Abstract
Cadmium (Cd) is a toxic heavy metal that is widespread and nephrotoxic, but the mechanism of its toxicity is not well understood. Alpha-lipoic acid (α-LA) has a protective effect on Cd-induced oxidative stress, but the underlying mechanism is also not clear. This study aimed to confirm that Cd causes renal damage and to explore the potential underlying mechanism of α-LA to the kidney. Rats were randomly divided into four groups: control group, Cd group (50 mg/L CdAc2), Cd+α-LA group (50 mg/L CdAc2 + 50 mg/kg body wt/day α-LA), and α-LA group (50 mg/kg body wt/day). The rats were exposed to Cd via drinking water and α-LA in the form of gavage at the same time every day. After 12 weeks, the activity of antioxidant enzymes and the level of Cd in the kidney were analyzed. Renal damage was evaluated based on histopathological and ultrastructure examinations. The apoptosis index was determined based on the results of western blotting and qRT-PCR. Our results indicate that accumulation of Cd causes serious kidney damage and α-LA has a protective effect against Cd-induced oxidative stress and apoptosis. Further, the findings indicate that the antioxidant, Cd chelation, and antiapoptotic activities of α-LA are the key factors that alleviate nephrotoxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium is a highly toxic non-essential heavy metal pollutant due to its high mobility in soil, plant, and water systems (Manquian-Cerda et al. 2016). In addition, this element has more than one target organs in the body; in particular, the kidney is considered to be the main target of Cd-induced toxicity (Wlostowski et al. 2008; Wang et al. 2011). When present in high amounts, Cd is retained in the kidney and has a half-life of 20–30 years in humans, and thus, it has serious effects on health (Jarup and Akesson 2009). At the cellular level, Cd has been associated with oxidative stress and apoptosis (El-Habit and Abdel Moneim 2014); furthermore, some studies conducted over the last decade have demonstrated that Cd induces oxidative stress and then mediates apoptosis, a phenomenon that everyone recognizes, and there is not only one apoptosis pathway that will be induced in the process (Duan et al. 2016). It has been reported that the amount of Cd accumulated in the human body may increase in the future because excess Cd cannot be degraded and is accumulated via the food chain (Wang et al. 2016). Although Cd is known to induce oxidative stress in the kidney, the underlying mechanism of the oxidative damage is not fully understood. Some studies have demonstrated that excessive apoptosis induced by oxidative stress may be an important mechanism of oxidative damage (Pathak et al. 2013); however, very little related research has been conducted on kidney cells.
As a very strong antioxidant, alpha lipoic acid (α-LA) is a fatty acid that found naturally in plants and animals (Hu et al. 2016). α-LA has been shown to be a potent antioxidant and metal chelator which has the function of repair oxidatively damaged proteins, as well as involved in regeneration of antioxidants (Atukeren et al. 2010). Many studies on the antioxidative effects of α-LA have been performed at the cell level and played an important role in treatment of oxygen-related diseases, but very little research has been conducted at the molecular level, especially its impact on apoptosis. Previous researches indicated that α-LA has a protective effect on oxidative stress that induced by Cd (Zou et al. 2015; Shi et al. 2016); nevertheless, the mechanism of underlies is not very clear. Therefore, in this paper, we have performed animal experiments to further verify the role of α-LA in Cd-induced kidney damage in rats. We tried to determine whether α-LA exerts its protective effects by inhibiting excessive apoptosis of renal cells.
Material and methods
Chemicals and antibodies
All of the chemicals were the highest grade available. α-LA and cadmium acetate (CdAc2) were purchased from Sigma-Aldrich (St. Louis, MO, USA). PARP, cleaved caspase-3, cleaved caspase-9, caspase-12, caspase-7, caspase-8, β-actin, bcl-2, and bax were obtained from Cell Signaling Technology (Boston, MA, USA). The bicinchoninic acid protein assay kit and enhanced chemiluminescence (ECL) were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All the antioxidant enzyme detection kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Other reagents and chemicals in local procurement and all the reagents were of analytical grade.
Animals and experimental design
Twenty-four female SD rats (body weight, 60–70 g) were purchased from the medical center of Jiangsu University (Jiangsu, China). Before the experiments were started, all the rats were acclimated for 1 week under well-controlled temperature and light conditions, with the temperature set at 23 ± 2 °C and a 12/12-h light/dark cycle (lights on from 07:00 to 19:00). The rats were housed in polypropylene cages, with three rats in each cage. Procedures involving the rats and their care were conducted in the Guidelines for Ethical Control and Supervision in the Care and Use of Animals.
A total of 24 rats were randomly assigned into four groups of six animals each as follows: group 1, control group (this group was given free access to double-distilled water and food); group 2, Cd group (in this group, Cd was added to drinking water at a concentration of 50 mg/L); group 3, Cd+α-LA group (in this group, Cd was added to drinking water at a concentration of 50 mg/L CdAc2, and 50 mg/kg body weight α-LA was administered via gavage); and group 4, α-LA group (in this group, 50 mg/kg body weight α-LA was administered via gavage). The rats in the Cd+α-LA group and α-LA group underwent gavage of α-LA; in addition, the rats in the control group and Cd group were given double-distilled water by gavage at the same time every day. Body weight, the amount of water consumed, and food intake were recorded every day. The breeding environment was kept clean and dry, and the temperature was controlled in the optimum range. Rats were examined daily for 12 weeks and sacrificed by cervical dislocation 24 h after the last treat.
Biochemical assays
We collected blood samples from the jugular vein in rats with rats under anesthesia at 4, 8, and 12 weeks after starting treatment. Then, the samples were immediately biochemical assayed and analyzed through automatic methods and equipments (American Beckman Coulter AU480 automatic biochemical analyzer, Beckman Coulter, USA).
Oxidative stress assessment
The renal cortex was extracted to determine the concentrations of the antioxidant enzymes present. The procedures were conducted in accordance with the instructions provided in the kit from Nanjing Jiancheng Bioengineering Institute. The detection kit can be used to measure the levels of superoxide dismutase (Cu/Zn-SOD and Mn-SOD Assay Kit with WST-8), glutathione peroxidase (GSH-Px, DTNB colorimetric method), catalase (CAT, ammonium molybdate colorimetry), glutathione (GSH, DTNB colorimetric method), and malondialdehyde (MDA, thiobarbituric acid colorimetric method).
Assessment of the levels of Cd present in the kidney of the rats
An adequate amount of rat renal cortical tissue was sheared off and dried. Then, 5 mL of concentrated nitric acid was added and the tissue was digested in a microwave digestion instrument. Once the digestion was complete, the volume of the solution was adjusted to 10 mL by adding double-distilled water, and atomic absorption spectroscopy (Optima 7300 DV, PerkinElmer, USA) was used to determine the Cd levels in the kidney.
Histological analysis
For light microscopy analysis, the tissue samples were taken from kidney of rat and fixed in 10% buffered formalin for 24 h at 4 °C. Then, a series of ethanol were used for dehydration of the kidney samples, after which they were immersed in xylol and embedded in paraffin with an automatic processor. The paraffin blocks were sectioned to 4-μm thickness with a rotary microtome. The obtained tissue sections were collected on glass slides and stained with hematoxylin and eosin. All the samples were viewed and photographed using a fluorescence microscope (Leica 2500; Leica Corporation, Germany).
For transmission electron microscopy analysis, the kidney samples were fixed in 2.5% glutaraldehyde fixative for 12 h and then post-fixed in 1% osmium tetroxide fixative for 0.5 h at 4 °C. Then, the samples were rinsed with PBS, dehydrated in an ethanol series of 5–100%, and embedded in Epon 812. Ultramicrotome was used to obtain ultrathin sections and then dyed with uranyl acetate and lead citrate. After completion of dyed, these ultrathin sections were observed by transmission electron microscopy using a Tecnai 12 (FEI, USA). Randomly selected three samples from each group for transmission electron microscopy analysis.
Western blot analysis
Kidney proteins were extracted by ultrasonication with the radio-immunoprecipitation assay lysis buffer (Beijing Applygen Technologies Inc) and a protease inhibitor cocktail. Homogenates were centrifuged for 10 min (1500×g, 4 °C). After centrifugation, the protein concentration of the supernatant was assayed using the Pierce bicinchoninic acid protein assay kit (Beyotime, Jiang Su, China). Total proteins (20–40 μg) were separated on 8–12% SDS-polyacrylamide gels and transferred to 0.22 μm PVDF membranes. Then, the membrane was incubated with blocking solution containing 5% non-fat milk in TBST before it was incubated with primary antibodies against caspase-9, caspase-3, PARP, bcl-2, bax, Fas, FasL, caspase-8, caspase-12, or β-actin (1:1000 dilution) overnight at 4 °C. Then, the membranes were incubated with the appropriate secondary antibodies (1:5000 dilution). After that, all the membranes were washed six times with “’TBST, shaking for 5 min each time, and then the protein bands detection were performed by enhanced chemiluminescence reagents. Finally, the proteins were detected using the Image Lab software (Bio-Rad, Hercules, CA, USA), and the proteins levels were determined by standard scanning densitometry and the density of each band was normalized to its respective β-actin.
RNA extraction, cDNA synthesis, and quantitative real-time PCR
Total RNA was extracted from kidney tissue using the Trizol® reagent according to the instruction. About 900 ng RNA was used to synthesize cDNA by using the PrimeScript® RT reagent kit with gDNA Eraser according to the manufacturer’s instructions. Gene expression level was measured using a real-time PCR system (Applied Biosystems 7500, USA). Following the kit instructions, the reaction was performed with a SYBR® Premix Taq™II kit (Takara, Japan) and the primers were designed using the Primer Premier 5 software with β-actin as the endogenous control (Table 1). The qRT-PCR reaction was performed in triplicate for each sample, and the mean value was considered to represent the messenger RNA (mRNA) level. Analysis of the relative mRNA levels was carried out using the 2−△△CT method.
Statistical analysis
Experiments were performed at least three times with similar results. The mean ± SD value was determined by one-way ANOVA using the SPSS 22.0 statistical software (SPSS, Chicago, USA). The results were considered significant at P < 0.05 and highly significant at P < 0.01.
Results
Effects of cadmium on body weight and food/water consumption of rats
The data of body weight is shown in Fig. 1. During the test, the rats had no obvious symptoms and no death. The weight of rats in the early stage of the test (0–5 weeks) had no significant change between each group but in the late test the body weight growth trend in Cd group began to significantly lower than the control group; moreover, the body weight growth trend in Cd+α-LA group significantly higher compared with the Cd group.
The results of food consumption are shown in Table 2. During the test, the food consumption of rats is normal; no poisoning occurred. In the early stage of the test, the food consumption had no obvious change between each group, but in the late test, the food intake of rats in Cd group began to decline compared to control group. In addition, we find that the food intake of rats has a tendency to increase when co-treated with Cd and α-LA, compared to Cd group. The water consumption data of rats are presented in Table 3. As we can see in the table, the amount of water consumed in Cd group and Cd+α-LA group was generally less than the control group or α-LA group. Moreover, the results showed an interesting phenomenon that the water consumption of rats in Cd+α-LA group significant lower than Cd group.
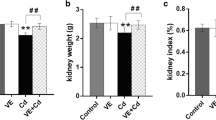
Determination of the absolute/relative kidney weight
None of the rats died or exhibited any sign of toxicity, and pathological changes in the kidney, if present, were not visible to the naked eye in any of the rats. The absolute/relative kidney weights are presented in Table 4. The relative weight of the kidney is represented by the viscera index (OSI). The results indicate that there was a significant increase in the absolute kidney weights and kidney OSI after exposure to Cd (P < 0.05). However, α-LA treatment was associated with a decrease in kidney weight and OSI, but the difference was not significant compared with the Cd group.
Assessment of renal function in rats
In order to evaluate the renal function, we measured the concentration of creatinine and blood urea. The determination results are shown in Table 5. The data indicated that compared to the control group, the creatinine concentrations and serum urea were higher in Cd group significantly, at the time of 4 and 12 weeks. On the contrary, the serum urea and creatinine levels were significantly lower in Cd+α-LA group than in Cd group. No significant changes were found between Control group and α-LA group.
Level of oxidative stress in the rat kidney
The oxidative stress level is shown in Table 6 and Fig. 2. Compared to control group, the activities of CuZn/Mn-SOD, CAT, GSH-Px, and the level of GSH were significantly lower in the Cd group, but the MDA content was highly marked, whereas the activity of antioxidants and the level of GSH were higher than Cd group significantly in Cd+α-LA group; in the meantime, the MDA content presents the tendency of decrease. In α-LA group compared with control group, there was no significantly difference on these indicators.
MDA levels and activities of antioxidative enzymes in the renal cortex of rats. (a) Effect of Cd on the MAD level in the renal cortex of rats. (b) GSH content in the renal cortex of rats. (c) CuZn/Mn-SOD activity in the renal cortex of rats. (d) CAT activity in the renal cortex of rats. (e) GSH-Px activity in the renal cortex of rats. Single asterisk and single number sign indicate a statistically significant difference at P < 0.05. Double asterisks and double number signs indicate a statistically significant difference at P < 0.01. “ns” indicates that there was no significant difference compared to the control group
Levels of Cd in kidney tissue
The results of atomic absorption spectrometry, as presented in Table 7, showed that Cd accumulation was significantly higher in the renal cortex of the Cd group than in the control group. Moreover, the Cd content was markedly lower in the Cd+α-LA group than in Cd group. These findings indicate that α-LA can reduce the accumulation of Cd in the kidney, probably because of its ability to chelate metal ions.
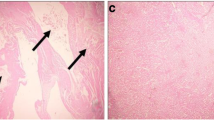
Histopathological observations
As we can see from the results of paraffin section, the renal tissue of control group (Fig. 3a, e) and the α-LA group (Fig. 3d, h) showed a normal organization structure, but the histopathological findings in the Cd group rats revealed typical changes associated with Cd accumulation, including inflammatory cell infiltration, tubular cell necrosis, regeneration, protein cast formation, congestion, and hemorrhage (Fig. 3b, f). In the Cd+α-LA group, as shown in Fig. 3c, g, inflammatory cell infiltration and changes in the pathological features of the glomerulus are not obviously visible, and the tubular cell necrosis rate is also lower than that in the Cd group.
Histopathological changes in the kidneys of rats. a, e Kidney sections of the control group showed no obvious changes. b, f Kidney sections of the Cd group showed inflammatory cell infiltration, tubular cell necrosis, protein cast formation, congestion, and hemorrhage. c, g Kidney sections of the co-treatment group showed a significant reduction in tubular cell necrosis and protein cast formation compared with the Cd group. A slight dilatation of Bowman’s space was also observed. d, h Kidney sections of the α-LA group showed renal tubular cells presenting with small dark nuclei and reduced cytoplasm. No obvious pathological changes were observed in the glomeruli
Ultrastructure examination
As we can see in the pictures of the sections derived from the rats in control group, the nuclear membranes were intact and chromatin was evenly; in the meantime, nuclear pores were clear (Fig. 4a). Moreover, the complete mitochondrial structure and the mitochondrial ridge are clearly visible (Fig. 4e). But, the morphologic changes were obvious in the Cd group. The significant alterations were nuclear pycnosis, severe deformation, fusion of the chromatin edge, and the presence of a large number of vacuoles in cells (Fig. 4b). In addition, the mitochondrial matrix is not visible; the mitochondrial cristae are ruptured, deformed, blurred, and even invisible; and expansion and formation of a cavity are observed (Fig. 4f). In the Cd+α-LA group, mild nuclear pycnosis is observed; chromatin distribution is relatively uniform; the degree of the aggregation of the edge of the nuclear membrane is reduced (Fig. 4c); the mitochondrial morphology is basically intact; and the mitochondrial matrix density is higher but swelling and cracking are dramatically reduced (Fig. 4g) compared with the Cd group. Moreover, in the α-LA group, apart from slight puckering of the nucleus and a small degree of the aggregation of the chromatin edge, no obvious pathological changes were observed (Fig. 4d) and no obvious anomalies were observed in the mitochondria (Fig. 4h).
Expression of apoptosis-related proteins in the kidney tissue
We analyzed the activity of the iconic protein PARP, bcl-2, cleaved caspase-3, caspase-9, and bax in the mitochondrial apoptosis pathway (Figs. 5 and 6). The results show that the cleaved PARP, cleaved caspase-3, caspase-9, and bax levels were markedly increase in the Cd group, while the bcl-2/bax ratio was decrease. Moreover, a significant decrease in cleaved caspase-3, caspase-9, cleaved PARP, and bax levels in Cd+α-LA group were observed; there was also an increase in bcl-2 level compared with Cd group, as well as the bcl-2/bax ratio was increased.
Expression level of relevant proteins in the mitochondrial apoptosis pathway. PARP (a) and cleaved caspase-3 and cleaved caspase-9 (c) protein levels were assessed by western blot analysis. b, d, e The results of quantitative analysis, with the images depicting the results of three independent experiments (mean ± SEM, n = 3). *P < 0.05; **P < 0.01 vs. the control group by one-way ANOVA. ## P < 0.01 vs. the Cd group by one-way ANOVA
The protein levels of bcl-2 and bax. a Expression levels of bax and bcl-2 in the renal cortical cells of rats was detected by western blot analysis. Blots for bcl-2 and bax (b) were semi-quantified using the Image LabTM software. Data are expressed as mean ± SD (n = 3) values. *P < 0.05; **P < 0.01 vs. the control group by one-way ANOVA. ## P < 0.01 vs. the Cd group by one-way ANOVA
We measured the expression of iconic proteins present in the endoplasmic reticulum apoptosis pathway and in the death receptor apoptosis pathway (Figs. 7 and 8). The levels of cleaved caspase-7, caspase-8, caspase-12, Fas, and FasL were obviously higher in the Cd group than in control group. However, there was a significant decrease in the expression of cleaved caspase-7, caspase-8, caspase-12, Fas, and FasL in the Cd+α-LA group compared with Cd group.
Expression level of relevant proteins expressed in the endoplasmic reticulum apoptotic pathway. a Western blot analysis of caspase-12 and caspase-7 expression in the renal cortical cells of rats. Blots for caspase-12 and caspase-7 (b, c) were semi-quantified using the Image LabTM software. Data are expressed as the mean ± SD (n = 3) values. *P < 0.05; **P < 0.01 vs. the control group by one-way ANOVA. ## P < 0.01 vs. the Cd group by one-way ANOVA
The expression level of relevant proteins in the death receptor apoptotic pathway. (a) Western blot analyses of Fas, FasL, and cleaved caspase-8 in the renal cortical cells of rats. Blots for Fas, FasL, and cleaved caspase-8 (b) were semi-quantified using the Image LabTM software. Data are expressed as the mean ± SD (n = 3) values. *P < 0.05; **P < 0.01 vs. the control group by one-way ANOVA. # P < 0.05; ## P < 0.01 vs. the Cd group by one-way ANOVA
Effect of Cd and α-LA on Fas and FasL mRNA expression
qRT-PCR was used to analyze the transcriptional level of Fas and FasL in the kidney (Fig. 9). Cd exposure induced an increase in the mRNA expression of Fas and FasL. However, the level of Fas and FasL mRNA expression was significantly lower (P < 0.01) in the Cd +α-LA group than in the Cd group.
Expression of Fas and FasL mRNA in the renal cortical cells of rats (n = 8). The mRNA expression of Fas and FasL was examined by real-time PCR analysis. β-Actin was used as a reference gene to normalize values. The relative expression ratio was calculated using the 2−△△CT method. Error bars indicate standard deviation. Values represent the mean ± SD. Statistical significance was analyzed using a factorial ANOVA. *P < 0.05; **P < 0.01 vs. the control group by one-way ANOVA. ##P < 0.01 vs. the Cd group by one-way ANOVA
Discussion
The results of this study demonstrate that Cd has a great influence on rat renal function; in the meantime, Cd exposure causes the decrease of rat body weight and food intake. Compared with the control group, the amount of food intake and the water intake all show the tendency of decline in α-LA group; the reason may be that α-LA has the effect of suppressing appetite, but we are not sure of the decline of water intake due to the loss of appetite. In addition, the water consumption in Cd+α-LA group was significantly lower than Cd group; the reason we guess is the α-LA reaction with Cd in the body and the produced water, so the demand for external water decreased. Moreover, after treatment with Cd, the blood urea nitrogen and creatinine all elevated. After produced in the liver, the urea goes into the blood and then discharged through the kidney by the urine (Klein et al. 2012). If the kidney or liver damage, urea transport would be disturbed and lead to a decrease in blood urea level (Golli et al. 2016). In this study, we have shown that α-LA may indeed have an in vitro protective effect on renal cells exposed to Cd, and this effect may involve inhibition of the apoptotic pathways induced by Cd.
As ingestion is the main route via which animals are exposed to environmental Cd (Thijssen et al. 2007), we used drinking water as the source of Cd in this experiment. The Cd concentration used was based on the results of previous research (Wang et al. 2009a; Chwelatiuk et al. 2006; Antonio Garcia and Corredor 2004). During the experiment, no significant change in body weight was observed in any of the rats, and the Cd groups did not exhibit any poisoning symptoms. However, the renal weight of the Cd group rats indicated an increase in renal tissue hypertrophy, which might indicate inflammation of the kidney (Morales et al. 2006). In the Cd+α-LA group, the absolute weight and relative weight of the kidneys decreased, but the difference relative to the Cd group was not significant. Thus, α-LA may play a role in kidneys. The insignificant results obtained in this study may be related to the dose and treatment time. In agreement with other reported studies, our study has also shown that α-LA plays a role in chelating metal ions. This is evidenced by the significantly lower rate of Cd accumulation in the kidney in the Cd+α-LA group than in the Cd group.
In recent years, many studies have shown that α-LA is a powerful antioxidant on account of α-LA has the effect of chelating metal ions, eliminating ROS and regenerating antioxidant enzymes in the body (Chen et al. 2016). Besides, α-LA being absorbed in the body and to the organizations by the blood and then into the cellular mitochondria further play a role, and in the systemic circulation, α-LA is a cofactor of cell dehydrogenase complex system such as pyruvate dehydrogenase and alpha ketone glutaric acid dehydrogenase (Shi et al. 2016). However, studies have reported that when α-LA is administered intraperitoneally at a dose of 100 mg/kg for 14 days, it induces oxidative stress and cytotoxicity in SD rats; thus, high doses of α-LA could have pro-oxidant effects and might induce oxidative stress and cytotoxicity (Cakatay and Kayali 2005; Kayali et al. 2007). Our results showed that 50 mg/kg α-LA gavage significantly ameliorated the Cd-induced oxidative damage but did not have any other adverse effects; thus, the 50 mg/kg α-LA gavage was not pro-oxidant to the rats. This is an interesting phenomenon that α-LA has the function of antioxidant but sometimes exhibit the ability of pro-oxidant as well; the reason may be associated with different types of oxidant stress, different physiological circumstances, and the working concentration of α-LA (Cakatay 2006).
Studies have shown that excessive ROS will bring adverse effect to the body through oxidative damage, and many studies have reported that oxidative stress plays a very important role in nephrotoxicity induced by Cd (Hu et al. 2016; Erboga et al. 2016). Cd, as an inducer of peroxide, can stimulate the production of excess free radicals. Excess free radicals have many deleterious effects such as DNA damage and apoptosis, and when the generation of ROS exceeds an organism’s capacity to neutralize these molecules, it will cause oxidative stress (Xia et al. 2016). α-LA has the function of scavenging free radicals and chelating metal ion, so it may play a protective role in Cd-induced oxidative stress. Antioxidant enzyme system is the first line of defense to oxidative stress as they are involved in the metabolism of oxygen radicals to produce innocuous byproducts (Ugusman et al. 2011). MDA is considered to be one of the biomarkers of oxidative stress in recent years because the MDA content is a momentous indicator of lipid peroxidation, and it is also a sensitive diagnostic index of diagnosis in cell oxidative damage (Zhang et al. 2014). GSH is a non-enzymatic antioxidant, and it can inhibit free radical formation; in addition, GSH play a vital role on defense against ROS (Liu et al. 2008; Loro et al. 2012). In addition, other antioxidant enzymes, such as GSH, uric acid, CAT, GSH-Px, and CuZn/Mn-SOD, are also believed to be a part of anti-ROS defense systems (Xia et al. 2016). Therefore, in order to evaluate the damage caused in the renal cortical cells of rats, we measured the levels of CuZn/Mn-SOD, MDA, GSH, GSH-Px, and CAT. The results showed that α-LA protected renal cortical cells from Cd-induced injury by increasing the activity of CuZn/Mn-SOD, CAT, and GSH-Px and the GSH content. Besides, α-LA have a protection for renal by reducing the MDA content. The underlying mechanism may involve α-LA-attenuated ROS generation in renal cortical cells. In the last 10 years, some studies indicate that antioxidants have the certain protective effect on heavy metal toxicity (Zhang et al. 2012); α-LA as an antioxidant is also one of the hot researches.
We also performed histological and ultrastructural analysis in order to verify the protective effects of α-LA on Cd-induced injury in renal cortical cells. The histological and ultrastructural observations were indicative of kidney injury after Cd exposure. Moreover, we found that Cd-induced tissue damage was significantly ameliorated in the Cd+α-LA group. Studies have reported that α-LA can protect cells against oxidative stress and cytotoxicity induced by Cd (Xu et al. 2015). In agreement with their findings, our results also showed that α-LA protects rat renal cortical tissue from Cd-induced damage. However, it was not clear whether this protective effect was brought about via inhibition of renal cell apoptosis by α-LA. Therefore, we analyzed the expression of apoptosis-related proteins in the different groups.
Studies have reported that the apoptosis induced by Cd will affect the structure and function of mitochondria and endoplasmic reticulum (Hajnoczky et al. 2006; Wang et al. 2009b). Moreover, some studies have showed that apoptosis induced by Cd may be caused by intracellular calcium ion imbalances (Xie et al. 2010). The bcl-2 is an important protein in apoptotic pathways and plays an important regulatory role in the process. On the contrary, bax is a promote apoptosis protein that relate to the release of cytochrome C from mitochondrion (Adefolaju et al. 2014). The death receptor pathway may be the key apoptotic pathway associated with Cd-induced damage in organisms; in addition, Fas and FasL are the important proteins in the death receptor pathway with the function of mediating apoptosis (Gregory et al. 2011). There are some studies that have shown that the effects of Cd in renal cell apoptosis (Gao et al. 2013), but it is unclear whether the Fas/FasL pathway is involved in Cd-induced kidney tissue injury and whether α-LA plays a protective role.
In this study, we used western blot analysis to detect the expression of apoptosis related proteins and we observed an increase in the level of cleaved PARP, Fas, FasL, and cleaved caspase-3, caspase-7, caspase-8, caspase-9, and caspase-12 in the Cd group. The results indicate that Cd-induced renal injury in rat may by activating the mitochondrial apoptosis pathway, death receptor pathway and endoplasmic reticulum apoptotic pathway in the renal cortical cells. Moreover, the activity of those pro-apoptotic proteins were found to be decreased in the Cd+α-LA group, Furthermore, qRT-PCR analyses showed that the mRNAs of Fas and FasL in the kidney of the Cd group were significantly higher than those in the Cd+α-LA group. These findings show that α-LA inhibits Cd-induced apoptosis via all three apoptosis pathways. In addition, the western blotting results for bcl-2 and bax also confirm these findings.
In conclusion, our results indicate that the accumulation of Cd will result in serious oxidative damage in the kidney of rats. Moreover, α-LA seems to have a protective effect against Cd-induced renal damage, oxidative stress, and apoptosis. The antioxidant, Cd chelation and antiapoptotic activities of α-LA can be considered as key factors associated with the alleviation of nephrotoxicity. Therefore, α-LA is an effective agent for the prevention of renal injury and dysfunction induced by Cd.
Abbreviations
- α-LA:
-
Alpha-lipoic acid
- PARP:
-
Poly-ADP-ribose polymerase
- ECL:
-
Enhanced chemiluminescence
- DTNB:
-
5,5′-Dithiobis-(2-nitrobenzoic acid)
- PVDF:
-
Polyvinylidene fluoride
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- CuZn/Mn-SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- GSH-Px:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- MDA:
-
Malondialdehyde
- OSI:
-
Viscera index
- ROS:
-
Reactive oxygen species
References
Adefolaju GA, Theron KE, Hosie MJ (2014) Effects of HIV protease, nucleoside/non-nucleoside reverse transcriptase inhibitors on Bax, Bcl-2 and apoptosis in two cervical cell lines. Biomed Pharmacother 68:241–251
Antonio Garcia T, Corredor L (2004) Biochemical changes in the kidneys after perinatal intoxication with lead and/or cadmium and their antagonistic effects when coadministered. Ecotoxicol Environ Saf 57:184–189
Atukeren P, Aydin S, Uslu E, Gumustas M, Cakatay U (2010) Redox homeostasis of albumin in relation to alpha-lipoic acid and dihydrolipoic acid. Oxidative Med Cell Longev 3:206–213
Cakatay U (2006) Pro-oxidant actions of alpha-lipoic acid and dihydrolipoic acid. Med Hypotheses 66:110–117
Cakatay U, Kayali R (2005) Plasma protein oxidation in aging rats after alpha-lipoic acid administration. Biogerontology 6:87–93
Chen K, Yan B, Wang F, Wen F, Xing X, Tang X, Shi Y, G. LE (2016) Type 1 5′-deiodinase activity is inhibited by oxidative stress and restored by alpha-lipoic acid in HepG2 cells. Biochem Biophys Res Commun 472:496–501
Chwelatiuk E, Wlostowski T, Krasowska A, Bonda E (2006) The effect of orally administered melatonin on tissue accumulation and toxicity of cadmium in mice. J Trace Elem Med Biol 19:259–265
Duan P, Hu C, Butler HJ, Quan C, Chen W, Huang W, Tang S, Zhou W, Yuan M, Shi Y, Martin FL, Yang K (2016) Effects of 4-nonylphenol on spermatogenesis and induction of testicular apoptosis through oxidative stress-related pathways. Reprod Toxicol 62:27–38
El-habit OH, Abdel Moneim AE (2014) Testing the genotoxicity, cytotoxicity, and oxidative stress of cadmium and nickel and their additive effect in male mice. Biol Trace Elem Res 159:364–372
Erboga M, Kanter M, Aktas C, Sener U, Fidanol Erboga Z, BozdemirDonmez Y, Gurel A (2016) Thymoquinone ameliorates cadmium-induced nephrotoxicity, apoptosis, and oxidative stress in rats is based on its anti-apoptotic and anti-oxidant properties. Biol Trace Elem Res 170:165–172
GAO D, Xu ZE, Zhang X, Zhu C, Wang Y, Min W (2013) Cadmium triggers kidney cell apoptosis of purse red common carp (Cyprinus carpio) without caspase-8 activation. Developmental & Comparative Immunology 41:728–737
Golli NE, Jrad-Lamine A, Neffati H, Dkhili H, Rahali D, Dallagi Y, El May MV, El Fazaa S (2016) Impact of e-cigarette refill liquid exposure on rat kidney. Regul Toxicol Pharmacol 77:109–116
Gregory MS, Hackett CG, Abernathy EF, Lee KS, Saff RR, Hohlbaum AM, Moody KS, Hobson MW, Jones A, Kolovou P, Karray S, Giani A, John SW, Chen DF, Marshak-Rothstein A, Ksander BR (2011) Opposing roles for membrane bound and soluble Fas ligand in glaucoma-associated retinal ganglion cell death. PLoS One 6:e17659
Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M (2006) Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium 40:553–560
Hu H, C. W, Jin Y, Meng Q, Liu Q, Liu K, Sun H (2016) Alpha-lipoic acid defends homocysteine-induced endoplasmic reticulum and oxidative stress in HAECs. Biomed Pharmacother 80:63–72
Jarup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208
Kayali R, Cakatay U, Kiziler AR, Aydemir B (2007) Effect of alpha-lipoic acid supplementation on trace element levels in serum and in postmitotic tissue in aged rats. Med Chem 3:297–300
Klein JD, Blount MA, Sands JM (2012) Molecular mechanisms of urea transport in health and disease. Pflugers Arch 464:561–572
Liu N, Yan B, YQ. L, Q. W, L. W (2008) Effects of Cd2+ on glutathione system of hepatopancreas and gills in freshwater crab Sinopotamon yangtsekiense. Huan Jing Ke Xue 29:2302–2307
Loro VL, Jorge MB, Silva KR, Wood CM (2012) Oxidative stress parameters and antioxidant response to sublethal waterborne zinc in a euryhaline teleost Fundulus heteroclitus: protective effects of salinity. Aquat Toxicol 110-111:187–193
Manquian-Cerda K, Escudey M, Zuniga G, Arancibia-Miranda N, Molina M, Cruces E (2016) Effect of cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plantlets grown in vitro. Ecotoxicol Environ Saf 133:316–326
Morales AI, Vicente-Sanchez C, Jerkic M, Santiago JM, Sanchez-Gonzalez PD, Perez-Barriocanal F, Lopez-Novoa JM (2006) Effect of quercetin on metallothionein, nitric oxide synthases and cyclooxygenase-2 expression on experimental chronic cadmium nephrotoxicity in rats. Toxicol Appl Pharmacol 210:128–135
Pathak N, Mitra S, Khandelwal S (2013) Cadmium induces thymocyte apoptosis via caspase-dependent and caspase-independent pathways. J Biochem Mol Toxicol 27:193–203
Shi, C., Zhou, X., Zhang, J., Wang, J., Xie, H. & Wu Z. 2016. α-Lipoic acid protects against the cytotoxicity and oxidative stress induced by cadmium in HepG2 cells through regeneration of glutathione by glutathione reductase via Nrf2/ARE signaling pathway. Environmental Toxicology and Pharmacology.
Thijssen S, Cuypers A, Maringwa J, Smeets K, Horemans N, Lambrichts I, Van Kerkhove E (2007) Low cadmium exposure triggers a biphasic oxidative stress response in mice kidneys. Toxicology 236:29–41
Ugusman A, Zakaria Z, Hui CK, Nordin NA (2011) Piper sarmentosum inhibits ICAM-1 and Nox4 gene expression in oxidative stress-induced human umbilical vein endothelial cells. BMC Complement Altern Med 11:31
Wang L, Chen D, Wang H, Liu Z (2009a) Effects of lead and/or cadmium on the expression of metallothionein in the kidney of rats. Biol Trace Elem Res 129:190–199
Wang L, Wang H, Li J, Chen D, Liu Z (2011) Simultaneous effects of lead and cadmium on primary cultures of rat proximal tubular cells: interaction of apoptosis and oxidative stress. Arch Environ Contam Toxicol 61:500–511
Wang SH, Shih YL, Lee CC, Chen WL, Lin CJ, Lin YS, Wu KH, Shih CM (2009b) The role of endoplasmic reticulum in cadmium-induced mesangial cell apoptosis. Chem Biol Interact 181:45–51
Wang T, Wang Q, Song R, Zhang Y, Yang J, Wang Y, Yuan Y, Bian J, Liu X, GU J, Zhu J, Liu Z (2016) Cadmium induced inhibition of autophagy is associated with microtubule disruption and mitochondrial dysfunction in primary rat cerebral cortical neurons. Neurotoxicol Teratol 53:11–18
Wlostowski T, Krasowska A, Bonda E (2008) Joint effects of dietary cadmium and polychlorinated biphenyls on metallothionein induction, lipid peroxidation and histopathology in the kidneys and liver of bank voles. Ecotoxicol Environ Saf 69:403–410
Xia L, Chen S, Dahms HU, Ying X, Peng X (2016) Cadmium induced oxidative damage and apoptosis in the hepatopancreas of Meretrix meretrix. Ecotoxicology 25:959–969
Xie Z, Zhang Y, Li A, P L, Ji W, Huang D (2010) Cd-induced apoptosis was mediated by the release of Ca2+ from intracellular Ca storage. Toxicol Lett 192:115–118
Xu Y, Zhou X, Shi C, Wang J, Wu Z (2015) α-Lipoic acid protects against the oxidative stress and cytotoxicity induced by cadmium in HepG2 cells through regenerating glutathione regulated by glutamate-cysteine ligase. Toxicol Mech Methods 25:596–603
Zhang H, Cai C, Shi C, Cao H, Han Z, Jia X (2012) Cadmium-induced oxidative stress and apoptosis in the testes of frog Rana limnocharis. Aquat Toxicol 122-123:67–74
Zhang H, Pan L, Tao Y (2014) Antioxidant responses in clam Venerupis philippinarum exposed to environmental pollutant hexabromocyclododecane. Environ Sci Pollut Res Int 21:8206–8215
Zou H, Liu X, Han T, Hu D, Yuan Y, Gu J, Bian J, Liu Z (2015) Alpha-lipoic acid protects against cadmium-induced hepatotoxicity via calcium signalling and gap junctional intercellular communication in rat hepatocytes. J Toxicol Sci 40:469–477
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No. 2016YFD0501208) and the National Natural Science Foundation of China (Nos. 31101866, 31372495, and 31302058). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We are thankful to Dr. Maozhi Hu of Testing Center of Yangzhou University for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Procedures involving the rats and their care were conducted in the Guidelines for Ethical Control and Supervision in the Care and Use of Animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Tongwang Luo and Gang Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Luo, T., Liu, G., Long, M. et al. Treatment of cadmium-induced renal oxidative damage in rats by administration of alpha-lipoic acid. Environ Sci Pollut Res 24, 1832–1844 (2017). https://doi.org/10.1007/s11356-016-7953-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7953-x