Abstract
Cadmium (Cd), a widely distributed heavy metal, is extremely toxic to the kidney. Vitamin E (VE) is an important antioxidant in the body. It is known that VE exerts a protective effect on renal oxidative damage caused by Cd, but the effect and mechanism of VE on apoptosis are not fully understood. Thus, we conducted this study to explore the protective effect of VE on Cd-induced renal apoptosis and to elucidate its potential mechanism. Thirty-two 9-week-old male Sprague-Dawley rats were randomly divided into four groups, namely control, VE (100 mg/kg VE), Cd (5 mg/kg CdCl2), and VE + Cd (100 mg/kg VE + 5 mg/kg CdCl2), and received intragastric administration of Cd and/or VE for 4 weeks. The results showed that Cd exposure significantly reduced the weight of the body and kidney, elevated the accumulation of Cd in the kidney as well as the levels of BUN and Scr in serum, caused renal histological alterations, decreased the GSH and T-AOC contents and antioxidant enzyme (SOD, CAT, GSH-PX) activities, and increased renal MDA content. And the increased number of TUNEL-positive cells by Cd was accompanied by upregulated mRNA and protein expressions of apoptotic regulatory molecules (Bax, Caspase-3, GRP94, GRP78, Caspase-8) and downregulated Bcl-2 expressions. However, the combined treatment of Cd and VE could restore the above parameters to be close to those in the control rats. In conclusion, VE supplement could alleviate Cd-induced rat renal damage and oxidative stress through enhancing the antioxidant defense system and inhibiting apoptosis of renal cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd), a superfluous trace element and naturally occurring heavy metal, is related to many industrial processes [1]. It is an environmental and occupational pollutant and ranks seventh on the priority list of hazardous substances issued by the Agency for Toxic Substances and Disease Registry (ATSDR) [2]. Humans are inevitably exposed to Cd in a variety of ways, such as ingestion of food and water contaminated with Cd and inhalation of Cd-contaminated cigarette smoke, as well as occupational risks from mining, electroplating processes, and the production of batteries and electronic products [3, 4].
Cd has a long biological half-life of 10–30 years, and therefore, its content accumulates in organisms with age [5, 6]. Cd can cause serious toxicity to multiple tissues, such as nephrotoxicity, hepatotoxicity, and reproduction toxicity, or even cancer [7,8,9]. The kidney is the main target organ for Cd toxicity, and Cd deposits in proximal tubular epithelial cells via glomerular filtration, impairing the structure and function of the kidney [5, 10]. Previous studies have shown that oxidative stress and apoptosis were involved in Cd-induced nephrotoxicity [11,12,13].
Oxidative stress is essentially an imbalance of oxidation and antioxidant levels in the body due to the consumption of antioxidants or the accumulation of reactive oxygen species (ROS) [14]. Exposure to Cd induces excessive ROS production, such as superoxide anions, hydrogen peroxides, and hydroxyl radicals, which causes damage to DNA, proteins, and lipids and leads to oxidative stress through depleting GSH and inhibiting the antioxidant defense system [10]. Cd is a non-redox metal and cannot generate free radicals directly. However, it can lead to excessive ROS production and oxidative damage [15] via disrupting the redox balance in vivo in a variety of ways, including replacing redox active metals (such as iron and copper) from some cellular proteins, exhausting free radical scavengers, decreasing the activity of antioxidant enzymes, and inhibiting the electron transport chain.
Apoptosis is considered a programmed cell death that can regulate cell growth and development [5], and the disorder of apoptosis can lead to the occurrence of various diseases. Numerous studies in vivo and in vitro have shown that Cd induces cell apoptosis of the testis, liver, and kidney [7,8,9, 16, 17], and the underlying mechanisms include (1) excessive ROS generation, directly attacking the mitochondrial membrane, decreasing mitochondrial membrane potential, releasing apoptotic factors, and then activating caspase to cause apoptosis [12]; (2) destroyed calcium homeostasis and increased concentration of calcium ions, leading to endoplasmic reticulum stress (ERS) and ER-mediated apoptosis [18]; and (3) activation of death receptor pathways to induce apoptosis [19].
Vitamin E (VE), an essential trace element and a direct scavenger for superoxide radicals and hydroxyl radicals, protects cell membranes and lipoproteins from lipid peroxidation primarily by disrupting lipid-free radical chain reactions [20]. The available data have indicated that VE has a protective effect on the toxicity of Cd through enhancing antioxidant capability [21,22,23,24,25]. VE decreases the level of lipid peroxidation by increasing the level of glutathione in the small intestine [23], improves the activity of antioxidant enzymes, and attenuates oxidative stress in the liver and kidney of rats exposed to Cd [24, 25]. However, studies on the protective effect of VE on Cd-induced apoptosis and its mechanism are limited. Thus, we conducted this study to explore the possible mechanism of VE against Cd-induced oxidative stress and apoptosis of the kidney by histopathological techniques, atomic absorption spectrophotometry, biochemical method, TUNEL assay, relative real-time fluorescent quantitative PCR (RT-qPCR), and Western blotting analysis. The outcomes from the present study may provide references for further study on the mechanism of VE mitigating renal damages caused by Cd.
Materials and Methods
Chemicals
Cadmium chloride (99.99%, CdCl2) was purchased from Sigma-Aldrich (USA, 202908), and Vitamin E (95%, α-tocopherol) was obtained from ACROS (Belgium, 428120250). Commercial kits for testing biochemical parameters of renal function and oxidative stress were purchased from Nanjing Jiancheng Bioengineering Institute of China (Nanjing, China). All chemicals and biochemicals used in the study were of analytical grade. The primary antibodies used for Western blotting are as follows: Bcl-2 (Abcam, ab196495), Bax (Abcam, ab32503), Caspase-3 (Abcam, ab184787), GRP94 (Cell Signaling Technology, 20292T), GRP78 (Abcam, ab108613), Caspase-8 (Cell Signaling Technology, 4790T), and β-actin (Cell Signaling Technology, 4970T).
Animals and Treatments
Thirty-two 9-week-old healthy male Sprague-Dawley rats weighing 280–300 g were purchased from Dashuo Biological Technology Company (Chengdu, China). Animals were kept in separate cages, raised under standard (25 ± 2 °C and 12-h light-dark cycle) and specific pathogen-free conditions, and fed with a standard pellet diet or water ad libitum. After 7 days of acclimatization, the animals were randomly divided into four groups, namely control, VE, Cd, and VE + Cd groups. The VE group was treated with VE at a dose of 100 mg/kg body weight (b.w), and the Cd group was given CdCl2 at a dose of 5 mg/kg b.w, and the VE + Cd group received VE (100 mg/kg b.w) 1 hour before and Cd (5 mg/kg b.w) intoxication for 28 days. VE dissolved in corn oil [26] or Cd dissolved in fresh distilled water was applied for each rat in a daily oral gavage administration. The control group was treated similarly with the same volume of corn oil. The doses, routes, and periods of administration of VE and Cd were based on previous studies [27, 28]. The experimental animals and procedures were implemented in accordance with the agreement approved by the Animal Care and the Ethics Committee of Sichuan Agricultural University (Approval No:2012–024, Chengdu, China).
Measurement of the Body Weight, Kidney Weight, and Kidney Index
Rats were weighed weekly, and at the end of the treatment, rats were anesthetized with chloral hydrate and sacrificed by cervical decapitation, and then the renal tissues were removed and weighed. Kidney index was calculated by the following formula:
Estimation of Serum Biochemical Parameters (BUN and Scr)
At the 28th day of the experiment, rats in each group were anesthetized, and blood was taken from the orbital vein of rats. Serum samples were obtained after being centrifuged at 3000×g for 10 min for subsequent detection. BUN and Scr levels in serum samples collected above were determined in accordance with the instructions of the commercial kits purchased from Nanjing Jiancheng Bioengineering Institute of China (C013-2, C011-2, Nanjing, China).
Detection of Cd and VE Contents in the Kidney
The collected kidney tissues were dried and wet-ashed and then estimated by atomic absorption spectrophotometry for Cd contents. The specific methods and operations referred to the previous study [29]. The Cd concentrations were expressed as μg/g of wet kidney tissue. The contents of VE in the kidney were detected according to the instructions of biological reagent kits of Nanjing Jiancheng Bioengineering Institute of China (A008, Nanjing, China).
Histopathological Observation
The collected kidney tissues were immediately fixed in 4% buffered formaldehydehyde and then were dehydrated in alcohol, routinely paraffin embedded, and sectioned at 5 μm. Slices were stained with hematoxylin and eosin (H&E) and then were examined under light microscope. Histopathological scoring was performed to evaluate the severity of renal tissue damage. All sections were scored for the degree of histopathological changes, including tubular degeneration, swollen glomerulus, narrowed capsular space, tubular pyknotic nuclei, hyperemia, and hemolysis. The scores were expressed as 0 (no findings) or 1–4 (the higher the score, the more severe the pathological damage). The above histopathological evaluation was mainly based on the reference 28 and performed by a single person.
Assessment of Oxidative Stress Parameters in the Kidney
At the end of the experiment, after the rats were euthanized, the collected kidney tissues were diluted with ice-cold saline and homogenized through a homogenizer to obtain a homogenate, which was centrifuged at 3000×g for 10 min; and then the supernatant was obtained to determine the amount of total protein by Bradford’s method [30]. The activities of renal SOD, CAT, and GSH-PX and contents of GSH, MDA, and T-AOC were assayed using biochemical method according to the biochemical reagent kits (A001-3, A007-1, A005, A006, A003-1, A015-2, Nanjing Jiancheng Bioengineering Institute, China).
TUNEL Assay
Apoptosis was detected by the terminal dUTP nick-end labeling (TUNEL) assay, which detected DNA fragmentation in the nucleus during apoptotic cell death in situ and followed the instructions of the apoptosis detection kit (MK1017 Boster, Wuhan, China). For each section, five fields of 0.064 mm2 (corresponding to five fields at × 400 magnification) of the renal cortex and medulla were analyzed, and the TUNEL-positive cells were counted by Image-Pro Plus 5.1 (USA) image analysis software. Results were expressed as the average of TUNEL-positive cells per 0.064 mm2 area.
Quantitative Real-Time PCR (qRT-PCR) Analysis
The collected kidney tissues were ground with liquid nitrogen and stored at −80 °C. The renal total RNA was extracted through RNAiso Plus according to the manufacturer’s recommendations. Then, the complementary DNA (cDNA) was synthesized with PrimeScript™ RT reagent kit (RR047A, Takara, Japan) according to the manufacturer’s instructions. qRT-PCR was performed with SYB® Premix Ex TaqTM II kit (TAKARA BIO INC.) on LightCycler® 480 Real-Time PCR System. The primer information used for qPCR is listed in Table 1. Rat β-actin was used for the normalization of gene expression. The mRNA relative abundance was calculated with the 2−ΔΔCt method [31].
Western Blotting Analysis
RIPA lysis buffer and BCA protein assay reagent were used to extract proteins and determine protein concentration, respectively. The obtained protein samples were subjected to SDS-PAGE gels and transferred to nitrocellulose filter membranes. After blocking with 5% skimmed milk at room temperature, the membranes were incubated overnight at 4 °C with primary antibody against Bcl-2 (diluted 1:2000), Bax (diluted 1:2000), Caspase-3 (diluted 1:2000), GRP94 (diluted 1:1000), GRP78 (diluted 1:2000), Caspase-8 (diluted 1:1000), and β-actin (diluted 1:1000). And then the membranes were incubated with horseradish peroxidase conjugated secondary antibody. Finally, the membranes were washed with TBST, and protein bands were detected on a ChemiDoc XRS using ECL kit. Then, the expression of proteins is shown to be relative to that of β-actin using quantity analysis.
Statistical Analysis
The significance of difference was analyzed by variance analyses (LSD or Dunnett’s T3) using SPSS 22.0 software (IBM Corp, Armonk, NY, USA) for windows. The results were expressed as means ± standard deviation. Statistically significant differences were considered at P < 0.05 or P < 0.01.
Results
Body Weight, Kidney Weight, and Kidney Index
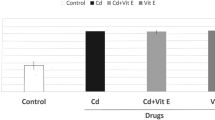
The effects of Cd and VE on body weight in rats are shown in Fig. 1. At the 28th day of the experiment, the body weight of the Cd group was significantly decreased (P < 0.01) compared with that of the control group. However, the body weight of rats in the VE + Cd group was significantly increased (P < 0.01) compared with that in the Cd group and showed no significant difference from the control group (P > 0.05).
Furthermore, at the 28th day of the experiment, compared with that in the control group, the kidney weight in the Cd group was significantly decreased (P < 0.01). But, this value in the VE + Cd group was significantly increased (P < 0.01) when compared with that in the Cd group and displayed no significant difference (P > 0.05) in comparison to that in the control group. In addition, there was no significant difference (P > 0.05) in the kidney index among the four groups.
Cd and VE Concentrations in the Kidney
As shown in Table 2, a significant accumulation (P < 0.01) of Cd in the kidney of the Cd group was observed when compared with that of the control group. Co-treatment with Cd and VE did not cause any significant change (P > 0.05) in Cd accumulation in the kidney when compared with the Cd group. No significant difference (P > 0.05) in Cd accumulation was found between the control group and VE group. Table 1 also showed that the intake of VE resulted in a significant higher level (P < 0.01) of VE in the kidney of the VE and VE + Cd groups in comparison with that of the control and Cd groups. There was no significant difference (P > 0.05) in the VE level of the kidney between the control and Cd groups.
Serum Biochemical Parameters (BUN and Scr)
In comparison with the control group, the levels of BUN and Scr in the Cd and Cd + VE groups were significantly increased (P < 0.01), but no significant difference was noted in the VE group (P > 0.05). Results also showed that BUN and Scr levels of the VE + Cd group were significantly decreased (P < 0.05 or P < 0.01) compared with those of the Cd group. The results are displayed in Fig. 2.
Histopathological Changes in the Kidney
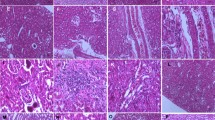
Histopathological observation showed normal renal tissue structure in the control group and VE group (Fig. 3a–b and c–d). In comparison with that of the control group, histopathological alterations were mainly observed in the renal cortex and medulla of the Cd group, including swollen glomerulus along with narrowed capsular space (Fig. 3e), cytoplasmic granule degeneration in the epithelial cells of some renal tubules in the cortex (Fig. 3e), pyknotic nuclei with condensed chromatin in the epithelial cells of proximal convoluted tubules and collecting ducts (Fig. 3e–f), and hemolysis and hyperemia in some areas. The histopathological alterations mentioned above in the VE + Cd group were obviously alleviated as compared with those in the Cd group (Fig. 3g–h). The histopathological scores of the kidney are shown in Table 3.
Histopathological changes in the kidney. Note: (a–b) control group, (c–d) VE group, (e–f) Cd group, (g–h) VE + Cd group. HE staining, × 400, scale bar = 50 μm. PT proximal convoluted tubule, DT distal convoluted tubule, G glomerulus, SG swollen glomerulus, CS capsular space, NCS narrowed capsular space, GD granule degeneration, HE hemolysis, HY hyperemia, CD collecting duct, PN pyknotic nuclei
Oxidative Damage Parameters in the Kidney
As shown in Fig. 4, the renal MDA contents in the Cd and VE + Cd groups were significantly increased (P < 0.01) as compared with those in the control group. Conversely, the contents of renal GSH and T-AOC and activities of SOD, CAT, and GSH-PX in the Cd group and VE + Cd group were all significantly decreased (P < 0.05 or P < 0.01), except CAT activity in the VE + Cd group, in comparison to those in the control group. Importantly, the combination of VE and Cd reversed Cd-induced changes of oxidative stress parameters. The MDA content decreased, and GSH and T-AOC contents as well as SOD and GSH-PX activities increased in the VE + Cd group compared with those in the Cd group (P < 0.05 or P < 0.01). No significant alterations (P > 0.05) were found between the control and VE groups in all these values.
Changes of oxidative damage parameters in the kidney. Note: a MDA contents, b GSH contents, c SOD activities, d CAT activities, e GSH-PX activities, f T-AOC contents. Values are presented with the means ± standard deviation (n = 8). *P < 0.05, **P < 0.01 compared with the control group. #P < 0.05, ##P < 0.01 between the Cd group and VE + Cd group
The Renal Cell Apoptosis by TUNEL Assay
According to Fig. 5, TUNEL-positive cells with yellow or brown nuclei are epithelial cells of uriniferous tubule, mainly distributed in the renal cortex. The number of TUNEL-positive cells in the renal cortex and medulla of the Cd and VE + Cd groups was significantly higher than those in the control group (P < 0.01), while no significant difference (P > 0.05) was noted between the VE and control groups. Moreover, in comparison to that of the Cd group, the number of TUNEL-positive cells in the renal cortex and medulla of the VE + Cd group was significantly declined (P < 0.05) (Fig. 6).
The mRNA Expression Levels of Renal Cell Apoptotic Regulatory Molecules
The mRNA expression levels of renal cell apoptotic regulatory molecules by qRT-PCR are shown in Fig. 7. Compared with those in the control group, the mRNA expression levels of cell apoptotic regulatory molecules such as Bax, Caspase-3, GRP94, GRP78, and Caspase-8 in the Cd and VE + Cd groups were significantly increased (P < 0.05 or P < 0.01), while Bcl-2 expression levels were significantly decreased (P < 0.01). Moreover, in comparison with the Cd group, the VE + Cd group showed a significant decrease (P < 0.05 or P < 0.01) in these parameters mentioned above except for Bcl-2 expression which was increased (P < 0.01). There was no significant difference in these parameters between the VE and control groups (P > 0.05).
The mRNA expression levels of cell apoptotic regulatory molecules in the kidney. Note: a Bcl-2, Bax, Caspase-3; b GRP94, GRP78, Caspase-8. Values are presented with the means ± standard deviation (n = 8). *P < 0.05, **P < 0.01 compared with the control group. #P < 0.05, ## P < 0.01 between the Cd group and VE + Cd group
The Protein Expression Levels of Renal Cell Apoptotic Regulatory Molecules
As shown in Fig. 8, compared with those in the control group, the protein expression levels of Bax, Caspase-3, GRP94, GRP78, and Caspase-8 in the Cd and VE + Cd groups (except for GRP94 protein expression level in the VE + Cd group) were significantly elevated (P < 0.05 or P < 0.01), while Bcl-2 protein expression level was significantly decreased (P < 0.01). Remarkably, the protein expression levels of these factors (Bax, Caspase-3, GRP94, GRP78, and Caspase-8) in the VE + Cd group were significantly lower than those in the Cd group (P < 0.01), and Bcl-2 protein expression level in the VE + Cd group was significantly higher (P < 0.01) than that in the Cd group. No significant differences (P > 0.05) in these values were found between the control and VE groups.
The protein expression levels of cell apoptotic regulatory molecules. Note: a–b The representative band pictures of cell apoptotic regulatory molecules, c–d relative protein expression of cell apoptotic regulatory molecules (relative to β-actin). Values are presented with the means ± standard deviation (n = 8). *P < 0.05, **P < 0.01 compared with the control group. ##P < 0.01 between the Cd group and VE + Cd group
Discussion
Cd Exposure Caused Renal Damages of Rat
Cd is a widely existing environmental pollutant that has high toxicity to the kidney [2, 32,33,34,35]. It is known that, after absorption, Cd enters the liver and binds with metallothionein (MT) to form Cd-MT complexes, which then reaches the kidney via blood circulation [6]. The complexes can be filtered by the glomerulus and reabsorbed into the proximal tubular epithelial cells [36,37,38]. Inside the renal tubules, Cd-MT complexes are degraded by lysosomes and release Cd2+ ions, which are combined with sulfhydryl group (–SH)-containing proteins and impair the kidney [1, 6, 39]. Cd can produce ROS and result in oxidative stress, which is one of the important mechanisms of Cd-induced renal injury [3]. Multiple pathophysiological events have implicated Cd-induced nephrotoxicity. Body weight and renal weight are important indexes in experimentally induced renal toxicity and helpful to understand the prognosis of diseases [28]. Renal damage is directly reflected in increased Cd accumulations along with BUN and Scr levels and its pathological changes [35, 40]. MDA, GSH, and T-AOC contents as well as SOD, CAT, and GSH-PX activities are widely used to evaluate Cd-induced renal oxidative stress [24, 28, 41,42,43]. Our present results showed that, in line with previous reports [28, 34, 40, 44, 45], Cd exposure significantly led to renal damages, which was represented with reduced weights of the body and kidney, increased levels of serum BUN and Scr, as well as histopathological lesions and oxidative stress of the kidney. However, unlike earlier reports, in our study, Cd exposure caused tissue damage not only in the renal cortex but also in the renal medulla, such as obvious hyperemia in the renal medulla and pyknotic nuclei in the epithelial cells of collecting ducts. Moreover, Nazima et al. [28] found that albino Wistar rats which received intragastric administration of CdCl2 (5 mg/kg) for 4 weeks showed changes in glomerular atrophy, dilatation of the renal capsule, and tubular degeneration and necrosis. Tripathi and Srivastav [46] reported that no tissue structure changes were observed in the kidney of rat treated with 5 mg/kg CdCl2 within 2 weeks; after 4 weeks, the glomeruli were swollen, and the renal capsule was narrowed; after 6 weeks, a few glomeruli shrank, and the urinary space enlarged; after 8 weeks, the glomeruli were further atrophic, and the mesangium of Bowman’s capsule was thickened, and the renal tubules were markedly dilated and extensively denatured. And Jihen et al. [45] observed obvious renal tubular necrosis and glomerular dilatation in rats exposed to Cd (200 ppm) for 35 days. It can be seen that the reports of renal histopathological changes induced by Cd were different, which may be related to the time, dosage, route, and animal strain of Cd exposure.
A growing amount of evidence has shown that apoptosis is also an important factor for Cd-induced nephrotoxicity [9, 12, 32, 47]. One of the biochemical hallmarks of apoptosis is the generation of free 3-hydroxyl termini on DNA via cleavage of chromatin into fragments, and the TUNEL method can label exposed termini of DNA to detect apoptosis in situ [48]. Our results showed that Cd exposure significantly increased the number of TUNEL-positive cells both in the cortex and medulla in the kidney of rats, consistent with previous reports [32, 49, 50]. It is noteworthy that the TUNEL-positive cells were mainly distributed in the cortex but also appeared in the renal medulla area (chiefly the epithelial cells of collecting tubules), which coincides with some pyknotic nuclei with condensed chromatin in the epithelial cells of collecting ducts observed in HE staining (because pyknotic nuclei are one of the morphological characteristics of apoptotic cells). The above phenomena indicated that Cd exposure can lead to excessive apoptosis of both the renal cortex and medulla.
Apoptosis is a complex phenomenon, and previous studies have shown that Cd-induced apoptosis involves multiple pathways (including mitochondrial pathway, endoplasmic reticulum pathway, and death receptor pathway) and can be mediated by multiple genes [51,52,53]. Bcl-2 family proteins (such as Bax and Bcl-2) and Caspase-3 play an important role in the intrinsic mitochondrial-mediated intrinsic apoptosis pathway. Among them, pro-apoptotic protein Bax can change mitochondrial permeability and cause apoptosis, while anti-apoptotic protein Bcl-2 can inhibit this process [7, 11]. Caspase-3 as an effector or executioner caspase can cause changes in the morphology and biochemistry of apoptotic cells [54]. The chaperone (GRP94 and GRP78) located in the endoplasmic reticulum can mitigate ERS damage to cells by increasing the folding capacity, otherwise long-term ERS will trigger apoptosis [47, 55]. Caspase-8 is a key protease of death receptor apoptosis pathway and can activate Caspase-3 and initiate apoptosis [56].
A large number of studies in vitro and in vivo have shown that Cd exposure can respectively induce apoptosis of nephrocytes [12], testicular cells [7], and neurons [4] by mitochondrial pathway and renal tubular cell by endoplasmic reticulum and death receptor pathways [53, 57]. In the present study, except Bcl-2 downregulated, all apoptotic regulatory molecules mentioned above were upregulated in the kidney of rat exposed to Cd, further suggesting that Cd-induced apoptosis of renal cells was related to mitochondrial, endoplasmic reticulum, and death receptor pathways.
VE Alleviated Cd-Caused Renal Injuries of Rat
VE, as an antioxidant, is a kind of lipid-soluble vitamin characterized by protecting cell membranes and lipoproteins against lipid peroxidation through breaking the lipid radical chain reaction and also exhibits free radical scavenging properties [14, 20, 58]. The available information indicates that VE has an antioxidant effect on the renal cytotoxicity induced by Cd [24, 40, 42]. Our data suggested that VE could mitigate renal damage and oxidative stress and ameliorate weight loss induced by Cd, coinciding with early reports [24, 40, 42, 59, 60]. Since weight loss caused by Cd is at least partially related to kidney toxicity leading to excessive loss of water, salts, and proteins [28], the protective effect of VE on the renal damage in the Cd-toxic rats may be one of the reasons why VE can improve the weight loss caused by Cd.
Our findings showed that VE supplied at 100 mg/kg for 4 weeks could not affect the Cd accumulation in the rat kidney exposed to 5 mg/kg Cd via intragastric administration. Similar to our results, Rambeck et al. found that VE supplementation did not affect the Cd accumulation in the chicken kidney [61]. However, the report by Kim et al. showed that continuous supplementation of VE with 40 and 400 mg/kg diet for 10 and 20 weeks significantly decreased the content of renal Cd of the rat treated with 50 ppm Cd2+ in the drinking water [59]. Tandon et al. also reported that the renal content of Cd in the rats intraperitoneally administrated with Cd was significantly dropped in the rats co-exposed to Cd (intraperitoneally) and VE (intramuscularly) for 7 days [62]. This discrepancy may be due to the duration of VE supplementation, exposure route, and time of administered Cd.
Remarkably, based on the number of TUNEL-positive cells and the expression levels of molecules associated with apoptosis pathways in the kidney from the Cd and VE + Cd group, we found that supplementation of VE has an inhibitory effect on Cd-induced apoptosis, which may be related to the regulation of VE on the mitochondrial, endoplasmic reticulum, and death receptor apoptosis pathways. A growing amount of evidence has shown that VE has anti-apoptotic effect on various pathological conditions. For example, in gentamicin-induced nephrotoxicity of rats, VE can exert anti-apoptotic effects by enhancing Bcl-2 and reducing Bax gene expressions [63, 64]. VE can also reduce the number of apoptosis of spermatogonia and primary spermatocytes and thus alleviate testicular toxicity caused by DZN [65]. More importantly, there is evidence showing the protective role of VE against the pro-apoptotic effect of Cd through mitochondrial pathway in rat testes [7]. Previous study has shown that Cd caused oxidative stress through depleting GSH and inhibiting the antioxidant defense system can induce cell apoptosis [28], and our present research showed that the co-administration of VE and Cd could relieve the oxidative stress induced by Cd, which could contribute to the anti-apoptotic effect of VE after Cd exposure. Consequently, there is logical reason to infer that VE, as an antioxidant, is capable of effectively scavenging free radicals and weakening oxidative stress caused by Cd through enhancing antioxidant defense system and inhibiting renal apoptosis. However, the specific anti-apoptotic mechanism of VE needs further research and confirmation.
In summary, supplement VE with 100 mg/kg for 4 weeks could restore body weight, alleviate rat renal damage and oxidative stress induced by Cd (5 mg/kg, intragastric administration), and protect against excessive apoptosis through mitochondrial, endoplasmic reticulum, and death receptor apoptosis pathways. This study may be helpful to better understand the molecular mechanism of VE protection against Cd toxicity.
Data Availability
All data used to support the findings of this study are included within the article.
References
Bhattacharya S (2018) The role of medicinal plants and natural products in melioration of cadmium toxicity. Orient Pharm Exp Med 18(3):177–186. https://doi.org/10.1007/s13596-018-0323-0
Wang XY, Wang ZY, Zhu YS, Zhu SM, Fan RF, Wang L (2018) Alleviation of cadmium-induced oxidative stress by trehalose via inhibiting the Nrf2-Keap1 signaling pathway in primary rat proximal tubular cells. J Biochem Mol Toxicol 32(1):1–6. https://doi.org/10.1002/jbt.22011
Pallio G, Micali A, Benvenga S, Antonelli A, Marini HR, Puzzolo D, Macaione V, Trichilo V, Santoro G, Irrera N, Squadrito F, Altavilla D, Minutoli L (2019) Myo-inositol in the protection from cadmium-induced toxicity in mice kidney: an emerging nutraceutical challenge. Food Chem Toxicol 132:1–9. https://doi.org/10.1016/j.fct.2019.110675
Al Olayan EM, Aloufi AS, AlAmri OD, El-Habit OH, Abdel Moneim AE (2020) Protocatechuic acid mitigates cadmium-induced neurotoxicity in rats: role of oxidative stress, inflammation and apoptosis. Sci Total Environ 723:137969. https://doi.org/10.1016/j.scitotenv.2020.137969
Shi L, Cao H, Luo J, Liu P, Wang T, Hu G, Zhang C (2017) Effects of molybdenum and cadmium on the oxidative damage and kidney apoptosis in duck. Ecotoxicol Environ Saf 145:24–31. https://doi.org/10.1016/j.ecoenv.2017.07.006
Mezynska M, Brzóska MM (2018) Environmental exposure to cadmium—a risk for health of the general population in industrialized countries and preventive strategies. Environ Sci Pollut Res 25(4):3211–3232. https://doi.org/10.1007/s11356-017-0827-z
Amanpour P, Khodarahmi P, Salehipour M (2019) Protective effects of vitamin E on cadmium-induced apoptosis in rat testes. Naunyn Schmiedeberg's Arch Pharmacol 393:349–358. https://doi.org/10.1007/s00210-019-01736-w
Gong ZG, Wang XY, Wang JH, Fan RF, Wang L (2019) Trehalose prevents cadmium-induced hepatotoxicity by blocking Nrf2 pathway, restoring autophagy and inhibiting apoptosis. J Inorg Biochem 192:62–71. https://doi.org/10.1016/j.jinorgbio.2018.12.008
Almeer RS, AlBasher GI, Alarifi S, Alkahtani S, Ali D, Abdel Moneim AE (2019) Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci Rep 9(1):5825. https://doi.org/10.1038/s41598-019-42368-7
Ge J, Zhang C, Sun YC, Zhang Q, Lv MW, Guo K, Li JL (2019) Cadmium exposure triggers mitochondrial dysfunction and oxidative stress in chicken (Gallus gallus) kidney via mitochondrial UPR inhibition and Nrf2-mediated antioxidant defense activation. Sci Total Environ 689:1160–1171. https://doi.org/10.1016/j.scitotenv.2019.06.405
Wang C, Nie G, Yang F, Chen J, Zhuang Y, Dai X, Liao Z, Yang Z, Cao H, Xing C, Hu G, Zhang C (2020) Molybdenum and cadmium co-induce oxidative stress and apoptosis through mitochondria-mediated pathway in duck renal tubular epithelial cells. J Hazard Mater 383:1–10. https://doi.org/10.1016/j.jhazmat.2019.121157
Zhuang J, Nie G, Yang F, Dai X, Cao H, Xing C, Hu G, Zhang C (2019) Cadmium induces cytotoxicity through oxidative stress-mediated apoptosis pathway in duck renal tubular epithelial cells. Toxicol in Vitro 61:1–10. https://doi.org/10.1016/j.tiv.2019.104625
Fan R, Hu PC, Wang Y, Lin HY, Su K, Feng XS, Wei L, Yang F (2018) Betulinic acid protects mice from cadmium chloride-induced toxicity by inhibiting cadmium-induced apoptosis in kidney and liver. Toxicol Lett 299:56–66. https://doi.org/10.1016/j.toxlet.2018.09.003
Nishad P, Mohini (2017) Effect of vitamin E supplementation on heavy metal induced renal toxicity in rat model. Int J Food Sci Nutr 2(3):77–79.
Nair AR, DeGheselle O, Smeets K, Van Kerkhove E, Cuypers A (2013) Cadmium-induced pathologies: where is the oxidative balance lost (or not)? Int J Mol Sci 14(3):6116–6143. https://doi.org/10.3390/ijms14036116
Chen J, Shaikh ZA (2009) Activation of Nrf2 by cadmium and its role in protection against cadmium-induced apoptosis in rat kidney cells. Toxicol Appl Pharmacol 241(1):81–89. https://doi.org/10.1016/j.taap.2009.07.038
Rana SVS (2008) Metals and apoptosis: recent developments. J Trace Elem Med Biol 22(4):262–284. https://doi.org/10.1016/j.jtemb.2008.08.002
Biagioli M, Pifferi S, Ragghianti M, Bucci S, Rizzuto R, Pinton P (2008) Endoplasmic reticulum stress and alteration in calcium homeostasis are involved in cadmium-induced apoptosis. Cell Calcium 43(2):184–195. https://doi.org/10.1016/j.ceca.2007.05.003
Kiran Kumar KM, Naveen Kumar M, Patil RH, Nagesh R, Hegde SM, Kavya K, Babu RL, Ramesh GT, Sharma SC (2016) Cadmium induces oxidative stress and apoptosis in lung epithelial cells. Toxicol Mech Methods 26(9):658–666. https://doi.org/10.1080/15376516.2016.1223240
Eder K, Siebers M, Most E, Scheibe S, Weissmann N, Gessner DK (2017) An excess dietary vitamin E concentration does not influence Nrf2 signaling in the liver of rats fed either soybean oil or salmon oil. Nutr Metab (Lond) 14(1):71. https://doi.org/10.1186/s12986-017-0225-z
Sen Gupta R, Sen Gupta E, Dhakal BK, Thakur AR, Ahnn J (2004) Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol Cell 17(1):132–974. https://doi.org/10.1242/jcs.00946
Ognjanović BI, Marković SD, Pavlović SZ, Zikić RV, Stajn AŠ, Saičić ZS (2006) Combined effects of coenzyme Q10 and vitamin E in cadmium induced alterations of antioxidant defense system in the rat heart. Environ Toxicol Pharmacol 22(2):219–224. https://doi.org/10.1016/j.etap.2006.03.008
Bolkent S, Koyuturk M, Bulan OK, Tunali S, Yanardag R, Tabakoglu AO (2007) The effects of combined alpha-tocopherol, ascorbic acid, and selenium against cadmium toxicity in rat intestine. J Environ Pathol Toxicol Oncol 26(1):21–27. https://doi.org/10.1615/JEnvironPatholToxicolOncol.v26.i1.30
Kara H, Cevik A, Konar V, Dayangac A, Servi K (2008) Effects of selenium with vitamin E and melatonin on cadmium-induced oxidative damage in rat liver and kidneys. Biol Trace Elem Res 125(3):236–244. https://doi.org/10.1007/s12011-008-8168-x
Nemmiche S, Chabane-Sari D, Guiraud P (2007) Role of α-tocopherol in cadmium-induced oxidative stress in Wistar rat's blood, liver and brain. Chem Biol Interact 170(3):221–230. https://doi.org/10.1016/j.cbi.2007.08.004
Weng D, Lu Y, Wei Y, Liu Y, Shen P (2007) The role of ROS in microcystin-LR-induced hepatocyte apoptosis and liver injury in mice. Toxicology 232(1):15–23. https://doi.org/10.1016/j.tox.2006.12.010
El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH (2004) Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and β-carotene. Food Chem Toxicol 42(10):1563–1571. https://doi.org/10.1016/j.fct.2004.05.001
Nazima B, Manoharan V, Miltonprabu S (2015) Grape seed proanthocyanidins ameliorates cadmium-induced renal injury and oxidative stress in experimental rats through the up-regulation of nuclear related factor 2 and antioxidant responsive elements. Biochim Biol Cell 93(3):210–226. https://doi.org/10.1139/bcb-2014-0114
Elkhadragy MF, Al-Olayan EM, Al-Amiery AA, Abdel Moneim AE (2017) Protective effects of Fragaria ananassa extract against cadmium chloride-induced acute renal toxicity in rats. Biol Trace Elem Res 181(2):378–387. https://doi.org/10.1007/s12011-017-1062-7
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1006/abio.1976.9999
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔ C T method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Erboga M, Kanter M, Aktas C, Sener U, Fidanol Erboga Z, Bozdemir Donmez Y, Gurel A (2016) Thymoquinone ameliorates cadmium-induced nephrotoxicity, apoptosis, and oxidative stress in rats is based on its anti-apoptotic and anti-oxidant properties. Biol Trace Elem Res 170(1):165–172. https://doi.org/10.1007/s12011-015-0453-x
Bao RK, Zheng SF, Wang XY (2017) Selenium protects against cadmium-induced kidney apoptosis in chickens by activating the PI3K/AKT/Bcl-2 signaling pathway. Environ Sci Pollut Res 41:1–12. https://doi.org/10.1007/s11356-017-9422-6
Sanjeev S, Bidanchi RM, Murthy MK, Gurusubramanian G, Roy VK (2019) Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats. Environ Sci Pollut Res 26(1):20631–20653. https://doi.org/10.1007/s11356-019-05420-7
Wongmekiat O, Peerapanyasut W, Kobroob A (2018) Catechin supplementation prevents kidney damage in rats repeatedly exposed to cadmium through mitochondrial protection. Naunyn Schmiedeberg's Arch Pharmacol 391(4):385–394. https://doi.org/10.1007/s00210-018-1468-6
Rana MN, Tangpong J, Rahman MM (2018) Toxicodynamics of lead, cadmium, mercury and arsenic- induced kidney toxicity and treatment strategy: a mini review. Toxicol Rep 5:704–713. https://doi.org/10.1016/j.toxrep.2018.05.012
Rinaldi M, Micali A, Marini H, Adamo EB, Puzzolo D, Pisani A, Trichilo V, Altavilla D, Squadrito F, Minutoli L (2017) Cadmium, organ toxicity and therapeutic approaches. A review on brain, kidney and testis damage. Curr Med Chem 24(35):3879–3893. https://doi.org/10.2174/0929867324666170801101448
Rani A, Kumar A, Lal A, Pant M (2014) Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res 24(4):378–399. https://doi.org/10.1080/09603123.2013.835032
Sabolić I, Breljak D, Skarica M, Herak-Kramberger CM (2010) Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals 23(5):897–926. https://doi.org/10.1016/S0378-1127(01)00623-5
Prabu SM, Shagirtha K, Renugadevi J (2011) Reno-protective effect of naringenin in combination with vitamins C and E on cadmium induced oxidative nephrotoxicity in rats. J Pharm Res 325(6):1921–1926
El-Boshy ME, Risha EF, Abdelhamid FM, Mubarak MS, Hadda TB (2015) Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepatorenal damage in rats. J Trace Elem Med Biol 29:104–110. https://doi.org/10.1016/j.jtemb.2014.05.009
Adi PJ, Burra SP, Vataparti AR, Matcha B (2016) Calcium, zinc and vitamin E ameliorate cadmium-induced renal oxidative damage in albino Wistar rats. Toxicol Rep 3:591–597. https://doi.org/10.1016/j.toxrep.2016.07.005
Ognjanović BI, Marković SD, Pavlović SZ, Zikić RV, Stajn AS, Saicić ZS (2008) Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: protective effect of selenium. Physiol Res 57(3):403–411. https://doi.org/10.1088/0967-3334/29/1/010
Evcimen M, Aslan R, Gulay MS (2018) Protective effects of polydatin and grape seed extract in rats exposed to cadmium. Drug Chem Toxicol 2:1–9. https://doi.org/10.1080/01480545.2018.1480629
Jihen EH, Imed M, Fatima H, Abdelhamid K (2008) Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver and kidney of the rat: histology and Cd accumulation. Food Chem Toxicol 46(11):3522–3527. https://doi.org/10.1016/j.fct.2008.08.037
Tripathi S, Srivastav AK (2011) Cytoarchitectural alterations in kidney of Wistar rat after oral exposure to cadmium chloride. Tissue Cell 43(2):131–136. https://doi.org/10.1016/j.tice.2011.01.001
Fujiwara Y, Lee J, Tokumoto M, Satoh M (2012) Cadmium renal toxicity via apoptotic pathways. Biol Pharm Bull 35(11):1892–1897. https://doi.org/10.1248/bpb.b212014
Loo DT (2011) In situ detection of apoptosis by the TUNEL assay: an overview of techniques. Methods Mol Biol 682:3–13. https://doi.org/10.1007/978-1-60327-409-8_1
Aktoz T, Kanter M, Uz YH, Aktaş C, Erboğa M, Atakan İH (2011) Protective effect of quercetin against renal toxicity induced by cadmium in rats. Balkan Med J 29(1):56–61. https://doi.org/10.5152/balkanmedj.2011.014
Aoyagi T, Hayakawa K, Miyaji K, Ishikawa H, Hata M (2003) Cadmium nephrotoxicity and evacuation from the body in a rat modeled subchronic intoxication. Int J Urol 10(6):332–338. https://doi.org/10.1046/j.1442-2042.2003.00627.x
Wang Y, Wu Y, Luo K, Liu Y, Zhou M, Yan S, Shi H, Cai Y (2013) The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem Toxicol 58(6):61–67. https://doi.org/10.1016/j.fct.2013.04.013
Shao CC, Li N, Zhang ZW, Su J, Li S, Li JL, Xu SW (2014) Cadmium supplement triggers endoplasmic reticulum stress response and cytotoxicity in primary chicken hepatocytes. Ecotoxicol Environ Saf 106:109–114. https://doi.org/10.1016/j.ecoenv.2014.04.033
Liu G, Yuan Y, Long M, Luo T, Bian J, Liu X, Gu J, Zou H, Song R, Wang Y, Wang L, Liu Z (2017) Beclin-1-mediated autophagy protects against cadmium-activated apoptosis via the Fas/FasL pathway in primary rat proximal tubular cell culture. Sci Rep 7(1):977. https://doi.org/10.1038/s41598-017-00997-w
Slee EA, Adrain C, Martin SJ (2001) Executioner caspase-3, −6, and −7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276(10):7320–7326. https://doi.org/10.1074/jbc.M008363200
Jing G, Wang JJ, Zhang SX (2012) ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res 2012:589589–589511. https://doi.org/10.1155/2012/589589
Palai TK, Mishra SR (2014) Caspases: an apoptosis mediator. J Adv Vet Anim Res 2(1):18–22. https://doi.org/10.5455/javar.2015.b52
Yokouchi M, Hiramatsu N, Hayakawa K, Kasai A, Takano Y, Yao J, Kitamura M (2007) Atypical, bidirectional regulation of cadmium-induced apoptosis via distinct signaling of unfolded protein response. Cell Death Differ 14(8):1467–1474. https://doi.org/10.1038/sj.cdd.4402154
Böhm V (2018) Vitamin E. Antioxidants 7(3):44. https://doi.org/10.3390/antiox7030044
Kim MJ, Hong JH, Rhee SJ (2003) Effect of vitamin E on cadmium accumulation and excretion in chronic cadmium poisoned rats. Korean J Nutr 36(7):691–698
Khalil MH, Helal AF, Abd elghfar AM (2017) Counteracting effect of selenium and vitamin E to cadmium toxicity in rats. Middle East J Appl Sci 7(4):681–702
Rambeck WA, Kollmer WE (2010) Modifying cadmium retention in chickens by dietary supplements. J Anim Physiol Anim Nutr 63(1–5):66–74. https://doi.org/10.1111/j.1439-0396.1990.tb00118.x
Tandon SK, Singh S, Dhawan M (1992) Preventive effect of vitamin E in cadmium intoxication. Biomed Environ Sci 5(1):39–45
Kandeil MAM, Hassanin KMAA, Mohammed ET, Safwat GM, Mohamed DS (2018) Pumpkin and vitamin E as potent modulators of apoptosis in gentamicin-induced rat nephrotoxicity. Asian J Biochem 13(1):1–8. https://doi.org/10.3923/ajb.2018.1.8
Kandeil MAM, Hassanin KMA, Mohammed ET, Safwat GM, Mohamed DS (2018) Wheat germ and vitamin E decrease BAX/BCL-2 ratio in rat kidney treated with gentamicin. Beni-Suef Univ J Basic Appl Sci 7(3):257–262. https://doi.org/10.1016/j.bjbas.2018.02.001
Rahimi Anbarkeh F, Nikravesh MR, Jalali M, Sadeghnia HR, Sargazi Z (2019) The effect of diazinon on cell proliferation and apoptosis in testicular tissue of rats and the protective effect of vitamin E. Int J Fertil Steril 13(2):154–160. https://doi.org/10.22074/ijfs.2019.5612
Acknowledgements
This work was supported by the National Key Research and Development Project (2018YFD0501800), the Sichuan Science and Technology Program (2018NZ0002, 2019YFQ0012), and the Sichuan beef cattle innovation team of National Modern Agricultural Industry Technology System (SCCXTD-2020-13).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, J., Xie, S., Chen, Z. et al. Protective Effect of Vitamin E on Cadmium-Induced Renal Oxidative Damage and Apoptosis in Rats. Biol Trace Elem Res 199, 4675–4687 (2021). https://doi.org/10.1007/s12011-021-02606-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02606-4