, Froehlich K (1995) Chemical and isotopic investigation of groundwater in Tahta region. Sohag-Egypt Environmental Geochemistry and Health 17:147–153. doi:10.1007/bf00126083
Ayers RS, Westcot DW (1985) Water quality for agriculture vol 29. Food and Agriculture Organization of the United Nations Rome
Boateng TK, Opoku F, Acquaah SO, Akoto O (2016) Groundwater quality assessment using statistical approach and water quality index in Ejisu-Juaben Municipality. Ghana Environmental Earth Sciences 75:1–14. doi:10.1007/s12665-015-5105-0
El Arabi N (2012) Environmental management of groundwater in Egypt via artificial recharge extending the practice to soil aquifer treatment (SAT) vol 1. 2012, vol 3.
El-Haddad A, El-Shater A (1988) Sediments characteristics as a controlling factor of pollution of the groundwater from disposed of wastes, Sohag, Egypt. Sohag Pure & Appl Sci Bull Fac Sci 4:145–162
Farrag Ah (2005) The hydraulic and hydrochemical impacts of the nile system on the groundwater in Upper Egypt vol 8, March 2005 edn. Ass. Univ. Bull. Environ. Res., Ass. Univ
Gebrehiwot AB, Tadesse N, Jigar E (2011) Application of water quality index to assess suitability of groundwater quality for drinking purposes in Hantebet watershed. Tigray, Northern Ethiopia ISABB Journal of Food and Agriculture Science 1:22–30
Hassan HA, Rasheedy AA (2007) The Nile River and Egyptian Foreign Policy Interest African Sociological Review/Revue Africaine de Sociologie 11
Ibrahim M, Ali M, Kotb M (2010) Soil properties as affected by different land management practices in the Sohag Region. South Egypt New York Science Journal 3:8–19
Johnston K, Ver Hoef JM, Krivoruchko K, Lucas N (2001) Using ArcGIS geostatistical analyst vol 380. Esri Redlands
Kumar M, Kumari K, Ramanathan A, Saxena R (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab. India Environmental Geology 53:553–574. doi:10.1007/s00254-007-0672-3
MaCalister C, Ie PP, Tindimugaya C, Ayenew T, Ibrahim ME, Meguid MA (2012) Overview of groundwater in the Nile river basin
Melegy AA, Shaban AM, Hassaan MM, Salman SA (2014) Geochemical mobilization of some heavy metals in water resources and their impact on human health in Sohag Governorate. Egypt Arabian Journal of Geosciences 7:4541–4552. doi:10.1007/s12517-013-1095-y
Mulat AG, Moges SA (2014) Assessment of the impact of the Grand Ethiopian Renaissance Dam on the performance of the High Aswan Dam Journal of Water Resource and Protection 2014
Organization WH (2004) Manganese in drinking-water: Background document for development of WHO Guidelines for Drinking-water Quality
Organization WH (2011) Guidelines for drinking-water quality. World Health Organization, Geneva
Rajankar PN, Tambekar DH, Wate SR (2010) Groundwater quality and water quality index at Bhandara District. Environ Monit Assess 179:619–625. doi:10.1007/s10661-010-1767-y
Ramakrishnaiah C, Sadashivaiah C, Ranganna G (2009) Assessment of water quality index for the groundwater in Tumkur Taluk. Karnataka State, India Journal of Chemistry 6:523–530
Ravikumar P, Aneesul Mehmood M, Somashekar RK (2013) Water quality index to determine the surface water quality of Sankey tank and Mallathahalli lake, Bangalore urban district, Karnataka, India. Appl Water Sci 3:247–261. doi:10.1007/s13201-013-0077-2
Sadat-Noori SM, Ebrahimi K, Liaghat AM (2014) Groundwater quality assessment using the Water Quality Index and GIS in Saveh-Nobaran aquifer, Iran. Environ. Earth Sci. 71(9):3827–3843. doi:10.1007/s12665-013-2770-8
Shabbir R, Ahmad SS (2015) Use of geographic information system and water quality index to assess groundwater quality in Rawalpindi and Islamabad Arabian. Journal for Science and Engineering 40:2033–2047. doi:10.1007/s13369-015-1697-7
Singh AK, Mondal G, Kumar S, Singh T, Tewary B, Sinha A (2008) Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin. India Environmental geology 54:745–758
Singh P, Khan I (2011) Ground water quality assessment of Dhankawadi ward of Pune by using GIS. International Journal of Geomatics and Geosciences 2:688–703
Srinivas P, Pradeep Kumar G, Srinivatas Prasad A, Hemalatha T (2011) Generation of groundwater quality index map—a case study. Civil and Environmental Research 1:9–21
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110. doi:10.1007/s00254-005-1243-0
Sumathi VR, Natesan U, Sarkar C (2008) GIS-based approach for optimized siting of municipal solid waste landfill Waste Management 28:2146–2160 doi:http://dx.doi.org/10.1016/j.wasman.2007.09.032
Tahlawi ME, Mohamed M, Boghdadi G, Rabeiy R, Saleem H (2014) Groundwater quality assessment to estimate its suitability for different uses in Assiut Governorate, Egypt https://www.researchgate.net/publication/305688706
Trivedi P, Bajpai A, Thareja S (2009) Evaluation of water quality: physico–chemical characteristics of Ganga River at Kanpur by using correlation study. Nature and Science 1:91–94
Vasanthavigar M et al (2010) Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess 171:595–609
Vasudevan S, Lakshmi J, Sozhan G (2009) Studies on the removal of iron from drinking water by electrocoagulation–A clean process. CLEAN–Soil, Air, Water 37(1):45–51
Walaa E (2014) Groundwater management in Egypt vol 11, Jul-Aug. 2014 edn. Journal of Mechanical and Civil Engineering
Wilcox LV (1955) Classification and use of irrigation waters vol November. US Department of Agriculture, Washington, DC
Youssef AM, Omer AA, Ibrahim MS, Ali MH, Cawlfield JD (2011) Geotechnical investigation of sewage wastewater disposal sites and use of GIS land use maps to assess environmental hazards: Sohag, Upper Egypt. Arab J Geosci 4:719–733. doi:10.1007/s12517-009-0069-6
Acknowledgments
The author thanks the Groundwater Sector (GWS) of Upper Egypt (belonging to Ministry of Water Resources and Irrigation). It provides this work with the data of groundwater samples and locations of each sample. I am greatly appreciating the help of Eng. Essam Hamdan (working at GWS, Upper Egypt) for his help in administrating the information of the supplied data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Rabeiy, R.E. Assessment and modeling of groundwater quality using WQI and GIS in Upper Egypt area. Environ Sci Pollut Res 25, 30808–30817 (2018). https://doi.org/10.1007/s11356-017-8617-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8617-1