Abstract
One of the main issues in arid and semi-arid areas like Iran is water shortage and environmental deterioration. This study aimed to address the groundwater quality for drinking, irrigation, and industrial consumption by integrating the water quality indices with the geographic information system (GIS) for Fasarud Plain groundwater quality interpretation in southern Iran. Hence, 186 borehole water samples were collected in wet and dry seasons, and all these samples were subjected to lab experiments for pH, EC, TDS, TH, and major cations and anions using standard techniques. The outcomes of the Piper diagram indicated that in both seasons, the water type in the research region is Ca-Mg (HCO3)2, CaCl2, and mixed. In this study, the suitability of groundwater for drinking was determined by using WHO guidelines and Water Quality Index (WQI). The results indicated that despite the decrease in total dissolved solids in the wet season, the concentration of high ionic strength inorganic components such as nitrate and bicarbonate increase and results in raised electrical conductivity. The spatial distribution maps for the most water quality variables revealed that the not-permissible values were found, during both seasons, in the south, southwest, and northeast parts of the aquifer. According to WQI in both seasons, most plain areas have excellent and good quality water for drinking purposes, except the regions at south and west of the study area with poor quality (10.26% and 8.62% in the dry and wet seasons, respectively). The classification of water for irrigation based on the US salinity diagram showed the medium-to-high salinity and low SAR class in both seasons. In addition, based on the corrosivity ratio, the groundwater quality in most areas of the plain is in an unsafe zone in terms of industrial uses in both seasons (81.97% and 88.63% in the dry and wet seasons, respectively). In general, the reduction of water quality for potable, agricultural, and industrial uses from the dry season to the wet season shows the destructive effect of anthropogenic activities such as agriculture on groundwater quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The distribution of freshwater resources is inconvenient all over the world, and freshwater accessibility is becoming rare day by day due to the development of communities and various anthropogenic operations. With decreasing fresh surface water resources, groundwater is used to encounter the requirements of diverse parts (Saha and Paul 2019). Spatial variability in groundwater quality due to local geologic formations and man-made parameters guarantees the assessment of the groundwater quality for usages such as domestic, agricultural, and industrial uses. Assessing water quality for the intended usage requires designating the groundwater chemical compounds and improvement proceedings to restore water quality. If it deteriorates, it is necessary to recognize potential sources of groundwater pollution (Annapoorna and Janardhana 2015; Tolera et al. 2020). Groundwater quality in areas facing water shortage in southern Iran is decreasing due to contaminants from natural origins and man-made point and non-point sources (Rezaei et al. 2017; Bahrami et al. 2020a). The potential sources of groundwater pollution have been expanded in this area in the last decades because of anthropogenic activities’ growth. Excessive withdrawal from a groundwater aquifer for agricultural and industrial usage has not only decreased groundwater levels but also damaged the quality of drinking water. Agriculture is one of the main reasons for the deterioration of groundwater via soil erosion and chemical effluent (Barakat et al. 2020). Due to the increment in water quality vulnerability, particularly with human activities, evaluation of groundwater quality has become inevitable in water resources management. The usual water quality evaluation is to investigate the water quality variables concerning their standard amounts under an intended aim. The principal parameters to evaluate groundwater quality competency for agricultural usage are sodicity and salinity (Sreedevi et al. 2018). In addition to the World Health Organization guidelines, the Water Quality Index (WQI) approach is extensively utilized for groundwater quality evaluation throughout the globe due to its capability to represent drinking water quality information. This index ranks the combined effects of individual water quality parameters concerning the overall water quality for targeted citizens and decision-makers (Radouane et al. 2021). On the other, the geographic information system (GIS) plays a fundamental role in natural resources management. Using geostatistics models, the spatial analysis extension of GIS permits interpolation of the groundwater quality parameters at unknown locations from known values to make a continuous surface, which helps comprehend the distribution of water quality parameters of the study area. (Bhunia et al. 2018; Batarseh et al. 2021) Many authors have exploited the GIS program to estimate the groundwater spatial distribution (Mosaferi et al. 2014; Verma et al. 2016; Gharahi and Zamani-Ahmadmahmoodi 2020; Zarei et al. 2021; Asha and Paul 2019).
In addition, many researchers have developed different water quality indices for the assessment of the propriety of water resources for various uses (Barakat et al. 2018, 2020; Ponsadailakshmi et al. 2018; Sunkari et al. 2020). Saha and Paul (2016) used several interdisciplinary methods to demonstrate a complex datasheet consisting of multiple water quality variables of various seasons into a beneficial model. They assessed the water competency for different usages like direct ingestion, domestic utilization, and agricultural and industrial operations. Rezaei et al. (2017) investigated geochemical processes governing groundwater chemistry with specific respect to nitrate and fluoride enrichment in groundwater in southern Iran. Aleem et al. (2018) investigated the spatial variation of aquifer water quality for potable and agricultural uses in an industrial region of Pakistan using the GIS software. Barakat et al. (2020) stated that the detailed assessment of the groundwater contamination condition in a district by various techniques has formed it feasible to specify the groundwater quality predetermined for different consumptions. The outputs contribute to water resource determiners to administer logically the groundwater resources as one of the main challenges for the progress of semi-arid regions. In an industrial zone in Pakistan, Ullah et al. (2022) investigated groundwater quality using a GIS-based Water Quality Index. The outcomes indicated that a larger proportion had poor drinking quality due to direct releases of toxins by industries. It was observed that 87% of the water samples demonstrated an unsuitable status of groundwater for drinking goals in terms of pH, EC, Fe, Mn, Cu, and Cr. These findings were used to construct wastewater treatment plant facilities, reduce pollution loads on the sewer system and river, and contaminant seepage rates into groundwater. Atta et al. (2022) assessed the suitability of groundwater quality around Ismailia Canal, Egypt, for drinking purposes and generated the WQI maps to help decision-makers and local authorities to avoid groundwater contamination and to facilitate the selection safely future development areas. They concluded that most of the observation wells are located within desirable and max. allowable limits. Taşan (2023) determined the suitability of groundwater for irrigation in Turkey. In the estimation of all irrigation water quality parameters, the artificial neural network (ANN) model performed much higher in comparison with the adaptive neuro-fuzzy inference system (ANFIS) model. Spatial distribution maps were generated for measured and ANN model-estimated irrigation water quality indices using the IDW interpolation method.
Reduction in the quantity and quality of groundwater in recent years and numerous subsidence have caused the migration of residents from arid and semi-arid southern areas of Iran. Hence, the main aim of this research was to evaluate the temporal and spatial physicochemical characteristics of the groundwater in the Fasarud plain, located in a semi-arid region of southern Iran. Due to population density in this part of the country and reliance on groundwater to meet drinking, agricultural, and industrial requirements, such a study has not been conducted so far. Therefore, the competency of the groundwater for potable, irrigation, and industrial uses was assessed using GIS software. To reach this objective, 186 groundwater specimens were gathered within wet and dry seasons and analyzed for different quality variables.
Materials and methods
Study area
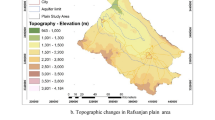
The specimens were gathered from 31 wells, mostly used for irrigation, at Fasarud Plain, Darab County, Iran, two times in June 2017 (dry season) and March 2018 (wet season). The Fasarud region is located in the Fars Province (the south-eastern zone of Iran) within E 54°13′ and E 54°32′ and N 28°39′ to N 28°49′, with a total area of 7500 km2, an altitude of 1180 m above sea level, mean annual temperature of about 25 °C, and the mean annual rainfall of 350 mm (Fig. 1). Based on the Demarthen index, the climate of the area is semi-arid (Zarei and Moghimi 2019).
Sampling and analysis
To assess the groundwater quality of Fasarud Plain, specimens taken from 31 wells located throughout the plain in dry and wet months (June and March) were used. Overall, taking into account three iterations, 186 water samples were collected. Every well was pumped around 15 min before each sampling. The samples were gathered below the water table in the polythene container that was cleaned three times with the water to be sampled before sampling. The collected specimens were kept at 4 °C prior to analysis and carried to the laboratory. The acidity (pH), total dissolved solids (TDS), and electrical conductivity (EC) were specified in situ immediately after sampling, and the other parameters, including sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), bicarbonate (HCO3−), chloride (Cl–), nitrate (NO3−), and sulfate (SO42−) were measured based on the APHA methods in the laboratory within 24 h (APHA 2005). The validity of chemical analysis findings was carried out by the ionic balance validation and by replicating the analysis for a similar specimen. The correctness of the outputs was assessed by computing the charge balance error (CBE). The experiment results are regarded as trusty, just if the CBE is fewer than 5% (Bahrami et al. 2020b). Based on the Ca2+ and Mg2+ concentration, total hardness (TH) was calculated as mg/L as CaCO3 (Sreedevi et al. 2018):
A correlation matrix was used to recognize the better relationship between the different physicochemical parameters of the groundwater samples. In addition, the major cations and anions in meq/L were plotted on a trilinear Piper diagram to evaluate the hydrochemistry of groundwater.
Groundwater data and GIS analysis
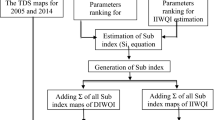
At first, the normality of each parameter data set was analyzed using Shapiro–Wilk's statistical test in SPSS Software. Then, the analytical data were fed into the ArcGIS10.2 program to create spatial distribution maps for the chosen variables that exhibit significant variation. For plotting these maps, the inverse distance weighted (IDW) geostatistical wizard was applied for non-normal data sets. While for normal data sets, various geostatistical wizards (such as Kriging, and Co-Kriging with Circular, Gaussian, Spherical, and lots of other variograms) were tested to find an appropriate interpolation method. Then, the error criterion and root mean square error (RMSE) was used to assess their accuracy and precision (Radouane et al. 2021; Mokarram et al. 2020). Based on these maps, water quality was evaluated for potable, agricultural, and industrial uses. Finally, area percentages of each parameter class were extracted from the spatial maps.
Assessment of groundwater for drinking uses
The suitability of groundwater quality of the plain was investigated for drinking use based on the WHO guidelines (WHO 2011) and Water Quality Index (WQI). A helpful tool for groundwater assessment and management to the citizens and policymakers is WQI. The index is expressed as a grading displaying the compound impact of multiple water quality variables (Ameur et al. 2016). Mapping this index aids scientists, policymakers, and the general public to easily distinguish the regions of high and low water quality for treatment before different uses (Tlili-Zrelli et al. 2018).
The WQI is computed in three steps as at the first step, each of the 10 variables is allotted a weight (wi) based on its relative influence on the whole water quality for potable uses (Table 1). In the next step, the relative weight (Wi) is determined from the following formula:
where wi is the weight of each variable and n is the number of parameters. The allocated weights to the variables range within 1 and 5 (Table 1) and are based on the health influences of the variables. The highest weight (5) is allocated to salinity and nitrate because of their great significance in water quality evaluation and the health consequences of the high concentration of these chemical variables in water (Srinivasamoorthy et al. 2008; Yidana et al. 2010).
In the third step, the following equation was applied to calculate the WQI:
where Ci is each chemical variable concentration in each water specimen in mg/L. Si is the standard of the World Health Organization for each of the major variables in potable water (Asadi et al. 2019).
The calculated WQI quantities are categorized into five kinds, from “excellent water” to “unsuitable water for potable” (Ramkrisahniah et al. 2009). In this research, a total of ten chemical variables (TDS, pH, Na+, K+, Ca2+, Mg2+, Cl−, SO42−, HCO3−, and NO3−) of 186 water specimens were applied to compute WQI for evaluation of the compatibility of the Fasarud plain groundwater for domestic (potable) purpose.
Assessment of groundwater for agricultural uses
Irrigation water quality is mostly assessed by the US salinity diagram according to electrical conductivity (EC) and sodium adsorption ratio (SAR). In this study, SAR is calculated from the following formula, where the concentrations are as meq/L (Sreedevi et al. 2018):
Assessment of groundwater for industrial uses
To determine the appropriateness of groundwater for industrial uses, the corrosivity ratio (CR) is applied as it indicates the corrosion level in the metal tubes through which water flows from one module to the next. This index is defined as the ratio of alkaline earths to saline salts (all units in mg/L). The water is inappropriate for industrial purposes if CR > 1 (Patel et al. 2016).
Results and discussion
GIS analysis and geostatistical model selection
The results of the geostatistical model selection showed that in non-normal data sets (such as PH and TDS), the IDW model with power equal to 2 and in normal data sets (such as EC and TH), the Spherical and Gaussian models were used (Table 2).
Evaluation of groundwater quality for drinking uses
The results of determining the groundwater quality suitability of the plain for drinking use according to WHO guidelines (WHO 2011) are presented in Table 3. The quantities of the chemical variables of the groundwater in the Fasarud Plain reveal that pH varies from 6.41 to 7.45 in the dry season and 6.64 to 7.80 in the wet season with mean values of 7.06 and 7.18, respectively. According to WHO (2011) standards, almost all the samples are in the suggested range for human utilization (6.5 to 8.5). Therefore, the groundwater samples in both seasons are categorized as slightly acidic and slightly alkaline according to the pH values with an increase in the wet season. The spatial distribution of pH indicated the permissible values of this parameter (6.5–8.5) in the whole plain in both the wet and dry seasons (Fig. 2). Only in the dry season 0.02% of the area had desirable pH (pH < 6.5).
The EC of the aquifer water samples varied from 428.40 to 4788.00 with a mean of 1170.93 µmho/cm during the dry season and from 394.80 to 5544.00 with a mean of 1239.41 µmho/cm throughout the wet season. Water TDS ranged from 253.31 to 3420.75 with an average of 871.06 mg L−1 for the dry season and from 222.23 to 3219.67 with an average of 795.73 mg L−1 for the wet season. The maximum quantities of TDS were observed in the dry season (June), while the minimum quantities were observed in the wet season (March). This designates that a lower content of total dissolved solids present in the groundwater contributes to electrical conductivity. The spatial distribution of EC in dry season indicated that 76.14% of the plain area is in the desirable limit of EC (< 1400 µmhos/cm), 0.05% of the area has permissible values of this parameter (EC = 1400 µmhos/cm), and 23.81% of the area located at south, southwest, and east of the plain is not permissible in terms of EC (> 1400 µmhos/cm). While in the wet season, the percent of area related to desirable values of EC decreased to 73.44%, the permissible percent was constant (0.05%), and the area percent with not permissible EC increased to 26.51%. The spatial distribution of TDS in the dry season revealed that 3.49% of the area is in the desirable range (TDS < 600 mg L−1), 64.37% of the area is in the permissible range (600–1000 mg L−1), and 32.14% of the studied area has not permissible values of TDS (> 1000 mg L−1). In the wet season, 8.17%, 64.83%, and 27% of the plain are in desirable, permissible, and not permissible limits of TDS, respectively. These results revealed that in contrast to EC, water quality in terms of TDS for drinking is improved from the dry season to the wet season. Because despite the TDS reduction in the wet season, the concentration of components affecting water electrical conductivity increases (Hasan et al. 2017).
The TH of the aquifer water specimens ranged from 130.05 to 1471.39 with an average of 467.22 mg L−1 as CaCO3 in the dry season and from 140.31 to 1392.22 with an average of 432.99 22 mg L−1 as CaCO3 throughout the wet season. The spatial distribution of TH in the dry season demonstrated that 72.16% of the plain area is in the desirable limit of total hardness (< 100 mg L−1 as CaCO3) and the rest of the area (27.84%), located at south, southwest, and east of the plain, is in the permissible limit of TH (100–500 mg L−1 as CaCO3). The water quality in terms of TH improved from the dry season to the wet season, as the area percentage related to desirable and permissible limits of TH obtained 79.46% and 20.54%, respectively (Muthukumaravel et al. 2010).
The findings demonstrated that the order of dominance of anions and cations is HCO3− > SO42− > Cl− > NO3− and Ca2+ > Na+ > Mg2+ > K+, respectively, in the dry season and HCO3− > Cl− > SO42− > NO3− and Na+ > Ca2+ > Mg2+ > K+ in the wet season. Most parameters have more concentration in the dry season, contrary to nitrate. The higher concentration of nitrate in the wet season is indicative of anthropogenic processes such as agronomy, industry, urban growth, and enhancing the extraction of water resources. The spatial distribution of cations and anions is represented in Fig. 3 and Fig. 4, respectively. It can be observed in Fig. 3 that in the dry season, 9.47% of the plain area has the desirable concentration of Ca (< 75 mg L−1), 81.34% of the area is in the permissible range (75–200 mg L−1), and 9.19% of the plain has a not-permissible range of Ca for potable water (> 200 mg L−1). But in the wet season, the area percentage with a desirable amount of Ca increases to 44.82%, and permissible and not permissible classes decrease to 47.62% and 7.56%, respectively.
The percentage of land area related to different classes of Mg, Na, and K in wet and dry seasons is almost equal. As most area of the studied plain is within the desirable and permissible limits of these cations. In the southern and eastern areas of the plain, the concentration of Mg and Na is in the not permissible class.
As can be observed in Fig. 4, in the dry season, 76.04% of the plain area has the desired amount of HCO3 (< 300 mg L−1), 2.21% of the area is in the permissible range (HCO3 = 300 mg L−1), and 21.75% of the plain has a not-permissible range of HCO3 for potable water (> 300 mg L−1). But in the wet season, the area percentage with desirable and permissible amounts of HCO3 decreases to 47.65% and 1%, respectively, and the not-permissible class increases to 51.35%. Areas with a not-permissible class range are in the western half of the plain.
Unlike bicarbonate, the concentration of SO4 in the dry season is more than in the wet season. In the dry season, 67.55% of the plain area has the desirable concentration of SO4 (< 200 mg L−1), 11.24% of the area is in the permissible range (200–400 mg L−1), and 21.21% of the plain has a not-permissible range of SO4 for potable water (> 400 mg L−1). But in the wet season, the area percentage with a desirable amount of SO4 increases to 82.33% while permissible and not permissible classes decrease to 5.5% and 12.17%, respectively.
The percentage of land area related to different classes of Cl in the wet and dry seasons is almost equal. The percentage of desirable (< 250 mg L−1), permissible (250–400 mg L−1), and not permissible (> 400 mg L−1) classes are 76.90%, 12.07%, and 11.03%, respectively, in the dry season and are 78.76%, 9.02%, and 12.22%, respectively, in the wet season.
The spatial distribution of NO3 showed that in the dry season, its concentration is within the desirable limit (< 50 mg L−1) almost all over the plain. But in the wet season, the desirable class percentage decreases to 64.67%, while 5.47% of the region has the permissible limit (= 50 mg L−1) and 29.87% of the plain is within the not-permissible range (> 50 mg L−1).
The most usually found groundwater pollutant, particularly in unconfined aquifers, is nitrate originating from both point and non-point sources (Esmaeili et al. 2014). The non-point sources of nitrate pollution are agronomic operations (fertilizer and manure utilization, leguminous plants, watering with groundwater comprising nitrogen compositions) and decaying vegetation (Anayah and Almasri 2009). Nitrate is transported approximately as a non-reactive composition in the unsaturated zone mostly due to its high solvability and its trend to be repulsed by negatively charged soil matrices.
The correlation matrix represented the better-paired relationship among the physicochemical variables (Table 4). A strong correlation between Ca2+ and Mg2+ with TH in both seasons reveals that these parameters are water hardness factors. In addition, such a strong correlation between SO4 and Cl is a demonstrator of permanent hardness. A positive and significant correlation of CI− with Na+ and Ca2+ in the specimens is indicative of the dominance of these soluble salts. To know the possible originations of Na+ and Cl− in the water samples, the relationship between these ions was assessed by plotting the scatter plot of Na+ versus Cl− (Panno et al. 2006). According to Fig. 5, most of the points that fall under the 1:1 line demonstrate that the concentration of Cl− has decreased, which might show silicate mineral dissolution and cation exchange.
The positive and significant correlation between EC and TDS, especially in the wet season (r = 0.946), might be because of ions in TDS that conduct electricity. In addition, a significant positive correlation between EC and Na+, K+, Ca2+, Mg2+, Cl−, and SO42− ions shows the high mobility of ions. The strong correlation between Mg2+ and Cl− can be attributed to domestic wastewater entering groundwater in the considered region. A significant and positive correlation between Mg2+ and SO42− can be a result of using organic and chemical fertilizers in agronomic operations in this area.
In addition, the results of the spatial distribution of WQI presented in Fig. 6 and Table 5 indicate that, in both seasons, most of the aquifer area has excellent and good quality water for drinking purposes. Only limited areas, mainly in the south of the plain, have poor-quality drinking water. In addition, in none of the seasons, water quality falls in the classes of very poor and unsuitable for potable. The reason for the decrease in water quality for potable in the wet season can be ascribed to the increment in ionizable ions and, consequently, the increase in electrical conductivity.
Evaluation of groundwater quality for irrigation uses
The primary source of irrigation in this region is groundwater throughout the year because of the incompetent surface water and irregular rainfall. A large amount of groundwater extraction happens from moderate to deep wells. The groundwater appropriateness for irrigation is ascertained by different values of various ions. Groundwater always contains some soluble salts, and the excess amount of soluble salts may be detrimental to many plants and also destroys the soil characteristics over a long period.
The SAR quantities in the research area range between 0.46 and 6.24 with a mean of 1.80 during the dry season and from 0.36 to 8.77 with a mean of 1.74 during the wet season. Figure 7 and Table 6 represent the sampling boreholes on the US salinity diagram.
These results indicate that within both seasons, the water samples fall mainly into C2S1 and C3S1 limits. 48.39% and 35.48% of groundwater specimens place into doubtful class (C3S1) within wet and dry seasons, respectively, showing water of medium-to-high salinity and low SAR class, without the hazard of soil destabilization. This groundwater can be applied for watering crops possessing well salt toleration on soils with good permeability, with salinity control (Wilcox 1955; Gouaidia et al. 2012). Overall, the water quality is more appropriate for irrigation purposes in the dry season (Table 7). The reason can be attributed to the lack of runoff with rainfall origin and no occurrence of agricultural effluents.
The spatial distribution of the irrigation suitability classes indicates that the water quality in none of the seasons is in the excellent class for watering crops (Fig. 8). In the dry season, the groundwater quality in the south, east, and west of the aquifer is in doubtful and unsuitable classes, which increases during the wet season and the irrigation period.
Assessment of groundwater quality for industrial purposes
The CR values in the study area varied between 0.23 and 9.46 with a mean of 2.51 during the dry season and from 0.10 to 16.50 with a mean of 1.84 within the wet season. The spatial distribution maps of the CR classes for the considered area are illustrated in Fig. 9. As in the dry season, 18.03% of the area is in the safe zone limit, and 81.97% of the plain has an unsafe zone for industrial uses (Table 8). These values in the wet season are 11.37% and 88.63% of the area for safe and unsafe zones. These results indicate that in both seasons, the groundwater quality in most areas of the plain is in an unsafe zone in terms of industrial uses. In addition to small sporadic areas throughout the plain, the northern and northwestern parts of the plain also have safe groundwater for industrial uses.
Hydro-chemical characteristics of groundwater
A Piper diagram was depicted to assess the hydro-chemical characteristics of cations and anions represented in Fig. 10. The results in both seasons show that the most dominant cation is Ca, and the most dominant anion is HCO3. Based on the Piper diagram for groundwater in both seasons, alkaline earth metals exceed alkali metals. In the other words, Ca-Mg (HCO3)2, CaCl2, and mixed types of water are found in the studied aquifer (Aleem et al. 2018). The dominance of Ca-HCO3 facies is because of the carbonate dissolution process relevant to the presence of limestone and dolomitic limestone outcropping in the research region (Prabha et al. 2013).
Conclusion
Pollution from point and nonpoint sources generated by human activities has damaged groundwater quality in recent years, causing socioeconomic and health troubles. Recognizing and managing groundwater quality are necessary to maintain freshwater resources in arid and semi-arid regions like Iran. The Water Quality Index (WQI), the US salinity diagram, and the corrosivity ratio were developed to evaluate groundwater resources in Fasarud Plain, southern Iran, for their drinking, irrigation, and industry suitability. These indices and GIS mapping illustrate spatial distribution along with other water quality parameters, such as anions and cations. The results demonstrated that the water type in the research region is Ca-Mg (HCO3)2, CaCl2, and mixed. In addition, the groundwater quality for various uses decreased from the dry season to the wet season. Based on WQI in both seasons, most plain areas have excellent and good quality water for drinking purposes. The spatial variation of groundwater quality indicated that groundwater with poor quality is seen in the south and west of the evaluation region (10.26% and 8.62% in the dry and wet seasons, respectively).
For irrigation purposes, the water has medium-to-high salinity and low SAR without the hazard of soil destabilization in both seasons. This water is appropriate for watering crops with good salt tolerance on soils with good permeability, along with salinity control. According to the spatial variation of groundwater quality, 7.06% and 8.05% of the area in the south of the plain has unsuitable water for agricultural uses. In addition, in terms of industrial uses in both seasons, the groundwater quality in most regions of the plain is in an unsafe zone. So that limited areas in the north and northwestern of the study area have safe water for industrial purposes.
In general, the reduction of water quality for potable, agricultural, and industrial uses from the dry season to the wet season shows the destructive effect of anthropogenic activities such as agriculture on groundwater quality. This research discovered that the usage of an integrated approach of GIS and the water quality indices is incredibly helpful in fetching groundwater quality and with a clear view of the geographic area of groundwater quality; this result has significant consequences for regional decision-makers for improving groundwater management and protection.
Availability of data and materials
Data cannot be made publicly available; readers should contact the corresponding author for details.
References
Aleem M, Cao JS, Chao L, Arslan MA, Wu Y, Muhammad IN, Wasif SA, Noman AB (2018) Evaluation of groundwater quality in the vicinity of Khurrianwala Industrial Zone Pakistan. Water 10(10):1321. https://doi.org/10.3390/w10101321
American Public Health Association and American Public Health Association (APHA) (2005) Standard methods for the examination of water and wastewater, APHA. American water works association and water environment federation, 21st edn. American Public Health Association, Washington, DC, USA, Vancouver
Ameur M, Hamzaoui-Azaza F, Gueddari M (2016) Nitrate contamination of Sminja aquifer groundwater in Zaghouan, northeast Tunisia: WQI and GIS assessments. Desalin Water Treat 57(50):23698–23708. https://doi.org/10.1080/19443994.2015.1137495
Anayah FM, Almasri MN (2009) Trends and occurrences of nitrate in the groundwater of the West Bank Palestine. Appl Geogr 29:588–601
Annapoorna H, Janardhana MR (2015) Assessment of groundwater quality for drinking purpose in rural areas surrounding a Defunct Copper Mine. Aquat Proc 4:685–692
Asadi E, Isazadeh M, Samadianfard S, Ramli MF, Mosavi A, Nabipour N, Shamshirband S, Hajnal E, Chau K-W (2019) Groundwater quality assessment for sustainable drinking and irrigation. Sustainability 12(1):177. https://doi.org/10.3390/su12010177
Asha P, Paul B (2019) Groundwater quality assessment in an industrial hotspot through interdisciplinary techniques. Environ Monit Assess 191(Suppl 2):326. https://doi.org/10.1007/s10661-019-7418-z
Atta HS, Omar MAS, Tawfik AM (2022) Water quality index for assessment of drinking groundwater purpose case study: area surrounding Ismailia Canal, Egypt. J Eng Appl Sci 69:83. https://doi.org/10.1186/s44147-022-00138-9
Bahrami M, Khaksar E, Khaksar E (2020a) Spatial variation assessment of groundwater quality using multivariate statistical analysis (Case Study: Fasa Plain, Iran). J Groundw Sci Eng 8:230–243
Bahrami M, Zarei AR, Rostami F (2020b) Temporal and spatial assessment of groundwater contamination with nitrate-by-nitrate pollution index (NPI) and GIS (case study: Fasarud Plain, southern Iran). Environ Geochem Health. https://doi.org/10.1080/10807039.2019.1584029
Barakat A, Meddah R, Afdali M, Touhami F (2018) Physicochemical and microbial assessment of spring water quality for drinking supply in Piedmont of B eni-Mellal Atlas (Morocco). Phys Chemis Earth, Parts 104:39–46
Barakat A, Hilali A, Baghdadi ME, Touhami F (2020) Assessment of shallow groundwater quality and its suitability for drinking purpose near the Béni-Mellal wastewater treatment lagoon (Morocco). Hum Ecol Risk Assess Int J 26(6):1476–1495
Batarseh M, Imreizeeq E, Tilev S, Al AM, Suleiman W, Al Remeithi AM, Al Tamimi MK, Al AM (2021) Assessment of groundwater quality for irrigation in the arid regions using irrigation water quality index (IWQI) and GIS-Zoning maps: Case study from Abu Dhabi Emirate. UAE. Groundw Sustain Dev 14:100611. https://doi.org/10.1016/j.gsd.2021.100611
Bhunia GS, Keshavarzi A, Shit PK, Omran EE, Bagherzadeh A (2018) Evaluation of groundwater quality and its suitability for drinking and irrigation using GIS and geostatistics techniques in semiarid region of Neyshabur, Iran. Appl Water Sci 8:168. https://doi.org/10.1007/s13201-018-0795-6
Esmaeili A, Moore F, Keshavarzi B (2014) Nitrate contamination in irrigation groundwater Isfahan, Iran. Environ Earth Sci 72:2511–2522. https://doi.org/10.1007/s12665-014-3159-z
Gharahi N, Zamani-Ahmadmahmoodi R (2020) Evaluation of groundwater quality for drinking purposes: a case study from the Beheshtabad Basin, Chaharmahal and Bakhtiari Province, Iran. Environ Earth Sci 79:82. https://doi.org/10.1007/s12665-020-8816-9
Gouaidia L, Gueffaifia O, Boudoukha A, Hemila ML, Martin C (2012) Evaluation of the salinity of groundwater used in irrigation and risks of soil degradation: example of the Meskiana plain (North-East Algeria). Physio-G′Eo 6:141–160
Hasan M, Shang Y, Akhter G, Jin W (2017) Evaluation of groundwater suitability for drinking and irrigation purposes in Toba Tek Singh District, Pakistan. Irrig Drainage Syst Eng 6:185
Mokarram M, Ghasemi MM, Zarei AR (2020) Evaluation of the soil fertility for corn production (Zea Mays) using the multiple-criteria decision analysis (MCDA). Model Earth Syst Environ 6:2251–2262
Mosaferi M, Pourakbar M, Shakerkhatibi M, Fatehifar E, Belvasi M (2014) Quality modeling of drinking groundwater using GIS in rural communities, northwest of Iran Iranian. J Environ Health Sci Eng 12:99. https://doi.org/10.1186/2052-336X-12-99
Muthukumaravel S, Padmanabhan V, Boopathidoss PS, Sadanandane C, Srinivasan R, Gunasekaran K, Sabesan S, Balaraman K (2010) Invasion of toxic marine cyanobacteria in to the tsunami affected coastal villages of southern India. J Commun Dis 42:135–138
Panno S, Hackley K, Hwang H, Greenberg S, Krapac I, Landsberger S, O’Kelly D (2006) Characterization and identification of Na-Cl sources in ground water. Groundwater 44(2):176–187. https://doi.org/10.1111/j.1745-6584
Patel P, Raju NJ, Reddy BCSR, Suresh U, Gossel W, Wycisk P (2016) Geochemical processes and multivariate statistical analysis for the assessment of groundwater quality in the Swarnamukhi River basin Andhra Pradesh, India. Environ Earth Sci 75(7):1–24
Ponsadailakshmi S, Sankari SG, Prasanna SM, Madhurambal G (2018) Evaluation of water quality suitability for drinking using drinking water quality index in Nagapattinam district Tamil Nadu in Southern India. Groundw Sustain Dev 6:43–49
Prabha S, Kumar M, Kumar A, Das P, Ramanathan AL (2013) Impact assessment of textile effluent on groundwater quality in the vicinity of Tirupur industrial area, Southern India. Environ Earth Sci 70:3015–3022
Radouane EM, Chahlaoui A, Maliki A, Boudellah A (2021) Assessment and modeling of groundwater quality by using water quality index (WQI) and GIS technique in Meknes aquifer (Morocco). Geol Ecol Landsc. https://doi.org/10.1080/24749508.2021.1944797
Ramkrisahniah CR, Sadashlvaiah C, Ranganna G (2009) Assessment of water quality index for the groundwater in Tumkur Taluk Karnataka State, India. E J Chemis 6(2):523–530
Rezaei M, Nikbakht M, Shakeri A (2017) Geochemistry and sources of fluoride and nitrate contamination of groundwater in Lar area, south Iran. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-9108-0
Saha P, Paul B (2019) Groundwater quality assessment in an industrial hotspot through interdisciplinary techniques. Environ Monit Assess 191:1–20
Sreedevi PD, Sreekanth PD, Shakeel Ahmed DV (2018) Evaluation of groundwater quality for irrigation in a semi-arid region of South India. Sustain Water Res Manage. https://doi.org/10.1007/s40899-018-0279-8
Srinivasamoorthy K, Chidambaram M, Prasanna MV, Vasanthavigar M, John PA, Anandha P (2008) Identification of major sources controlling groundwater chemistry from a hard rock terrain a case study from Mettur taluk Salem District, Tamil Nadu, India. J Earth Syst Sci 117(1):49–58
Sunkari ED, Abu M, Zango MS, Lomoro Wani AM (2020) Hydrogeochemical characterization and assessment of groundwater quality in the Kwahu-Bombouaka Group of the Voltaian Supergroup Ghana. J Afr Earth Sci 169:103899. https://doi.org/10.1016/j.jafrearsci.2020.103899
Taşan S (2023) Estimation of groundwater quality using an integration of water quality index, artificial intelligence methods and GIS: Case study, Central Mediterranean Region of Turkey. Appl Water Sci 13:15. https://doi.org/10.1007/s13201-022-01810-4
Tlili-Zrelli B, Gueddari M, Bouhlila R (2018) Spatial and temporal variations of water quality of mateur aquifer (Northeastern Tunisia): suitability for irrigation and drinking purposes. J Chem. https://doi.org/10.1155/2018/2408632
Tolera MB, Choi H, Chang SW, Chung IM (2020) Groundwater quality evaluation for different uses in the lower Ketar Watershed Ethiopia. Environ Geochem Health 42:3059–3078. https://doi.org/10.1007/s10653-019-00508-y
Ullah AS, Rashid H, Khan SN, Akbar MU, Arshad A, Rahman MM, Mustafa S (2022) A Localized Assessment of groundwater quality status using gis-based water quality index in industrial zone of faisalabad Pakistan. Water 14(20):3342. https://doi.org/10.3390/w14203342
Verma DK, Bhunia GS, Shit PK, Kumar S, Mandal J, Padbhushan R (2016) Spatial variability of groundwater quality of Sabour block, Bhagalpur district (Bihar, India). Appl Water Sci. https://doi.org/10.1007/s13201-016-0380-9
Water Health Organization (2011) Guidelines for Drinking-Water Quality, World Health Organization, 4th edn. Recommendations, Geneva
Wilcox L., (1955), Classification and Use of Irrigation Waters; FAO: Rome, Italy.
Yidana SM, Banoeng-Yakubo B, Akabzaa TM (2010) Analysis of groundwater quality using multivariate and spatial analyses in the Keta Basin Ghana. J Afr Earth Sci 58(2):220–234
Zarei AR, Moghimi MM (2019) Environmental assessment of semi-humid and humid regions based on modeling and forecasting of changes in monthly temperature. Int J Environ Sci Technol 16:1457–1470
Zarei AR, Moghimi MM, Koohi E (2021) Sensitivity assessment to the occurrence of different types of droughts using GIS and AHP techniques. Water Resour Manage 35(11):3593–3615
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
MB, and ARZ prepared the data, analyzed the data, and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared there is no conflict of interest associated with this work.
Ethical approval
This material is the authors' own original work, which has not been previously published elsewhere.
Consent to participate
Not applicable.
Research involving human and animal participants
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bahrami, M., Zarei, A.R. Assessment and modeling of groundwater quality for drinking, irrigation, and industrial purposes using water quality indices and GIS technique in fasarud aquifer (Iran). Model. Earth Syst. Environ. 9, 3907–3921 (2023). https://doi.org/10.1007/s40808-023-01725-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40808-023-01725-2